Supercritical CO2 Extraction of Natural Compounds from Capuchin (Tropaeolum majus) Leaves and Seeds
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Supercritical CO2 Extraction
2.3. Oil Characterization
2.4. Statistical Analysis
2.5. Kinetics of the Extraction—Model of the Sovová
3. Results and Discussions
3.1. Extraction Yield
3.2. Mathematic Modeling of the Extraction
3.3. Extract Components
3.3.1. Capuchin Seeds
3.3.2. Capuchin Leaves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melo, A.C.; Costa, S.C.A.; Castro, A.F.; Souza, A.N.V.; Sato, S.W.; Lívero, F.A.R.; Lourenço, E.L.B.; Baretta, I.P.; Lovato, E.C.W. Hydroethanolic Extract of Tropaeolum majus Promotes Anxiolytic Effects on Rats. Rev. Bras. Farmacogn. 2018, 28, 589–593. [Google Scholar] [CrossRef]
- Ribeiro, W.S.; Barbosa, J.A.; Costa, L.C.; Bruno, R.L.A.; Almeida, E.I.B.; Silva, K.R.G.; Braga Júnior, J.M.; Bezerra, A.K.D. Conservação e Fisiologia Pós-Colheita de Folhas de Capuchinha (Tropaeolum majus L.). Rev. Bras. Plantas Med. 2011, 13, 598–605. [Google Scholar] [CrossRef]
- Butnariu, M.; Bostan, C. Antimicrobial and Anti-Inflammatory Activities of the Volatile Oil Compounds from Tropaeolum majus L. (Nasturtium). Afr. J. Biotechnol. 2011, 10, 5900–5909. [Google Scholar] [CrossRef]
- Barboza, L.N.; Prando, T.B.L.; Dalsenter, P.R.; Gasparotto, F.M.; Gasparotto, F.; Jacomassi, E.; Araújo, V.D.O.; Lourenço, E.L.B.; Gasparotto Junior, A. Prolonged Diuretic Activity and Calcium-Sparing Effect of Tropaeolum majus: Evidence in the Prevention of Osteoporosis. Evid. Based Complement. Altern. Med. 2014, 2014, 958291. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.; Kolberg, T.; Engels, I.; Frank, U. In-Vitro-Untersuchungen Zur Antibakteriellen Wirksamkeit Einer Kombination Aus Kapuzinerkressenkraut (Tropaeoli majoris Herba) Und Meerrettichwurzel (Armoraciae rusticanae Radix). Arzneimittelforschung 2011, 56, 842–849. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal Models of Anxiety Disorders and Stress. Rev. Bras. Psiquiatr. 2013, 35, S101–S111. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.C.M.; dos Santos, W.L.; Leal, I.C.R.; Maria das Graças, B.Z.; Feirhmann, A.C.; Cabral, V.F.; Macedo, E.N.; Cardozo-Filho, L. Extraction of Essential Oil from Cyperus articulatus L. Var. Articulatus (Priprioca) with Pressurized CO2. J. Supercrit. Fluids 2014, 88, 134–141. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Janda, K.; Watychowicz, K.; Łukasiak, J.; Wolska, J. Garden Nasturtium (Tropaeolum majus L.)—A Source of Mineral Elements and Bioactive Compounds. Rocz. Panstw. Zakl. Hig. 2018, 69, 119–126. [Google Scholar]
- Vrca, I.; Ramić, D.; Fredotović, Ž.; Smole Možina, S.; Blažević, I.; Bilušić, T. Chemical Composition and Biological Activity of Essential Oil and Extract from the Seeds of Tropaeolum majus L. Var. Altum. Food Technol. Biotechnol. 2022, 60, 533–542. [Google Scholar] [CrossRef]
- Junior, A.G.; Prando, T.B.L.; Gebara, K.S.; Gasparotto, F.M.; dos Reis Lívero, F.A.; de Lima, D.P.; da Silva Gomes, R.; Lourenço, E.L.B. Protective Cardiorenal Effects of Tropaeolum majus L. In Rats With Renovascular Hypertension. J. Young Pharm. 2017, 9, 251–257. [Google Scholar] [CrossRef]
