Mathematical Modelling of Drying of Hydrogels via Finite Element Method and Texture Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Starch–Alginate Suspensions and Ionic Gelling Material
2.3. Convective Drying of Hydrogels
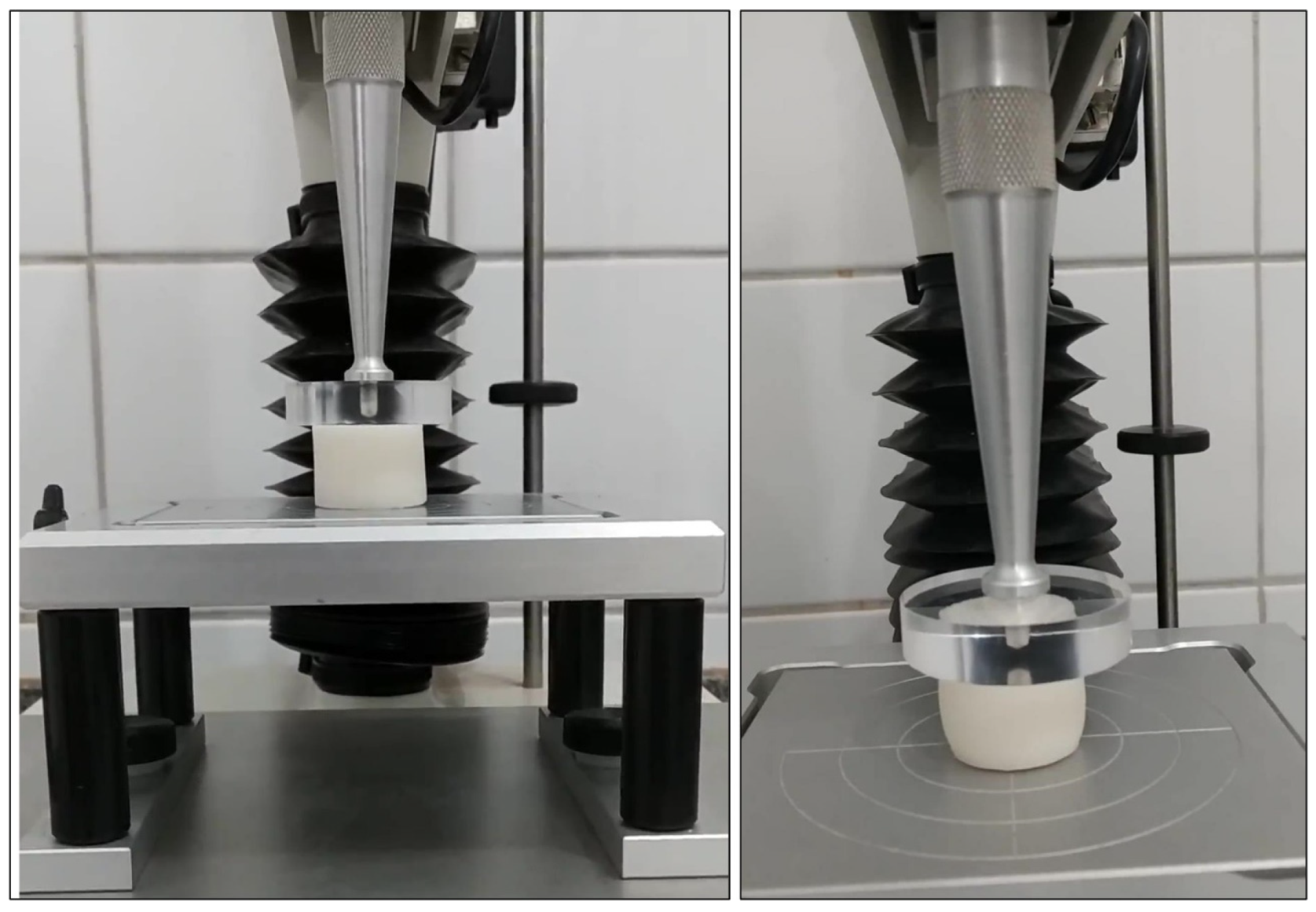
2.4. Texture Analysis
2.5. Evaluation of Mechanical Coefficients
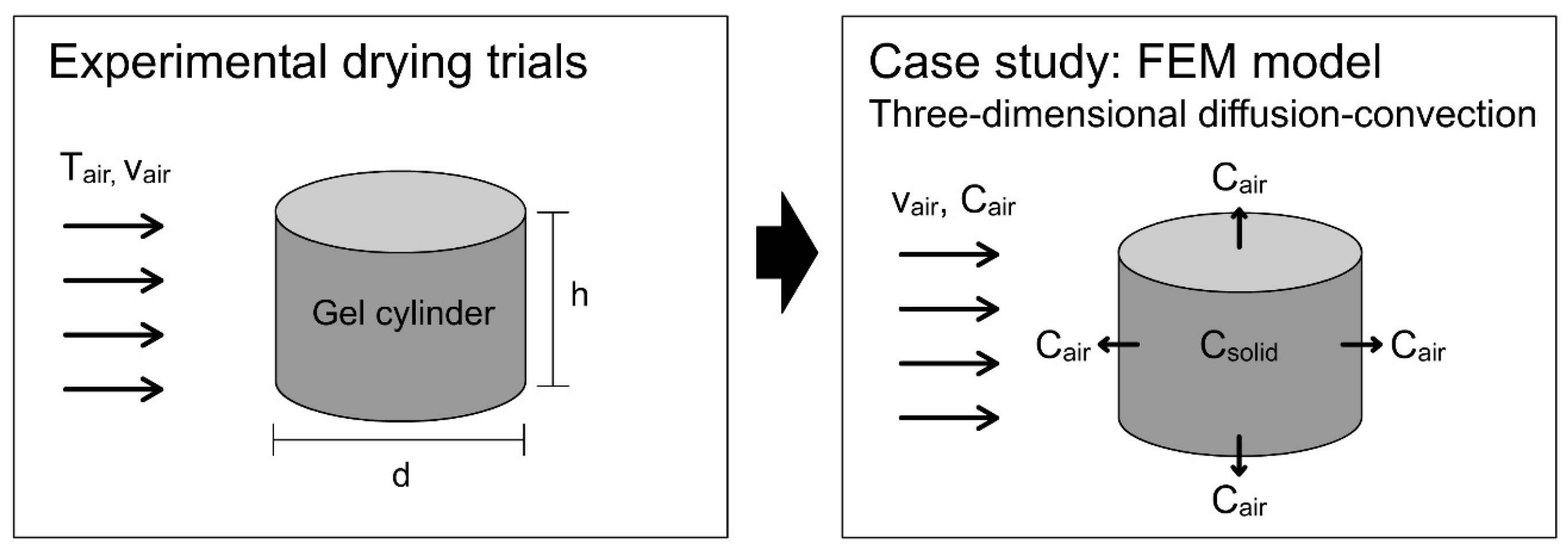
2.6. Mathematical Modelling of Convective Drying via FEM
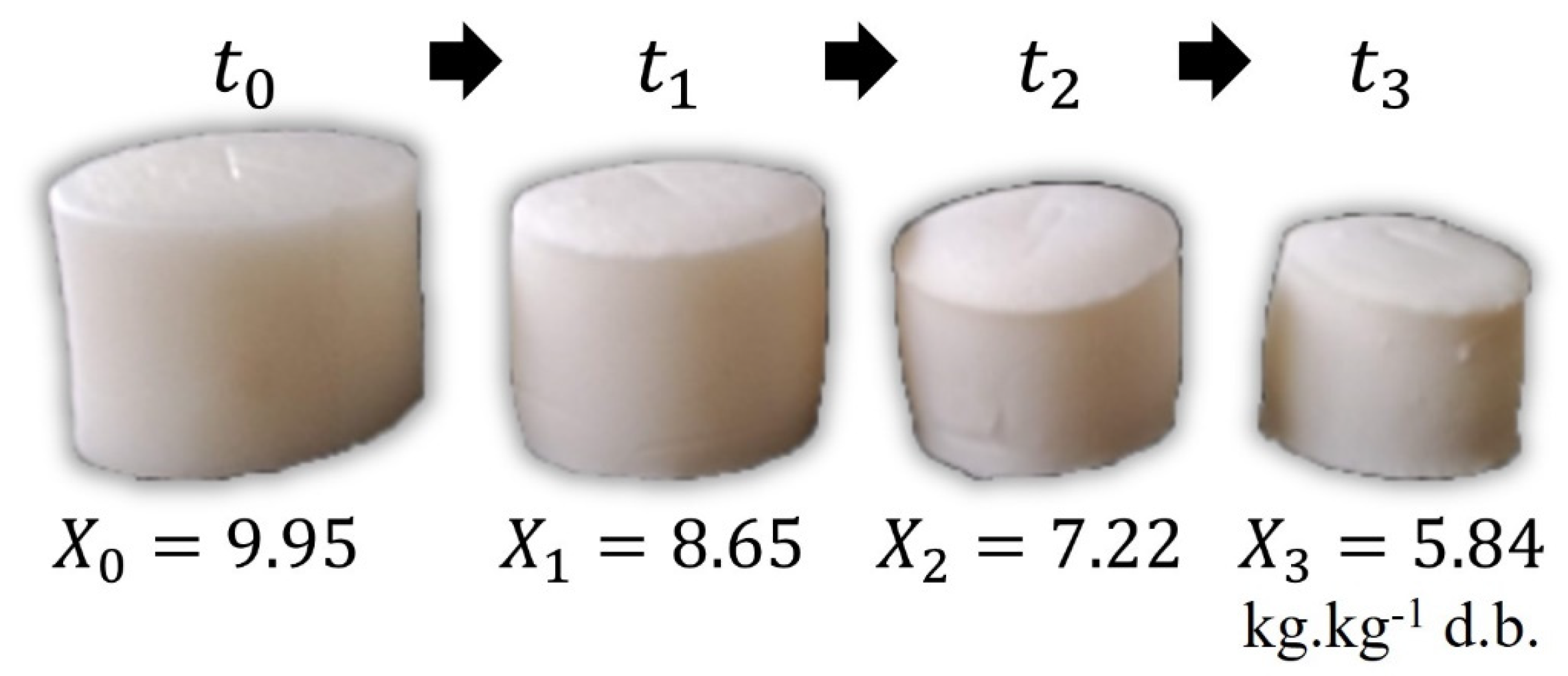
2.7. Evaluation of Shrinkage of Hydrogels during Drying
2.8. Statistical Measurements
3. Results and Discussion
3.1. Drying Experiments on Cornstarch–Alginate Hydrogels
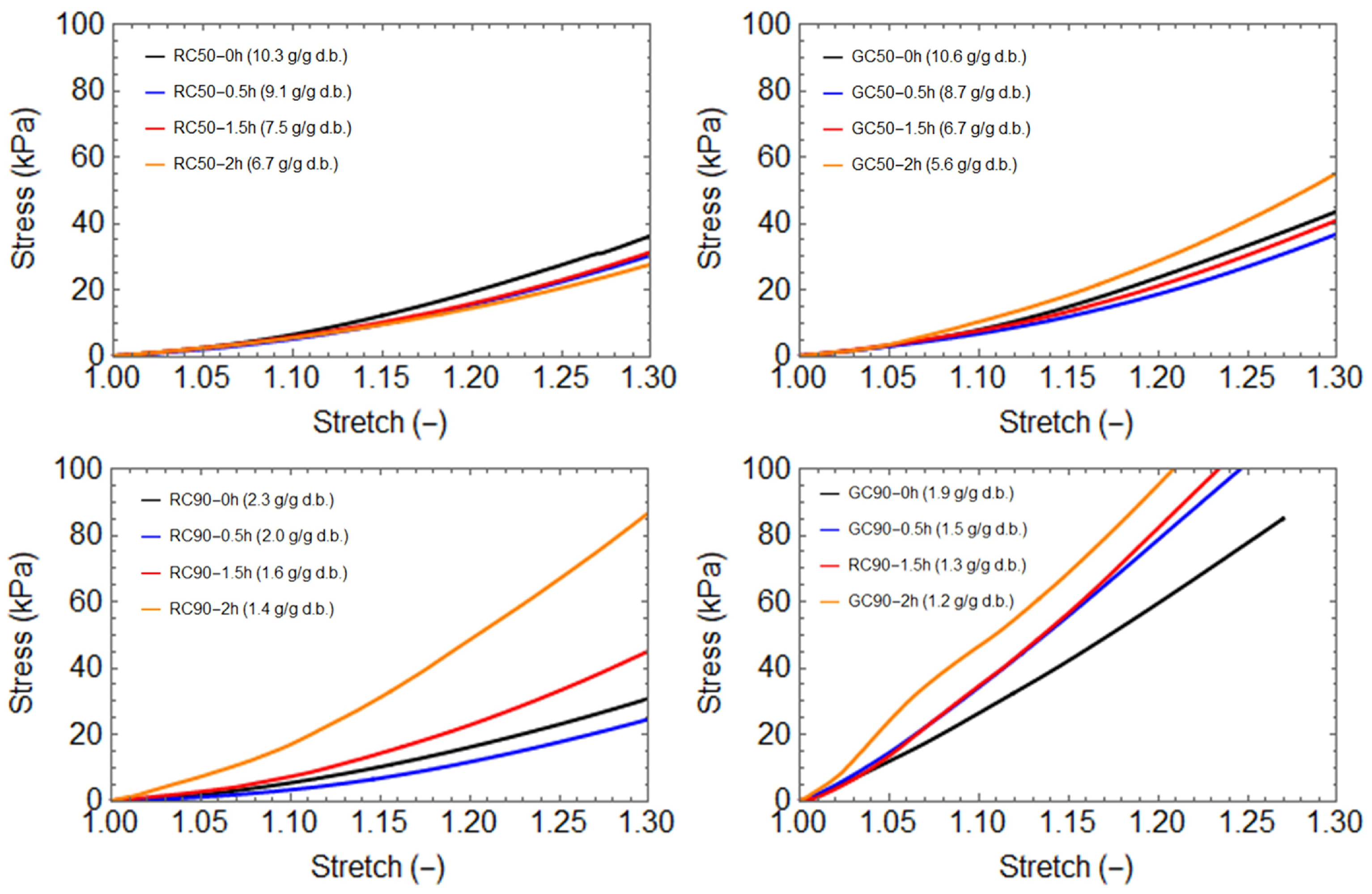
3.2. Texture Analysis
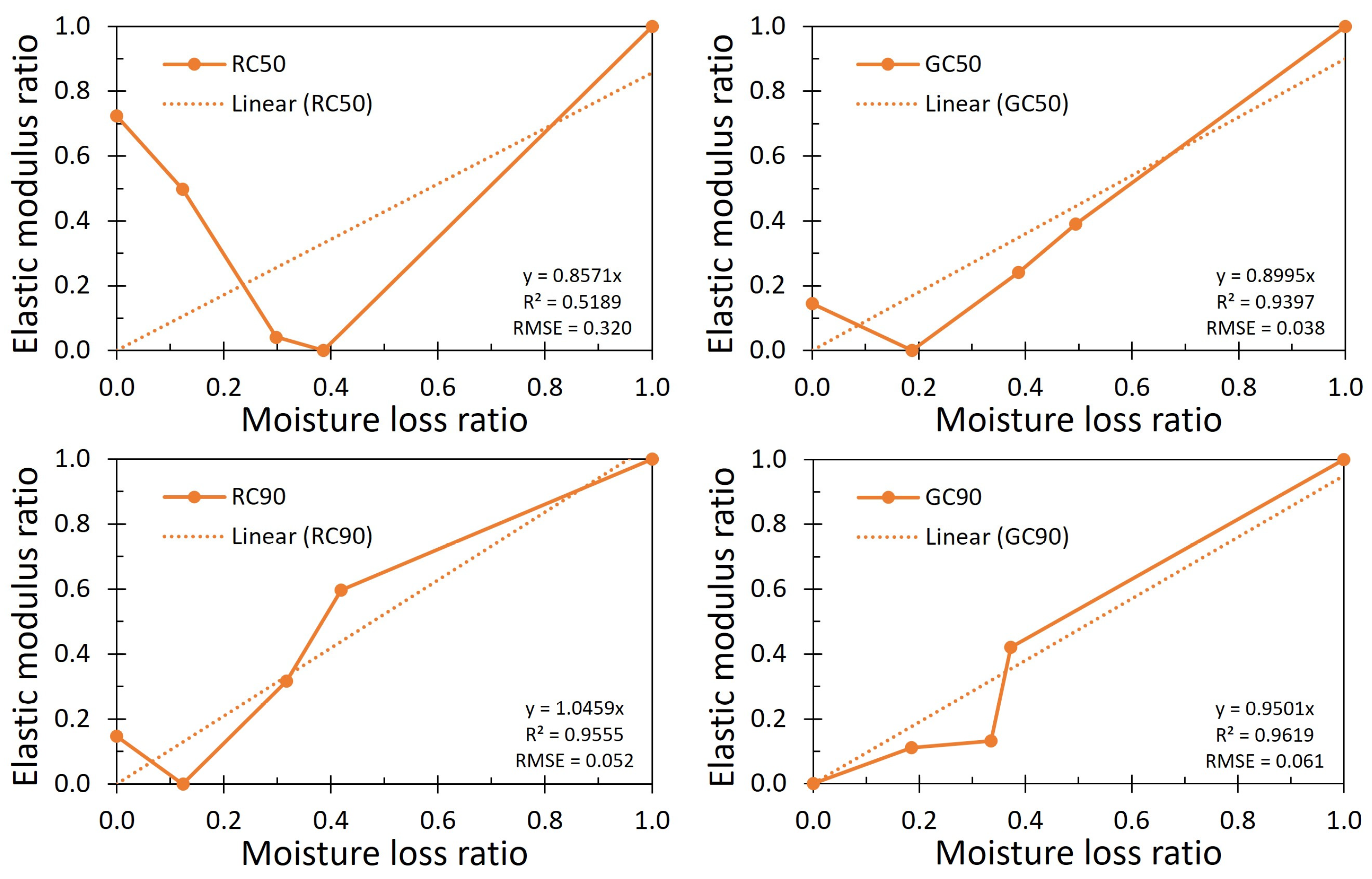
3.3. Evaluation of the Stiffening Coefficient (β−1)
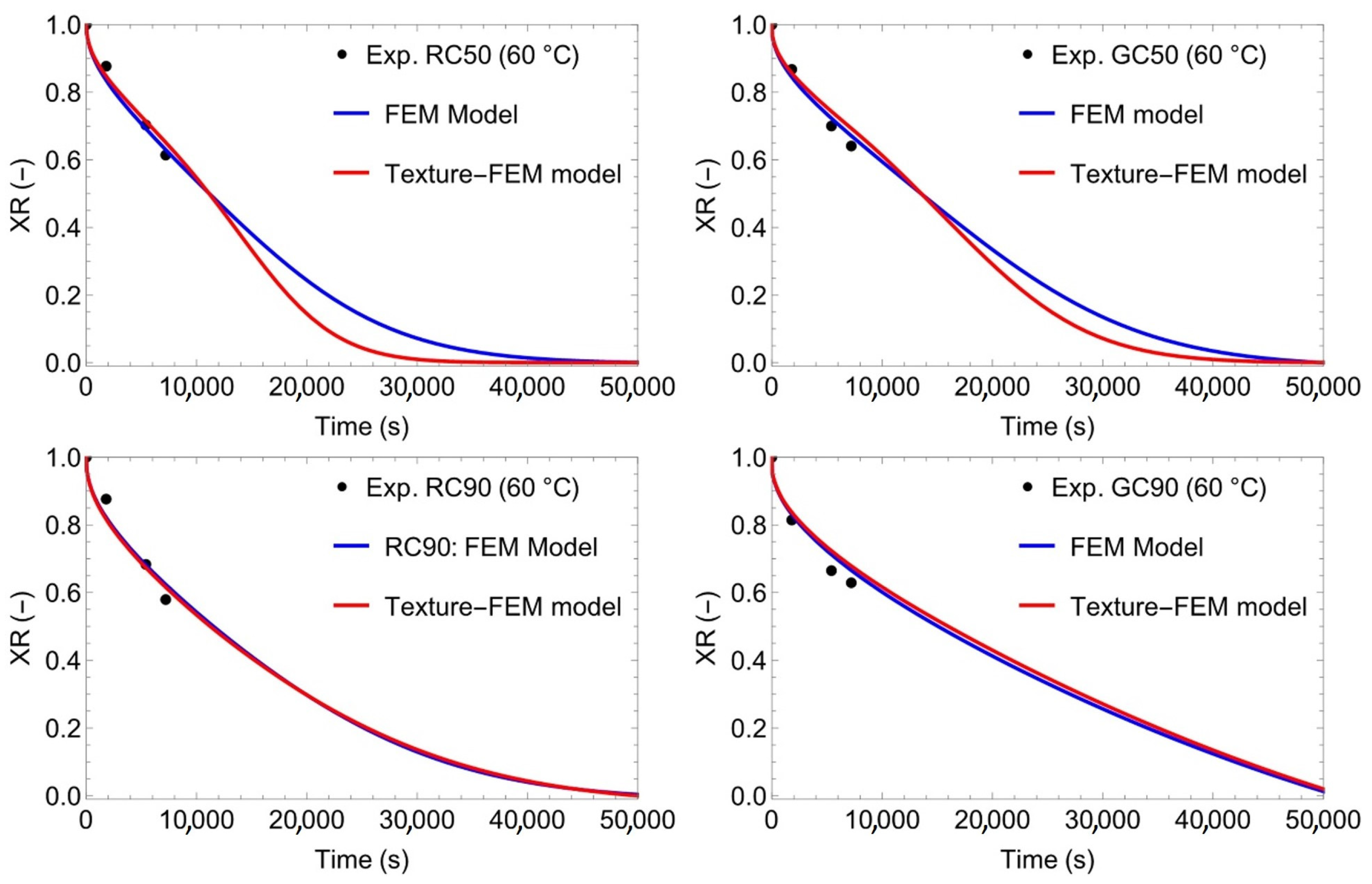
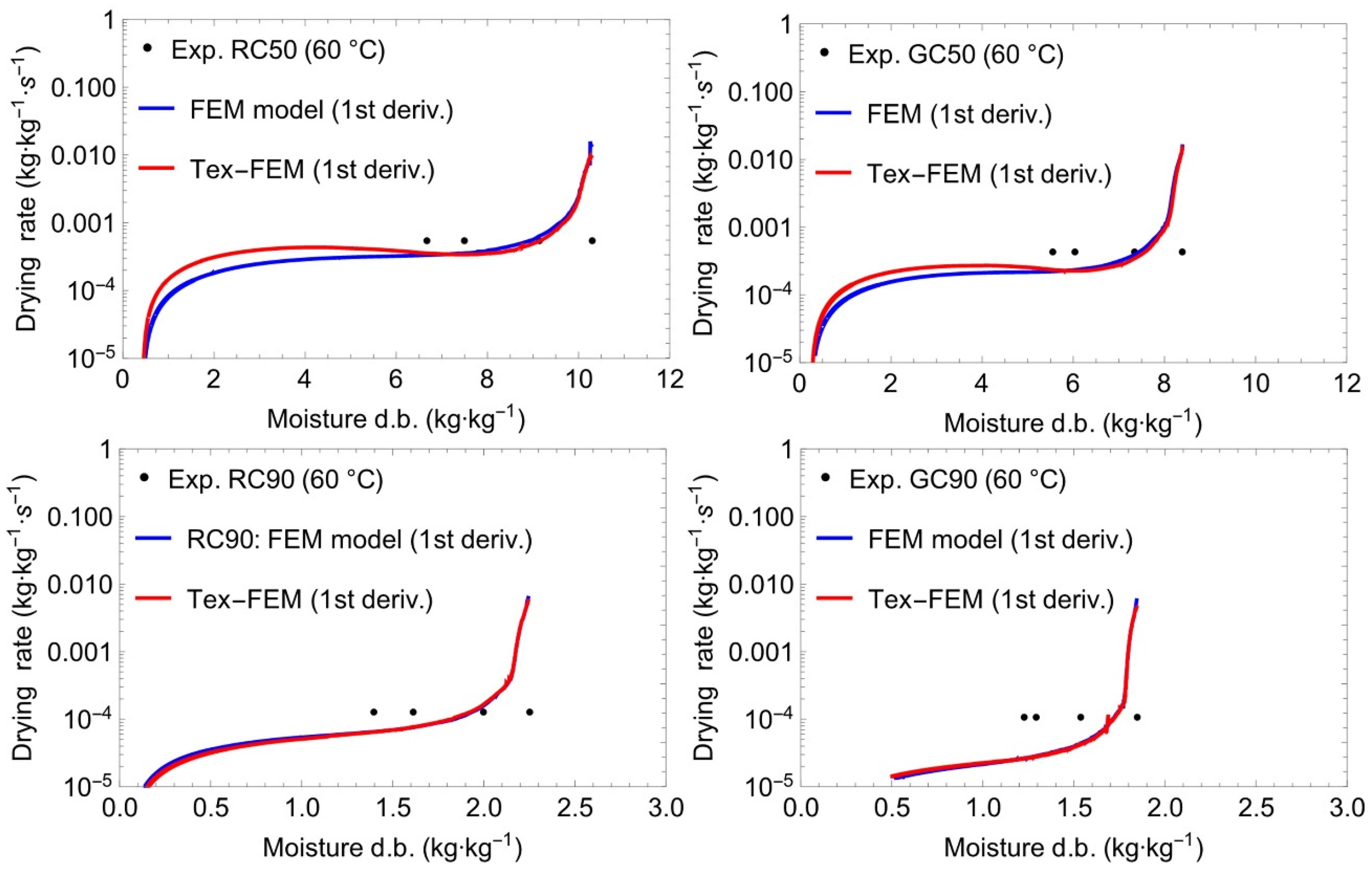
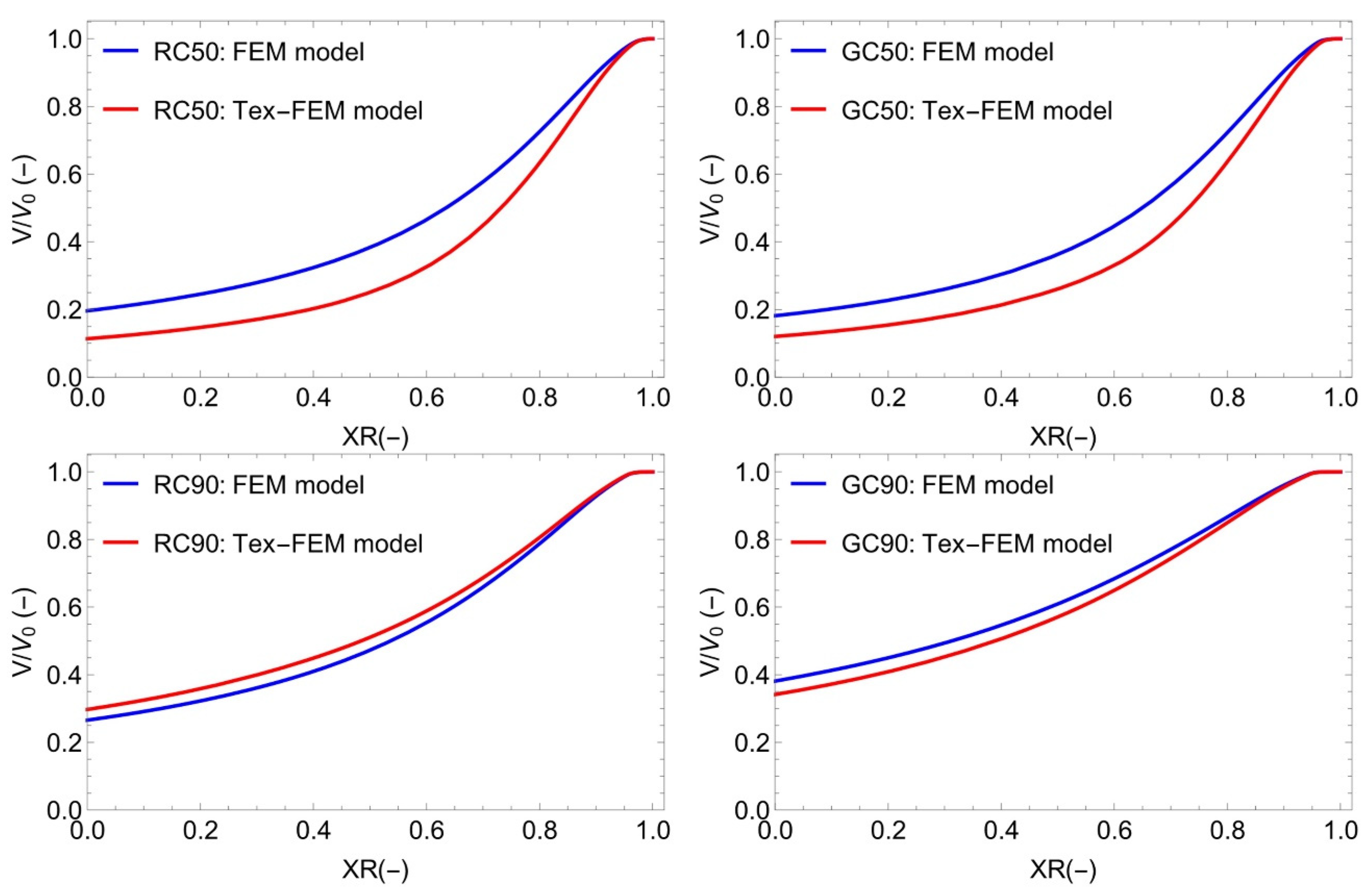
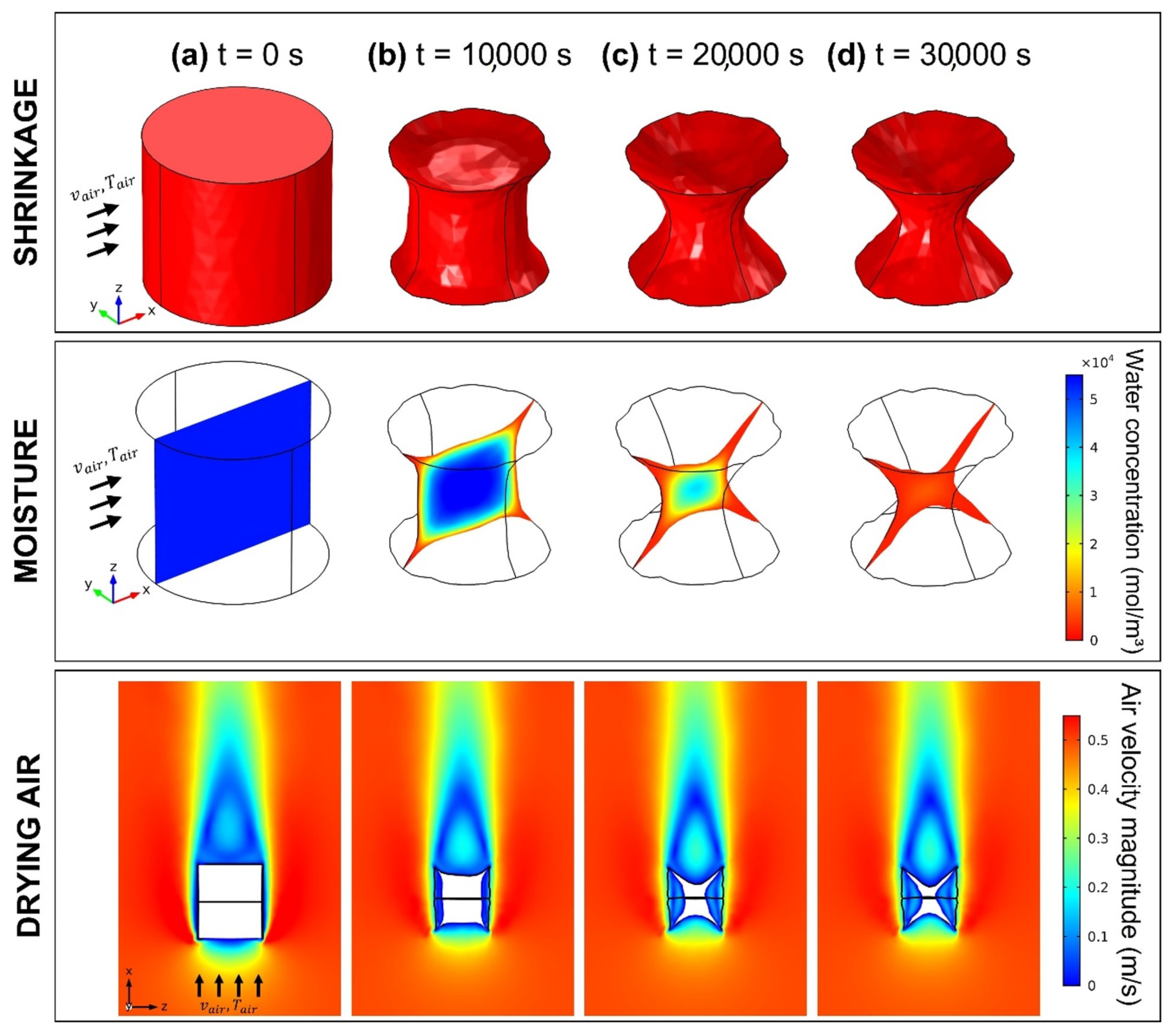
3.4. Modelling and Simulation of Convective Drying of Cornstarch–Alginate Hydrogels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | Description |
| Area (m2) | |
| Water concentration (mol∙m−3) | |
| Sample diameter (m) | |
| Diffusivity of water in air domain (m2∙s−1) | |
| Effective diffusivity of water in solid domain (m2∙s−1) | |
| Elasticity modulus or Young’s modulus (Pa) | |
| Applied force during uniaxial compression (N) | |
| Sample height during uniaxial compression (m) | |
| Initial sample height (m) | |
| Total normal molar flux (mol∙m−2∙s−1) | |
| Partition coefficient (dimensionless) | |
| Water molar mass (kg∙mol−1) | |
