Synergistic Effects of Pea Protein on the Viscoelastic Properties of Sodium Alginate Gels: Findings from Fourier Transform Infrared and Large-Amplitude Oscillatory Shear Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Small-Amplitude Oscillation Shear Measurements
2.3. Large-Amplitude Oscillatory Shear Measurements
2.3.1. Experimental Procedure of LAOS
2.3.2. Processing LAOS Data
2.4. Fourier Transform Infrared
2.5. Statistical Analysis
3. Results and Discussion
3.1. Thermal Gelation Behavior of Alginate and Pea Protein Paste Mixtures
3.2. Linear Viscoelastic Properties of Pea Protein and Alginate Gel
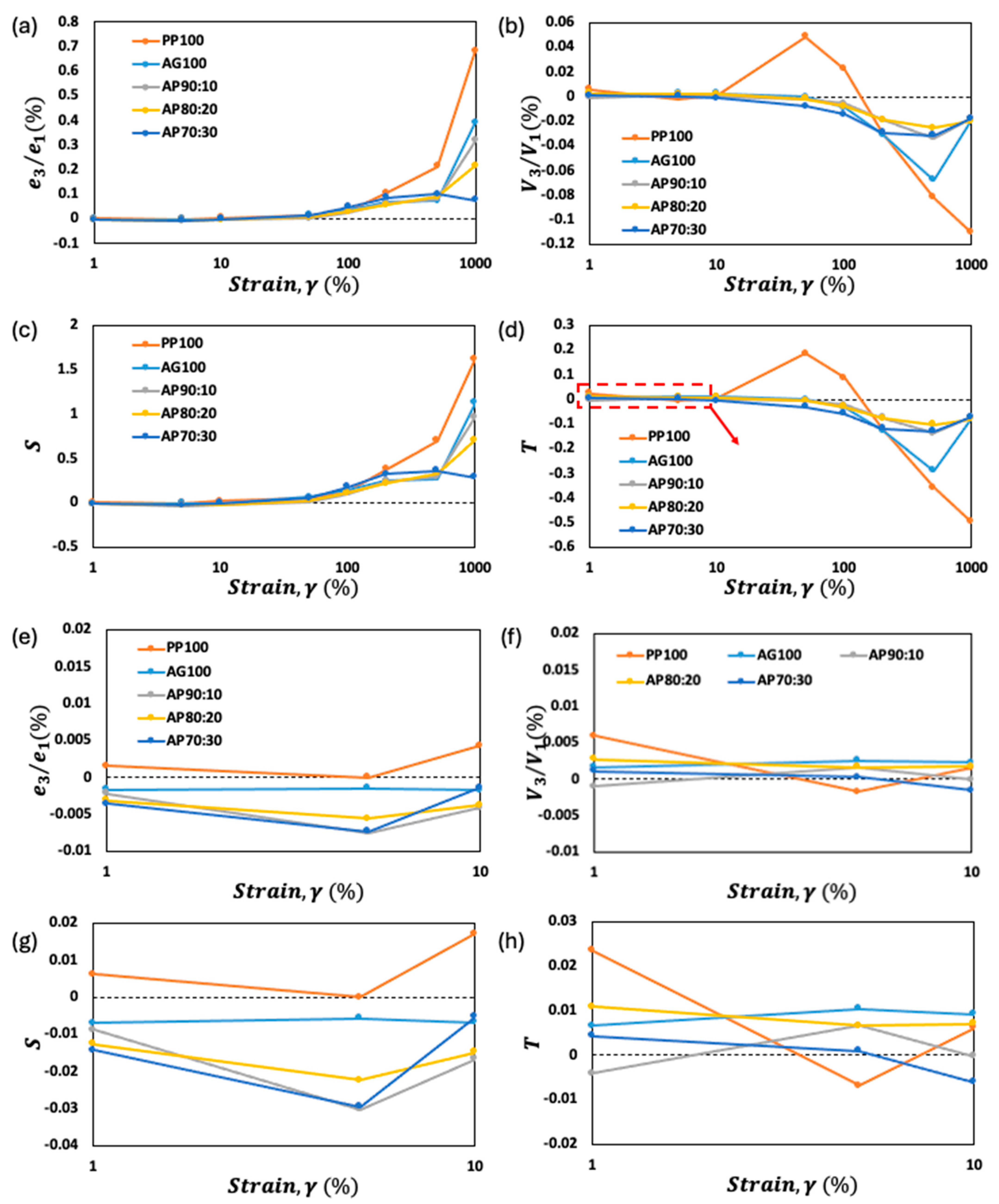
3.3. Nonlinear Viscoelastic Properties: Lissajous–Bowditch Curves
3.4. Analysis of Chebyshev Coefficients for Mixed Pea Protein and Alginate Gel
3.5. Fourier Transform Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorling, D. World population prospects at the UN: Our numbers are not our problem? In The Struggle for Social Sustainability; Policy Press: Bristol, UK, 2021; pp. 129–154. [Google Scholar]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and supply of high-quality food protein for human consumption: Sustainability, challenges, and innovations. Ann. New York Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.W.; Jakeman, P.M. Separating the wheat from the chaff: Nutritional value of plant proteins and their potential contribution to human health. Nutrients 2020, 12, 2410. [Google Scholar] [CrossRef] [PubMed]
- Estell, M.; Hughes, J.; Grafenauer, S. Plant protein and plant-based meat alternatives: Consumer and nutrition professional attitudes and perceptions. Sustainability 2021, 13, 1478. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Carvajal-Piñero, J.M.; Ramos, M.; Jiménez-Rosado, M.; Perez-Puyana, V.; Romero, A. Development of pea protein bioplastics by a thermomoulding process: Effect of the mixing stage. J. Polym. Environ. 2019, 27, 968–978. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Mession, J.L.; Blanchard, C.; Mint-Dah, F.V.; Lafarge, C.; Assifaoui, A.; Saurel, R. The effects of sodium alginate and calcium levels on pea proteins cold-set gelation. Food Hydrocoll. 2013, 31, 446–457. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Stability of 3D printing using a mixture of pea protein and alginate: Precision and application of additive layer manufacturing simulation approach for stress distribution. J. Food Eng. 2021, 288, 110127. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, A.; Qiu, C.; Liu, Q.; Yang, Y.; Bian, S.; Zeng, F.; Jin, Z. A combined enzymatic and ionic cross-linking strategy for pea protein/sodium alginate double-network hydrogel with excellent mechanical properties and freeze-thaw stability. Food Hydrocoll. 2022, 131, 107737. [Google Scholar] [CrossRef]