- Frei, P.; Nadegger, C.; Vollmar, A.M.; Müller, T.; Moser, S. Structural Characterization, and Antioxidative and Anti-Inflammatory Activities of Phylloxanthobilins in Tropaeolum majus, a Plant with Relevance in Phytomedicine. Planta Med. 2024, 90, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.S.; Costa, P.T.; Soares, A.M.S.; Fontes, A.L.; Pintado, M.E.; Vidigal, S.S.M.P.; Pimentel, L.L.; Rodríguez-Alcalá, L.M. Novel Lipids to Regulate Obesity and Brain Function: Comparing Available Evidence and Insights from QSAR In Silico Models. Foods 2023, 12, 2576. [Google Scholar] [CrossRef]
- Botelho, J.R.S.; Medeiros, N.G.; Rodrigues, A.M.C.; Araújo, M.E.; Machado, N.T.; Guimarães Santos, A.; Santos, I.R.; Gomes-Leal, W.; Carvalho, R.N. Black Sesame (Sesamum indicum L.) Seeds Extracts by CO2 Supercritical Fluid Extraction: Isotherms of Global Yield, Kinetics Data, Total Fatty Acids, Phytosterols and Neuroprotective Effects. J. Supercrit. Fluids 2014, 93, 49–55. [Google Scholar] [CrossRef]
- Pederssetti, M.M.; Palú, F.; da Silva, E.A.; Rohling, J.H.; Cardozo-Filho, L.; Dariva, C. Extraction of Canola Seed (Brassica napus) Oil Using Compressed Propane and Supercritical Carbon Dioxide. J. Food Eng. 2011, 102, 189–196. [Google Scholar] [CrossRef]
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780080518176. [Google Scholar]
- Zougagh, M.; Valcárcel, M.; Ríos, A. Supercritical Fluid Extraction: A Critical Review of Its Analytical Usefulness. TrAC Trends Anal. Chem. 2004, 23, 399–405. [Google Scholar] [CrossRef]
- Sihvonen, M. Advances in Supercritical Carbon Dioxide Technologies. Trends Food Sci. Technol. 1999, 10, 217–222. [Google Scholar] [CrossRef]
- Lima, J.C.; de Araújo, P.C.C.; dos Santos Croscato, G.; de Almeida, O.; Cabral, V.F.; Ferreira-Pinto, L.; Cardozo-Filho, L. Experimental Phase Equilibrium Data for Rotenone in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2019, 64, 2357–2362. [Google Scholar] [CrossRef]
- Nurhaslina, C.R.; Andi Bacho, S.; Mustapa, A.N. Review on Drying Methods for Herbal Plants. Mater. Today Proc. 2022, 63, S122–S139. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardising Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Garcia, V.A.D.S.; Cabral, V.F.; Zanoelo, É.F.; da Silva, C.; Filho, L.C. Extraction of Mucuna Seed Oil Using Supercritical Carbon Dioxide to Increase the Concentration of L-Dopa in the Defatted Meal. J. Supercrit. Fluids 2012, 69, 75–81. [Google Scholar] [CrossRef]
- Gonçalves, R.M.; Lemos, C.O.T.; Leal, I.C.R.; Nakamura, C.V.; Cortez, D.A.G.; da Silva, E.A.; Cabral, V.F.; Cardozo-Filho, L. Comparing Conventional and Supercritical Extraction of (-)-Mammea A/BB and the Antioxidant Activity of Calophyllum Brasiliense Extracts. Molecules 2013, 18, 6215–6229. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.O.; Camacho, F.P.; Ferreira-Pinto, L.; Giufrida, W.M.; Vieira, A.M.S.; Visentaine, J.V.; Vedoy, D.R.L.; Cardozo-Filho, L. Extraction and Phase Behaviour of Moringa Oleifera Seed Oil Using Compressed Propane. Can. J. Chem. Eng. 2016, 94, 2195–2201. [Google Scholar] [CrossRef]
- Weiß, C.H. StatSoft, Inc., Tulsa, OK.: STATISTICA, Version 8. AStA Adv. Stat. Anal. 2007, 91, 339–341. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. Design of Experiments. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2010; pp. 1–22. [Google Scholar]