| Mass (kg) | |
| Pressure (Pa) | |
| Universal gas constant (J∙mol−1∙K−1) | |
| Air temperature (°C or K) | |
| Time (s) | |
| Energy required to compress the gels (J) | |
| Volume (m3) | |
| Shrinkage (dimensionless) | |
| Air velocity (m∙s−1) | |
| Normal velocity of moving boundary (m∙s−1) | |
| Moisture content in wet basis (kg∙kg−1) | |
| Moisture content in dry basis (kg∙kg−1) | |
| Moisture ratio (dimensionless) | |
| Ogden constant (dimensionless) | |
| Stiffening coefficient (dimensionless) | |
| Strain (m∙m−1) | |
| Stretch ratio (dimensionless) | |
| Initial shear modulus (Pa) | |
| Density (kg∙m−3) | |
| Stress (Pa) | |
| GC50 | Dried samples containing 50% of gelatinized cornstarch |
| GC90 | Dried samples containing 90% of gelatinized cornstarch |
| RC50 | Dried samples containing 50% of native cornstarch |
| RC90 | Dried samples containing 90% of native cornstarch |
References
- Ratner, B.D. The Biocompatibility of Implant Materials. In Biocompatibility of Clinical Implant Materials; Williams, D.F., Ed.; CRC Press: Boca Raton, FL, USA, 1981; p. 272. ISBN 0849366259. [Google Scholar]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. PH-Sensitive Freeze-Dried Chitosan-Polyvinyl Pyrrolidone Hydrogels as Controlled Release System for Antibiotic Delivery. J. Control Release 2000, 68, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Picone, C.S.F.; Cunha, R.L. Influence of PH on Formation and Properties of Gellan Gels. Carbohydr. Polym. 2011, 84, 662–668. [Google Scholar] [CrossRef]
