Recent Progress in Non-Aqueous Biocatalysis of Immobilized Enzymes
Abstract
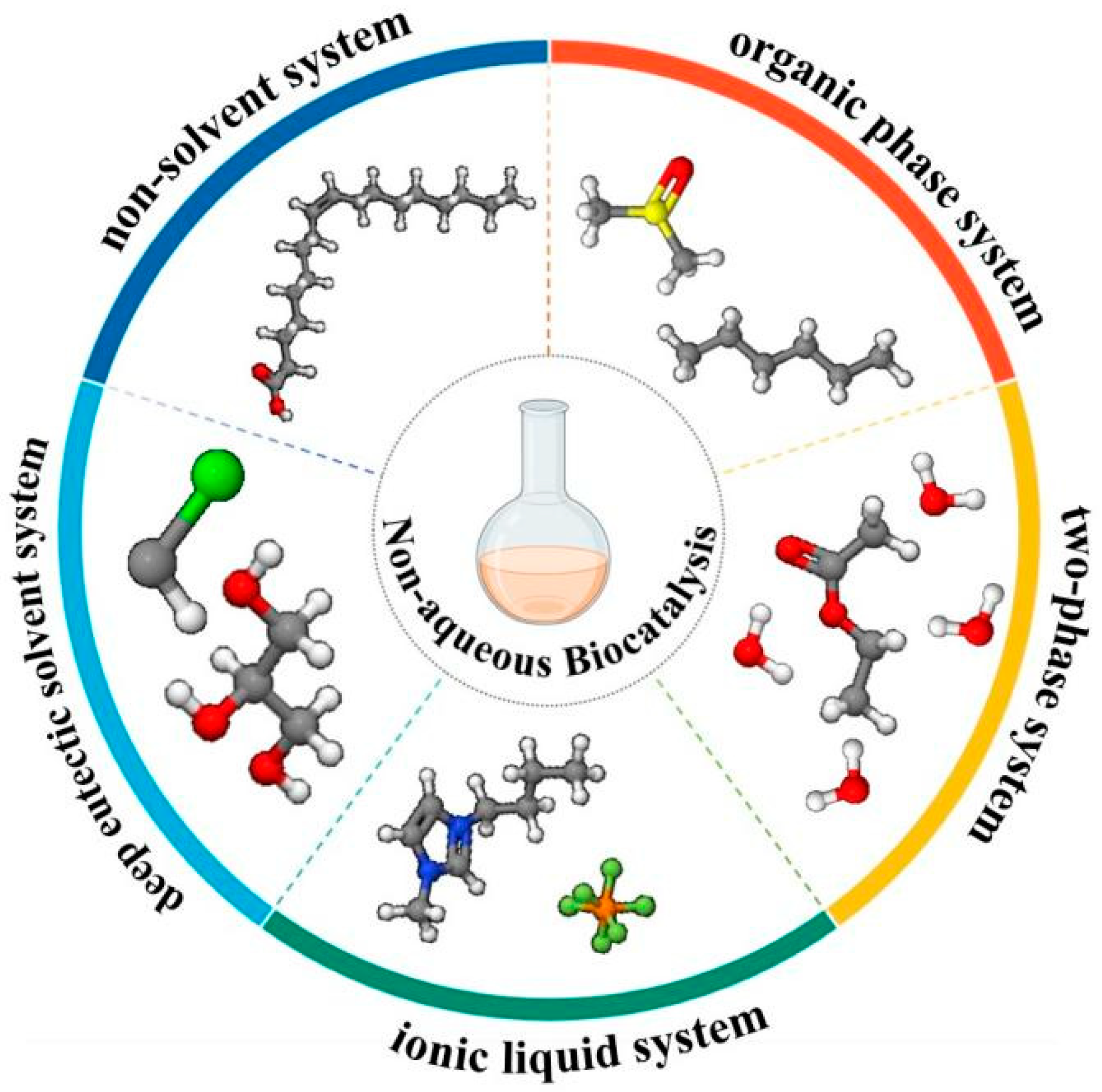
1. Introduction
2. Organic Phase System
3. Two-Phase System
4. Ionic Liquid System
5. Deep Eutectic Solvent System
| Deep Eutectic Solvent System | Molar Ratio | Carrier | Catalyst | Substrate | Product | Conversion (%) | Ref. |
|---|---|---|---|---|---|---|---|
| ChCl/Glycerol | 1:2 | XAD1180 resin | MAS1 lipase | Glycerol n-3 PUFA | Triacylglycerols | 55.80 | [98] |
| ChCl/Glc | 1:2 | Chitosan micro-spheres | β-D-glucosidase | Tyrosol | Salidroside | >50 | [99] |
| ChCl/Glycerol–DMSO | 1:2 | PD-MNP | Aspergillus niger lipase | Dihydromyricetin | DMY-16-acetate | 91.6 | [100] |
| ChCl/Glyceeol | 1:2 | Cross-linking aggregates | Pseudomonas stutzeri lipase | Benzoic acid | Glyceryl α-monobenzoate | >20 | [101] |
| ChCl/Glyceeol | 1:3 | Cross-linking aggregates | Lipase | Benzoic acid | Glyceryl α-monobenzoate | 50 | [102] |
| ChCl/Urea | 1:2 | PA@MNCC | Papain | N-(benzyloxycarbonyl)-alanyl methyl ester (Z-Ala-OMe) | N-(benzyloxycarbonyl)-alanyl-histidine | 68.40 | [103] |
| ChCl/Glycol | 7:3 (v/v) | Magnetic nano-crystalline cellulose | Penicillin acylase | 7-ACCA | Cefaclor | 84 | [104] |
| ChCl/Glycerol | 1:2 | Acrylic resin | Novozym 435 lipase | Waste oil Ethanol | Fatty acid ethyl ester | 93.33 | [105] |
| (-)-Menthol/Decanoic acid | - | Acrylic resin | Candida antarctica lipase B | Glucose | Glucose monodecanoate | - | [106] |
| Chcl/Glycerol | 1:2 | Acrylic resin | Novozym 435 lipase | Waste oil Butyl-3-Methylimidazolium hexafluorophosphate | Biodiesel | 44 | [107] |
| Chcl/Glycerol | 1:2 | Acrylic resin | Novozym 435 lipase | Soybean oil | Biodiesel | 88 | [108] |
| ChOAc/Glycerol | 1:1.5 | Acrylic resin | Novozym 435 lipase | Miglyol 812 | Biodiesel | 97 | [93] |
6. Non-Solvent System
7. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Winkler, C.K.; Schrittwieser, J.H.; Kroutil, W. Power of Biocatalysis for Organic Synthesis. ACS Cent. Sci. 2021, 7, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primer 2021, 1, 46. [Google Scholar] [CrossRef]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, B.A.; Hyster, T.K. Emerging strategies for expanding the toolbox of enzymes in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, S.; Romero, E.O.; Pyser, J.B.; Yazarians, J.A.; Narayan, A.R.H. Chemoenzymatic Total Synthesis of Natural Products. Acc. Chem. Res. 2021, 54, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.N.; Howard, R.M.; Kumar, R.; Thompson, M.P.; Truppo, M.D.; Turner, N.J. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2018, 2, 409–421. [Google Scholar] [CrossRef]
- Stout, C.N.; Renata, H. Reinvigorating the Chiral Pool: Chemoenzymatic Approaches to Complex Peptides and Terpenoids. Acc. Chem. Res. 2021, 54, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Fryszkowska, A.; Devine, P.N. Biocatalysis in drug discovery and development. Curr. Opin. Chem. Biol. 2020, 55, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Lewis, J.C. Introduction: Biocatalysis in Industry. Chem. Rev. 2018, 118, 1–3. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef]
- Cai, X.; Lin, L.; Shen, Y.; Wei, W.; Wei, D.Z. Functional expression of a novel methanol-stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent-free system. BMC Biotechnol. 2020, 20, 36. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, S.; Lee, J.; Choi, T.S.; Rhie, K.; Khim, J.S. Evaluation of residual toxicity of hypochlorite-treated water using bioluminescent microbes and microalgae: Implications for ballast water management. Ecotoxicol. Environ. Saf. 2019, 167, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Parandi, E.; Safaripour, M.; Mosleh, N.; Saidi, M.; Nodeh, H.R.; Oryani, B.; Rezania, S. Lipase enzyme immobilized over magnetic titanium graphene oxide as catalyst for biodiesel synthesis from waste cooking oil. Biomass Bioenergy 2023, 173, 106794. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.B. Recent Advances in Enzyme Immobilisation Strategies: An Overview of Techniques and Composite Carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Golombek, F.; Castiglione, K. Polymersomes as Nanoreactors Enabling the Application of Solvent-Sensitive Enzymes in Different Biphasic Reaction Setups. Biotechnol. J. 2020, 15, 1900561. [Google Scholar] [CrossRef]