- Leelapunnawut, S.; Ngamwonglumlert, L.; Devahastin, S.; Derossi, A.; Caporizzi, R.; Chiewchan, N. Effects of texture modifiers on physicochemical properties of 3D-printed meat mimics from pea protein isolate-alginate gel mixture. Foods 2022, 11, 3947. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.E.; Turgeon, S.L. Formation and functional properties of protein–polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Razi, S.M.; Motamedzadegan, A.; Shahidi, A.; Rashidinejad, A. The effect of basil seed gum (BSG) on the rheological and physicochemical properties of heat-induced egg albumin gels. Food Hydrocoll. 2018, 82, 268–277. [Google Scholar] [CrossRef]
- López, D.N.; Galante, M.; Alvarez, E.M.; Risso, P.H.; Boeris, V. Effect of the espina corona gum on caseinate acid-induced gels. LWT-Food Sci. Technol. 2017, 85, 121–128. [Google Scholar] [CrossRef]
- Sun, W.; Yang, Y.; Wang, T.; Liu, X.; Wang, C.; Tong, Z. Large amplitude oscillatory shear rheology for nonlinear viscoelasticity in hectorite suspensions containing poly (ethylene glycol). Polymer 2011, 52, 1402–1409. [Google Scholar] [CrossRef]
- Ewoldt, R.H.; Hosoi, A.E.; McKinley, G.H. New measures for characterizing nonlinear viscoelasticity in large amplitude oscillatory shear. J. Rheol. 2008, 52, 1427–1458. [Google Scholar] [CrossRef]
- Precha-Atsawanan, S.; Uttapap, D.; Sagis, L.M. Linear and nonlinear rheological behavior of native and debranched waxy rice starch gels. Food Hydrocoll. 2018, 85, 1–9. [Google Scholar] [CrossRef]
- Helgerud, T.; Gåserød, O.; Fjæreide, T.; Andersen, P.O.; Larsen, C.K. Alginates. In Food Stabilisers, Thickeners and Gelling Agents; John Wiley & Son: Hoboken, NJ, USA, 2009; pp. 50–72. [Google Scholar]
- Cho, K.S.; Hyun, K.; Ahn, K.H.; Lee, S.J. A geometrical interpretation of large amplitude oscillatory shear response. J. Rheol. 2005, 49, 747–758. [Google Scholar] [CrossRef]
- Joyner, H.S. Nonlinear (large-amplitude oscillatory shear) rheological properties and their impact on food processing and quality. Annu. Rev. Food Sci. Technol. 2021, 12, 591–609. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
- Shen, Y.; Du, Z.; Wu, X.; Li, Y. Modulating molecular interactions in pea protein to improve its functional properties. J. Agric. Food Res. 2022, 8, 100313. [Google Scholar] [CrossRef]
- Tanger, C.; Müller, M.; Andlinger, D.; Kulozik, U. Influence of pH and ionic strength on the thermal gelation behaviour of pea protein. Food Hydrocoll. 2022, 123, 106903. [Google Scholar] [CrossRef]
- Ren, W.; Xia, W.; Gunes, D.Z.; Ahrné, L. Heat-induced gels from pea protein soluble colloidal aggregates: Effect of calcium addition or pH adjustment on gelation behavior and rheological properties. Food Hydrocoll. 2024, 147, 109417. [Google Scholar] [CrossRef]
