Pine Nutshells and Their Biochars as Sources of Chemicals, Fuels, Activated Carbons, and Electrode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydroethanolic Extraction and Antioxidant Activity
2.3. Chemical Fractionation
2.4. Thermogravimetric Analysis
2.5. Production of Biochars and Activated Carbons
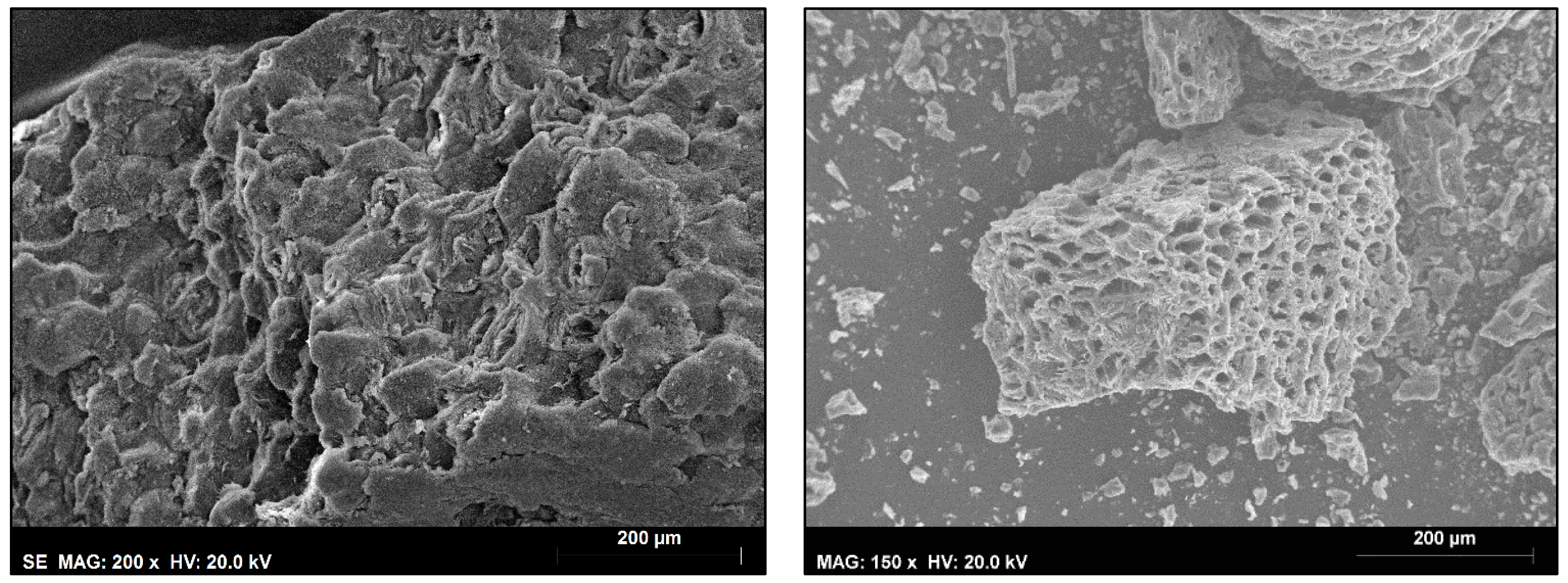
2.6. Scanning Electron Microscopy
2.7. Physical Nitrogen Adsorption
2.8. Water Retention and Nutrient Leaching
2.9. Electrochemical Characterization
3. Results and Discussion
3.1. Hydroethanolic Extraction and Cell-Based Antioxidant Activity
| Pine Biomass Component | Extract Yield (%) | Source |
| Pine nutshell | 1.2 | This work |
| Pine nutshell | 5 | [1] |
| Pinecone | 7.0 | [22] |
| Pine bark | 18.8 | [23] |
| Pine needle | 10.9 | [24] |
| Antioxidant Activity | ||
| TBARS (EC50, μg/mL) | 1100 ± 500 | This work |
| Trolox reference (μg/mL) | 5.4 ± 0.3 |
3.2. Chemical Fractionation
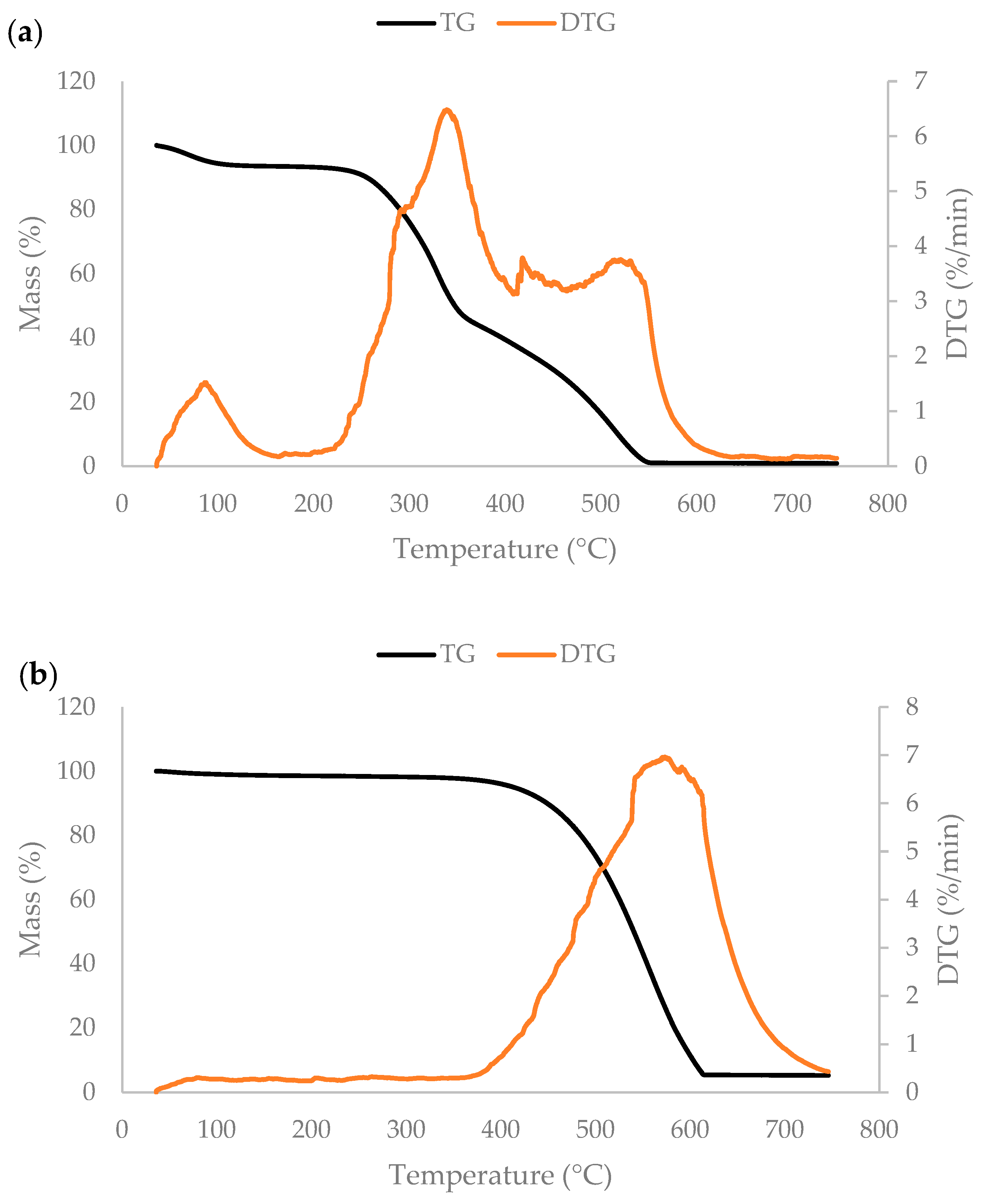
3.3. Thermogravimetric Analysis and Pseudocomponent Kinetic Modeling
3.4. Thermochemical Fractionation
3.5. Biochar Properties and Applications
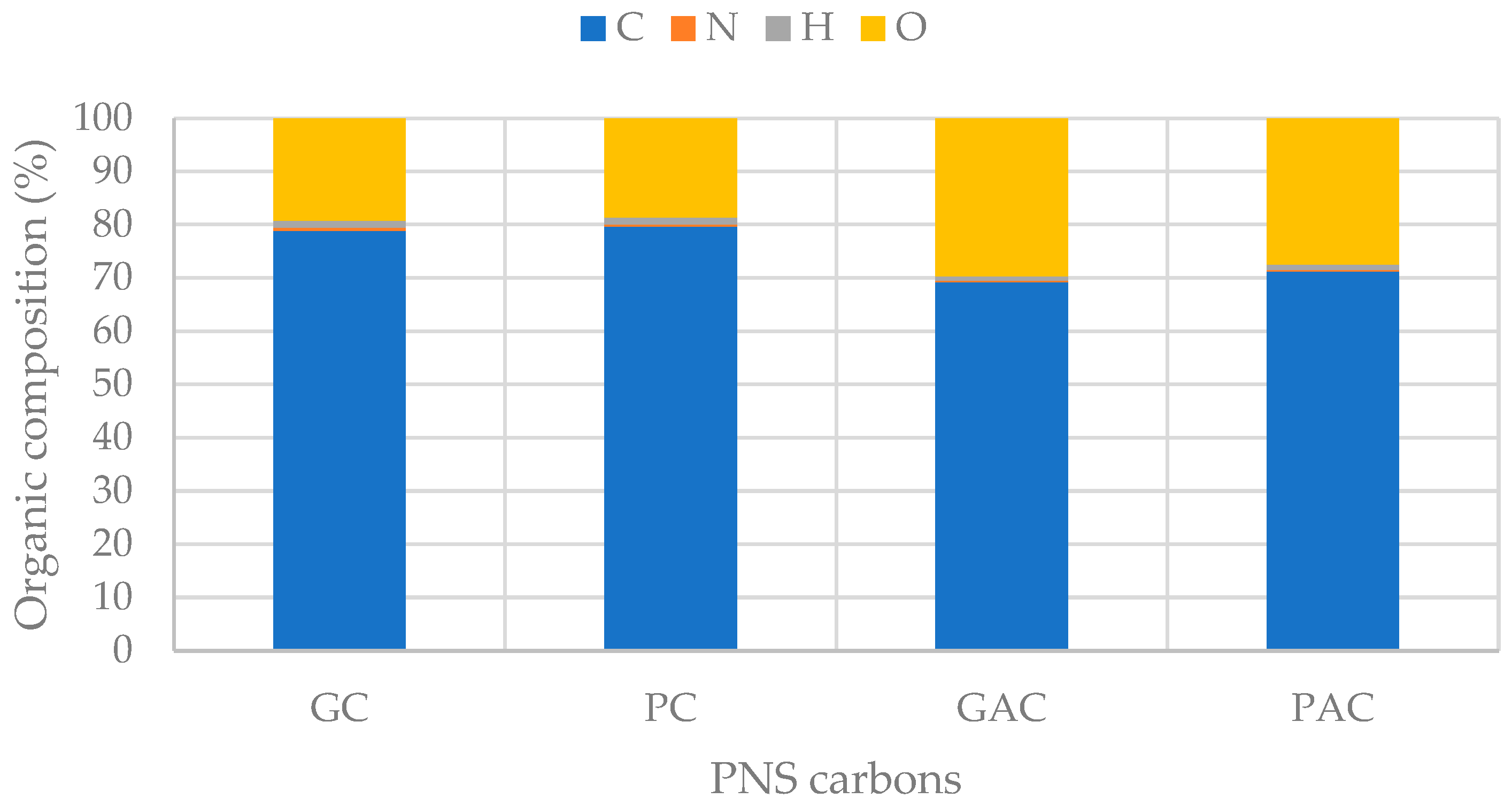
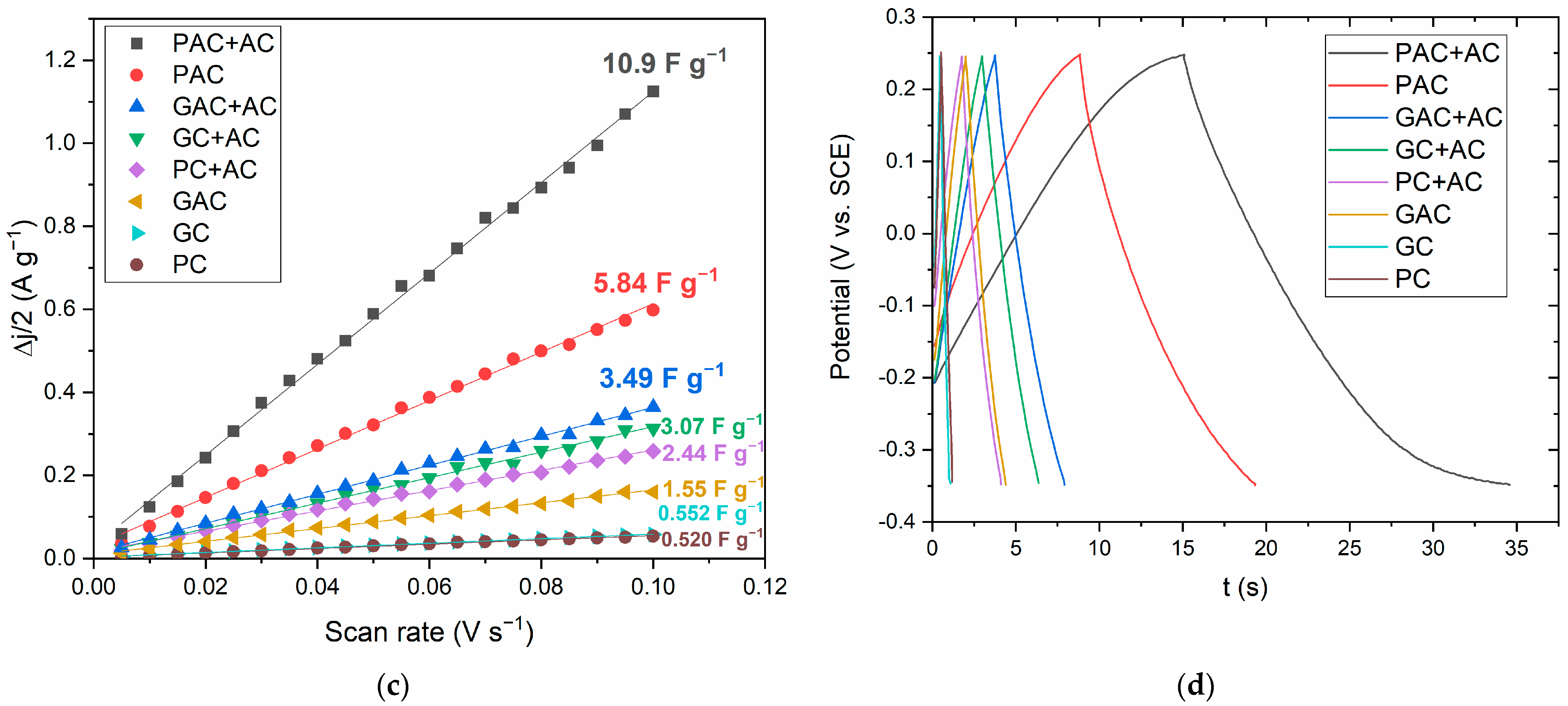
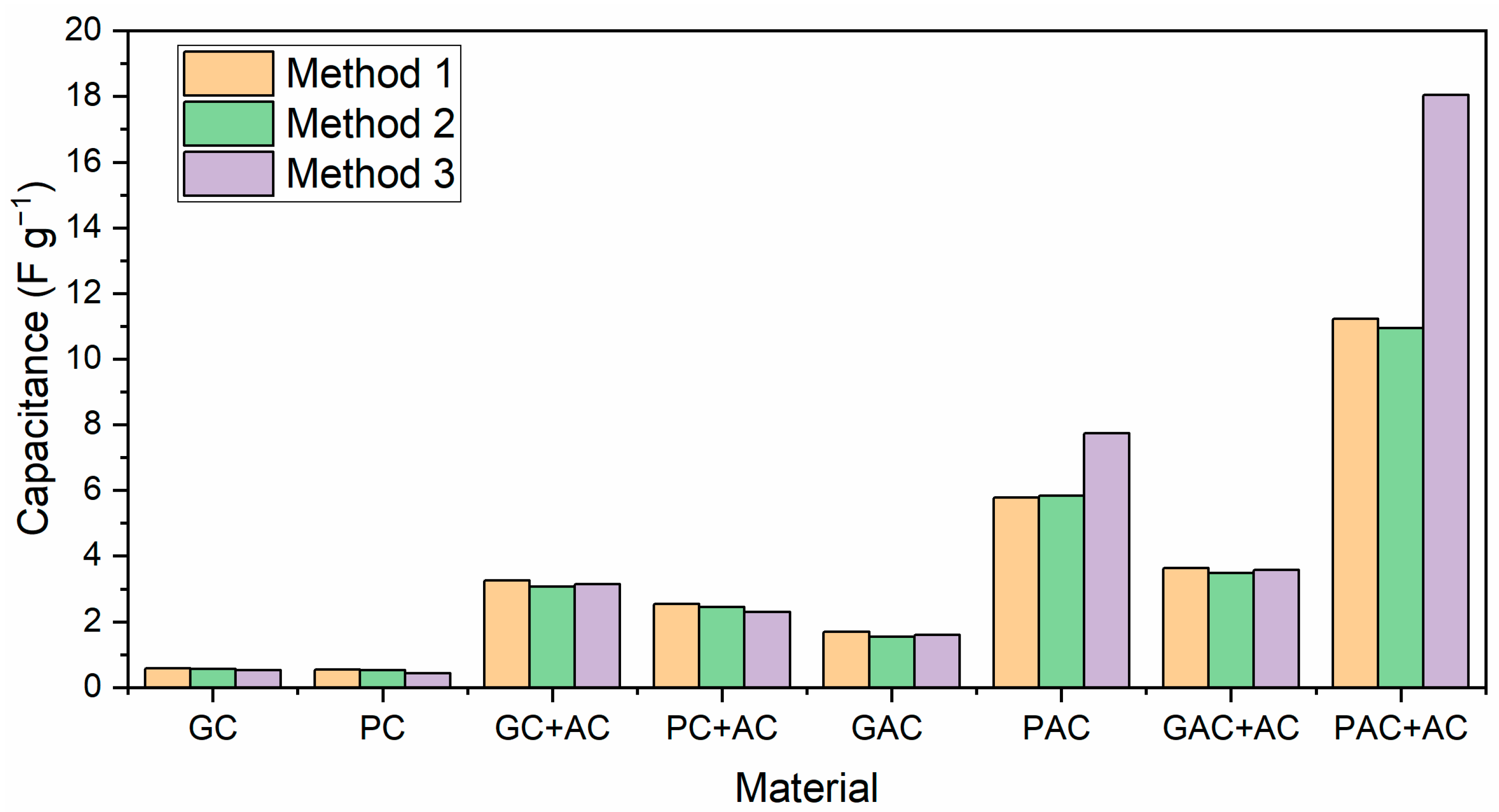
3.6. Electrochemical Properties of PNS Biochars and Activated Carbons
4. Conclusions
- The antioxidant activity of pine nutshells is weak.
- Pyrolytic kinetic modeling may be used to estimate the lignin and polysaccharide contents of pine nutshells.
- Moderate-temperature slow pyrolysis of pine nutshells resulted in a 23% biochar yield.
- Pine nutshell biochars are potential soil amendment and adsorbent materials with a porous structure, 36% water retention capacity, and 151 mg/L potassium leaching.
- Single-step steam activation of pine nutshell biochars resulted in an oxygen-enhanced activated carbon with a specific surface area between 438 and 467 m2/g.
