A Comparison of the Effects of Low-Temperature Vacuum Drying and Other Methods on Cauliflower’s Nutritional–Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Study Materials and Drying Procedure
2.2. Determination of Drying Parameters
2.3. Determination of Nutritional Parameters
2.4. Determination of Color Parameters
2.5. Extraction Procedure for Antioxidant Compounds
2.6. Determination of Total Phenolic and Flavonoid Contents
2.7. Determination of Antioxidant Potential by Two Assays
2.8. Determination of Antiproliferative Potential
2.9. Statistical Analyses
3. Results and Discussion
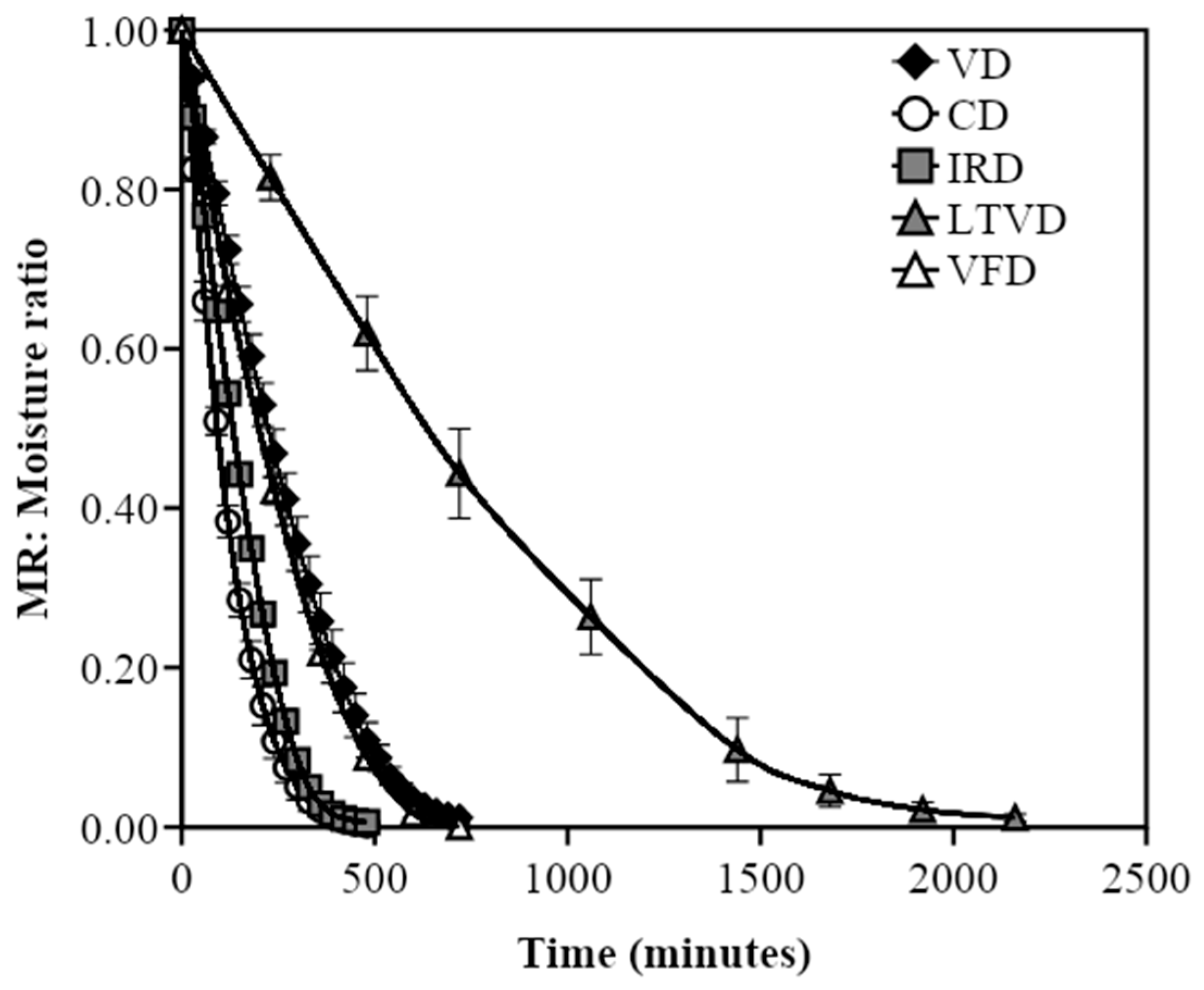
3.1. Comparative Characteristics in Dried Cauliflower
3.2. Nutritional Parameters of Dried Cauliflower
3.3. Color Parameters of Dried Cauliflower
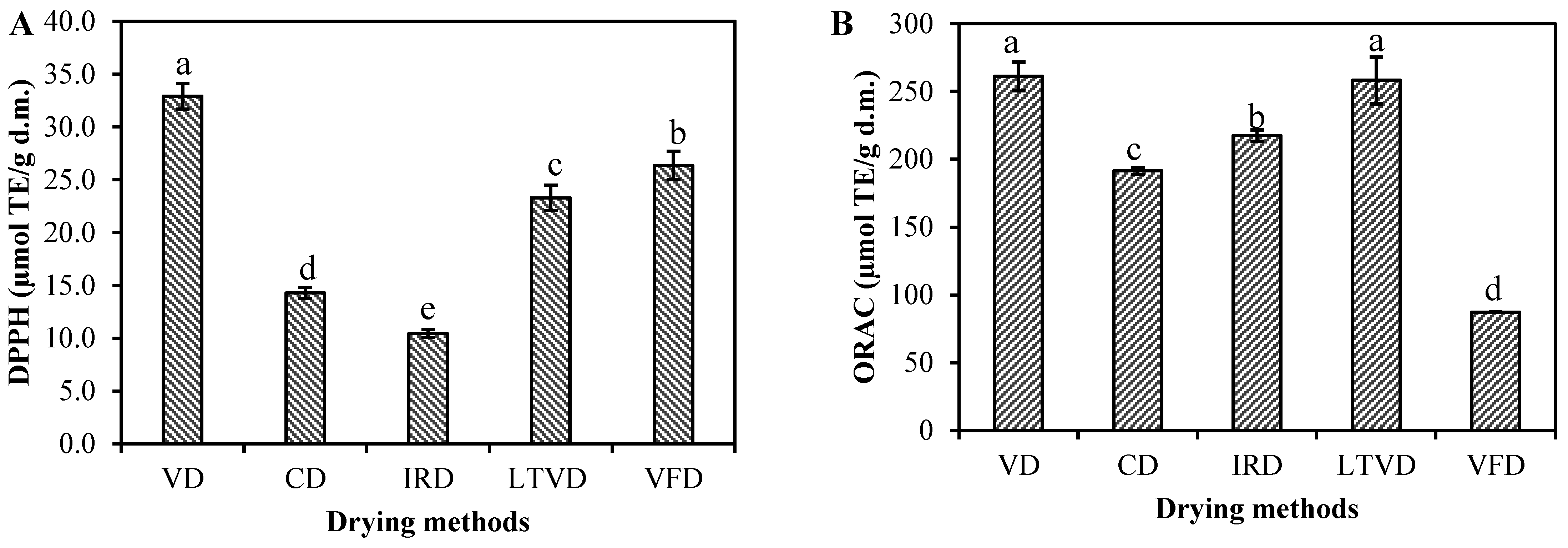
3.4. Antioxidant Potential of Dried Cauliflower
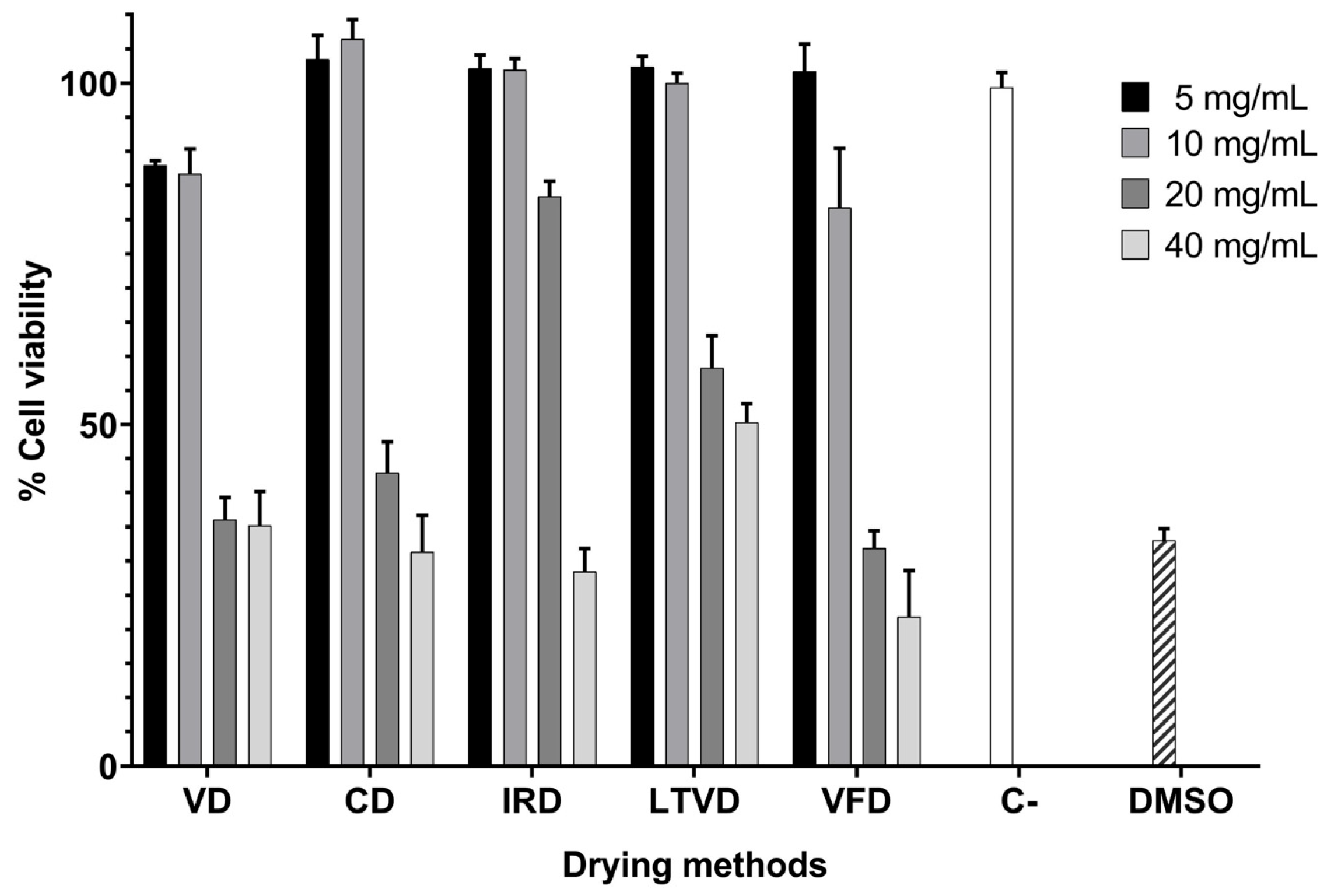
3.5. Antiproliferative Potential of Dried Cauliflower
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, F.A.; Ali, R.F.M. Bioactive compounds and antioxidant activity of fresh and processed white cauliflower. Biomed. Res. Int. 2013, 2013, 367819. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, T.A.A.; Yasmin, L.; Arfin, M.S.; Rahman, A.; Molla, M.M.; Sabuz, A.A.; Afroz, M. Preservation of postharvest quality of fresh cut cauliflower through simple and easy packaging techniques. Appl. Food Res. 2022, 2, 100125. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Pacetti, D.; Lenti, L.; Fiorini, D.; Lucci, P.; Frega, N.G.; Falcone, P.M. Cauliflower by-products as functional ingredient in bakery foods: Fortification of pizza with glucosinolates, carotenoids and phytosterols. Curr. Res. Food Sci. 2023, 6, 100437. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Sehgal, V.K.; Arora, S. Optimization of drying process parameters for cauliflower drying. J. Food Sci. Technol. 2013, 50, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, J.; Srivastava, A.K.; Mishra, B.B.; Suprasanna, P.; Variyar, P.S. Glucosinolates breakdown and enhanced nitrile formation in gamma irradiated minimally processed cauliflower (Brassica oleracia). Radiat. Phys. Chem. 2023, 205, 110672. [Google Scholar] [CrossRef]