- Carrea, G.; Riva, S. (Eds.) Organic Synthesis with Enzymes in Non-Aqueous Media; Wiley Online Books; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2024, 6, 185–205. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Brask, J.; Fjerbaek, L. Enzymatic biodiesel production: Technical and economical considerations. Eur. J. Lipid Sci. Technol. 2008, 110, 692–700. [Google Scholar] [CrossRef]
- Anbu, P.; Hur, B.K. Isolation of an organic solvent-tolerant bacterium Bacillus licheniformis PAL05 that is able to secrete solvent-stable lipase. Biotechnol. Appl. Biochem. 2014, 61, 528–534. [Google Scholar] [CrossRef]
- Romero, C.M.; Pera, L.M.; Loto, F.; Vallejos, C.; Castro, G.; Baigori, M.D. Purification of an organic solvent-tolerant lipase from Aspergillus niger MYA 135 and its application in ester synthesis. Biocatal. Agric. Biotechnol. 2012, 1, 25–31. [Google Scholar] [CrossRef]
- Leykun, S.; Johansson, E.; Vetukuri, R.R.; Ceresino, E.B.; Gessesse, A. A thermostable organic solvent-tolerant lipase from Brevibacillus sp.: Production and integrated downstream processing using an alcohol-salt-based aqueous two-phase system. Front. Microbiol. 2023, 14, 1270270. [Google Scholar] [CrossRef] [PubMed]
- Heipieper, H.J.; Neumann, G.; Cornelissen, S.; Meinhardt, F. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl. Microbiol. Biotechnol. 2007, 74, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kanwar, S.S. Organic Solvent Tolerant Lipases and Applications. Sci. World J. 2014, 2014, 625258. [Google Scholar] [CrossRef] [PubMed]
- Sardessai, Y.; Bhosle, S. Tolerance of bacteria to organic solvents. Res. Microbiol. 2002, 153, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kanwar, S.S.; Dogra, P.; Chauhan, G.S. Gallic Acid-Based Alkyl esters Synthesis in a Water-Free System by Celite-Bound Lipase of Bacillus licheniformis SCD11501. Biotechnol. Prog. 2015, 31, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Zeferino, G.L.; Ruiz-Lopez, I.I.; Oliart-Ros, R.; Sanchez-Otero, M.G. Improved expression and immobilization of Geobacillus thermoleovorans CCR11 thermostable recombinant lipase. Biotechnol. Appl. Biochem. 2017, 64, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, M.; Ponticorvo, E.; Cirillo, C.; Castaldo, R.; De Pasquale, S.; Gentile, G.; Sarno, M. Wax esters from waste fish oil catalysed by immobilized Candida rugosa lipase. Process Biochem. 2023, 130, 386–400. [Google Scholar] [CrossRef]
- Hinzmann, A.; Adebar, N.; Betke, T.; Leppin, M.; Gröger, H. Front Cover: Biotransformations in Pure Organic Medium: Organic Solvent-Labile Enzymes in the Batch and Flow Synthesis of Nitriles. Eur. J. Org. Chem. 2019, 2019, 6888. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, M.; Ren, L.; Zhou, H.; Wei, P. Synthesis of phytosterol esters catalyzed by immobilized lipase in organic media. Chin. J. Catal. 2013, 34, 2255–2262. [Google Scholar] [CrossRef]
- Koutinas, M.; Yiangou, C.; Osório, N.M.; Ioannou, K.; Canet, A.; Valero, F.; Ferreira-Dias, S. Application of commercial and non-commercial immobilized lipases for biocatalytic production of ethyl lactate in organic solvents. Bioresour. Technol. 2018, 247, 496–503. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, H.; Xu, M.; Huang, K.; Zhang, T.; Duan, J. Immobilized lipase catalytic synthesis of phenolamides and their potential against α-glucosidase. J. Biotechnol. 2021, 334, 51–57. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.C.M.; dos Santos, K.P.; Freire, R.M.; Barreto, A.C.H.; Fechine, P.B.A.; Goncalves, L.R.B. Production of flavor esters catalyzed by lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Eng. 2017, 34, 681–690. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Sheng, J.; Sun, M. Immobilization of Yarrowia lipolytica Lipase on Macroporous Resin Using Different Methods: Characterization of the Biocatalysts in Hydrolysis Reaction. BioMed Res. Int. 2015, 2015, 139179. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhang, Y.; Zheng, L.; Huang, H.; Wang, Z. Synthesis of 2-Ethylhexyl Palmitate Catalyzed by Enzyme Under Microwave. Appl. Biochem. Biotechnol. 2018, 185, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Lin, W.; Yue, K.; Ling, X.; Jing, K.; Lu, Y.; Tang, S.; Fan, E. Biocatalytic synthesis of vitamin A palmitate using immobilized lipase produced by recombinant Pichia pastoris. Eng. Life Sci. 2017, 17, 768–774. [Google Scholar] [CrossRef]
