Production and Application of a New Biosurfactant for Solubilisation and Mobilisation of Residual Oil from Sand and Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism, Maintenance Medium, and Growth Medium
2.2. Growth of Inoculum
2.3. Production Media and Culture Conditions
2.4. Isolation of Biosurfactant
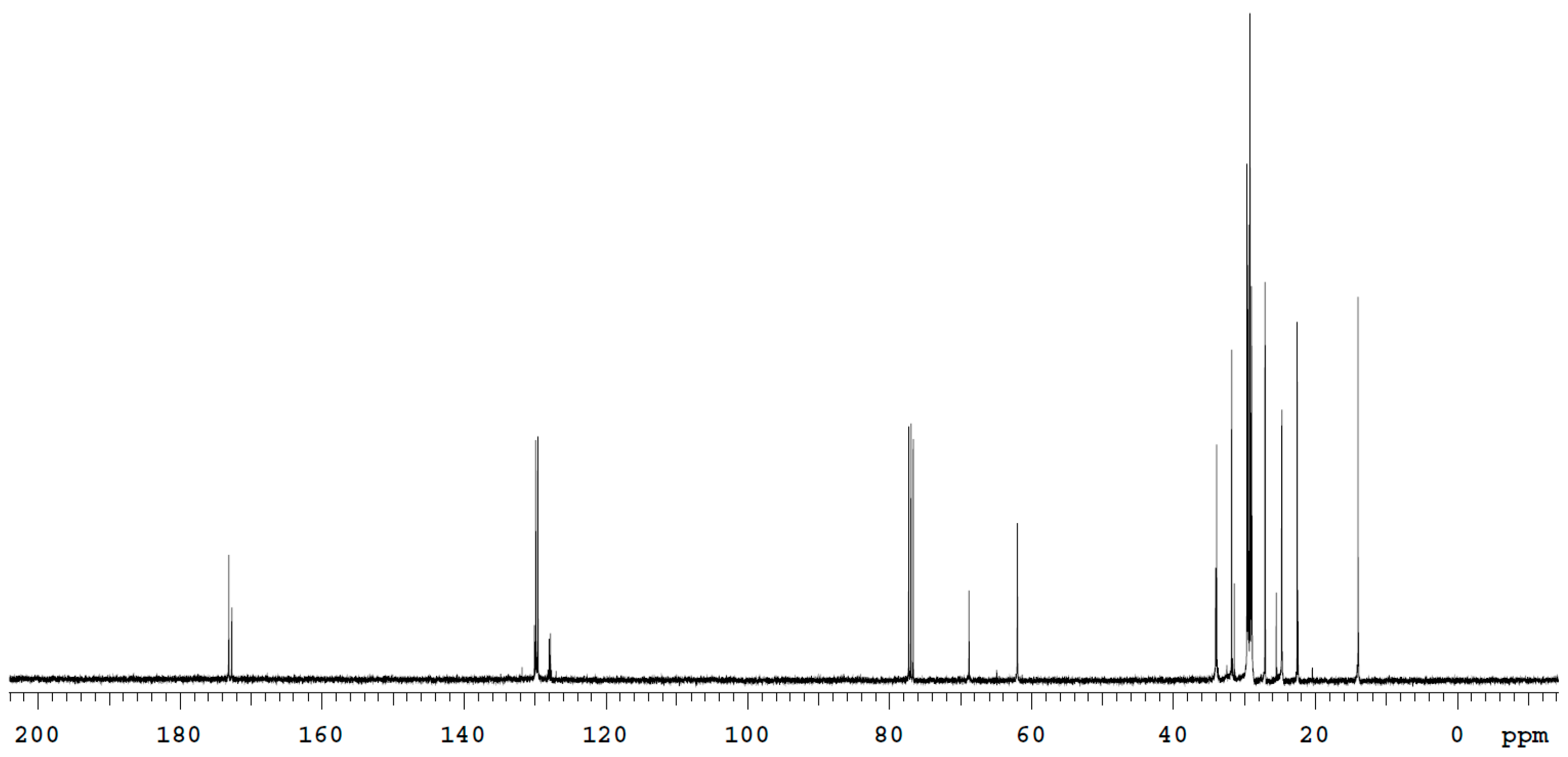
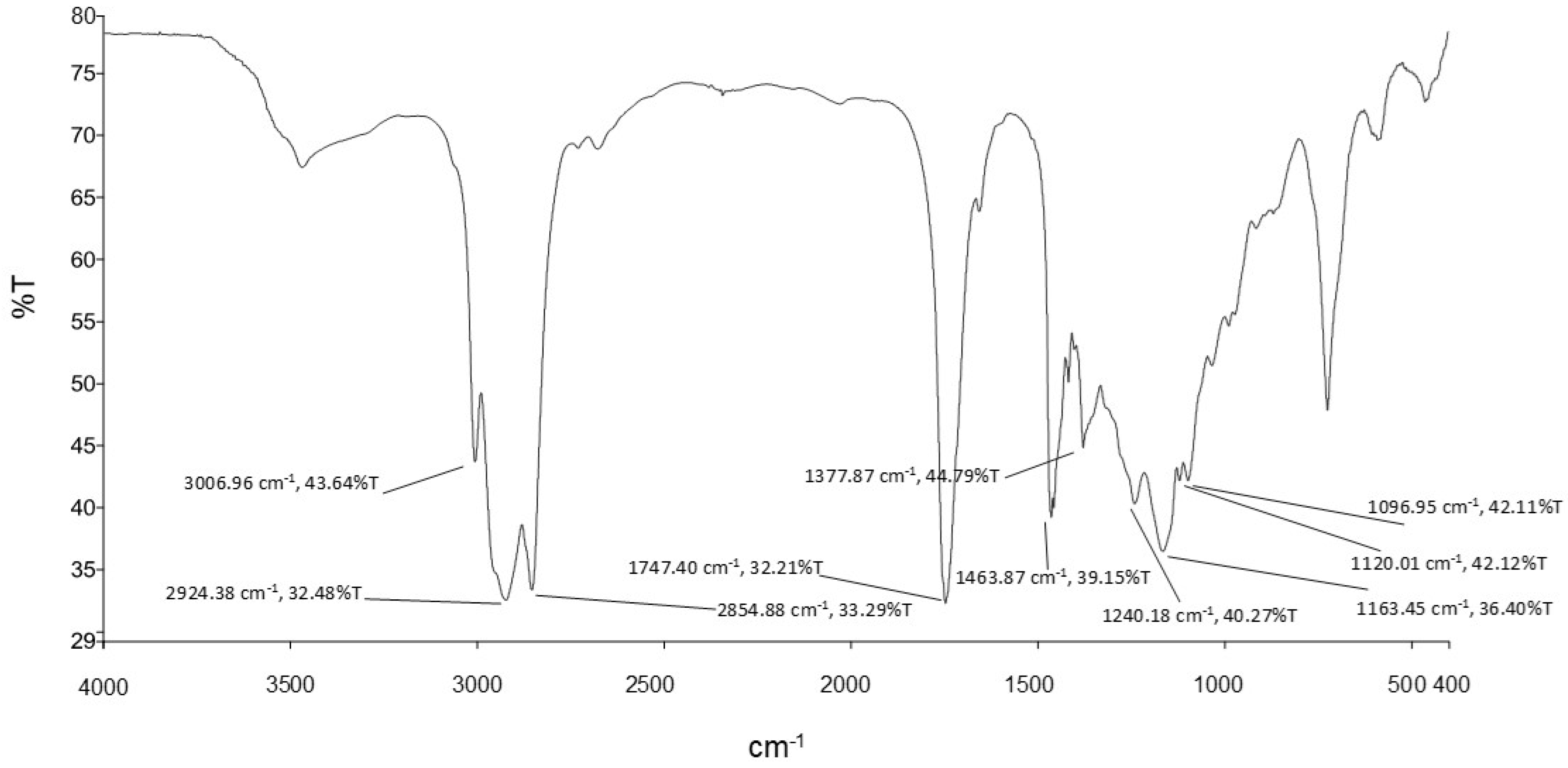
2.5. Biosurfactant Characterisation
2.6. Surface Tension
2.7. Emulsification Index (E24)
2.8. Effect of Environmental Factors
2.9. Determination of Critical Micelle Concentration
2.10. Toxicity Test with Artemia salina as Indicator
2.11. Ecotoxicity Tests with Danio rerio as Indicator
2.12. Remediation Experiment with Oil Derivative Adsorbed to Sand—Kinetic Test
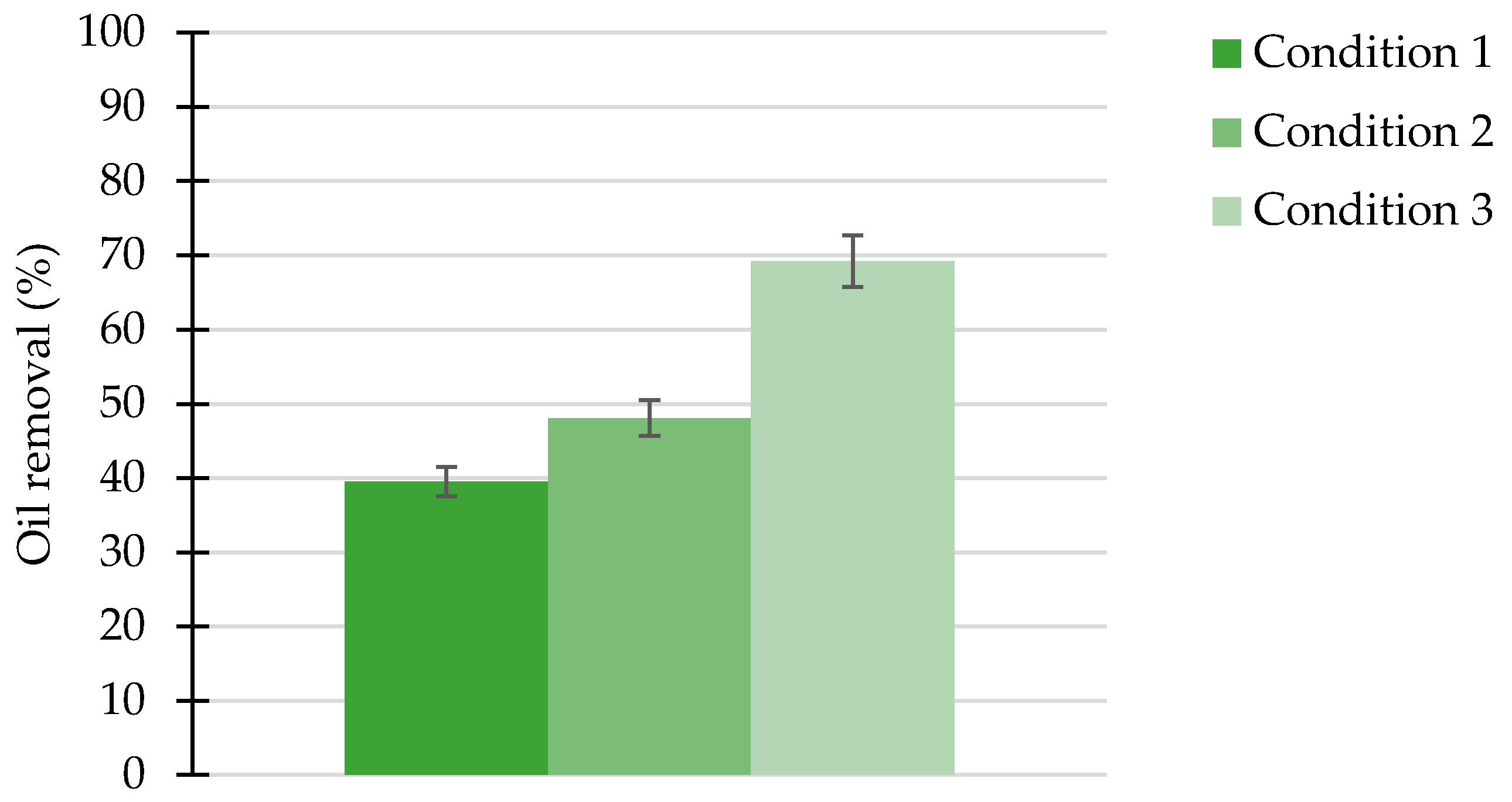
2.13. Removal of Oil Derivative Adsorbed to Sand in Packed Columns—Static Test
2.14. Analysis of Oil Derivative Removed from Sand
2.15. Remediation Experiment of Oil Derivative in Seawater
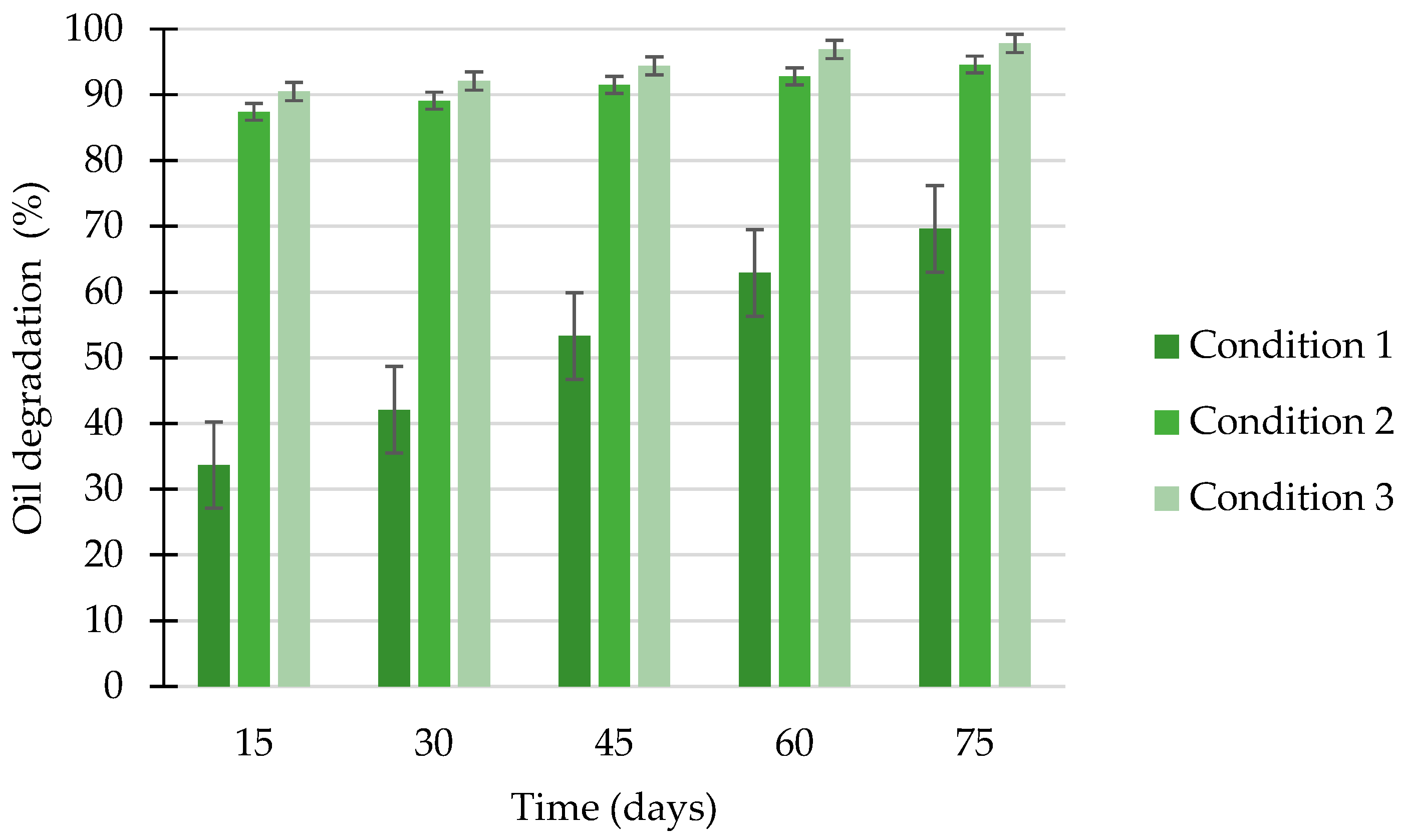
2.16. Calculation of Degradation Efficiency of Oil Derivative from Seawater
2.17. Statistical Analysis
3. Results and Discussion
3.1. Biosurfactant Production, Isolation, and Emulsifying Capacity
3.2. Biosurfactant Characterisation
3.3. Critical Micelle Concentration
3.4. Stability of Biosurfactant
3.5. Toxicity of Biosurfactants to Artemia salina
3.6. Ecotoxicity of Biosurfactant to Danio rerio (Zebrafish)
3.7. Remediation of Oil Derivative Adsorbed to Sand by Biosurfactant—Kinetic Test
3.8. Removal of Oil Derivative Adsorbed to Sand by Biosurfactant in Packed Columns—Static Test
3.9. Remediation of Seawater Contaminated with Spilled Oil Derivative
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorat, B.N.; Sonwani, R.K. Current technologies and future perspectives for the treatment of complex petroleum refinery wastewater: A review. Bioresour. Technol. 2022, 355, 127263. [Google Scholar] [CrossRef]
- Wei, Z.; Wei, Y.; Liu, Y.; Niu, S.; Xu, Y.; Park, J.-H.; Wang, J.J. Biochar-based materials as remediation strategy in petroleum hydrocarbon-contaminated soil and water: Performances, mechanisms, and environmental impact. J. Environ. Sci. 2024, 138, 350–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Y.; Zhong, S.; Zhang, L. AOPs-based remediation of petroleum hydrocarbons contaminated soils: Efficiency, influencing factors and environmental impacts. Chemosphere 2020, 246, 125726. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Rajabi, H.; Sharifipour, M. Geotechnical properties of hydrocarbon-contaminated soils: A comprehensive review. Bull. Eng. Geol. Environ. 2019, 78, 3685–3717. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, 1913–1922. [Google Scholar] [CrossRef]
- Jabbar, N.M.; Alardhi, S.M.; Mohammed, A.K.; Salih, I.K.; Albayati, T.M. Challenges in the implementation of bioremediation processes in petroleum-contaminated soils: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100694. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, A.; Fouillaud, M.; Dufossé, L.; Haq, I.u.; Mukhtar, H. Microbial Cell Factories: Biodiversity, Pathway Construction, Robustness, and Industrial Applicability. Microbiol. Res. 2024, 15, 247–272. [Google Scholar] [CrossRef]