- Thu, B.; Bruheim, P.; Espevik, T.; Smidsrød, O.; Soon-Shiong, P.; Skjåk-Bræk, G. Alginate Polycation Microcapsules: I. Interaction between Alginate and Polycation. Biomaterials 1996, 17, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological Interactions between Polysaccharides and Divalent Cations: The Egg-Box Model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Microencapsulation of Betacyanin from Dragon Fruit Peel by Complex Coacervation: Physicochemical Characteristics, Thermal Stability, and Release Profile of Microcapsules. Food Biosci. 2022, 49, 1011882. [Google Scholar] [CrossRef]
- Toprakçı, İ.; Şahin, S. Encapsulation of Olive Leaf Antioxidants in Microbeads: Application of Alginate and Chitosan as Wall Materials. Sustain. Chem. Pharm. 2022, 27, 100707. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsøyen, E. Alginate as a Source of Dietary Fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef]
- Paxman, J.R.; Richardson, J.C.; Dettmar, P.W.; Corfe, B.M. Daily Ingestion of Alginate Reduces Energy Intake in Free-Living Subjects. Appetite 2008, 51, 713–719. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Sousa Mendes, D.; Asquieri, E.R.; Batista, R.D.; de Morais, C.C.; Ramirez Ascheri, D.P.; de Macêdo, I.Y.L.; de Souza Gil, E. Microencapsulation of Jabuticaba Extracts (Myrciaria Cauliflora): Evaluation of Their Bioactive and Thermal Properties in Cassava Starch Biscuits. LWT 2021, 137, 110460. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Altintas, Z.; Sharma, S. Effect of Alginate-Microencapsulated Hydrogels on the Survival of Lactobacillus Rhamnosus under Simulated Gastrointestinal Conditions. Foods 2021, 10, 1999. [Google Scholar] [CrossRef]