- Jahangiri, A.; Møller, A.H.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Adlercreutz, P.; Dalsgaard, T.K. Hydrophilization of bixin by lipase-catalyzed transesterification with sorbitol. Food Chem. 2018, 268, 203–209. [Google Scholar] [CrossRef]
- Braia, N.; Merabet-Khelassi, M.; Aribi-Zouioueche, L. Efficient access to both enantiomers of 3-(1-hydroxyethyl)phenol by regioselective and enantioselective CAL-B–catalyzed hydrolysis of diacetate in organic media by sodium carbonate. Chirality 2018, 30, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Carrea, G. Biocatalysis in water-organic solvent two-phase systems. Trends Biotechnol. 1984, 2, 102–106. [Google Scholar] [CrossRef]
- Bühler, B.; Bollhalder, I.; Hauer, B.; Witholt, B.; Schmid, A. Use of the two-liquid phase concept to exploit kinetically controlled multistep biocatalysis. Biotechnol. Bioeng. 2003, 81, 683–694. [Google Scholar] [CrossRef]
- Antonini, E.; Carrea, G.; Cremonesi, P. Enzyme catalysed reactions in water—Organic solvent two-phase systems. Enzyme Microb. Technol. 1981, 3, 291–296. [Google Scholar] [CrossRef]
- Oppermann, S.; Stein, F.; Kragl, U. Ionic liquids for two-phase systems and their application for purification, extraction and biocatalysis. Appl. Microbiol. Biotechnol. 2011, 89, 493–499. [Google Scholar] [CrossRef]
- Maciejewski, H. Ionic Liquids in Catalysis. Catalysts 2021, 11, 367. [Google Scholar] [CrossRef]
- Thomas, E.; Creighton, W.H. (Eds.) Proteins: Structures Molecular Properties, 2nd ed.; Freeman: New York, NY, USA, 1992; ISBN 0-7167-7030-X. [Google Scholar]
- Tooney, N.M. Biophysical chemistry—Part I: The conformation of biological, macromolecules, Cantor and Schimmel, W.H. Freeman and Company, San Francisco, 1980, 341 pp. J. Polym. Sci. Polym. Lett. Ed. 1980, 18, 643–644. [Google Scholar] [CrossRef]
- Xiang, L.; Kaspar, F.; Schallmey, A.; Constantinou, I. Two-Phase Biocatalysis in Microfluidic Droplets. Biosensors 2021, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Grundtvig, I.P.R.; Heintz, S.; Krühne, U.; Gernaey, K.V.; Adlercreutz, P.; Hayler, J.D.; Wells, A.S.; Woodley, J.M. Screening of organic solvents for bioprocesses using aqueous-organic two-phase systems. Biotechnol. Adv. 2018, 36, 1801–1814. [Google Scholar] [CrossRef]
- Ramsden, W.; Gotch, F. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation)—Preliminary account. Proc. R. Soc. Lond. 1997, 72, 156–164. [Google Scholar]
- Xu, C.; Sun, Y.; Sun, Y.; Cai, R.; Zhang, S. High Internal Phase Pickering Emulsion Stabilized by Lipase-Coated ZIF-8 Nanoparticles towards Recyclable Biphasic Biocatalyst. Catalysts 2023, 13, 383. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, M.; Zhang, X.; Xin, H.; Yang, H. Pickering Emulsion as an Efficient Platform for Enzymatic Reactions without Stirring. ACS Sustain. Chem. Eng. 2016, 4, 6838–6843. [Google Scholar] [CrossRef]
- Mathys, R.G.; Schmid, A.; Witholt, B. Integrated two-liquid phase bioconversion and product-recovery processes for the oxidation of alkanes: Process design and economic evaluation. Biotechnol. Bioeng. 1999, 64, 459–477. [Google Scholar] [CrossRef]
- Aghababaie, M.; Beheshti, M.; Razmjou, A.; Bordbar, A.K. Covalent immobilization of Candida rugosa lipase on a novel functionalized Fe3O4@SiO2 dip-coated nanocomposite membrane. Food Bioprod. Process. 2016, 100, 351–360. [Google Scholar] [CrossRef]
- Li, W.; Lin, S.; Lan, D.; Wang, Y. Efficient enzymatic synthesis of vitamin E succinate using an organic solvent-stable immobilized lipase. J. Am. Oil. Chem. Soc 2024. Early View. [Google Scholar] [CrossRef]
- Li, H.; Pang, Y.; Wang, X.; Cao, X.; He, X.; Chen, K.; Li, G.; Ouyang, P.; Tan, W. Phospholipase D encapsulated into metal-surfactant nanocapsules for enhancing biocatalysis in a two-phase system. RSC Adv. 2019, 9, 6548–6555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, L.; Wu, G.; Wang, X.; Jin, Q.; Qi, X.; Zhang, H. A novel immobilized enzyme enhances the conversion of phosphatidylserine in two-phase system. Biochem. Eng. J. 2021, 172, 108035. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, B.; Li, C.; Li, X.F.; Li, K.B.; Shen, Y.H. Immobilization of Candida rugosa Lipase on Glutaraldehyde-Activated Fe3O4@Chitosan as a Magnetically Separable Catalyst for Hydrolysis of Castor Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1700373. [Google Scholar] [CrossRef]