- Purohit, S.R.; Rana, S.S.; Idrishi, R.; Sharma, V.; Ghosh, P. A review on nutritional, bioactive, toxicological properties and preservation of edible flowers. Future Foods 2021, 4, 100078. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics. J. Agric. Food Chem. 2003, 51, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Bidchol, A.M.; Wilfred, A.; Abhijna, P.; Harish, R. Free radical scavenging activity of aqueous and ethanolic extract of Brassica oleracea L. var. italica. Food Bioprocess Technol. 2011, 4, 1137–1143. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Song, L.; Wang, X.; Zheng, X.; Huang, D. Polyphenolic antioxidant profiles of yellow camellia. Food Chem. 2011, 129, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.F.; Sajak, A.A.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compost. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Jin, C.-Y.; Xu, D.; Zeng, F.-K.; Zhao, Y.-C.; Yang, Y.-C.; Gao, G.-Q.; Wen, G.-H.; Liu, G. A simple method to prepare raw dehydrated potato flour by Low-Temperature Vacuum Drying. Int. J. Food Eng. 2017, 13, 20170127. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, K.; Wang, Y.; Dai, B.; Liu, S.; Li, Y. Moisture diffusion and shrinkage characteristics of broad bean during low-temperature vacuum drying. Int. J. Food Prop. 2020, 23, 2217–2230. [Google Scholar] [CrossRef]
- Ramli, A.S.; Basrawi, F.; Ibrahim, T.K.; Yusof, M.H.B.; Oumer, A.N.; Johari, N.A.; Muhamad, A.; Mamat, M.R.; Habib, K. Experimental analysis on a novel low-temperature vacuum drying with induced nucleation technique for dewatering stingless bees honey. Dry. Technol. 2018, 2, 149–155. [Google Scholar] [CrossRef]
- Do, L.T.K.; Vu, L.T.K.; Phan, D.T.A.; Dzung, N.T. Mathematical modeling and optimization of low-temperature vacuum drying for banana. Carpathian J. Food Sci. Technol. 2021, 13, 47–61. [Google Scholar]
- Vega-Gálvez, A.; Stucken, K.; Cantuarias, C.; Lamas, F.; García, V.; Pastén, A. Antimicrobial properties of papaya (Vasconcellea pubescens) subjected to low-temperature vacuum dehydration. Innov. Food Sci. Emerg. Technol. 2021, 67, 102563. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Uribe, E.; Pastén, A.; Vega, M.; Poblete, J.; Bilbao-Sainz, C.; Chiou, B.-S. Low-temperature vacuum drying as novel process to improve papaya (Vasconcellea pubescens) nutritional-functional properties. Future Foods 2022, 5, 100117. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; Pasten, A.; Cantuarias, C.; Stucken, K.; García, V.; Rodríguez, A.; Valenzuela-Barra, G.; Delporte, C. Effect of high- and low-temperature drying methods on fatty acid profile, antimicrobial and anti-inflammatory traits of papaya (Vasconcellea pubescens). ACS Food Sci. Technol. 2023, 3, 77–84. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Uribe, E.; Pasten, A.; Camus, J.; Rojas, M.; Garcia, V.; Araya, M.; Valenzuela-Barra, G.; Zambrano, A.; Goñi, M.G. Low-temperature vacuum drying on broccoli: Enhanced anti-inflammatory and anti-proliferative properties regarding other drying methods. Foods 2023, 12, 3311. [Google Scholar] [CrossRef] [PubMed]
- Mejías, N.; Vega-Galvez, A.; Gomez-Perez, L.S.; Pasten, A.; Uribe, E.; Cortés, A.; Valenzuela-Barra, G.; Camus, J.; Delporte, C.; Bernal, G. Health-promoting properties of processed red cabbage (Brassica oleracea var. capitata f. rubra): Effects of drying methods on bio-compound retention. Foods 2024, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Analytical Chemists). Official Method of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 1. [Google Scholar]
- Dini, I.; Tenore, G.C.; Dini, A. Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT-Food Sci. Technol. 2010, 43, 447–451. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- King, V.A.-E.; Zall, R.R.; Ludington, D.C. Controlled low-temperature vacuum dehydration a new approach for low-temperature and low-pressure food drying. J. Food Sci. 1989, 54, 1573–1579. [Google Scholar] [CrossRef]
- Espinoza, O.; Bond, B. Vacuum drying of wood—State of the art. Curr. For. Rep. 2016, 2, 223–235. [Google Scholar] [CrossRef]
- Luqmani, B.; Brookes, A.; Moore, A.; Vale, P.; Pidou, M.; McAdam, E.J. Transitioning through the vapour-liquid equilibrium for low energy thermal stripping of ammonia from wastewater: Enabling transformation of NH3 into a zero-carbon fuel. Water Res. 2024, 248, 120856. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.C.; Schmidt, F.C. Intensification of freeze-drying rate of coffee extract by vacuum freezing. Innov. Food Sci. Emerg. Technol. 2022, 78, 103022. [Google Scholar] [CrossRef]