- Donati, I.; Holtan, S.; Mørch, Y.A.; Borgogna, M.; Dentini, M.; Skjåk-Bræk, G. New hypothesis on the role of alternating sequences in calcium− alginate gels. Biomacromolecules 2005, 6, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Khalil HP, S.A.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong EW, N.; Syakir, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Roopa, B.S.; Bhattacharya, S. Alginate gels: II. Stability at different Processing conditions. J. Food Process Eng. 2010, 33, 466–480. [Google Scholar] [CrossRef]
- O’Kane, F.E.; Vereijken, J.M.; Gruppen, H.; Van Boekel, M.A. Gelation behavior of protein isolates extracted from 5 cultivars of Pisum sativum L. J. Food Sci. 2005, 70, C132–C137. [Google Scholar] [CrossRef]
- Ortiz, S.M.; Puppo, M.C.; Wagner, J.R. Relationship between structural changes and functional properties of soy protein isolates–carrageenan systems. Food Hydrocoll. 2004, 18, 1045–1105. [Google Scholar] [CrossRef]
- Panaras, G.; Moatsou, G.; Yanniotis, S.; Mandala, I. The influence of functional properties of different whey protein concentrates on the rheological and emulsification capacity of blends with xanthan gum. Carbohydr. Polym. 2011, 86, 433–440. [Google Scholar] [CrossRef]
- Ma, Y.; Su, D.; Wang, Y.; Li, D.; Wang, L. Effects of concentration and NaCl on rheological behaviors of konjac glucomannan solution under large amplitude oscillatory shear (LAOS). LWT 2020, 128, 109466. [Google Scholar] [CrossRef]
- Sagis, L.M.; Fischer, P. Nonlinear rheology of complex fluid–fluid interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 520–529. [Google Scholar] [CrossRef]
- Qu, R.J.; Wang, Y.; Li, D.; Wang, L.J. Rheological behavior of nanocellulose gels at various calcium chloride concentrations. Carbohydr. Polym. 2021, 274, 118660. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Li, D.; Wang, L.J. Freeze-thaw stability and rheological properties of soy protein isolate emulsion gels induced by NaCl. Food Hydrocoll. 2022, 123, 107113. [Google Scholar] [CrossRef]
- Ptaszek, P.; Kabziński, M.; Ptaszek, A.; Kaczmarczyk, K.; Kruk, J.; Bieńczak, A. The analysis of the influence of xanthan gum and apple pectins on egg white protein foams using the large amplitude oscillatory shear method. Food Hydrocoll. 2016, 54, 293–301. [Google Scholar] [CrossRef]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. LWT-Food Sci. Technol. 2015, 63, 612–619. [Google Scholar] [CrossRef]
- Duceac, I.A.; Stanciu, M.C.; Nechifor, M.; Tanasă, F.; Teacă, C.A. Insights on some polysaccharide gel type materials and their structural peculiarities. Gels 2022, 8, 771. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, D.; Toker, O.S. Large-amplitude oscillatory shear behavior of xanthan gum/locust bean gum mixture: Effect of preparation methods on synergistic interaction. J. Food Process Eng. 2022, 45, e14073. [Google Scholar] [CrossRef]