- Shi, T.Q.; Darvishi, F.; Cao, M.; Ji, B.; Ji, X.J. Design and construction of microbial cell factories for the production of fuels and chemicals. Front. Bioeng. Biotechnol. 2023, 11, 1198317. [Google Scholar] [CrossRef]
- Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Perspectives on the design of microbial cell factories to produce prenylflavonoids. Int. J. Food Microbiol. 2022, 367, 109588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Zhou, J.J.; Quan, C.S.; Xiu, Z.L. Advances in industrial microbiome based on microbial consortium for biorefinery. Bioresour. Biproc. 2017, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Pal, M.; Sharma, R.K. Pichia as yeast cell factory for production of industrially important bio-products: Current trends, challenges, and future prospects. J. Bioresour. Bioprod. 2023, 8, 108–124. [Google Scholar] [CrossRef]
- Bi, H.; Mulligan, C.N.; Zhang, B.; Biagi, M.; An, C.; Yang, X.; Lyu, L.; Chen, X. A review on recent development in the use of surface washing agents for shoreline cleanup after oil spills. Ocean Coast. Manag. 2023, 245, 106877. [Google Scholar] [CrossRef]
- Charoentanaworakun, C.; Assabumrungrat, S.; Soottitantawat, A. Techno-economic environmental analysis of sustainable anionic biosurfactant production from palm fatty acid distillate. ACS Omega 2023, 8, 45045–45055. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.C.; Bui, X.-T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Fact. 2021, 20, 120. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.-M. Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiol. Lett. 2010, 311, 140–146. [Google Scholar] [CrossRef]
- Jadav, S.; Sakthipriya, N.; Doble, M.; Sangwai, J.S. Effect of biosurfactants produced by Bacillus subtilis and Pseudomonas aeruginosa on the formation kinetics of methane hydrates. J. Nat. Gas Sci. Eng. 2017, 43, 156–166. [Google Scholar] [CrossRef]
- Negrete, P.S.; Ghilardi, C.; Pineda, L.R.; Pérez, E.; Herrera, M.L.; Borroni, V. Biosurfactant production by Rhodococcus ALDO1 isolated from olive mill wastes. Biocatal. Agric. Biotechnol. 2024, 57, 103106. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green surfactants (biosurfactants): A petroleum-free substitute for sustainability-comparison, applications, market, and future prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef] [PubMed]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K.; Dayarathne, H.N.P.; Jamal, A.; Aftab, M.N.; Mainali, B.; Choi, Y. Current advances in the classification, production, properties and applications of microbial biosurfactants—A critical review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef]
- Thapliyal, U.; Negi, S. Biosurfactants: Recent trends in healthcare applications. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Mouafo, H.T.; Pahane, M.M.; Mbarga, A.J.M.; Sokamte, A.T.; Somashekar, D.; Mbawala, A. Methods of purification and characterization of biosurfactants: An overview. J. Adv. Biol. Biotechnol. 2023, 26, 35–53. [Google Scholar] [CrossRef]
- Sales da Silva, I.G.; Almeida, F.C.G.; Rocha e Silva, N.M.P.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Soil bioremediation: Overview of technologies and trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, characterization and application of biosurfactant in various industries: A critical review on progress, challenges and perspectives. Environ. Technol. Innov. 2021, 24, 102090. [Google Scholar] [CrossRef]