- Pingniang, S.; Zhiyuan, L.; Kaizhong, H.; Qiong, X. An engineering study and analysis of the factors affecting evaporation rate under the vacuum drying process. Mode. Tradit. Chin. Med. Mater. Med. 2010, 12, 118–121. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Z.; Peng, X.; Yang, J.; Li, X.; Yuan, T.; Jia, X.; Liu, Y.; Abdullaev, O.; Jenis, J. Study on vacuum drying kinetics and processing of the Lonicera japonica Thunb. aqueous extracts. LWT-Food Sci. Technol. 2022, 167, 113868. [Google Scholar] [CrossRef]
- Shukla, B.D.; Singh, S.P. Osmo-convective drying of cauliflower, mushroom and greenpea. J. Food Eng. 2007, 80, 741–747. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Paniwnyk, L.; Lelas, V. Accelerated drying of button mushrooms, Brussels sprouts and cauliflower by applying power ultrasound and its rehydration properties. J. Food Eng. 2007, 81, 88–97. [Google Scholar] [CrossRef]
- Sahin, M.; Doymaz, I. Estimation of cauliflower mass transfer parameters during convective drying. Heat Mass Transfer 2017, 53, 507–517. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of water activity (aw) on microbial stability as a hurdle in food preservation. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.H., Jr., Schmidt, S.J., Labuza, T.P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; Chapter 14; pp. 323–355. [Google Scholar]
- Wang, Y.; Sun, J.; Ma, D.; Li, X.; Gao, X.; Miao, J.; Gao, W. Improving the contents of the active components and bioactivities of Chrysanthemum morifolium Ramat.: The effects of drying methods. Food Biosci. 2019, 29, 9–16. [Google Scholar] [CrossRef]
- Liu, B.; Tao, Y.; Manickam, S.; Li, D.; Han, Y.; Yu, Y.; Liu, D. Influence of sequential exogenous pretreatment and contact ultrasound-assisted air drying on the metabolic pathway of glucoraphanin in broccoli florets. Ultrason. Sonochem. 2022, 84, 105977. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M. 2015. Effect of food processing methods on the bioactive compound of cauliflower. Egypt. J. Agric. Res. 2015, 93, 117–131. [Google Scholar]
- Drabińska, N.; Jeż, M.; Nogueira, M. Variation in the accumulation of phytochemicals and their bioactive properties among the aerial parts of cauliflower. Antioxidants 2021, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Dou, J.; Lv, J.; Jin, N.; Jin, L.; Li, Z.; Zhang, B.; Tang, Z.; Yu, J. A comparative study on the nutrients, mineral elements, and antioxidant compounds in different types of Cruciferous vegetables. Agronomy 2022, 12, 3121. [Google Scholar] [CrossRef]