- Sovová, H. Rate of the Vegetable Oil Extraction with Supercritical CO2—I. Modelling of Extraction Curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Musolino, V.; Marrelli, M.; Perri, M.R.; Palermo, M.; Gliozzi, M.; Mollace, V.; Conforti, F. Centranthus ruber (L.) DC. and Tropaeolum majus L.: Phytochemical Profile, In Vitro Anti-Denaturation Effects and Lipase Inhibitory Activity of Two Ornamental Plants Traditionally Used as Herbal Remedies. Molecules 2022, 28, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Zha, X.; Mei, Y.; Xia, J.; Jiao, Z. Supercritical Carbon Dioxide Extraction of β-Carotene and α-Tocopherol from Pumpkin: A Box–Behnken Design for Extraction Variables. Anal. Methods 2017, 9, 294–303. [Google Scholar] [CrossRef]
- Mateus, L.S.; Dutra, J.M.; Favareto, R.; da Silva, E.A.; Ferreira Pinto, L.; da Silva, C.; Cardozo-Filho, L. Optimization Studies and Compositional Oil Analysis of Pequi (Caryocar brasiliense Cambess) Almonds by Supercritical CO2 Extraction. Molecules 2023, 28, 1030. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yi, C.; Ye, X.; Xue, S.; Jiang, Y.; Ma, Y.; Liu, D. Effects of Supercritical CO2 Fluid Parameters on Chemical Composition and Yield of Carotenoids Extracted from Pumpkin. LWT Food Sci. Technol. 2010, 43, 39–44. [Google Scholar] [CrossRef]
- Frohlich, P.C.; Santos, K.A.; Palú, F.; Cardozo-Filho, L.; da Silva, C.; da Silva, E.A. Evaluation of the Effects of Temperature and Pressure on the Extraction of Eugenol from Clove (Syzygium aromaticum) Leaves Using Supercritical CO2. J. Supercrit. Fluids 2019, 143, 313–320. [Google Scholar] [CrossRef]
- Wenceslau, B.R.; Santos, K.A.; da Silva, E.A.; Cardozo-Filho, L.; da Silva, C.; Favareto, R. Guariroba (Syagrus oleracea) Kernel Oil Extraction Using Supercritical CO2 and Compressed Propane and Its Characterization. J. Supercrit. Fluids 2021, 177, 105326. [Google Scholar] [CrossRef]
- Klein, E.J.; Johann, G.; da Silva, E.A.; Vieira, M.G.A. Mathematical Modeling of Supercritical CO2 Extraction of Eugenia Pyriformis Cambess. Leaves. Chem. Eng. Commun. 2021, 208, 1543–1552. [Google Scholar] [CrossRef]

 ) of capuchin leaves: 313.15 K (
) of capuchin leaves: 313.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa); 323.15 K (
, 28 MPa); 323.15 K ( , 25 MPa); 333.15 K (
, 25 MPa); 333.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa) with a constant flow rate of 2.0 mL.min−1.
, 28 MPa) with a constant flow rate of 2.0 mL.min−1.
 ) of capuchin leaves: 313.15 K (
) of capuchin leaves: 313.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa); 323.15 K (
, 28 MPa); 323.15 K ( , 25 MPa); 333.15 K (
, 25 MPa); 333.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa) with a constant flow rate of 2.0 mL.min−1.
, 28 MPa) with a constant flow rate of 2.0 mL.min−1.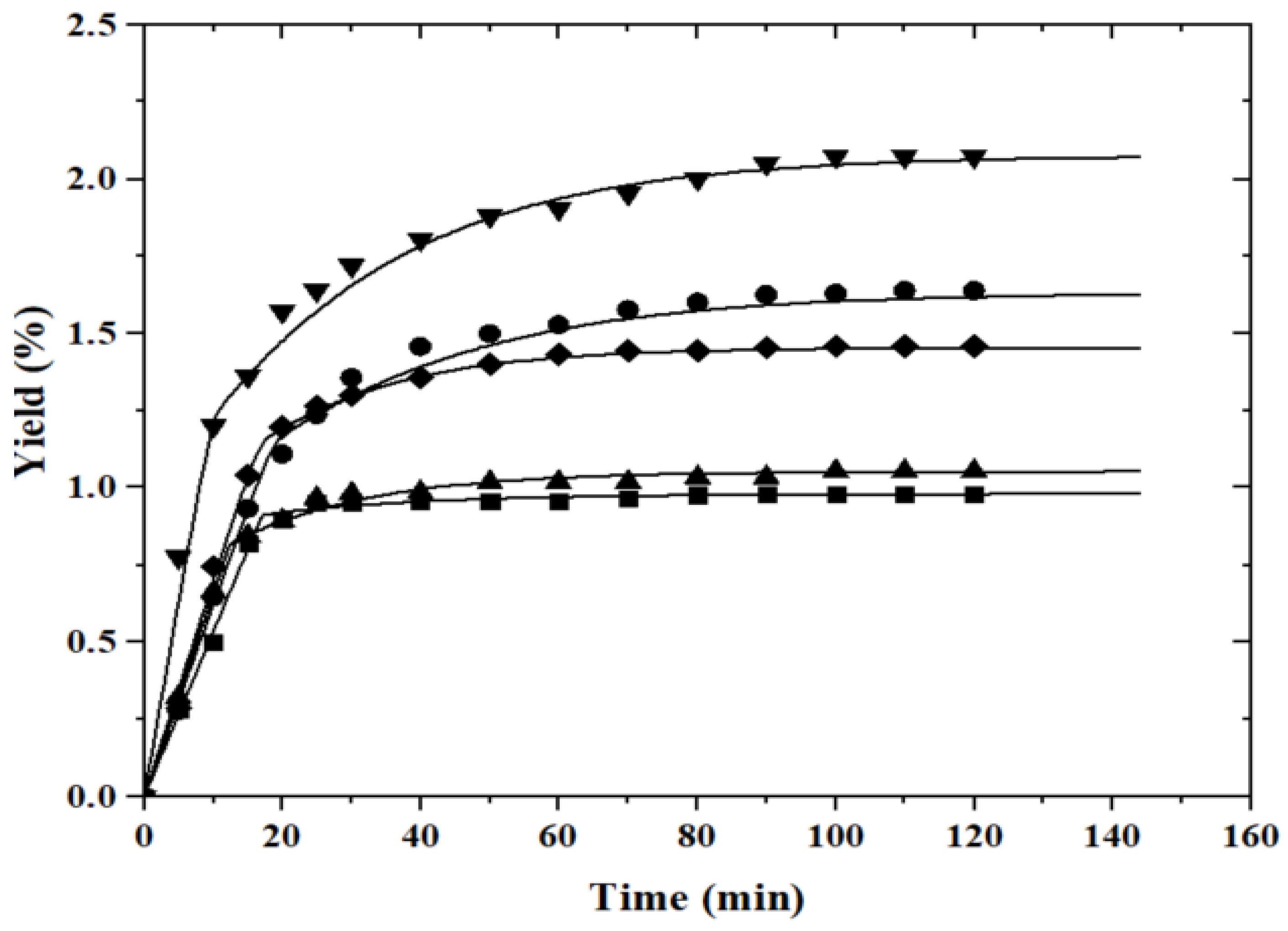
 ) of capuchin seeds: 313.15 K (
) of capuchin seeds: 313.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa); 323.15 K (
, 28 MPa); 323.15 K ( , 25 MPa); 333.15 K (
, 25 MPa); 333.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa) with a constant flow rate of 2.0 mL.min−1.