- Kanawal, H.H.; Khan, K.; Abdullah, M. Mass production and factors affecting biosurfactant productivity using bioreactors. In Green Sustainable Process for Chemical and Environmental Engineering and Science Microbially-Derived Biosurfactants for Improving Sustainability in Industry; Inamuddin, A.C.O., Asiri, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 379–398. [Google Scholar]
- Gaur, V.K.; Sharma, P.; Gupta, S.; Varjani, S.; Srivastava, J.K.; Wong, J.W.C.; Ngo, H.H. Opportunities and challenges in omics approaches for biosurfactant production and feasibility of site remediation: Strategies and advancements. Environ. Technol. Innov. 2022, 25, 102132. [Google Scholar] [CrossRef]
- Aslam, A.; Ishtaiq, M.; Badar, R.; Nazir, M.S.; Tahir, Z. Applications of biosurfactants in the production of industrially relevant bioproducts. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Microbially Derived Biosurfactants for Improving Sustainability in Industry; Inamuddin, A.C.O., Asiri, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 173–201. [Google Scholar]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Dolman, B.; Fuju, W.; Winterburn, J.B.; Ben, M. Integrated production and separation of biosurfactants. Process Biochem. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Treinen, C.; Noll, P.; Henkel, M.; Hausmann, R.; Alzate, C.A.C. Assessing the feasibility and sustainability of a surfactin production process: A techno-economic and environmental analysis. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.; Veras, B.O.; Ribeiro, B.G.; Aguiar, J.S.; Guerra, J.M.C.; Luna, J.M.; Sarubbo, L.A. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. PeerJ 2020, 8, e9064. [Google Scholar] [CrossRef] [PubMed]
- Meylheuc, T.; Van Oss, C.J.; Bellon-Fontaine, M.N. Adsorption of biosurfactants on solid surfaces and consequences regarding the bioahesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001, 91, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.G.; Goldenberg, B.G. Surface active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Han, Y.; Liu, E.; Liu, L.; Zhang, B.; Wang, Y.; Gui, M.; Wu, R.; Li, P. Rheological, emulsifying and thermostability properties of two exopolysaccharides produced by Bacillus amyloliquefaciens LPL061. Carbohydr. Polym. 2015, 115, 230–237. [Google Scholar] [CrossRef]
- Das, A.J.; Kumar, R. Production of biosurfactant from agro-industrial waste by Bacillus safensis J2 and exploring its oil recovery efficiency and role in restoration of diesel contaminated soil. Environ. Technol. Innov. 2019, 16, 100450. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, French, 2013. [Google Scholar]
- Alves, R.N.; Mariz, C.F., Jr.; Melo, A.M.K.; Cavalcanti, M.G.N.; Melo, T.J.B.; Arruda-Santos, R.H.; Zanardi-Lamardo, E.; Carvalho, P.S.M. Contamination and toxicity of surface Waters along rural and urban regions of the Capibaribe river in tropical Northeastern Brazil. Environ. Toxicol. Chem. 2021, 40, 3063–3077. [Google Scholar] [CrossRef] [PubMed]