- Tang, C.; Chai, Y.; Wang, C.; Wang, Z.; Min, J.; Wang, Y.; Qi, W.; Su, R.; He, Z. Pickering Emulsions Stabilized by Lignin/Chitosan Nanoparticles for Biphasic Enzyme Catalysis. Langmuir 2022, 38, 12849–12858. [Google Scholar] [CrossRef] [PubMed]
- Girelli, A.M.; Chiappini, V.; Amadoro, P. Immobilization of lipase on spent coffee grounds by physical and covalent methods: A comparison study. Biochem. Eng. J. 2023, 192, 108827. [Google Scholar] [CrossRef]
- Yang, Y.S.; Zhang, T.; Yu, S.C.; Ding, Y.; Zhang, L.Y.; Qiu, C.; Jin, D. Transformation of Geniposide into Genipin by Immobilized β-Glucosidase in a Two-Phase Aqueous-Organic System. Molecules 2011, 16, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Endres, F.; Zein El Abedin, S. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Meakin, P.; Sun, J.; Amini, N.; Forsyth, M. Pyrrolidinium Imides: A New Family of Molten Salts and Conductive Plastic Crystal Phases. J. Phys. Chem. B 1999, 103, 4164–4170. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Gomez, C.; Alvarez, E.; Markiv, B.; Garcia-Verdugo, E.; Luis, S.V. Green biocatalytic synthesis of biodiesel from microalgae in one-pot systems based on sponge-like ionic liquids. Catal. Today 2020, 346, 87–92. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Zhang, L.; Li, J.; Yang, L.; Gao, P.; Zhou, Z. Lipase-catalyzed synthesis of biodiesel in a hydroxyl-functionalized ionic liquid. Chem. Eng. Res. Des. 2018, 132, 199–207. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Q.; Ma, X.; Li, S.; Sun, W.; Liu, C. Synthesis of benzothiazoles catalyzed by [Bmim]PF6 ionic liquid in solvent-free condition. J. Catal. 2024, 429, 115274. [Google Scholar] [CrossRef]
- Yuan, J.; Dai, Y.; Yu, Y.; Wang, P.; Wang, Q.; Fan, X. Biocatalytic synthesis of poly(ε-caprolactone) using modified lipase in ionic liquid media. Eng. Life Sci. 2016, 16, 371–378. [Google Scholar] [CrossRef]
- Zhao, G.; Lang, X.; Wang, F.; Li, J.; Li, X. A one-pot method for lipase-catalyzed synthesis of chitosan palmitate in mixed lonic liquids and its characterization. Biochem. Eng. J. 2017, 126, 24–29. [Google Scholar] [CrossRef]
- Yang, H.; Han, X.; Saleh, A.S.M.; Shao, C.; Duan, Y.; Xiao, Z.G. Lipase-catalyzed Synthesis of Feruloylated Lysophospholipid in Toluene-Ionic Liquids and Its Antioxidant Activity. J. Oleo Sci. 2021, 70, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Z.; Wang, Z.; Guo, C.; Wang, C.; Zhao, R.; Wang, L. Lipase-Catalyzed Synthesis of Indolyl 4H-Chromenes via a Multicomponent Reaction in Ionic Liquid. Catalysts 2017, 7, 185. [Google Scholar] [CrossRef]
- Shin, D.W.; Mai, N.L.; Bae, S.W.; Koo, Y.M. Enhanced lipase-catalyzed synthesis of sugar fatty acid esters using supersaturated sugar solution in ionic liquids. Enzyme Microb. Technol. 2019, 126, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Findrik, Z.; Megyeri, G.; Gubicza, L.; Bélafi-Bakó, K.; Nemestóthy, N.; Sudar, M. Lipase catalyzed synthesis of glucose palmitate in ionic liquid. J. Clean. Prod. 2016, 112, 1106–1111. [Google Scholar] [CrossRef]
- Alvarez, E.; Rodriguez, J.; Villa, R.; Gomez, C.; Nieto, S.; Donaire, A.; Lozano, P. Clean Enzymatic Production of Flavor Esters in Spongelike Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 13307–13314. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Zhang, L.; Gu, S.; Wu, F. Lipase-catalyzed Synthesis of Caffeic Acid Phenethyl Ester in Ionic Liquids: Effect of Specific Ions and Reaction Parameters. Chin. J. Chem. Eng. 2013, 21, 1376–1385. [Google Scholar] [CrossRef]
- Kanojia, S.V.; Chatterjee, S.; Chattopadhyay, S.; Goswami, D. A chemoenzymatic synthesis of ceramide trafficking inhibitor HPA-12. Beilstein J. Org. Chem. 2019, 15, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Patiño, J.; Gutiérrez, M.C.; Carriazo, D.; Ania, C.O.; Parra, J.B.; Ferrer, M.L.; Del Monte, F. Deep eutectic assisted synthesis of carbon adsorbents highly suitable for low-pressure separation of CO2-CH4 gas mixtures. Energy Environ. Sci. 2012, 5, 8699–8707. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Liu, S.; Xing, J.; Zheng, Z.; Pi, Z.; Song, F.; Liu, Z. Enhanced one-step sample pretreatment method for extraction of ginsenosides from rat plasma using tailor-made deep eutectic mixture solvents. Anal. Methods 2019, 11, 1035–1042. [Google Scholar] [CrossRef]