, 28 MPa) with a constant flow rate of 2.0 mL.min−1.
 ) of capuchin seeds: 313.15 K (
) of capuchin seeds: 313.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa); 323.15 K (
, 28 MPa); 323.15 K ( , 25 MPa); 333.15 K (
, 25 MPa); 333.15 K ( , 22 MPa;
, 22 MPa;  , 28 MPa) with a constant flow rate of 2.0 mL.min−1.
, 28 MPa) with a constant flow rate of 2.0 mL.min−1.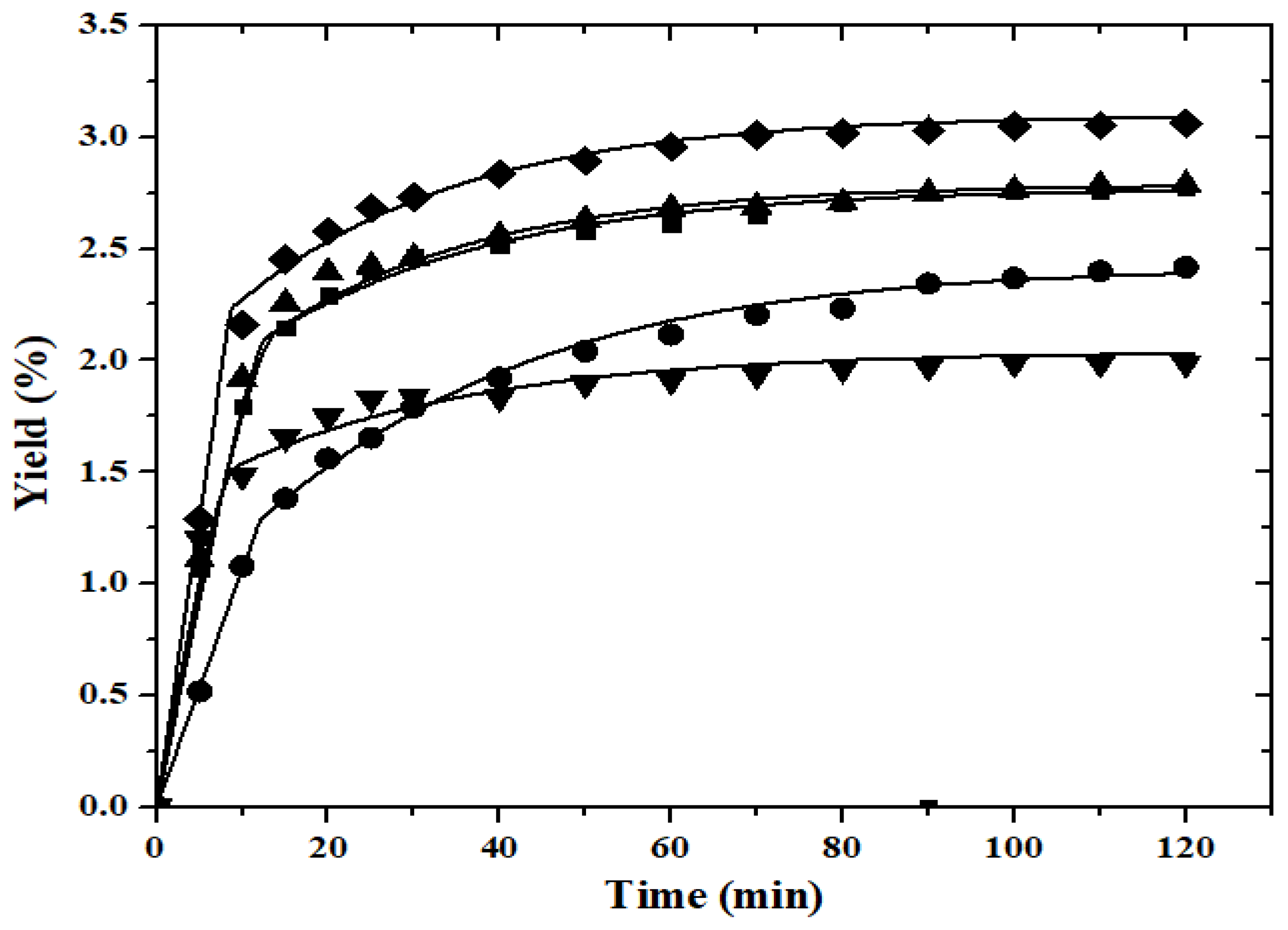
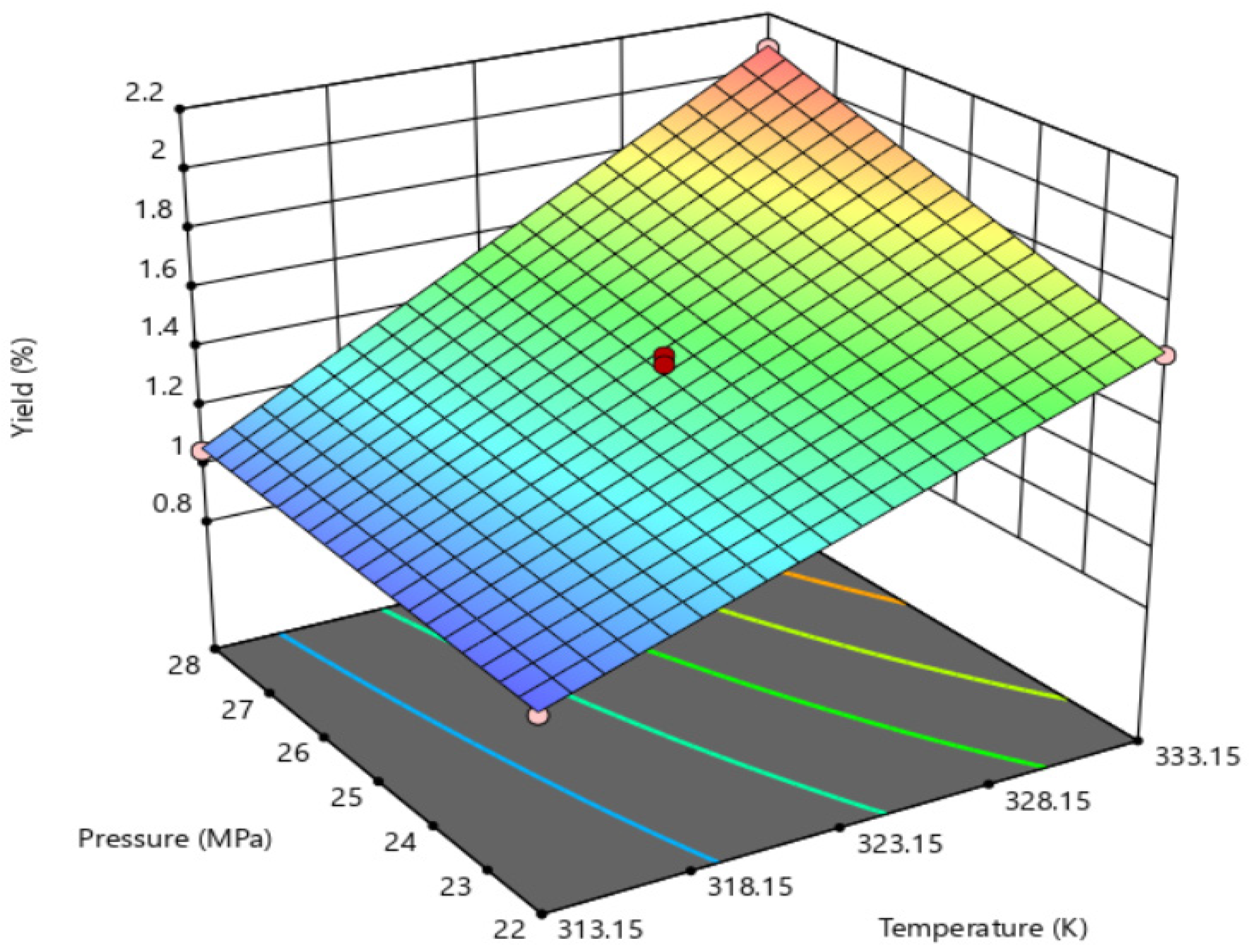
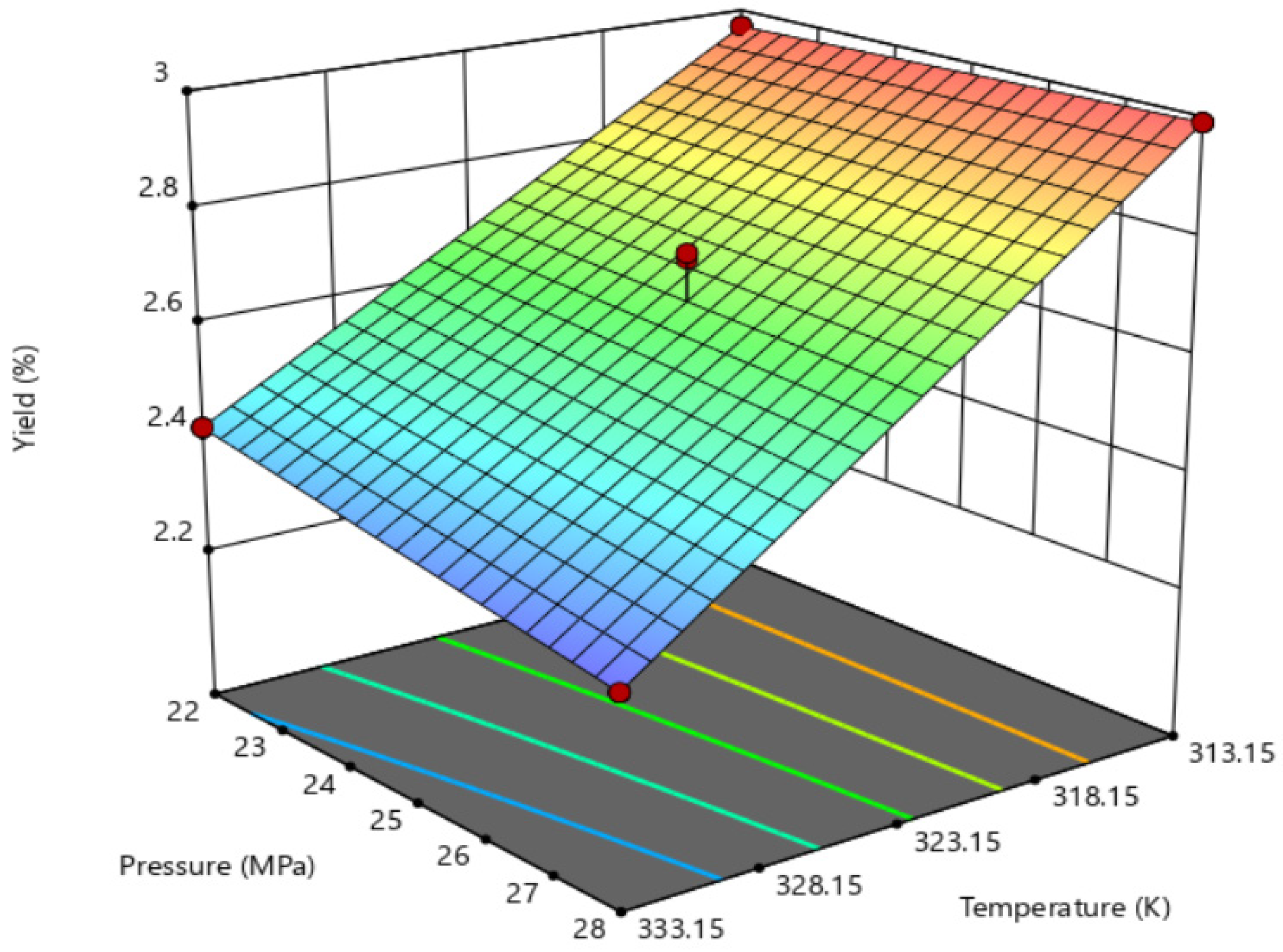

| Factors | Symbols | Units | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Temperature | T | K | 313.15 | 323.15 | 333.15 |
| Pressure | P | MPa | 22 | 25 | 28 |
| Run | Temperature (K) | Pressure (MPa) | Yield (wt%) |
|---|---|---|---|
| Capuchin Leaves | |||
| 1 | 313.15 | 22 | 0.98 |
| 2 | 333.15 | 22 | 1.64 |
| 3 | 313.15 | 28 | 1.05 |
| 4 | 333.15 | 28 | 2.10 |
| 5 | 318.15 | 25 | 1.51 |
| 6 | 318.15 | 25 | 1.39 |
| 7 | 318.15 | 25 | 1.48 |
| Capuchin Seeds | |||