- Tadesse, A.Y.; Mohammed, H.H.; Andersa, K.N. Proximate composition and selected phytochemical component of Dawrach (Raphanus raphanistrum L.) as affected by blanching temperature. Heliyon 2023, 9, e19240. [Google Scholar] [CrossRef]
- Cheng, R.; Liao, X.; Addou, A.M.; Quian, J.; Wang, S.; Cheng, Z.; Wang, L.; Huang, J. Effects of “nine steaming nine sun-drying” on proximate composition, oil properties and volatile compounds of black sesame seeds. Food Chem. 2021, 344, 128577. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Impact of infrared and dry air roasting on the oxidative stability, fatty acid composition, Maillard reaction products and other chemical properties of black cumin (Nigella sativa L.) seed oil. Food Chem. 2019, 295, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Raitio, R.; Orlien, V.; Skibsted, L.H. Storage stability of cauliflower soup powder: The effect of lipid oxidation and protein degradation reactions. Food Chem. 2011, 128, 371–379. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Bianchi, G.; Genna, A.; Summa, C. Antioxidant properties and lipidic profile as quality indexes of cauliflower (Brassica oleracea L. var. botrytis) in relation to harvest time. Food Chem. 2007, 100, 1019–1025. [Google Scholar] [CrossRef]
- Aksüt, B.; Polatci, H. The effect of drying processes on model and quantitative color quality characteristics of cauliflower (Brassica oleracea L.). Turk. J. Agr. Eng. Res. 2022, 3, 170–180. [Google Scholar] [CrossRef]
- Hodges, D.M.; Munro, K.D.; Forney, C.F.; McRae, K.B. Glucosinolate and free sugar content in cauliflower (Brassica oleracea var. botrytis cv. Freemont) during controlled-atmosphere storage. Postharvest Biol. Tec. 2006, 40, 123–132. [Google Scholar] [CrossRef]
- Kurek, M.A.; Finnseth, C.; Skipnes, D.; Rode, T.M. Impact of high-pressure processing (HPP) on selected quality and nutritional parameters of cauliflower (Brassica oleracea var. Botrytis). Appl. Sci. 2022, 12, 6013. [Google Scholar] [CrossRef]
- Xu, H.; Lei, M.; Li, J.; Zou, S.; Yin, W.; Jiang, Y.; Xianyu, D.; Li, D.; Zhao, C.; Yu, L. Effects of different drying methods on the physicochemical and functional properties of Pyracantha fortuneana (Maxim.) Li fruit. LWT-Food Sci. Technol. 2023, 187, 115383. [Google Scholar] [CrossRef]