- Olayemi, O.J.; Apeji, Y.E.; Isimi, C.Y. Formulation and Evaluation of Cyperus Esculentus (Tiger Nut) Starch-Alginate Microbeads in the Oral Delivery of Ibuprofen. J. Pharm. Innov. 2022, 17, 366–375. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of Various Polysaccharides (Alginate, Carrageenan, Gums, Chitosan) and Their Combination with Prebiotic Saccharides (Resistant Starch, Lactosucrose, Lactulose) on the Encapsulation of Probiotic Bacteria Lactobacillus Casei 01 Strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Okunlola, A.; Adewusi, S.A. Development of Theophylline Microbeads Using Pregelatinized Breadfruit Starch (Artocarpus Altilis) as a Novel Co-Polymer for Controlled Release. Adv. Pharm. Bull. 2019, 9, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Feltre, G.; Sartori, T.; Silva, K.F.C.; Dacanal, G.C.; Menegalli, F.C.; Hubinger, M.D. Encapsulation of Wheat Germ Oil in Alginate-gelatinized Corn Starch Beads: Physicochemical Properties and Tocopherols’ Stability. J. Food Sci. 2020, 85, 2124–2133. [Google Scholar] [CrossRef]
- Nicolin, D.J.; Defendi, R.O.; Rossoni, D.F.; de Matos Jorge, L.M. Mathematical Modeling of Soybean Drying by a Fractional-Order Kinetic Model. J. Food Process. Eng. 2018, 41, e12655. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Simulation of Food Drying Processes by Computational Fluid Dynamics (CFD); Recent Advances and Approaches. Trends Food Sci. Technol. 2018, 78, 206–223. [Google Scholar] [CrossRef]