| 1 | 313.15 | 22 | 2.97 |
| 2 | 333.15 | 22 | 2.42 |
| 3 | 313.15 | 28 | 3.00 |
| 4 | 333.15 | 28 | 2.29 |
| 5 | 318.15 | 25 | 2.74 |
| 6 | 318.15 | 25 | 2.75 |
| 7 | 318.15 | 25 | 2.75 |
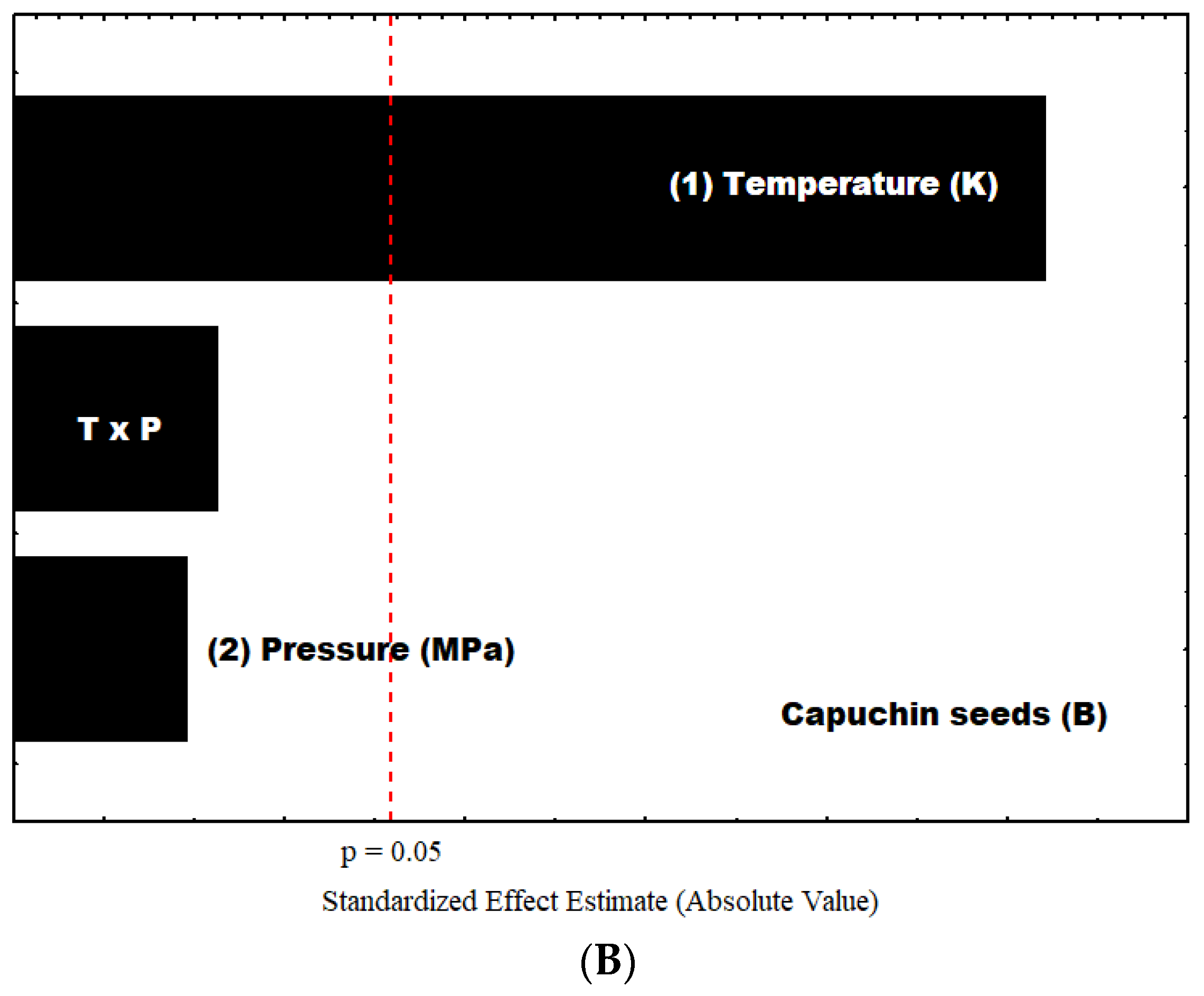
| Terms | Sum of Squares | Degrees of Freedom | Mean Squares | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|
| Capuchin Leaves | ||||||
| Model | 0.8133 | 3 | 0.2711 | 69.51 | 0.0142 | 0.9895 |
| T | 0.7140 | 1 | 0.7140 | 183.08 | 0.0054 | |
| P | 0.0650 | 1 | 0.0650 | 16.67 | 0.0551 | |
| T.P | 0.0342 | 1 | 0.0342 | 8.78 | 0.0976 | |
| Pure Error | 0.0078 | 2 | 0.0039 | |||
| Cor Total | 0.8219 | 6 | ||||
| Capuchin Seeds | ||||||
| Model | 0.3993 | 3 | 0.1331 | 36.93 | 0.0072 | 0.9736 |
| T | 0.3906 | 1 | 0.3906 | 108.40 | 0.0019 | |
| P | 0.0030 | 1 | 0.0030 | 0.8394 | 0.4271 | |
| T.P | 0.0056 | 1 | 0.0056 | 1.56 | 0.3001 | |
| Pure Error | 0.0001 | 2 | 0.0107 | 322.32 | 0.0031 | |
| Cor Total | 0.4101 | 6 | ||||
