Comparison of Light Intensity Effect on Microalgal Growth in Cactus-like and Cylindrical Photo Bioreactors
Abstract
:1. Introduction
2. Materials and Methods
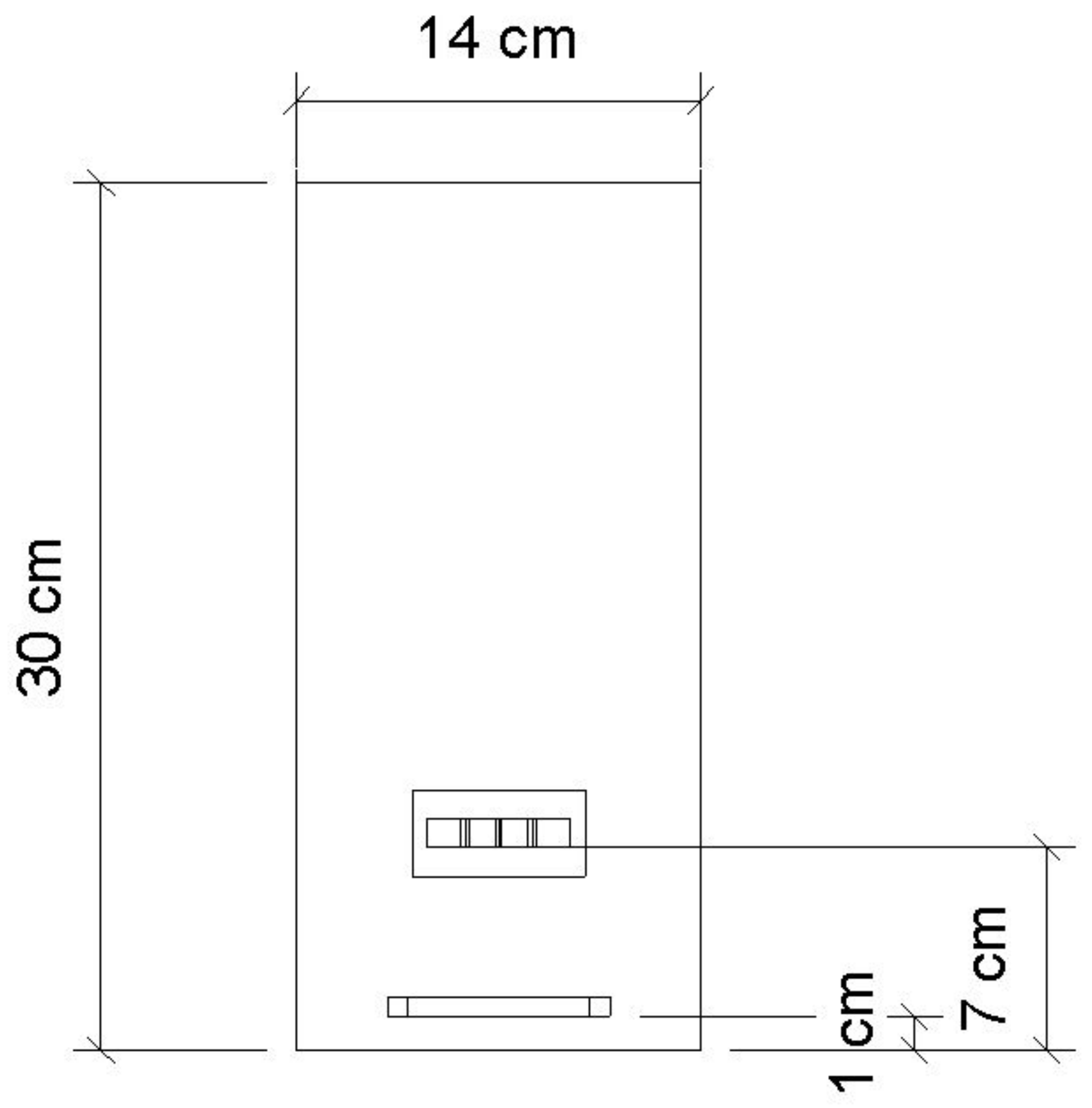
2.1. PBR Geometry Description
2.2. Cultivation Experiment
2.3. Light Intensity Measurement
2.4. Biomass Measurement
2.4.1. Specific Growth Rate
2.4.2. Productivity
2.4.3. Energy Efficiency
2.5. Statistical Analysis
3. Results and Discussion
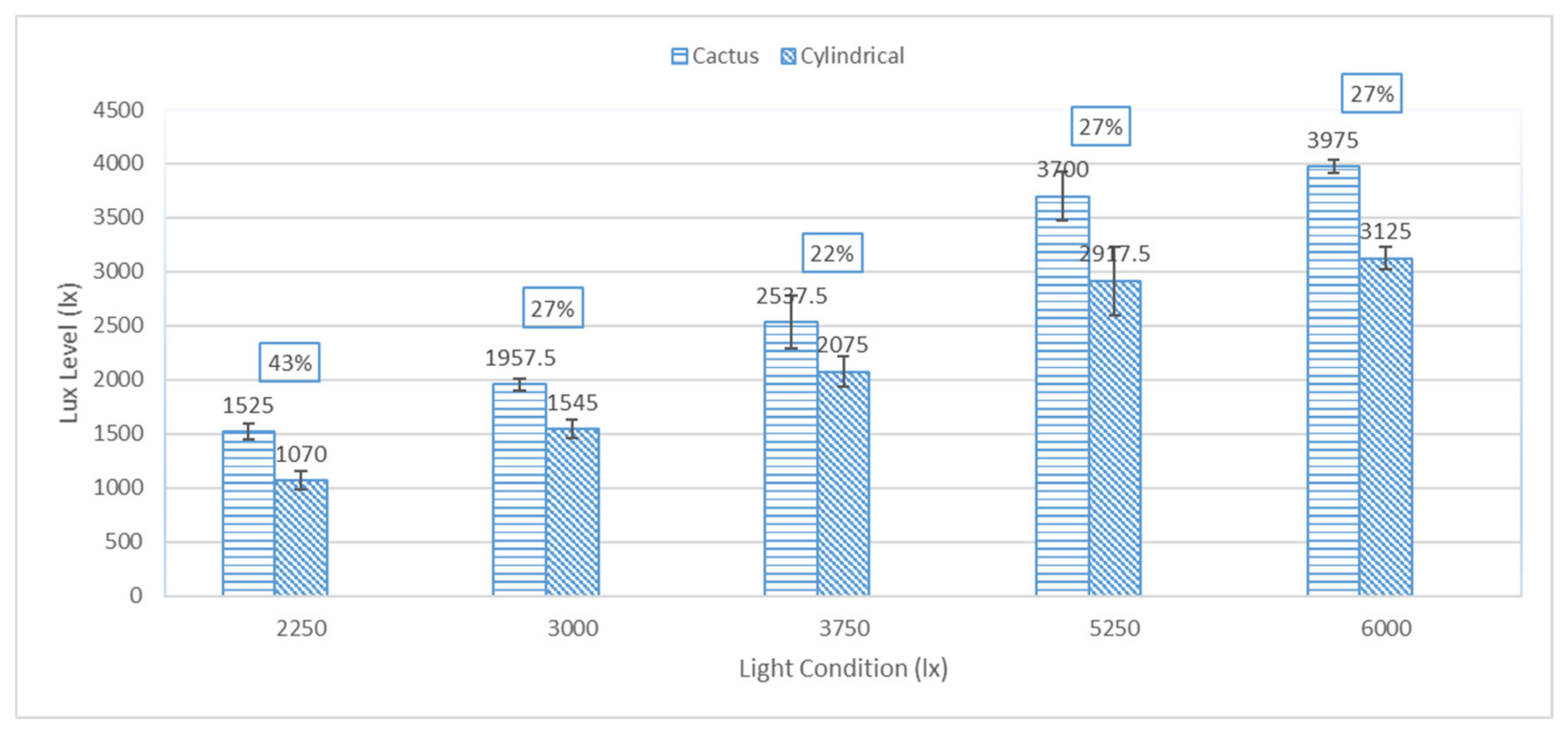
3.1. Irradiance
3.2. Biomass Production
3.2.1. Specific Growth Rate
3.2.2. Microalgae Productivity
3.2.3. Energy Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBR | Photobioreactors |
| TPBR | Tubular Photobioreactor |
| SVR | Surface Area-to-Volume Ratio |
| LDPE | Low-Density Polyethylene |
| PMMA | Poly (Methyl Methacrylate) |
| PLA | Polylactic Acid |
| FDM | Fused Deposition Modelling Technology |
| PPFD | Photosynthetic Photon Flux Density |
| CRI | Color Rendering Index |
References
- WHO. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/ (accessed on 19 December 2022).
- IEA. Global Energy and Climate Model. License: CC BY 4.0. 2022. Available online: https://www.iea.org/reports/global-energy-and-climate-model (accessed on 29 June 2024).
- UNEP. Pollution Action Note—Data you Need to Know. 7 September 2021. Available online: https://www.unep.org/ (accessed on 30 August 2022).