- Yue, Y.; Huang, Q.; Fu, Y.; Chang, J. A quick selection of natural deep eutectic solvents for the extraction of chlorogenic acid from herba artemisiae scopariae. RSC Adv. 2020, 10, 23403–23409. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem-Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, Q.; Yang, H.; Gao, W.; Li, P. pH-controlled reversible deep-eutectic solvent based enzyme system for simultaneous extraction and in-situ separation of isoflavones from Pueraria lobata. Sep. Purif. Technol. 2022, 292, 120992. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Row, K.H. Exploration of Mesoporous Stationary Phases Prepared Using Deep Eutectic Solvents Combining Choline Chloride with 1,2-Butanediol or Glycerol for Use in Size-Exclusion Chromatography. Chromatographia 2015, 78, 1321–1325. [Google Scholar] [CrossRef]
- Yang, W.; Gu, Q.; Zhou, J.; Liu, X.; Yu, X. High-Value Bioconversion of Ginseng Extracts in Betaine-Based Deep Eutectic Solvents for the Preparation of Deglycosylated Ginsenosides. Foods 2023, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, C.; Li, Q.; Li, Q.; He, Y.C. Efficient chemoenzymatic valorization of biobased D-fructose into 2,5-bis(hydroxymethyl)furan with deep eutectic solvent Lactic acid:Betaine and Pseudomonas putida S12 whole cells. Bioresour. Technol. 2022, 344, 126299. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, F.; Guaglio, A.; Conti, P.; Tamborini, L.; Gandolfi, R. Continuous-flow stereoselective reduction of prochiral ketones in a whole cell bioreactor with natural deep eutectic solvents. Green. Chem. 2022, 24, 950–956. [Google Scholar] [CrossRef]
- Harris, R.C. Physical Properties of Alcohol Based Deep Eutectic Solvents. Ph.D. Thesis, University of Leicester, Leicester, UK, 2009. [Google Scholar]
- Mero, A.; Mezzetta, A.; De Leo, M.; Braca, A.; Guazzelli, L. Sustainable valorization of cherry (Prunus avium L.) pomace waste via the combined use of (NA)DESs and bio-ILs. Green Chem. 2024, 26, 6109–6123. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Holmes, S. New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel. Org. Biomol. Chem. 2011, 9, 1908–1916. [Google Scholar] [CrossRef]
- Qi, J.; Qu, Y.; Zhou, M.; Su, Z.; Zhang, X.; Wei, R.; Xue, K.; Zhu, Z.; Meng, F.; Wang, Y. Phase behavior and molecular insights on the separation of dimethyl carbonate and methanol azeotrope by extractive distillation using deep eutectic solvents. Sep. Purif. Technol. 2023, 305, 122489. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Row, K.H. Exploration of deep eutectic solvent-based mesoporous silica spheres as high-performance size exclusion chromatography packing materials. J. Appl. Polym. Sci. 2015, 132, 42203. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem.-Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Qin, X.; Zhao, Z.; Yang, B.; Wang, Y. Immobilized MAS1 Lipase-catalyzed Synthesis of n − 3 PUFA-rich Triacylglycerols in Deep Eutectic Solvents. J. Oleo Sci. 2021, 70, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, Q.; Ma, Q.; Wu, Y.; Zhang, L. Salidroside synthesis via glycosylation by β-D-glucosidase immobilized on chitosan microspheres in deep eutectic solvents. Biocatal. Biotransform. 2024, 42, 227–240. [Google Scholar] [CrossRef]
- Cao, S.L.; Deng, X.; Xu, P.; Huang, Z.X.; Zhou, J.; Li, X.H.; Zong, M.H.; Lou, W.Y. Highly Efficient Enzymatic Acylation of Dihydromyricetin by the Immobilized Lipase with Deep Eutectic Solvents as Cosolvent. J. Agric. Food Chem. 2017, 65, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Ahumada, K.; Domínguez de María, P. Immobilization of Pseudomonas stutzeri lipase through Cross-linking Aggregates (CLEA) for reactions in Deep Eutectic Solvents. J. Biotechnol. 2021, 337, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Ahumada, K.; Domínguez de María, P. Immobilized lipase-CLEA aggregates encapsulated in lentikats® as robust biocatalysts for continuous processes in deep eutectic solvents. J. Biotechnol. 2020, 310, 97–102. [Google Scholar] [CrossRef]
- Xiong, J.; Cao, S.L.; Zong, M.H.; Lou, W.Y.; Wu, X.L. Biosynthesis of Alanyl-Histidine Dipeptide Catalyzed by Papain Immobilized on Magnetic Nanocrystalline Cellulose in Deep Eutectic Solvents. Appl. Biochem. Biotechnol. 2020, 192, 573–584. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, J.; Huang, Z.; Cao, S.; Zong, M.; Lou, W. Improving biocatalysis of cefaclor with penicillin acylase immobilized on magnetic nanocrystalline cellulose in deep eutectic solvent based co-solvent. Bioresour. Technol. 2019, 288, 121548. [Google Scholar] [CrossRef]