- Pine nutshell-activated carbons were used as electrode materials for supercapacitor applications, resulting in a capacitance value as high as 18 F g−1.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şen, A.U.; Correia, R.; Longo, A.; Nobre, C.; Alves, O.; Santos, M.; Gonçalves, M.; Miranda, I.; Pereira, H. Chemical Composition, Morphology, Antioxidant, and Fuel Properties of Pine Nut Shells within a Biorefinery Perspective. Biomass Convers. Biorefin. 2022, 14, 14505–14517. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure Reactions; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1984. [Google Scholar]
- Şen, A.U.; Pereira, H. State-of-the-Art Char Production with a Focus on Bark Feedstocks: Processes, Design, and Applications. Processes 2021, 9, 87. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, S.; Pan, N.; Hsieh, Y.-L. High Energy Density Supercapacitors from Lignin Derived Submicron Activated Carbon Fibers in Aqueous Electrolytes. J. Power Sources 2014, 270, 106–112. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.-H.; Kirkham, M.B. Multifunctional Applications of Biochar beyond Carbon Storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Junior, I.I.; Do Nascimento, M.A.; de Souza, R.O.M.A.; Dufour, A.; Wojcieszak, R. Levoglucosan: A Promising Platform Molecule? Green Chem. 2020, 22, 5859–5880. [Google Scholar] [CrossRef]
- Szostak, K.; Hodacka, G.; Długosz, O.; Pulit-Prociak, J.; Banach, M. Sorption of mercury in batch and fixed-bed column system on hydrochar obtained from apple pomace. Processes 2022, 10, 2114. [Google Scholar] [CrossRef]
- Modak, A.; Bhaumik, A. Porous carbon derived via KOH activation of a hypercrosslinked porous organic polymer for efficient CO2, CH4, H2 adsorptions and high CO2/N2 selectivity. J. Solid State Chem. 2015, 232, 157–162. [Google Scholar] [CrossRef]
- Şen, A.U.; Fonseca, F.G.; Funke, A.; Pereira, H.; Lemos, F. Pyrolysis Kinetics and Estimation of Chemical Composition of Quercus cerris Cork. Biomass Convers. Biorefin. 2020, 12, 4835–4845. [Google Scholar] [CrossRef]