- Beekhuijzen, M.; De Koning, C.; Flores-Guillén, M.E.; De Vries-Buitenweg, S.; Tobor-Kaplon, M.; Van De Waart, B.; Emmen, H. From cutting edge to guideline: A first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod. Toxicol. 2015, 56, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.N.; Mariz, C.F.; Paulo, D.V.; Carvalho, P.S.M. Toxicity of effluents from gasoline stations oil-water separators to early life stages of zebrafish Danio rerio. Chemosphere 2017, 178, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, 0146021. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Ndegwa, P.M.; Shoda, M.; Phae, C.-G. Bioremediation of oil-contaminated soil using Candida catenulate and food waste. Environ. Pollut. 2008, 156, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Mercadé, M.E.; Bosch, M.P.; Parra, J.L.; Espuny, M.J.; Manresa, M.A.; Guinea, J. Effect of the carbon source on biosurfactant production byPseudomonas aeruginosa 44T1. Biotechnol. Lett. 1989, 11, 871–874. [Google Scholar] [CrossRef]
- Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Bose, S.; Kumar, P.S.; Rangasamy, G. Exploring the role of biosurfactants in the remediation of organic contaminants with a focus on the mechanism- a review. J. Mol. Liq. 2024, 393, 123585. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Kaur, G.; Wang, H.; To, M.H.; Roelants, S.L.K.W.; Soetaert, W.; Lin, C.S.K. Efficient sophorolipids production using food waste. J. Clean. Prod. 2019, 232, 1–11. [Google Scholar] [CrossRef]
- Bacille, N.; Babonneau, F.; Banat, I.M.; Ciesielska, K.; Cuvier, A.; Devreese, B.; Everaert, B.; Lydon, H.; Marchant, R.; Mitchell, C.A.; et al. Development of a Cradle-to-Grave Approach for Acetylated Acidic Sophorolipid Biosurfactants. ACS Sustain. Chem. Eng. 2017, 5, 1186–1198. [Google Scholar] [CrossRef]
- Konishi, M.; Yoshida, Y.; Horiuchi, J. Efficient production of sophorolipids by Starmerella bombicola using a corncob hydrolysate medium. J. Biosci. Bioeng. 2014, 119, 317–322. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Castillejos, M.; Koh, A.; Gross, R.; Sánchez, A.; Font, X.; Gea, T. Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J. Clean. Prod. 2018, 172, 2735–2747. [Google Scholar] [CrossRef]
- Jadhav, J.V.; Pratap, A.P.; Kale, S.B. Evaluation of sunflower oil refinery waste as feedstock for production of sophorolipid. Process Biochem. 2019, 78, 15–24. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Sivapragasam, M.; Moniruzzaman, M.; Talukder, M.M.R.; Yusup, S.B.; Goto, M. Production of sophorolipids by Starmerella bombicola yeast using new hydrophobic substrates. Biochem. Eng. J. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Kamthan, M.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef]
- Marcelino, P.R.F.; Peres, G.F.D.; Terán-Hilares, R.; Pagnocca, F.C.; Rosa, C.A.; Lacerda, T.M.; Dos Santos, J.C.; Da Silva, S.S. Biosurfactants production by yeasts using sugarcane bagasse hemicellulosic hydrolysate as new sustainable alternative for lignocellulosic biorefineries. Ind. Crop. Prod. 2019, 129, 212–223. [Google Scholar] [CrossRef]
- Dierickx, S.; Maes, K.; Roelants, S.L.K.W.; Pomian, B.; Meulebroek, L.V.; De Maeseneire, S.L.; Vanhaecke, L.; Soetaert, W.K. A multi-omics study to boost continuous bolaform sophorolipid production. New Biotechnol. 2022, 66, 107–115. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Aiad, I.; Osman, M.E.; Abo-ELnasr, A.A.; Kobisy, A.S. Production of biosurfactants by Bacillus licheniformis and Candida albicans for application in microbial enhanced oil recovery. Egypt. J. Pet. 2016, 25, 293–298. [Google Scholar] [CrossRef]