- Suo, H.; Hao, X.; Zhang, G.; Zhang, Q.; Du, S. A kinetic study of the ultrasonically assisted ethyl esterification of fatty acids using an immobilized lipase catalyst and deep eutectic solvent. Int. J. Chem. Kinet. 2022, 54, 400–412. [Google Scholar] [CrossRef]
- Hollenbach, R.; Ochsenreither, K.; Syldatk, C. Enzymatic Synthesis of Glucose Monodecanoate in a Hydrophobic Deep Eutectic Solvent. Int. J. Mol. Sci. 2020, 21, 4342. [Google Scholar] [CrossRef]
- Merza, F.; Fawzy, A.; AlNashef, I.; Al-Zuhair, S.; Taher, H. Effectiveness of using deep eutectic solvents as an alternative to conventional solvents in enzymatic biodiesel production from waste oils. Energy Rep. 2018, 4, 77–83. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Crittle, T.D. Choline-based deep eutectic solvents for enzymatic preparation of biodiesel from soybean oil. J. Mol. Catal. B-Enzym. 2013, 85–86, 243–247. [Google Scholar] [CrossRef]
- Venturi, V.; Presini, F.; Trapella, C.; Bortolini, O.; Giovannini, P.P.; Lerin, L.A. Microwave-assisted enzymatic synthesis of geraniol esters in solvent-free systems: Optimization of the reaction parameters, purification and characterization of the products, and biocatalyst reuse. Mol. Divers. 2023. [Google Scholar] [CrossRef]
- Jawale, P.V.; Bhanage, B.M. Synthesis of propyl benzoate by solvent-free immobilized lipase-catalyzed transesterification: Optimization and kinetic modeling. Bioprocess. Biosyst. Eng. 2021, 44, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N.A.; Quirrenbach, C.G.; Corazza, M.L.; Voll, F.A.P. Enzymatic kinetics of cetyl palmitate synthesis in a solvent-free system. Biochem. Eng. J. 2018, 137, 116–124. [Google Scholar] [CrossRef]
- Choi, S.; Kim, B.H.; No, D.S.; Yoon, S.W.; Lee, M.W.; Im, D.J.; Kim, I.H. Lipase-catalyzed synthesis of 2-ethylhexyl palmitate in a solvent free system using step changes in temperature. Biochem. Eng. J. 2022, 177, 108261. [Google Scholar] [CrossRef]
- Nhivekar, G.S.; Rathod, V.K. Optimization of lipase-catalyzed synthesis of polyethylene glycol stearate in a solvent-free system. Green. Process Synth. 2019, 8, 30–37. [Google Scholar] [CrossRef]
- Gonzalez-Miranda, D.; Carballares, D.; Pedregal, T.; Fernandez-Lafuente, R.; Ladero, M.; Bolivar, J.M. Enzyme-Catalyzed Synthesis of Glycerol Carbonate in Solventless Liquid One Phase Conditions: Role of Reaction Medium Engineering on Catalytic Performance. Ind. Eng. Chem. Res. 2023, 62, 15798–15808. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Jiang, C.; Chang, M.; Huang, J.; Xie, D. Enzymatic synthesis of bornyl linoleate in a solvent-free system. Food Biosci. 2021, 41, 100947. [Google Scholar] [CrossRef]
- Yu, H.; Kim, S.; Chang, P.S. Lipase-catalyzed production of pyridoxine monolaurate in solvent-free bioreactor system. Food Chem. 2023, 399, 133949. [Google Scholar] [CrossRef] [PubMed]
- Nieto, S.; Villa, R.; Donaire, A.; Lozano, P. Ultrasound-assisted enzymatic synthesis of xylitol fatty acid esters in solvent-free conditions. Ultrason. Sonochem. 2021, 75, 105606. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, K.S.; Rathod, V.K. Green synthesis of amyl levulinate using lipase in the solvent free system: Optimization, mechanism and thermodynamics studies. Catal. Today 2021, 375, 120–131. [Google Scholar] [CrossRef]
- Lee, A.; Kim, H.; Choi, N.; Yoon, S.W.; Kim, Y.; Kim, H.R.; Kim, I.H. Preparation of diisononyl adipate in a solvent-free system via an immobilized lipase-catalyzed esterification. Enzyme Microb. Technol. 2019, 131, 109340. [Google Scholar] [CrossRef] [PubMed]
- Lăcătuş, M.A.; Dudu, A.I.; Bencze, L.C.; Katona, G.; Irimie, F.D.; Paizs, C.; Toşa, M.I. Solvent-Free Biocatalytic Synthesis of 2,5-bis-(Hydroxymethyl)Furan Fatty Acid Diesters from Renewable Resources. ACS Sustain. Chem. Eng. 2020, 8, 1611–1617. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, G.; Wang, S.; Nian, B.; Hu, Y. Study on the synthesis of pine sterol esters in solvent-free systems catalyzed by Candida rugosa lipase immobilized on hydrophobic macroporous resin. J. Sci. Food Agric. 2023, 103, 7849–7861. [Google Scholar] [CrossRef]
- Ye, R.; Hayes, D.G.; Burton, R.; Liu, A.; Harte, F.M.; Wang, Y. Solvent-Free Lipase-Catalyzed Synthesis of Technical-Grade Sugar Esters and Evaluation of Their Physicochemical and Bioactive Properties. Catalysts 2016, 6, 78. [Google Scholar] [CrossRef]