- Bastiani, F.H.; Sufredini, S.; Romio, A.P.; Nicolin, D.J. Modeling the Drying Kinetics of Alginate Particles Considering Shrinkage. J. Food Process. Eng. 2021, 44, e13760. [Google Scholar] [CrossRef]
- Achchaq, F.; Jomaa, W.; Godin, A.; Bontemps, S.; Puiggali, J.R. Temperature and Moisture Effects on the Failure Mode of Highly Shrinkable Raw Catalyst Supports. Procedia Struct. Integr. 2016, 2, 2283–2290. [Google Scholar] [CrossRef]
- Mayor, L.; Sereno, A.M. Modelling Shrinkage during Convective Drying of Food Materials: A Review. J. Food Eng. 2004, 61, 373–386. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Khalloufi, S.; Mondor, M.; Ratti, C. Shrinkage and Porosity Evolution during Air-Drying of Non-Cellular Food Systems: Experimental Data versus Mathematical Modelling. Food Res. Int. 2017, 103, 215–225. [Google Scholar] [CrossRef]
- Ratti, C. Shrinkage during Drying of Foodstuffs. J. Food Eng. 1994, 23, 91–105. [Google Scholar] [CrossRef]
- Silva Júnior, M.A.V.; Rabi, J.A.; Ribeiro, R.; Dacanal, G.C. Modeling of Convective Drying of Cornstarch-Alginate Gel Slabs. J. Food Eng. 2019, 250, 9–17. [Google Scholar] [CrossRef]
- Silva Júnior, M.A.V.; Leite, M.A.; Dacanal, G.C. Modelling of Convective Drying of Potatoes Polyhedrons. Int. J. Food Eng. 2023, 19, 605–617. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Nielsen, S.S. Moisture Content Determination BT—Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 105–115. ISBN 978-3-319-44127-6. [Google Scholar]