- Lira, I.R.A.S.; Santos, E.M.S.; Santos, J.C.V.; Silva, R.R.; Silva, Y.A.; Durval, I.J.B.; Guerra, J.C.M.; Sarubbo, L.A.; Luna, J.M. Production of biossurfactant by Candida guilliermondii and application in a mayonnaise emulsion. Chem. Eng. Trans. 2021, 87, 259–264. [Google Scholar] [CrossRef]
- Santos, J.C.V.; Santos, E.M.S.; Silva, Y.A.; Lira, I.R.A.S.; Silva, R.R.; Durval, I.J.B.; Sarubbo, L.A.; Luna, J.M. Application of Candida lipolytica biosurfactant for bioremediation of motor oil from contaminated environment. Chem. Eng. Trans. 2021, 86, 649–654. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar] [CrossRef]
- Rubio-Ribeaux, D.; Andrade, R.F.S.; Silva, G.S.; Holanda, R.S.; Pele, M.A.; Nunes, P.; Junior, J.C.V.; Resende-Stoianoff, M.A.; Campos-Takaki, G.M. Promising biosurfactant produced by a new Candida tropicalis UCP 1613 strain using substrates from renewable-resources. Afr. J. Microbiol. Res. 2017, 11, 981–991. [Google Scholar] [CrossRef]
- Camargo, F.P.; Menezes, A.J.; Tonello, P.S.; Santos, A.C.A.; Duarte, I.C.S. Characterization of biosurfactant from yeast using residual soybean oil under acidic conditions and their use in metal removal processes. FEMS Microbiol. Lett. 2018, 365, fny098. [Google Scholar] [CrossRef]
- Price, N.P.J.; Ray, K.J.; Vermillion, K.E.; Dunlap, C.A.; Kurtzman, C.P. Structural characterization of novel sophorolipid biosurfactants from a newly identified species of Candida yeast. Carbohydr. Res. 2012, 348, 33–41. [Google Scholar] [CrossRef]
- Daverey, A.; Pakshirajan, K. Sophorolipids from Candida bombicola using mixed hydrophilic substrates: Production, purification and characterization. Colloids Surf. B 2010, 79, 246–253. [Google Scholar] [CrossRef]
- Silveira, V.A.I.; Marim, B.M.; Hipólito, A.; Gonçalvez, M.C.; Mali, S.; Kobayashi, R.K.T.; Celligoi, M.A.P.C. Characterization and antimicrobial properties of bioactive packaging films based on polylactic acid-sophorolipid for the control of foodborne pathogens. Food Packag. Shelf Life 2020, 26, 100591. [Google Scholar] [CrossRef]
- Bacille, N.; Poirier, A.; Griel, P.L.; Pernot, P.; Pala, M.; Roelants, S.; Soetaert, W.; Stevens, C.V. Aqueous self-assembly of a wide range of sophorolipid and glucolipid microbial bioamphiphiles (biosurfactants): Considerations on the structure-properties relationship. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132518. [Google Scholar] [CrossRef]
- Roy, A.; Khan, M.R.; Mukherjee, A.K. Recent advances in the application of microbial biosurfactants in food industries: Opportunities and challenges. Food Control 2024, 163, 110465. [Google Scholar] [CrossRef]
- Ashish; Debnath, M. Application of biosurfactant produced by an adaptive strain of C. tropicalis MTCC230 in microbial enhanced oil recovery (MEOR) and removal of motor oil from contaminated sand and water. J. Petrol. Sci. Eng. 2018, 170, 40–48. [Google Scholar] [CrossRef]