- Alimi, A.; Assaker, I.B.; Mozaryn, J.; Ávila-Brande, D.; Castillo-Martínez, E.; Chtourou, R. Electrochemical Synthesis of MnO2/NiO/ZnO Trijunction Coated Stainless Steel Substrate as a Supercapacitor Electrode and Cyclic Voltammetry Behavior Modeling Using Artificial Neural Network. Int. J. Energy Res. 2022, 46, 17163–17179. [Google Scholar] [CrossRef]
- Mladenović, D.; Santos, D.M.F.; Bozkurt, G.; Soylu, G.S.P.; Yurtcan, A.B.; Miljanić, Š.; Šljukić, B. Tailoring Metal-Oxide-Supported PtNi as Bifunctional Catalysts of Superior Activity and Stability for Unitised Regenerative Fuel Cell Applications. Electrochem. Commun. 2021, 124, 106963. [Google Scholar] [CrossRef]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.S.; Thomas, S.; Wei, B. Supercapacitors from Activated Carbon Derived from Banana Fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Y.; Wang, F.; Wang, G.; Qi, P.; Guo, X.; Dai, B.; Yu, F. An Activated Carbon Derived from Tobacco Waste for Use as a Supercapacitor Electrode Material. New Carbon. Mater. 2017, 32, 592–599. [Google Scholar] [CrossRef]
- Li, M.; Xiao, H.; Zhang, T.; Li, Q.; Zhao, Y. Activated Carbon Fiber Derived from Sisal with Large Specific Surface Area for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 4716–4723. [Google Scholar] [CrossRef]
- Qin, L.; Hou, Z.; Zhang, S.; Zhang, W.; Jiang, E. Supercapacitive Charge Storage Properties of Porous Carbons Derived from Pine Nut Shells. J. Electroanal. Chem. 2020, 866, 114140. [Google Scholar] [CrossRef]
- Qin, L.; Hou, Z.; Lu, S.; Liu, S.; Liu, Z.; Jiang, E. Porous Carbon Derived from Pine Nut Shell Prepared by Steam Activation for Supercapacitor Electrode Material. Int. J. Electrochem. Sci. 2019, 14, 8907–8918. [Google Scholar] [CrossRef]
- Sjostrom, E. Wood Chemistry: Fundamentals and Applications; Gulf Professional Publishing: Houston, TX, USA; Academic Press: New York, NY, USA, 1993; ISBN 0126474818. [Google Scholar]