- Yu, J.; Santos, P.H.S.; Campanella, O.H. A Study to Characterize the Mechanical Behavior of Semisolid Viscoelastic Systems under Compression Chewing—Case Study of Agar Gel. J. Texture Stud. 2012, 43, 459–467. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Charalambides, M.N.; Williams, J.G. Determination of Large Deformation and Fracture Behaviour of Starch Gels from Conventional and Wire Cutting Experiments. J. Mater. Sci. 2009, 44, 4976–4986. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion Mass Transfer in Fluid Systems, 3rd ed.; Cambridge University Press: Cambrigde, UK, 2009; ISBN 9780521871211. [Google Scholar]
- Ajani, C.; Kumar, A.; Curcio, S.; Tekasakul, P. Parametric Study and Shrinkage Modelling of Natural Rubber Sheet Drying Using COMSOL Multiphysics. IOP Conf. Ser. Mater. Sci. Eng. 2017, 243, 012012. [Google Scholar] [CrossRef]
- Sun, M.L.; Wu, D.; Wang, M.Q.; Jin, S.F.; Wang, K.Z. Simulation Analysis of Fluid-Structure Interactions with Moving Mesh. Adv. Mater. Res. 2011, 305, 235–238. [Google Scholar] [CrossRef]
- Thussu, S.; Datta, A.K. Texture Prediction during Deep Frying: A Mechanistic Approach. J. Food Eng. 2012, 108, 111–121. [Google Scholar] [CrossRef]
- Nafo, W.; Al-Mayah, A. Characterization of PVA Hydrogels’ Hyperelastic Properties by Uniaxial Tension and Cavity Expansion Tests. Int. J. Non-Linear Mech. 2020, 124, 103515. [Google Scholar] [CrossRef]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical Behaviour of Alginate-Gelatin Hydrogels for 3D Bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]

| Sample | RC50 | RC90 | GC50 | GC90 |
|---|---|---|---|---|
| Corn starch (g/100 g d.b.) | 50 | 90 | 50 | 90 |
| Corn starch (g/100 g w.b.) | 5.2 | 33.2 | 5.2 | 33.2 |
| Water (g/100 g w.b.) | 86.6 | 61.1 | 86.6 | 61.1 |