- Picchi, V.; Migliori, C.; Lo Scalzo, R.; Campanelli, G.; Ferrari, V.; Di Cesare, L.F. Phytochemical content in organic and conventionally grown Italian cauliflower. Food Chem. 2012, 130, 501–509. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Kwak, J.-H. Chemical composition and antioxidant activity in different tissues of brassica vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.; Qin, Y.; Yan, J.; Li, R.; Yang, Q. The impact of different drying methods on the physical properties, bioactive components, antioxidant capacity, volatile components and industrial application of coffee peel. Food Chem. X 2023, 19, 100807. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.; Lamy, S.; Lord-Dufour, S.; Jackson, J.; Beaulieu, E.; Côté, M.; Moghrabi, A.; Barrette, S.; Gingras, D.; Béliveau, R. Antiproliferative and antioxidant activities of common vegetables: A comparative study. Food Chem. 2009, 112, 374–380. [Google Scholar] [CrossRef]
- Zafar, I.; Hussain, A.I.; Fatima, T.; Abdullah Alnasser, S.M.; Ahmad, A. Inter-varietal variation in phenolic profile, sugar contents, antioxidant, anti-proliferative and antibacterial activities of selected brassica species. Appl. Sci. 2022, 12, 5811. [Google Scholar] [CrossRef]
- Wall-Medrano, A.; Olivas-Aguirre, F.J. 11—Antioxidant phytochemicals in cancer prevention and therapy—An update. In Functional Foods in Cancer Prevention and Therapy; Kabir, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 195–220. [Google Scholar]
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Lee-Martínez, S.; Larrauri-Rodríguez, M.; Zaldívar-Lelo de Larrea, G.; Pérez-Serrano, R.M.; Camacho-Calderón, N. Isothiocyanate-rich extracts from cauliflower (Brassica oleracea Var. Botrytis) and radish (Raphanus sativus) inhibited metabolic activity and induced ROS in selected human HCT116 and HT-29 colorectal cancer cells. Int. J. Environ. Res. Public Health 2022, 19, 14919. [Google Scholar] [CrossRef]
- Boškovic, S.; Mimica-Dukić, N.; Četojević-Simin, D.; Orčić, D.; Karaman, M.; Bogavac, M.; Lozanov-Crvenković, Z.; Simin, N. Antioxidant, antimicrobial and antiproliferative activity of Brassica oleracea varieties broccoli, cauliflower and kohlrabi under organic and conventional cropping. J. Food Nutr. Res. 2023, 62, 224–235. [Google Scholar]