- Şen, U.; Esteves, B.; Pereira, H. Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review. Energies 2023, 16, 4848. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Ganzera, M.; Akgun, I.; Sevimli, C.; Korkmaz, K.S.; Bedir, E. Determination of Polyphenolic Constituents and Biological Activities of Bark Extracts from Different Pinus Species. J. Sci. Food Agric. 2009, 89, 1339–1345. [Google Scholar] [CrossRef]
- Serrano, J.; Goñi, I.; Saura-Calixto, F. Food Antioxidant Capacity Determined by Chemical Methods May Underestimate the Physiological Antioxidant Capacity. Food Res. Int. 2007, 40, 15–21. [Google Scholar] [CrossRef]
- Kostova, I.N.; Iossifova, T. Chemical Components of Fraxinus Ornus Bark—Structure and Biological Activity. Stud. Nat. Prod. Chem. 2002, 26, 313–349. [Google Scholar] [CrossRef]
- Dönmez, I.E.; Hafizoğlu, H.; Kilic, A.; Tümen, I.; Sivrikaya, H. Chemical Composition of Fourteen Different Coniferous Species Cones Growing Naturally in Turkey. Wood Res. 2012, 57, 339–344. [Google Scholar]
- Miranda, I.; Mirra, I.; Gominho, J.; Pereira, H. Fractioning of Bark of Pinus Pinea by Milling and Chemical Characterization of the Different Fractions. Maderas Cienc. Tecnol. 2017, 19, 185–194. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Comparative Evaluation of the Impact of Extraction Solvent and Time on the Yield and Antioxidant Potential of Pinus Densiflora Needle and Bark Extracts. Wood Sci. Technol. 2020, 54, 587–598. [Google Scholar] [CrossRef]
- Lee, J. Biological Conversion of Lignocellulosic Biomass to Ethanol. J. Biotechnol. 1997, 56, 1–24. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Iulianelli, A.; Di Paola, L.; Basile, M.; Basile, A. Ethanol from Biomass: Future and Perspectives. In Ethanol; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–59. ISBN 978-0-12-811458-2. [Google Scholar]
- Le Normand, M.; Moriana, R.; Ek, M. Isolation and Characterization of Cellulose Nanocrystals from Spruce Bark in a Biorefinery Perspective. Carbohydr. Polym. 2014, 111, 979–987. [Google Scholar] [CrossRef]