| Run | Z | W | r | S (goil.gsolvent−1) | tCER | tFER | KFa | KSa | ADD | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| (min) | (min) | (min−1) | (min−1) | (%) | ||||||
| Capuchin Leaves | ||||||||||
| 1 | 81.45 | 0.06 | 0.098 | 0.001 | 0.20 | 17.27 | 5.86 | 0.009 | 1.59 | 0.994 |
| 2 | 14.11 | 0.04 | 0.387 | 0.001 | 1.14 | 19.87 | 1.48 | 0.007 | 2.69 | 0.993 |
| 3 | 84.10 | 0.05 | 0.299 | 0.001 | 0.13 | 12.58 | 8.82 | 0.011 | 1.16 | 0.994 |
| 4 | 7.59 | 0.04 | 0.469 | 0.001 | 1.14 | 11.04 | 0.79 | 0.008 | 4.02 | 0.956 |
| 5–7 * | 11.46 | 0.05 | 0.300 | 0.001 | 1.27 | 17.88 | 1.20 | 0.011 | 1.60 | 0.997 |
| Capuchin Seeds | ||||||||||
| 1 | 6.34 | 0.034 | 0.27 | 0.002 | 0.64 | 5.55 | 0.48 | 0.64 | 1.85 | 0.989 |
| 2 | 13.61 | 0.034 | 0.53 | 0.001 | 0.89 | 15.04 | 1.03 | 0.89 | 1.38 | 0.997 |
| 3 | 2.73 | 0.033 | 0.24 | 0.002 | 1.17 | 4.99 | 0.21 | 1.17 | 1.70 | 0.987 |
| 4 | 2.72 | 0.035 | 0.27 | 0.002 | 0.84 | 3.40 | 0.20 | 0.84 | 2.62 | 0.943 |