- Velmurugan, M.; Baskaran, A.; Kumar, S.D.; Sureka, I.; Raj, E.A.; Emelda, J.; Sathiyamurthy, K. Screening, stability and antibacterial potential of rhamnolipids from Pseudomonas sp., isolated from hydrocarbon contaminated soil. J. Appl. Pharm. Sci. 2015, 5, 26–33. [Google Scholar] [CrossRef]
- Murawski, S.A.; Ainsworth, C.H.; Gilbert, S.; Hollander, D.J.; Paris, C.B.; Schlüter, M.; Wetzel, D.L. Modernizing protocols for aquatic toxicity testing of oil and dispersant. In Scenarios and Responses to Future Deep Oil Spills, 1st ed.; Mitchelmore, C.L., Griffitt, R.J., Coelho, G.M., Wetzel, D.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 239–252. [Google Scholar]
- Silva, I.A.; Almeida, F.C.G.; Souza, T.C.; Bezerra, K.G.O.; Durval, I.J.B.; Converti, A.; Sarubbo, L.A. Oil spills: Impacts and perspectives of treatment technologies with focus on the use of green surfactants. Environ. Monit. Assess. 2022, 194, 143. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.I.; Campos, J.M.; Meira, H.M.; Sarubbo, L.A.; Luna, J.M. A Biosurfactant from Candida bombicola: Its synthesis, characterization, and its application as a food emulsions. Foods 2022, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhattacharyya, A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J. Clean. Prod. 2017, 150, 127–134. [Google Scholar] [CrossRef]
- Yi, X.; Wei, Y.; Zhai, W.; Wang, P.; Liu, D.; Zhou, Z. Effects of three surfactants on the degradation and environmental risk of metolachlor in aquatic environment. Chemosphere 2022, 300, 134295. [Google Scholar] [CrossRef] [PubMed]
- Embry, M.R.; Belanger, S.E.; Braunbeck, T.A.; Galay-Burgos, M.; Halder, M.; Hinton, D.E.; Léonard, M.A.; Lillicrap, A.; Norbeg-King, T.; Whale, G. The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquat. Toxicol. 2010, 97, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, X.; Sun, M.; Wei, Z.; Wang, Y.; Gao, A.; Chen, D.; Zhao, X.; Feng, X. Exploring the effects of different types of surfactants on zebrafish embryos and larvae. Sci. Rep. 2015, 5, 10107. [Google Scholar] [CrossRef] [PubMed]
- Korbut, R.; Skjolding, L.M.; Mathiessen, H.; Jaafar, R.; Li, X.; Jørgensen, L.V.G.; Kania, P.W.; Wu, B.; Buchmann, K. Toxicity of the antiparasitic lipopeptide biosurfactant SPH6 to green algae, cyanobacteria, crustaceans and zebrafish. Aquat. Toxicol. 2022, 243, 106072. [Google Scholar] [CrossRef]
- El-Harbawi, M. Toxicity Measurement of Imidazolium Ionic Liquids Using Acute Toxicity Test. Procedia Chem. 2014, 9, 40–52. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Majdalawieh, A.F.; Abdullah, A.M.; Younes, N.; Da’as, S.I.; Radwan, A.B.; Sliem, M.H.; Ech-Cherif, H.; Pintus, G.; Nasrallah, G.K. AEO-7 surfactant is “super toxic” and induces severe cardiac, liver, and locomotion damage in zebrafish embryos. Environ. Sci. Eur. 2020, 32, 149. [Google Scholar] [CrossRef]
- Johann, S.; Seiler, T.-B.; Tiso, T.; Bluhm, K.; Blank, L.M.; Hollert, H. Mechanism-specific and whole-organism ecotoxicity of mono-rhamnolipids. Sci. Total Environ. 2016, 548–549, 155–163. [Google Scholar] [CrossRef]
- Bejarano, A.C. Critical review and analysis of aquatic toxicity data on oil spill dispersants. Environ. Toxicol. Chem. 2018, 37, 2989–3001. [Google Scholar] [CrossRef]
- CONAMA. Conselho Nacional de Meio Ambiente, Brasil. 2021. Available online: http://www.mma.gov.br/port/conama/estr.cfm/ (accessed on 3 March 2024).