- Song, Y.; Mobley, J.K.; Motagamwala, A.H.; Isaacs, M.; Dumesic, J.A.; Ralph, J.; Lee, A.F.; Wilson, K.; Crocker, M. Gold-Catalyzed Conversion of Lignin to Low Molecular Weight Aromatics. Chem. Sci. 2018, 9, 8127–8133. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.D.; Singh, G.; Majumdar, S.; Chanda, P. Challenges in Developing Strategies for the Valorization of Lignin—A Major Pollutant of the Paper Mill Industry. Environ. Sci. Pollut. Res. 2023, 30, 11119–11140. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.D.S.; Crestini, C.; Dahlstrand, C.; Furusjö, E.; Gioia, C.; Jedvert, K.; Henriksson, G.; Hulteberg, C.; Lawoko, M.; Pierrou, C. Kraft Lignin: A Valuable, Sustainable Resource, Opportunities and Challenges. ChemSusChem 2023, 16, e202300492. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Casimiro, F.M.; Vega-Aguilar, C.; Rodrigues, A.E. Lignin Valorization for Added-Value Chemicals: Kraft Lignin versus Lignin Fractions. ChemEngineering 2023, 7, 42. [Google Scholar] [CrossRef]
- Oliveira, R.C.P.; Mateus, M.; Santos, D.M.F. Chronoamperometric and Chronopotentiometric Investigation of Kraft Black Liquor. Int. J. Hydrogen Energy 2018, 43, 16817–16823. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2012; ISBN 0429109091. [Google Scholar]
- Sjöström, E.; Alén, R. Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Springer: Berlin, Germany, 1998; ISBN 354063102X. [Google Scholar]
- Mészáros, E.; Várhegyi, G.; Jakab, E.; Marosvölgyi, B. Thermogravimetric and Reaction Kinetic Analysis of Biomass Samples from an Energy Plantation. Energy Fuels 2004, 18, 497–507. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric Analysis as a New Method to Determine the Lignocellulosic Composition of Biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Esteves, B.; Sen, U.; Pereira, H. Influence of Chemical Composition on Heating Value of Biomass: A Review and Bibliometric Analysis. Energies 2023, 16, 4226. [Google Scholar] [CrossRef]