- Sose, M.T.; Bansode, S.R.; Rathod, V.K. Solvent free lipase catalyzed synthesis of butyl caprylate. J. Chem. Sci. 2017, 129, 1755–1760. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Yadav, G.D. Synthesis of geranyl acetate by transesterification of geraniol with ethyl acetate over Candida antarctica lipase as catalyst in solvent-free system. Flavour Fragr. J. 2019, 34, 288–293. [Google Scholar] [CrossRef]
- Cordeiro, E.d.S.; Henriques, R.O.; Deucher, E.M.; de Oliveira, D.; Lerin, L.A.; Furigo, A., Jr. Optimization, kinetic, and scaling-up of solvent-free lipase-catalyzed synthesis of ethylene glycol oleate emollient ester. Biotechnol. Appl. Biochem. 2021, 68, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Bayout, I.; Bouzemi, N.; Guo, N.; Mao, X.; Serra, S.; Riva, S.; Secundo, F. Natural flavor ester synthesis catalyzed by lipases. Flavour Fragr. J. 2020, 35, 209–218. [Google Scholar] [CrossRef]
- Chea, S.; Nguyen, K.T.; Rosencrantz, R.R. Microwave-Assisted Synthesis of 5′-O-methacryloylcytidine Using the Immobilized Lipase Novozym 435. Molecules 2022, 27, 4112. [Google Scholar] [CrossRef]
- Yuan, M.; Cong, F.; Zhai, Y.; Li, P.; Yang, W.; Zhang, S.; Su, Y.; Li, T.; Wang, Y.; Luo, W.; et al. Rice straw enhancing catalysis of Pseudomonas fluorescens lipase for synthesis of citronellyl acetate. Bioprocess. Biosyst. Eng. 2022, 45, 453–464. [Google Scholar] [CrossRef]
| Organic Phase System | Carrier | Time (h) | Catalyst | Substrate | Product | Conversion (%) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cyclohexane | Super-absorber | 24 | OxdB | N-octanaloxime | N-octanenitrile | >99 | - | [30] |
| N-hexane | Macro-porous resin | 10 | Candida rugosa lipase | Lauric acid Phytosterol | Phytosterol ester | 96.6 | 6 | [31] |
| N-hexane | Magnetic amino-functionalized hyper-cross-linked resin | 12 | Candida rugosa lipase | Waste fish oil | Wax ester | 94 | 10 | [29] |
| Chloroform | Acrylic resin | - | Novozym 435 lipase | Lactic acid Ethanol | Ethyl lactate | 88 | 5 | [32] |
| MTBE | Acrylic resin | 24 | Novozym 435 lipase | N-trans-4-coumaroyltyramine | Coumaroyltyramine | 65 | - | [33] |
| N-hexane | Iron magnetic nano-particles | 8 | Candida antarctica lipase B | Butyric acid Methanol | Methyl butyrate | 96.8 | 12 | [34] |
| Tert-butanol | Macro-porous resin | 3 | Yarrowia lipolytica lipase | P-Nitrophenyl laurate | Nitrophenol Lauric acid | - | 5 | [35] |
| N-heptane | Santa barbara amorphous-15 | 3 | Thermophilic lipase QLM | Palmitic acid 2-ethyl hexanol | 2-ethylhexyl palmitate | 99 | 10 | [36] |
| Petroleum ether | Macro-porous resin HPD826 | 6 | Candida antarctica lipase B | Vitamin A acetate Palmitic acid | Vitamin A palmitate | 84 | 15 | [37] |
| 2-methyl-2-butanol | Acrylic resin | 24 | Candida antarctica lipase B | Bixin Sorbitol | Sorbitol ester of norbixin | 50 | - | [38] |
| Tetrahydrofuran | Acrylic resin | 72 | Candida antarctica lipase B | 3-(1-acetoxyethyl) phenyl acetate | (S) and (R) enantiomers of 3-(1-hydroxyethyl) phenol | 50 | - | [39] |
| Two-Phase System | Ratio | Carrier | Catalyst | Substrate | Product | Conversion (%) | Ref. |
|---|---|---|---|---|---|---|---|
| N-heptane/Water | 3 | ZIF-8 | Candida antarctica Lipase B | Cinnamic acid | Benzyl cinnamate | 48.9 | [50] |
| Methanol/Water | 4:1 (v/v) | Nano-fibrous membrane | Candida antarctica lipase B | 2-Bromoethyl ketone Salicylaldehyde | Benzofuran-2-yl (phenyl) methanone | 88 | [53] |
| Acetonitrile/DMSO | 3:2 | XAD1180 resin | Lipase UM1 | Dihydromyricetin | Vitamin E succinate | 99 | [54] |
| Dichloromethane/Water | - | Metal-surfactant nano-capsules | Phospholipase D | Phosphatidylcholine | Phosphatidylserine | 91.9 | [55] |
| Ethyl acetate/Water | 1:1 | Mesoporous silica cube | Phospholipase D | Phosphatidylcholine | Phosphatidylserine | 91.2 | [56] |
| Castor oil/Water | 1.60:1 | Fe3O4@chitosan | Candida rugosa lipase | Castor oil | Ricinoleic acid | 46.8 | [57] |
| Paraffin oil/Water | 1:1 (v/v) | Lignin/Chitosan nano-particles | Candida rugosa lipase | P-nitrophenol palmitate | Nitrophenol Palmitic acid | 100 | [58] |
| Hexane/Water | 3:2 | Coffee ground | Candida rugosa lipase | 4-Nitrophenol palmitate | 4-nitrophenol | 74 | [59] |
| Ethyl acetate/Sodium acetate | 1:1 | Sodium Alginate | β-glucosidase | Genipin | Geniposide | 47.81 | [60] |
| Ionic Liquid System | Carrier | Catalyst | Substrate | Product | Conversion (%) | Ref. |
|---|---|---|---|---|---|---|
| [Bmim] PF6 | Acrylic resin | Novozym 435 lipase | ε-caprolactone | Poly (ε-caprolactone) | 97 | [69] |