| 5–7 * | 2.95 | 0.030 | 0.26 | 0.002 | 1.04 | 4.60 | 0.23 | 1.04 | 1.06 | 0.996 |
| Compound | Chemical Class | Peak Area (%) |
|---|---|---|
| Hexadecanoic acid | fatty acid | 9.87 ± 0.01 |
| Linoleic acid | fatty acid | 19.74 ± 0.09 |
| Oleic acid | fatty acid | 22.50 ± 0.25 |
| Tricosane | alkane | 1.02 ± 0.11 |
| Tetracosane | alkane | 2.74 ± 0.13 |
| Pentacosane | alkane | 3.12 ± 0.06 |
| Hexacosane | alkane | 3.88 ± 0.04 |
| Heptacosane | alkane | 4.20 ± 0.08 |
| Octacosane | alkane | 3.24 ± 0.19 |
| Unknown | - | 24.78 ± 0.30 |
| Nonacosane | alkane | 2.89 ± 0.15 |
| Triacontane | alkane | 2.02 ± 0.15 |
| Compound | Chemical Class | Peak Area (%) |
|---|---|---|
| Unknown | - | 2.31 |
| Hexadecanoic acid | fatty acid | 2.80 |
| Unknown | - | 2.09 |
| Oleic acid | alkane | 1.75 |
| Nonacosane | alkane | 17.68 |
| Octacosanol | alkane | 73.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa, G.; Souza, M.R.d.R.; Nascimento, E.S.; Rodrigues Bjerk, T.; Goncalves, J.E.; Moritz, C.M.F.; Sakai, O.A.; Silva, E.A.d.; Santos, R.J.d.; da Silva, E.A.; et al. Supercritical CO2 Extraction of Natural Compounds from Capuchin (Tropaeolum majus) Leaves and Seeds. Processes 2024, 12, 1566. https://doi.org/10.3390/pr12081566
Corrêa G, Souza MRdR, Nascimento ES, Rodrigues Bjerk T, Goncalves JE, Moritz CMF, Sakai OA, Silva EAd, Santos RJd, da Silva EA, et al. Supercritical CO2 Extraction of Natural Compounds from Capuchin (Tropaeolum majus) Leaves and Seeds. Processes. 2024; 12(8):1566. https://doi.org/10.3390/pr12081566
Chicago/Turabian StyleCorrêa, Gabriel, Michel Rubens dos Reis Souza, Eduardo Soares Nascimento, Thiago Rodrigues Bjerk, José Eduardo Goncalves, Cristiane Mengue Feniman Moritz, Otávio Akira Sakai, Erivaldo Antônio da Silva, Renivaldo José dos Santos, Edson Antônio da Silva, and et al. 2024. "Supercritical CO2 Extraction of Natural Compounds from Capuchin (Tropaeolum majus) Leaves and Seeds" Processes 12, no. 8: 1566. https://doi.org/10.3390/pr12081566