- Berninger, J.P.; Williams, E.S.; Brooks, B.W. An initial probabilistic hazard assessment of oil dispersants approved by the United States National Contingency Plan. Environ. Toxicol. Chem. 2011, 30, 1704–1708. [Google Scholar] [CrossRef]
- Nitschke, M.; Pastore, G.M. Biossurfactantes: Propriedades e aplicações. Quim. Nova 2002, 25, 772–776. [Google Scholar] [CrossRef]
- Parus, A.; Ciesielski, T.; Woźniak-Karczewska, M.; Ślachciński, M.; Owsianiak, M.; Ławniczak, Ł.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Basic principles for biosurfactant-assisted (bio)remediation of soils contaminated by heavy metals and petroleum hydrocarbons—A critical evaluation of the performance of rhamnolipids. J. Hazard. Mater. 2023, 443, 130171. [Google Scholar] [CrossRef]
- Oluwaseun, A.C.; Kola, O.J.; Mishra, P.; Singh, J.R.; Singh, A.K.; Cameotra, S.S.; Micheal, B.O. Characterization and optimization of a rhamnolipid from Pseudomonas aeruginosa C1501 with novel biosurfactant activities. Sustain. Chem. Pharm. 2017, 6, 26–36. [Google Scholar] [CrossRef]
- Zhao, F.; Li, P.; Guo, C.; Shi, R.-J.; Zhang, Y. Bioaugmentation of oil reservoir indigenous Pseudomonas aeruginosa to enhance oil recovery through in-situ biosurfactant production without air injection. Bioresour. Technol. 2018, 251, 295–302. [Google Scholar] [CrossRef]
- Kim, B.K.; Baek, K.; Ko, S.H.; Yang, J.W. Research and field experiences on electrokinetic remediation in South Korea. Sep. Purif. Technol. 2011, 79, 116–123. [Google Scholar] [CrossRef]
- Adamczak, M.; Bednarski, W. Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antarctica. Biotechnol. Lett. 2000, 22, 313–316. [Google Scholar] [CrossRef]
- Santos, E.M.S.; Lira, I.R.A.S.; Filho, A.A.P.S.; Meira, H.M.; Farias, C.B.B.; Rufino, R.D.; Sarubbo, L.A.; Luna, J.M. Application of the biosurfactant produced by Candida sphaerica as a bioremediation agent. Chem. Eng. Trans. 2020, 79, 451–456. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Ijah, U.J.J.; Manga, S.B.; Bilbis, L.S.; Umar, S. Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int. Biodeterior. Biodegrad. 2013, 81, 28–34. [Google Scholar] [CrossRef]
- Fernandes, P.L.; Rodrigues, E.M.; Paiva, F.R.; Ayupe, B.A.L.; McInerney, M.J.; Tótola, M.R. Biosurfactant, solvents and polymer production by Bacillus subtilis RI4914 and their application for enhanced oil recovery. Fuel 2016, 180, 551–557. [Google Scholar] [CrossRef]
- Kavitha, V.; Mandal, A.B.; Gnanamani, A. Microbial biosurfactant mediated removal and/or solubilization of crude oil contamination from soil and aqueous phase: An approach with Bacillus licheniformis MTCC 5514. Int. Biodeterior. Biodegrad. 2014, 94, 24–30. [Google Scholar] [CrossRef]
- Jain, R.M.; Mody, K.; Mishra, A.; Jha, B. Physicochemical characterization of biosurfactant and its potential to remove oil from soil and cotton cloth. Carbohydr. Polym. 2012, 89, 1110–1116. [Google Scholar] [CrossRef]
- Wang, X.; Cai, T.; Wen, W.; Zhang, Z. Effect of biosurfactant on biodegradation of heteroatom compounds in heavy oil. Fuel 2018, 230, 418–429. [Google Scholar] [CrossRef]
- Aparna, A.; Srinikethan, G.; Hedge, S. Effect of addition of biosurfactant produced by Pseudomonas ssp. on biodegradation of crude oil. In International Proceedings of Chemical, Biological & Environmental Engineering, 2nd ed.; World Scientific: Singapore, 2011; Volume 6, pp. 71–75. [Google Scholar]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Dirisala, V.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Saeki, H.; Sasaki, K.M.; Komatsu, O.; Miura, A.; Matsuda, H. Oil spill remediation by using the remediation agent JE1058BS that contains a biosurfactant produced by Gordonia sp. strain JE-1058. Bioresour. Technol. 2009, 100, 572–577. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Rojas, O.J.; Fingas, M.; Gupta, B.S. Cellulosic substrates for removal of pollutants from aqueous systems: A review. 3. Spilled oil and emulsified organic liquids. BioResources 2013, 8, 3038–3097. [Google Scholar] [CrossRef]
- Couto, C.R.A.; Jurelevicius, D.A.; Alvarez, V.M.; Elsas, J.D.; Seldin, L. Response of the bacterial community in oil-contaminated marine water to the addition of chemical and biological dispersants. J. Environ. Manag. 2016, 184, 473–479. [Google Scholar] [CrossRef]
- Pi, Y.; Bao, M.; Liu, Y.; Lu, T.; He, R. The contribution of chemical dispersants and biosurfactants on crude oil biodegradation by Pseudomonas sp. LSH-7′. J. Clean. Prod. 2017, 153, 74–82. [Google Scholar] [CrossRef]