| Sodium alginate (g/100 g w.b.) | 4.6 | 3.2 | 4.6 | 3.2 |
| Ethanol (g/100 g w.b.) | 3.6 | 2.5 | 3.6 | 2.5 |
| 1 Heating temperature (°C) | n.a. | n.a. | 80 | 80 |
| Sample | RC50 | GC50 | RC90 | GC90 |
|---|---|---|---|---|
| , Diameter (cm) | 2.60 ± 0.02 | 2.45 ± 0.04 | 2.56 ± 0.02 | 2.65 ± 0.03 |
| , Height (cm) | 2.26 ± 0.08 | 2.17 ± 0.06 | 2.08 ± 0.13 | 2.49 ± 0.19 |
| , Initial moisture (-) | 10.29 ± 0.06 | 8.41 ± 0.06 | 2.25 ± 0.03 | 1.85 ± 0.03 |
| , Final moisture (-) | 0.93 ± 0.05 | 0.48 ± 0.08 | 0.22 ± 0.04 | 0.18 ± 0.01 |
| , Solid density of wet hydrogel (g⋅cm−3) | 1055.3 ± 3.6 | 1061.2 ± 9.3 | 1186.7 ± 46.4 | 1160.7 ± 23.9 |
| , Solid density of dry hydrogel (g⋅cm−3) | 1849.4 ± 94.9 | 1928.6 ± 91.7 | 1657.5 ± 24.7 | 1826.9 ± 81.1 |
| m2⋅s−1) | 5.46 | 3.78 | 4.42 | 2.38 |
| Sample | Drying Time (h) | (kg∙kg−1 w.b.) | (kg∙kg−1 d.b.) | (Pa) | (-) | (Pa) | (103 J) |
|---|---|---|---|---|---|---|---|
| GC50 | 0 | 0.8937 ± 0.0007 | 8.41 ± 0.06 | 24,798.6 ± 66.1 | 7.61 ± 0.42 | 74,395.8 | 36.6 |
| GC50 | 0.5 | 0.8803 ± 0.0024 | 7.36 ± 0.16 | 21,104.9 ± 286.0 | 7.62 ± 0.05 | 63,314.7 | 28.7 |
| GC50 | 1.5 | 0.8576 ± 0.0050 | 6.03 ± 0.25 | 27,227.4 ± 1006.0 | 6.73 ± 0.89 | 81,682.1 | 34.5 |
| GC50 | 2.0 | 0.8472 ± 0.0095 | 5.56 ± 0.40 | 31,032.2 ± 2384.0 | 6.17 ± 0.33 | 93,096.6 | 39.3 |
| RC50 | 0 | 0.9114 ± 0.0005 | 10.29 ± 0.06 | 24,605.1 ± 4.3 | 7.84 ± 0.18 | 73,815.2 | 41.6 |
| RC50 | 0.5 | 0.9014 ± 0.0005 | 9.14 ± 0.05 | 22,572.3 ± 2377.0 | 6.55 ± 0.34 | 67,716.8 | 33.5 |
| RC50 | 1.5 | 0.8813 ± 0.0142 | 7.51 ± 1.03 | 18,443.0 ± 335.9 | 6.97 ± 0.10 | 55,329.1 | 33.2 |