- Chen, L.; Wen, C.; Wang, W.; Liu, T.; Liu, E.; Liu, H.; Li, Z. Combustion Behaviour of Biochars Thermally Pretreated via Torrefaction, Slow Pyrolysis, or Hydrothermal Carbonisation and Co-Fired with Pulverised Coal. Renew. Energy 2020, 161, 867–877. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, W.; Liang, S.; Bi, X.; Sun, R.; Pan, X. Comparative Study on the Co-Combustion Behavior of Torrefied Biomass Blended with Different Rank Coals. Biomass Convers. Biorefin. 2022, 14, 781–793. [Google Scholar] [CrossRef]
- Vakalis, S.; Moustakas, K. Modeling the Co-Combustion of Coal and Biocoal from the Novel Process of Frictional Pyrolysis for Reducing the Emissions of Coal Plants. Biomass Convers. Biorefin. 2021, 11, 2937–2945. [Google Scholar] [CrossRef]
- Huang, C.-W.; Li, Y.-H.; Xiao, K.-L.; Lasek, J. Cofiring Characteristics of Coal Blended with Torrefied Miscanthus Biochar Optimized with Three Taguchi Indexes. Energy 2019, 172, 566–579. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass Gasification Technology: The State of the Art Overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Chang, A.C.C.; Chang, H.-F.; Lin, F.-J.; Lin, K.-H.; Chen, C.-H. Biomass Gasification for Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 14252–14260. [Google Scholar] [CrossRef]
- Rios, M.L.V.; González, A.M.; Lora, E.E.S.; del Olmo, O.A.A. Reduction of Tar Generated during Biomass Gasification: A Review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Dinca, C.; Slavu, N.; Cormoş, C.-C.; Badea, A. CO2 Capture from Syngas Generated by a Biomass Gasification Power Plant with Chemical Absorption Process. Energy 2018, 149, 925–936. [Google Scholar] [CrossRef]
- Oreggioni, G.D.; Brandani, S.; Luberti, M.; Baykan, Y.; Friedrich, D.; Ahn, H. CO2 Capture from Syngas by an Adsorption Process at a Biomass Gasification CHP Plant: Its Comparison with Amine-Based CO2 Capture. Int. J. Greenh. Gas Control 2015, 35, 71–81. [Google Scholar] [CrossRef]