- Jin, L.; Garamus, V.M.; Liu, F.; Xiao, J.; Eckerlebe, H.; Willumeit-Römer, R.; Mu, B.; Zou, A. Interaction of a biosurfactant, surfactin with a cationic gemini surfactant in aqueous solution. J. Colloid Interface Sci. 2016, 481, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.U.H.; Moniruzzaman, M.; Sivapragasam, M.; Talukder, M.M.R.; Yusup, S.B.; Goto, M. A binary mixture of a biosurfactant and an ionic liquid surfactant as a green dispersant for oil spill remediation. J. Mol. Liq. 2019, 280, 111–119. [Google Scholar] [CrossRef]





| Medium | Components of Production Medium (g/L) |
|---|---|
| Medium 1 | Sunflower oil: 50; Glucose: 25; Yeast extract: 1; KH2PO4: 0.5; MgSO4·7H2O: 0.5; NaNO3: 3 |
| Medium 2 | Sunflower oil: 50; Sucrose: 25; Yeast extract: 1; KH2PO4: 0.5; MgSO4·7H2O: 0.5; NaNO3: 3 |
| Medium 3 | Canola oil: 12; Glucose: 100; Corn steep liquor: 5; K2HPO4: 1; (NH4)2SO4: 4; MgSO4·7H2O: 0.5 |
| Medium 4 | Canola oil: 12; Sucrose: 100; Corn steep liquor: 5; K2HPO4: 1; (NH4)2SO4: 4; MgSO4·7H2O: 0.5 |
| Medium 5 | Canola oil: 12; Glucose: 100; Corn steep liquor: 5 |
| Medium 6 | Canola oil: 12; Sucrose: 100; Corn steep liquor: 5 |
| Medium 7 | Crude cotton seed oil: 100; Glucose: 100; Urea: 1.5; K2HPO4: 1; Corn steep liquor: 4; NaCl: 0.1 |
| Medium 8 | Refined cotton seed oil: 100; Glucose: 100; Urea: 1.5; K2HPO4: 1; Corn steep liquor: 4; NaCl: 0.1 |
| Medium 9 | Crude cotton seed oil: 100; Sucrose: 100; Urea: 1.5; K2HPO4: 1; Corn steep liquor: 4; NaCl: 0.1 |
| Medium 10 | Refined cotton seed oil: 100; Sucrose: 100; Urea: 1.5; K2HPO4: 1; Corn steep liquor: 4; NaCl: 0.1 |
| Biosurfactant | Surface Tension (mN/m) | Yield (g/L) | Emulsification Index (%) |
|---|---|---|---|
| Medium 1 | 32.4 ± 0.2 | 8.1 | 83.3 ± 0.1 |
| Medium 2 | 32.8 ± 0.1 | 7.3 | 84.4 ± 0.4 |
| Medium 3 | 38.0 ± 0.1 | 11.4 | 77.0 ± 0.1 |
| Medium 4 | 32.7 ± 0.3 | 23.0 | 96.2 ± 0.1 |
| Medium 5 | 32.8 ± 0.5 | 7.7 | 90.2 ± 0.1 |
| Medium 6 | 32.9 ± 0.2 | 5.3 | 90.5 ± 0.1 |
| Medium 7 | 33.6 ± 0.6 | 5.4 | 72.9 ± 0.1 |
| Medium 8 | 33.1 ± 0.1 | 8.7 | 91.0 ± 0.1 |
| Medium 9 | 33.4 ± 0.4 | 10.1 | 89.7 ± 0.1 |
| Medium 10 | 31.3 ± 0.1 | 19.5 | 92.4 ± 0.5 |
| NaCl (v/v) | Surface Tension (%) | Emulsification Index (%) |
| 2 | 33.3 ± 0.02 | 95.5 ± 0.3 |
| 4 | 33.7 ± 0.03 | 95.4 ± 0.3 |
| 6 | 32.2 ± 0.01 | 96.2 ± 0.2 |
| 8 | 33.5 ± 0.02 | 96.2 ± 0.1 |
| 10 | 31.8 ± 0.02 | 96.2 ± 0.2 |
| 12 | 32.4 ± 0.01 | 96.9 ± 0.1 |
| Temperature (°C) | Surface Tension (mN/m) | Emulsification Index (%) |
| 0 | 33.7 ± 0.02 | 96.3 ± 0.2 |
| 5 | 33.9 ± 0.01 | 96.4 ± 0.2 |
| 28 | 32.7 ± 0.01 | 96.3 ± 0.4 |
| 70 | 32.8 ± 0.00 | 92.7 ± 0.2 |
| 100 | 32.8 ± 0.00 | 92.6 ± 0.4 |
| 120 | 33.5 ± 0.18 | 90.4 ± 0.3 |
| pH | Surface Tension (mN/m) | Emulsification Index (%) |
| 2 | 35.0 ± 0.02 | 94.4 ± 0.1 |
| 4 | 34.9 ± 0.03 | 94.5 ± 0.3 |
| 6 | 32.7 ± 0.01 | 95.5 ± 0.2 |
| 8 | 31.8 ± 0.01 | 95.5 ± 0.2 |
| 10 | 34.2 ± 0.01 | 96.4 ± 0.2 |
| 12 | 33.9 ± 0.01 | 97.6 ± 0.1 |
| Biosurfactant Concentration in Saline Water | Mortality of Brine Shrimp Larvae (%) |
|---|---|
| Cell-free broth | no mortality |
| ½ × CMC | no mortality |
| CMC | no mortality |
| 2 × CMC | no mortality |
| 3 × CMC | 10.000 ± 0.000 |
| 5 × CMC | 20.000 ± 0.000 |
| Concentration (mg/L) | Deaths 24 hpe | Deaths 48 hpe | Deaths 96 hpe | Mortality Rate (%) at End of Test (96 h) |
|---|---|---|---|---|
| 600 | 20 | - | - | 100 |
| 300 | 20 | - | - | 100 |
| 150 | 9 | 8 | 3 | 100 |
| 75 | 0 | 4 | 16 | 100 |
| 37.50 | 0 | 4 | 16 | 100 |
| 18.750 | 0 | 3 | 14 | 85 |
| 9.375 | 0 | 0 | 5 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, I.A.; Fortunato, J.G.L.A.; Almeida, F.C.G.; Alves, R.N.; Cunha, M.C.C.; Rufino, R.D.; Fernandes, M.L.B.; Sarubbo, L.A. Production and Application of a New Biosurfactant for Solubilisation and Mobilisation of Residual Oil from Sand and Seawater. Processes 2024, 12, 1605. https://doi.org/10.3390/pr12081605
Silva IA, Fortunato JGLA, Almeida FCG, Alves RN, Cunha MCC, Rufino RD, Fernandes MLB, Sarubbo LA. Production and Application of a New Biosurfactant for Solubilisation and Mobilisation of Residual Oil from Sand and Seawater. Processes. 2024; 12(8):1605. https://doi.org/10.3390/pr12081605
Chicago/Turabian StyleSilva, Ivison Amaro, José Gabriel Lima Alcântara Fortunato, Fabíola Carolina Gomes Almeida, Romulo Nepomuceno Alves, Maristela Casé Costa Cunha, Raquel Diniz Rufino, Mucio Luiz Banja Fernandes, and Leonie Asfora Sarubbo. 2024. "Production and Application of a New Biosurfactant for Solubilisation and Mobilisation of Residual Oil from Sand and Seawater" Processes 12, no. 8: 1605. https://doi.org/10.3390/pr12081605