- Tang, M.X.; Lei, Y.C.; Wang, Y.; Li, D.; Wang, L.J. Rheological and structural properties of sodium caseinate as influenced by locust bean gum and κ-carrageenan. Food Hydrocoll. 2021, 112, 106251. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Bie, P.; Xie, F.; Zheng, B. An insight into the structural evolution of waxy maize starch chains during growth based on nonlinear rheology. Food Hydrocoll. 2021, 116, 106655. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Li, L. Enhanced stability and mechanical strength of sodium alginate composite films. Carbohydr. Polym. 2017, 160, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.E.; Maugard, T. Complex coacervation of pea protein isolate and tragacanth gum: Comparative study with commercial polysaccharides. Innov. Food Sci. Emerg. Technol. 2021, 69, 102641. [Google Scholar] [CrossRef]
- Moreno, H.M.; Dominguez-Timon, F.; Díaz, M.T.; Pedrosa, M.M.; Borderías, A.J.; Tovar, C.A. Evaluation of gels made with different commercial pea protein isolate: Rheological, structural and functional properties. Food Hydrocoll. 2020, 99, 105375. [Google Scholar] [CrossRef]






| Ingredient | Concentrations of Constituting Components | (%W/W) | Concentration | Paste Material |
|---|---|---|---|---|
| Pea protein | Pea powder | 20 | 100 | Pea protein paste |
| Distilled water | 80 | |||
| Alginate | Sodium phosphate | 10 | 2.5 | Alginate gel solution |
| Calcium chloride | 10 | |||
| Sodium alginate | 80 | |||
| Distilled water | Distilled water | 100 | 97.5 |
| Gel | Ratio of the Mixture (%) | Code Name of the Sample |
|---|---|---|
| Pea protein gel | 100 | PP100 |
| Alginate gel | 100 | AG100 |
| Alginate and pea protein mixtures | 90:10 | AP90:10 |
| 80:20 | AP80:20 | |
| 70:30 | AP70:30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, W.B.; Jung, H.; Oyinloye, T.M. Synergistic Effects of Pea Protein on the Viscoelastic Properties of Sodium Alginate Gels: Findings from Fourier Transform Infrared and Large-Amplitude Oscillatory Shear Analysis. Processes 2024, 12, 1638. https://doi.org/10.3390/pr12081638
Yoon WB, Jung H, Oyinloye TM. Synergistic Effects of Pea Protein on the Viscoelastic Properties of Sodium Alginate Gels: Findings from Fourier Transform Infrared and Large-Amplitude Oscillatory Shear Analysis. Processes. 2024; 12(8):1638. https://doi.org/10.3390/pr12081638
Chicago/Turabian StyleYoon, Won Byong, Hwabin Jung, and Timilehin Martins Oyinloye. 2024. "Synergistic Effects of Pea Protein on the Viscoelastic Properties of Sodium Alginate Gels: Findings from Fourier Transform Infrared and Large-Amplitude Oscillatory Shear Analysis" Processes 12, no. 8: 1638. https://doi.org/10.3390/pr12081638
APA StyleYoon, W. B., Jung, H., & Oyinloye, T. M. (2024). Synergistic Effects of Pea Protein on the Viscoelastic Properties of Sodium Alginate Gels: Findings from Fourier Transform Infrared and Large-Amplitude Oscillatory Shear Analysis. Processes, 12(8), 1638. https://doi.org/10.3390/pr12081638