| [EMIM] Ac /[BMIM] [BF] | Acrylic resin | Novozym 435 lipase | Chitosan | Long-chain chitosan ester | - | [70] |
| [Bmim] [Tf2N] | Acrylic resin | Novozym 435 lipase | Ethyl ferulate Phosphatidylcholine | Feruloylated lysophospholipids | 50.79 | [71] |
| [EMIM] [BF4] | Santa Barbara Amorphous-15 | Mucor miehei lipase | Licylaldehyde Indole cyclohexane-1,3-dione | Indolyl 4H-Chromenes | 98 | [72] |
| [Bmim] [TfO] /[Bmim] [Tf2N] | Acrylic resin | Novozym 435 lipase | Glucose Fatty acid | Glucose fatty acid ester | 55 | [73] |
| [Bmim] [PF6] | Acrylic resin | Novozym 435 lipase | Palmitic acid Glucose | Glucose palmitate | - | [74] |
| [C16mim] [NTf2] /[Bmim] [Cl] | Acrylic resin | Novozym 435 lipase | Algal oil | Biodiesel | 100 | [66] |
| [C16tma] [NTf2] | Acrylic resin | Novozym 435 lipase | Aliphatic acids Alcohol | Flavor ester | 100 | [75] |
| [Emim] [Tf2N] | Acrylic resin | Novozym 435 lipase | Caffeic acid Phenylethanol | Caffeic acid Phenethyl ester | 63.75 | [76] |
| [Bmim] [PF6] | Acrylic resin | Novozym 435 lipase | Sterol | (1R,3R)-N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl) dodecanamid | 23 | [77] |
| [C1C3OHPyr] NTf2 | Acrylic resin | Novozym 435 lipase | Soybean oil | Biodiesel | 82.4 | [67] |
| Carrier | Catalyst | Substrate | Product | Conversion (%) | Ref. |
|---|---|---|---|---|---|
| Lewatit VP OC 1600 | Novozymes eversa transform 2.0 | 2-ethylhexyl alcohol Palmitic acid | 2-ethylhexyl palmitate | 97 | [112] |
| Polyacrylate beads | Fermase CALBex 10,000 | Polyethylene glycol 600 Stearic acid | Polyethylene glycol stearate | 86.98 | [113] |
| Octyl agarose | Candida rugosa lipase | Glycerol Ethylene carbonate | Glycerol carbonate | >99 | [114] |
| Lewatit VP OC 1600 | Novozym 435 lipase | Borneol Linoleic acid | Bornyl linoleate | 92.62 | [115] |
| Acrylic resin | Novozym 435 lipase | Geranyl ester | Polyhydroquinolines | 95 | [109] |
| Hydroxypropyl methylcellulose | Candida cylindracea lipase | N-propyl alcohol Vinyl benzoate | Propyl benzoate | 99 | [110] |
| Lewatit VP OC 1600 | Novozym 435 lipase | Lauric acid Pyridoxine | Pyridoxine monolaurate | 94.45 | [116] |
| Acrylic resin | Novozym 435 lipase | Free fatty acids Xylitol | Xylitol fatty acid esters | 95 | [117] |
| Polyacrylate beads | Fermase CALB™ 10,000 | Levulinic acid Amyl alcohol | Amyl levulinate | 73.20 | [118] |
| Lewatit VP OC 1600 | Thermomyces lanuginosus Eversa lipase | Adipic acid Isononyl alcohol | Diisononyl adipate | 100 | [119] |
| Acrylic resin | Novozym 435 lipase | 2,5-bis-(Hydroxymethyl) Furan | 2,5-bis-(Hydroxymethyl) Furan fatty acid | 97 | [120] |
| Acrylic resin | Candida rugosa lipase | Oleic acid | Pine sterol ester | 95.10 | [121] |
| Acrylic resin | Novozym 435 lipase | Sucrose Fructose | Sugar ester | 96.60 | [122] |
| Macro-porous ionexchange resin | Rhizomucor miehei lipozyme RM IM | Cetyl alcohol Palmitic acid | Cetyl palmitate | 100 | [111] |
| Macro-porous resin | Novozym 435 lipase | Caprylic acid N-butanol | Butyl caprylate | 92 | [123] |
| Acrylic resin | Novozym 435 lipase | Eraniol Ethyl acetate | Geranyl acetate | 83 | [124] |
| Micro-porous resins | Lipase NS 88,011 | Oleic acid Monoethylene glycol | Ethylene glycol oleate | 99% | [125] |
| Acrylic resin | Novozym 435 lipase | Methanol phenylacetic acid | Methyl phenylacetate | - | [126] |
| Acrylic resin | Novozym 435 lipase | Vinyl methacrylate | 5-O-methacryloylcytidine | 36 | [127] |
| Rice straw filaments | Pseudomonas fluorescens lipase | Citronellol Vinyl acetate | Citronelly acetate | 99.8 | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, L.; Chu, Y.; Wang, Y.; Chen, K.; Li, H. Recent Progress in Non-Aqueous Biocatalysis of Immobilized Enzymes. Processes 2024, 12, 1571. https://doi.org/10.3390/pr12081571
Ma J, Wang L, Chu Y, Wang Y, Chen K, Li H. Recent Progress in Non-Aqueous Biocatalysis of Immobilized Enzymes. Processes. 2024; 12(8):1571. https://doi.org/10.3390/pr12081571
Chicago/Turabian StyleMa, Jiayun, Luyao Wang, Yan Chu, Yitong Wang, Kequan Chen, and Hui Li. 2024. "Recent Progress in Non-Aqueous Biocatalysis of Immobilized Enzymes" Processes 12, no. 8: 1571. https://doi.org/10.3390/pr12081571
APA StyleMa, J., Wang, L., Chu, Y., Wang, Y., Chen, K., & Li, H. (2024). Recent Progress in Non-Aqueous Biocatalysis of Immobilized Enzymes. Processes, 12(8), 1571. https://doi.org/10.3390/pr12081571