- Pereira, H.; Ferreira, E. Scanning Electron Microscopy Observations of Insulation Cork Agglomerates. Mater. Sci. Eng. A 1989, 111, 217–225. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Gonnermann, H.M. Biochar Particle Size, Shape, and Porosity Act Together to Influence Soil Water Properties. PLoS ONE 2017, 12, e0179079. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Thomas, S.C. Biochar Particle Size and Post-Pyrolysis Mechanical Processing Affect Soil PH, Water Retention Capacity, and Plant Performance. Soil Syst. 2019, 3, 14. [Google Scholar] [CrossRef]
- Lyu, H.; Yu, Z.; Gao, B.; He, F.; Huang, J.; Tang, J.; Shen, B. Ball-Milled Biochar for Alternative Carbon Electrode. Environ. Sci. Pollut. Res. 2019, 26, 14693–14702. [Google Scholar] [CrossRef] [PubMed]
- Bruun, E.W.; Ravenni, G.; Müller-Stöver, D.; Petersen, C.T. Small Biochar Particles Added to Coarse Sandy Subsoil Greatly Increase Water Retention and Affect Hydraulic Conductivity. Eur. J. Soil Sci. 2023, 74, e13442. [Google Scholar] [CrossRef]
- Ntuli, V.; Hapazari, I. Sustainable Waste Management by Production of Activated Carbon from Agroforestry Residues. S. Afr. J. Sci. 2013, 109, 6. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable Biochar for Advanced Electrochemical/Energy Storage Applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Gao, J.; Sun, S. Changes of Biochar Physiochemical Structures during Tar H2O and CO2 Heterogeneous Reforming with Biochar. Fuel Process. Technol. 2017, 165, 72–79. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, K.; Jiang, K.; Zhang, F.; Zhu, G.; Xu, H. Progress of Synthetic Strategies and Properties of Heteroatoms-Doped (N, P, S, O) Carbon Materials for Supercapacitors. J. Energy Storage 2022, 56, 105995. [Google Scholar] [CrossRef]
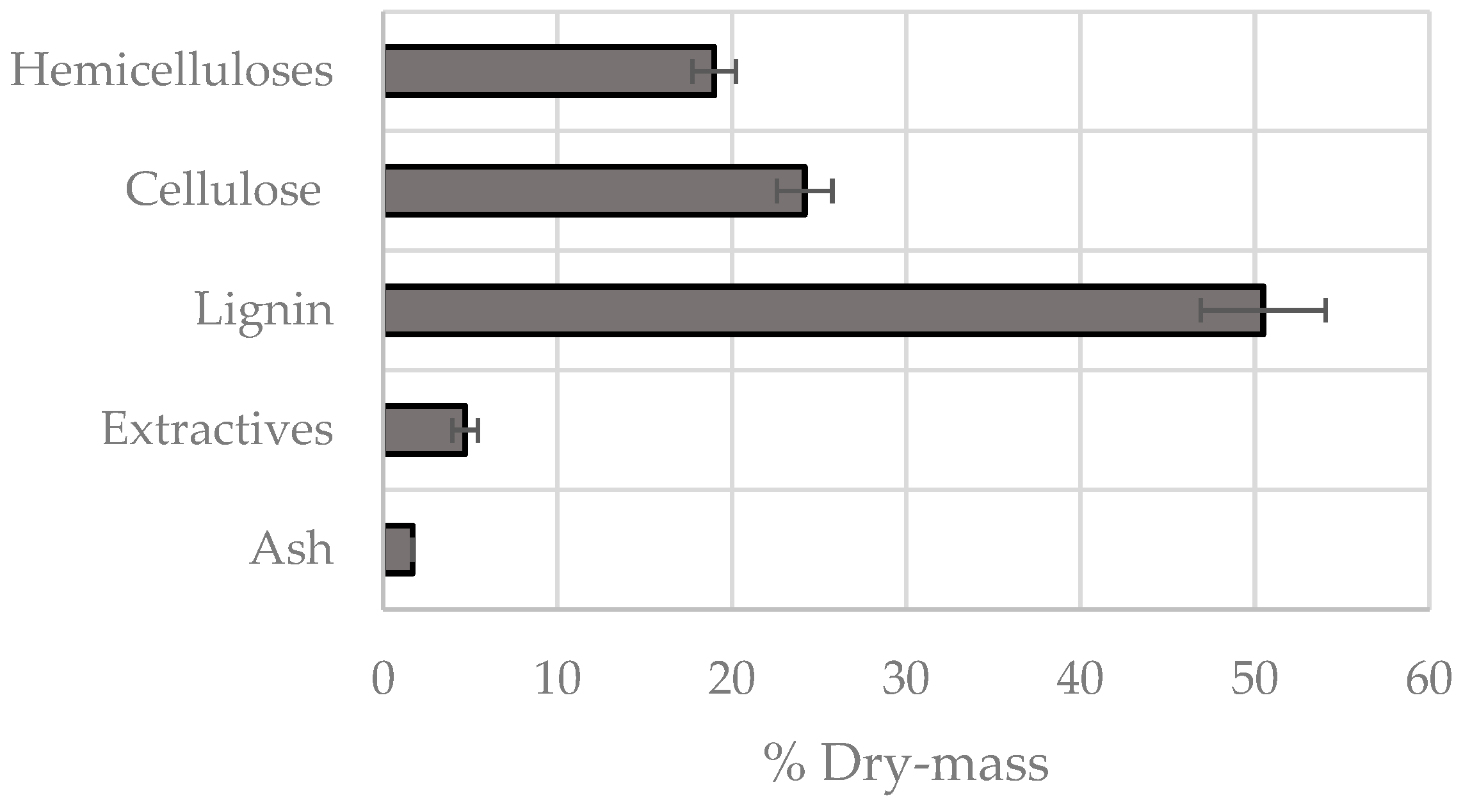

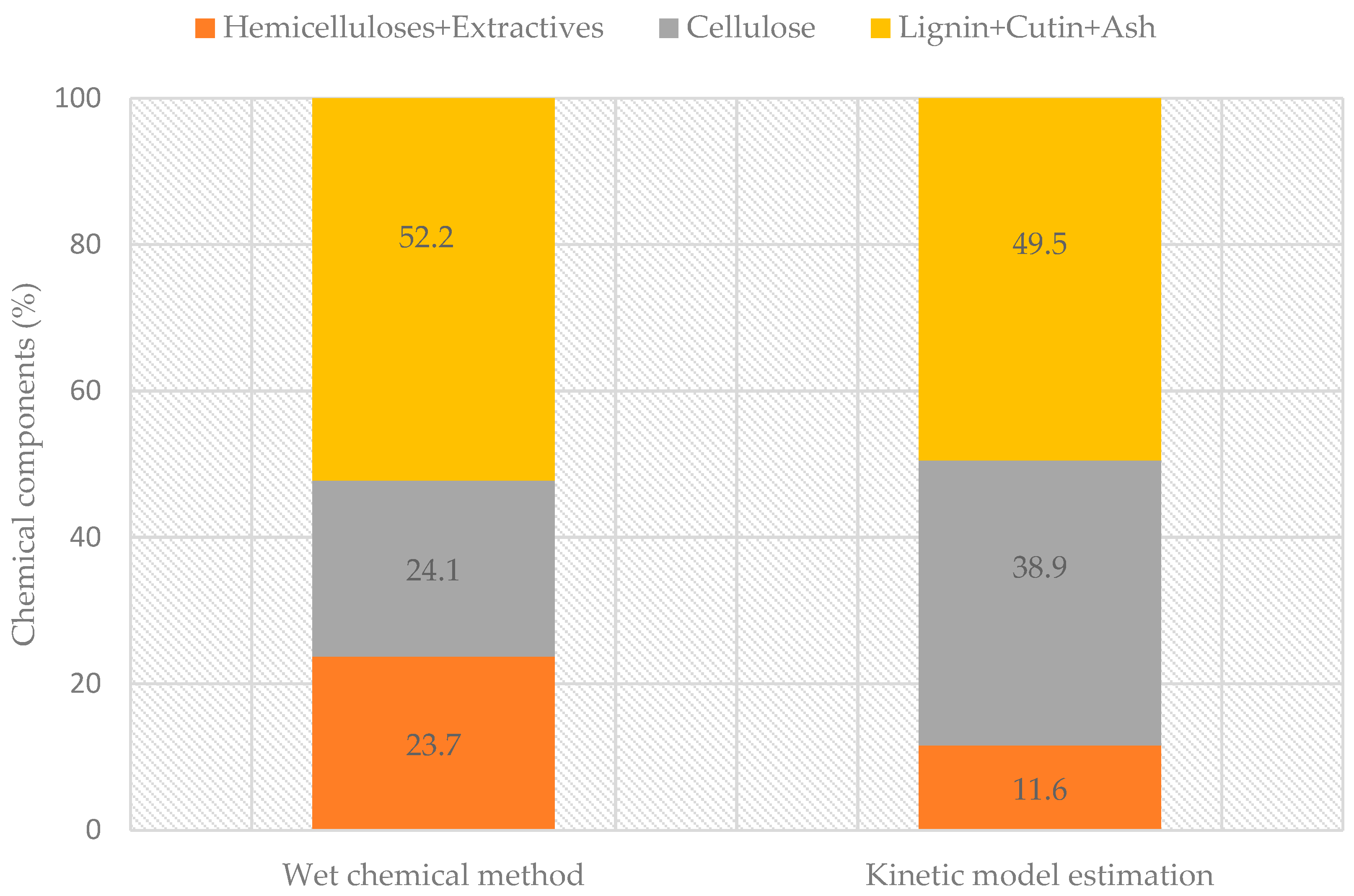

| Kinetic Model Components | PS1 | PS2 | PS3 | PS4 | Charcoal |
|---|---|---|---|---|---|
| Assignment | Moisture | Cellulose | Lignin + Cutin | Hemicellulose + Extractive | Residual Lignin + Ash |
| k (min−1) | 0.990 | 0.035 | 0.021 | 0.900 | – |
| Ea (kJ mol−1) | 10 | 165 | 19 | 147 | – |
| Mass ratio (%) | 4 | 37 | 25 | 11 | 22 |
| RMSE | 0.029 |