| Parameters | Drying Methods | ||||
|---|---|---|---|---|---|
| VD | CD | IRD | LTVD | VFD | |
| 1 Moisture | 11.65 ± 0.13 b | 9.05 ± 0.02 d | 10.31 ± 0.15 c | 12.87 ± 0.16 a | 8.88 ± 0.05 d |
| 2 Water activity | 0.4161 ± 0.0031 a | 0.3224 ± 0.0008 d | 0.3628 ± 0.0006 c | 0.4007 ± 0.0018 b | 0.2565 ± 0.0045 e |
| 3 Ash | 12.06 ± 0.21 a | 10.81 ± 0.17 b | 12.03 ± 0.18 a | 11.90 ± 0.10 a | 11.84 ± 0.10 a |
| 3 Crude fiber | 12.76 ± 0.45 a | 9.31 ± 0.51 b | 12.21 ± 0.93 a | 12.67 ± 1.20 a | 10.52 ± 0.97 b |
| 3 Fat | 2.69 ± 0.04 bc | 2.95 ± 0.05 b | 2.51 ± 0.15 c | 2.68 ± 0.11 bc | 3.66 ± 0.16 a |
| 3 Crude protein | 25.05 ± 0.11 ab | 21.79 ± 0.04 b | 23.35 ± 1.07 ab | 26.90 ± 0.57 a | 23.39 ± 0.17 ab |
| Saturated fatty acids (SFAs) | |||||
| 4 C12:0 | 0.01 ± 0.00 b | 0.11 ± 0.07 a | 0.02 ± 0.00 ab | 0.03 ± 0.00 ab | 0.03 ± 0.00 ab |
| 4 C14:0 | 0.16 ± 0.01 b | 0.11 ± 0.04 c | 0.18 ± 0.00 ab | 0.19 ± 0.00 ab | 0.22 ± 0.01 a |
| 4 C15:0 | 0.37 ± 0.01 ab | 0.31 ± 0.05 b | 0.39 ± 0.00 a | 0.40 ± 0.00 a | 0.39 ± 0.01 a |
| 4 C16:0 | 19.08 ± 0.10 a | 18.24 ± 0.42 b | 17.91 ± 0.09 b | 17.74 ± 0.12 b | 18.35 ± 0.39 b |
| 4 C17:0 | 0.83 ± 0.00 ab | 0.76 ± 0.03 c | 0.77 ± 0.04 bc | 0.75 ± 0.01 c | 0.85 ± 0.02 a |
| 4 C18:0 | 1.82 ± 0.02 a | 1.60 ± 0.02 b | 1.66 ± 0.06 b | 1.64 ± 0.09 b | 1.54 ± 0.01 b |
| 4 C20:0 | 0.43 ± 0.02 a | 0.24 ± 0.07 b | 0.35 ± 0.03 ab | 0.33 ± 0.05 ab | 0.34 ± 0.05 ab |
| 4 C22:0 | 0.19 ± 0.02 a | 0.12 ± 0.06 b | 0.14 ± 0.01 b | ND | ND |
| 4 C24:0 | 0.53 ± 0.09 a | 0.33 ± 0.15 a | 0.38 ± 0.02 a | 0.49 ± 0.16 a | 0.40 ± 0.08 a |
| Monounsaturated fatty acids (MUFAs) | |||||
| 4 C16:1 | 0.47 ± 0.01 a | 0.37 ± 0.08 b | 0.41 ± 0.02 b | ND | ND |
| 4 C17:1 | 0.30 ± 0.00 a | 0.27 ± 0.02 a | 0.29 ± 0.01 b | 0.30 ± 0.03 b | 0.28 ± 0.02 b |
| 4 C18:1 n9 c/C18:1 n9 t | 0.37 ± 0.01 a | 0.26 ± 0.04 a | 0.27 ± 0.05 a | 0.20 ± 0.01 b | 0.27 ± 0.02 b |
| 4 C20:1 n9 | 0.07 ± 0.01 a | 0.03 ± 0.00 a | 0.02 ± 0.00 a | ND | ND |
| 4 C24:1 n9 | 0.48 ± 0.03 a | 0.39 ± 0.18 a | 0.49 ± 0.06 a | 0.72 ± 0.18 a | 0.66 ± 0.03 a |
| 4 Unidentified MUFA | ND | 9.26 ± 0.05 a | 8.47 ± 0.04 b | 9.21 ± 0.00 a | 9.03 ± 0.31 a |
| Polyunsaturated fatty acids (PUFAs) | |||||