| RC50 | 2.0 | 0.8696 ± 0.0038 | 6.68 ± 0.22 | 18,069.4 ± 1463.0 | 6.24 ± 0.92 | 54,028.2 | 32.6 |
| GC90 | 0 | 0.6497 ± 0.0039 | 1.85 ± 0.03 | 93,982.3 ± 2721.0 | 3.82 ± 0.07 | 281,946.8 | 111.4 |
| GC90 | 0.5 | 0.6070 ± 0.0038 | 1.54 ± 0.02 | 108,094 ± 4876.0 | 4.41 ± 0.05 | 324,282.0 | 125.8 |
| GC90 | 1.5 | 0.5640 ± 0.0053 | 1.29 ± 0.03 | 110,686 ± 4391.0 | 4.69 ± 0.90 | 332,059.5 | 134.5 |
| GC90 | 2.0 | 0.5514 ± 0.0128 | 1.23 ± 0.06 | 147,374 ± 4249.0 | 3.13 ± 0.80 | 442,124.0 | 167.0 |
| RC90 | 0 | 0.6921 ± 0.0033 | 2.25 ± 0.03 | 17,327.8 ± 4945.0 | 8.38 ± 0.68 | 51,983.4 | 30.9 |
| RC90 | 0.5 | 0.6664 ± 0.0017 | 2.00 ± 0.02 | 10,182.7 ± 1362.0 | 8.78 ± 0.15 | 30,548.1 | 21.8 |
| RC90 | 1.5 | 0.6162 ± 0.0062 | 1.61 ± 0.04 | 25,589.3 ± 548.3 | 7.15 ± 0.61 | 76,767.9 | 50.1 |
| RC90 | 2.0 | 0.5830 ± 0.0096 | 1.40 ± 0.05 | 39,295 ± 20,990.0 | 7.11 ± 1.92 | 117,885.8 | 67.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.A.V., Júnior; Feltre, G.; Dacanal, G.C. Mathematical Modelling of Drying of Hydrogels via Finite Element Method and Texture Analysis. Processes 2024, 12, 1564. https://doi.org/10.3390/pr12081564
da Silva MAV Júnior, Feltre G, Dacanal GC. Mathematical Modelling of Drying of Hydrogels via Finite Element Method and Texture Analysis. Processes. 2024; 12(8):1564. https://doi.org/10.3390/pr12081564
Chicago/Turabian Styleda Silva, Marco Antônio Vasiliev, Júnior, Gabriela Feltre, and Gustavo Cesar Dacanal. 2024. "Mathematical Modelling of Drying of Hydrogels via Finite Element Method and Texture Analysis" Processes 12, no. 8: 1564. https://doi.org/10.3390/pr12081564