| Biochar Component | Composition of PNS (%) | Composition of PNS Biochar (%) |
|---|---|---|
| Moisture | 6.0 ± 0.5 | 1.1 ± 0.0 |
| Ash | 1.2 ± 0.4 | 5.7 ± 0.5 |
| VM | 75.1 ± 0.7 | 18.9 ± 1.9 |
| FC | 17.7 ± 0.4 | 74.3 ± 2.4 |
| FR | 0.24 | 3.74 |
| Estimated HHV (MJ/kg) | 20.7 | 23.4 |
| Estimated energy densification ratio | – | 1.13 |
| Tb (°C) | 544 | 612 |
| Biochar | Moisture Content (%) | Water Retention (%) |
|---|---|---|
| Granular (GC) | 4.57 ± 0.55 | 36.29 ± 0.05 |
| Powder (PC) | 3.62 ± 0.18 | 36.49 ± 1.24 |
| Component | Concentration (mg/L) | |
|---|---|---|
| GC | PC | |
| Al | 0.03 ± 0.01 | 0.01 ± 0.01 |
| B | 0.13 ± 0.01 | 0.16 ± 0.01 |
| Ca | 14.56 ± 0.62 | 17.34 ± 0.97 |
| Cu | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Fe | 0.05 ± 0.01 | 0.04 ± 0.01 |
| K | 150.55 ± 1.51 | 217.48 ± 4.61 |
| Mg | 8.59 ± 0.38 | 25.35 ± 1.48 |
| Mn | 0.12 ± 0.01 | 0.25 ± 0.01 |
| Na | 47.48 ± 1.08 | 49.45 ± 1.50 |
| P | 9.08 ± 0.21 | 28.98 ± 1.07 |
| Si | 22.90 ± 0.33 | 36.07 ± 0.78 |
| Sr | 0.22 ± 0.01 | 0.20 ± 0.01 |
| Zn | 0.03 ± 0.01 | 0.03 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șen, U.; Rodrigues, J.F.G.; Almeida, D.; Fernandes, Â.; Gonçalves, M.; Martins, M.; Santos, D.M.F.; Pereira, H. Pine Nutshells and Their Biochars as Sources of Chemicals, Fuels, Activated Carbons, and Electrode Materials. Processes 2024, 12, 1603. https://doi.org/10.3390/pr12081603
Șen U, Rodrigues JFG, Almeida D, Fernandes Â, Gonçalves M, Martins M, Santos DMF, Pereira H. Pine Nutshells and Their Biochars as Sources of Chemicals, Fuels, Activated Carbons, and Electrode Materials. Processes. 2024; 12(8):1603. https://doi.org/10.3390/pr12081603
Chicago/Turabian StyleȘen, Umut, João F. G. Rodrigues, Daiana Almeida, Ângela Fernandes, Margarida Gonçalves, Marta Martins, Diogo M. F. Santos, and Helena Pereira. 2024. "Pine Nutshells and Their Biochars as Sources of Chemicals, Fuels, Activated Carbons, and Electrode Materials" Processes 12, no. 8: 1603. https://doi.org/10.3390/pr12081603