| 4 C18:2 n6 c | 11.00 ± 0.17 a | 9.38 ± 0.08 d | 9.75 ± 0.08 c | 9.32 ± 0.03 d | 10.66 ± 0.07 b |
| 4 C18:3 n6 | 63.63 ± 0.21 a | 58.14 ± 0.13 b | 58.48 ± 0.20 b | 58.44 ± 0.23 b | 56.25 ± 0.44 c |
| 4 C20:2 | 0.11 ± 0.01 ab | 0.06 ± 0.02 ab | 0.09 ± 0.01 ab | 0.10 ± 0.02 ab | 0.13 ± 0.03 a |
| 4 C20:3 n3 | 0.15 ± 0.01 a | 0.09 ± 0.03 b | 0.13 ± 0.01 ab | 0.14 ± 0.01 a | 0.17 ± 0.02 a |
| Fresh | VD | CD | IRD | LTVD | VFD | |
|---|---|---|---|---|---|---|
| L* | 70.48 ± 0.33 e | 78.79 ± 0.68 c | 81.58 ± 0.23 b | 76.12 ± 0.36 d | 82.59 ± 0.20 b | 89.18 ± 0.31 a |
| a* | −3.68 ± 0.20 e | 0.52 ± 0.10 b | −0.14 ± 0.08 c | 3.01 ± 0.13 a | −0.25 ± 0.06 c | −3.12 ± 0.03 d |
| b* | 19.74 ± 0.30 cd | 30.82 ± 0.11 a | 20.16 ± 0.55 c | 24.67 ± 0.28 b | 19.19 ± 0.22 d | 10.49 ± 0.36 e |
| ΔE | - | 14.48 ± 0.18 b | 11.67 ± 0.23 d | 10.06 ± 0.46 e | 12.61 ± 0.22 c | 20.87 ± 0.10 a |
| Chroma | 20.09 ± 0.26 c | 30.82 ± 0.11 a | 20.16 ± 0.55 c | 24.86 ± 0.28 b | 19.19 ± 0.22 d | 10.95 ± 0.35 e |
| Hue | 100.61 ± 0.70 b | 89.04 ± 0.20 d | 90.40 ± 0.23 c | 83.04 ± 0.26 e | 90.75 ± 0.16 c | 106.58 ± 0.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasten, A.; Vega-Galvez, A.; Uribe, E.; Carvajal, M.; Mejías, N.; Araya, M.; Goñi, M.G. A Comparison of the Effects of Low-Temperature Vacuum Drying and Other Methods on Cauliflower’s Nutritional–Functional Properties. Processes 2024, 12, 1629. https://doi.org/10.3390/pr12081629
Pasten A, Vega-Galvez A, Uribe E, Carvajal M, Mejías N, Araya M, Goñi MG. A Comparison of the Effects of Low-Temperature Vacuum Drying and Other Methods on Cauliflower’s Nutritional–Functional Properties. Processes. 2024; 12(8):1629. https://doi.org/10.3390/pr12081629
Chicago/Turabian StylePasten, Alexis, Antonio Vega-Galvez, Elsa Uribe, Manuel Carvajal, Nicol Mejías, Michael Araya, and Maria Gabriela Goñi. 2024. "A Comparison of the Effects of Low-Temperature Vacuum Drying and Other Methods on Cauliflower’s Nutritional–Functional Properties" Processes 12, no. 8: 1629. https://doi.org/10.3390/pr12081629
APA StylePasten, A., Vega-Galvez, A., Uribe, E., Carvajal, M., Mejías, N., Araya, M., & Goñi, M. G. (2024). A Comparison of the Effects of Low-Temperature Vacuum Drying and Other Methods on Cauliflower’s Nutritional–Functional Properties. Processes, 12(8), 1629. https://doi.org/10.3390/pr12081629