- Ahmed, S.F.; Rafa, S.J.; Mehjabin, A.; Tasannum, N.; Ahmed, S.; Mofijur, M.; Lichtfouse, E.; Almomani, F.; Badruddin, I.A.; Kamangar, S. Bio-oil from microalgae: Materials, production, technique, and future. Energy Rep. 2023, 10, 3297–3314. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technol. Sustain. 2023, 2, 100060. [Google Scholar] [CrossRef]
- Murthy, G.S. Overview and assessment of algal biofuels production technologies. In Biofuels; Academic Press: Cambridge, MA, USA, 2011; pp. 415–437. [Google Scholar] [CrossRef]
- Hijazi, R.; Mounsef, J.R.; Kanaan, H.Y. Design considerations for photo-bioreactors: A review. In Proceedings of the 2020 5th International Conference on Renewable Energies for Developing Countries (REDEC), Marrakech, Morocco, 29–30 June 2020; IEEE: Piscataway Township, NJ, USA, 2020; pp. 1–7. [Google Scholar]
- Hijazi, R.; Mounsef, J.R.; Kanaan, H.Y. Design and Computational Fluid Dynamics Simulation of a Novel Stirred Photo-Bioreactor. In Proceedings of the IECON 2021–47th Annual Conference of the IEEE Industrial Electronics Society, Toronto, ON, Canada, 13–16 October 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Chen, P.; Chen, M.; Wang, Y.-F.; Li, L.; Chen, Y.; Wang, Q.; Wan, C.; Wang, Y.; Cheng, X.; Deng, Y.; et al. Review of the biological and engineering aspects of algae to fuels approach. Int. J. Agric. Biol. Eng. 2009, 2, 1–30. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Oncel, S.S.; Imamoglu, E. Computational fluid dynamics modelling of stirred tank photobioreactor for Haematococcus pluvialis production: Hydrodynamics and mixing conditions. Algal Res. 2020, 47, 101854. [Google Scholar] [CrossRef]
- Sergejevová, M.; Malapascua, J.R.; Kopecký, J.; Masojídek, J. Photobioreactors with internal illumination. In Algal Biorefineries: Volume 2: Products and Refinery Design; Springer: Cham, Switzerland, 2015; pp. 213–236. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Soejima, T.; Tanaka, H. An integrated solar and artificial light system for internal illumination of photobioreactors. J. Biotechnol. 1999, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Sato, T. A photobioreactor for microalgae cultivation with internal illumination considering flashing light effect and optimized light-source arrangement. Energy Convers. Manag. 2017, 133, 558–565. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Marconi, P.L.; Trentini, A.; Zawoznik, M.; Nadra, C.; Mercadé, J.M.; Novoa, J.G.S.; Orozco, D.; Groppa, M.D. Development and testing of a 3D-printable polylactic acid device to optimize a water bioremediation process. AMB Express 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhuang, X.; Tang, Z.; Chen, X. Polylactic acid (PLA): Research, development and industrialization. Biotechnol. J. 2010, 5, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Kadic, E.; Heindel, T.J. An Introduction to Bioreactor Hydrodynamics and Gas-Liquid Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Bitog, J.P.; Lee, I.B.; Lee, C.G.; Kim, K.S.; Hwang, H.S.; Hong, S.W.; Seo, I.H.; Kwon, K.S.; Mostafa, E. Application of computational fluid dynamics for modelling and designing photobioreactors for microalgae production: A review. Comput. Electron. Agric. 2011, 76, 131–147. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.C.B.G.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Mounsef, J.R.; Lteif, R. Pigment production by Scenedesmus dimorphus using different low-cost and alternative culture media. J. Chem. Technol. Biotechnol. 2022, 97, 287–294. [Google Scholar] [CrossRef]
- Waveform Lighting, Waveform Lighting, LLC. 2024. Available online: https://www.waveformlighting.com/horticulture/convert-lux-to-ppfd-online-calculator (accessed on 29 June 2024).
- Pozza, C.; Schmuck, S.; Mietzel, T. A Novel Photobioreactor with Internal Illumination Using Plexiglas Rods to Spread the Light and LED as a Source of Light for Wastewater Treatment Using Microalgae; ISA: London, UK, 2013. [Google Scholar]
- Doucha, J.; Lívanský, K. Production of high-density Chlorella culture grown in fermenters. J. Appl. Phycol. 2012, 24, 35–43. [Google Scholar] [CrossRef]
- Calvo, F.; Bula, A.; Di Mare, L.; Garcia, S. CFD simulation of multiphase (liquid–solid–gas) flow in an airlift column photobioreactor. Acta Mech. 2017, 228, 2413–2427. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Res. 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. The effects of light and temperature on microalgal growth and nutrient removal: An experimental and mathematical approach. RSC Adv. 2016, 6, 22896–22907. [Google Scholar] [CrossRef]
- Qiang, H.; Zarmi, Y.; Richmond, A. Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (Cyanobacteria). Eur. J. Phycol. 1998, 33, 165–171. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fu, C.C.; Liu, Y.C. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Sankar, V.; Daniel, D.K.; Krastanov, A. Carbon dioxide fixation by Chlorella minutissima batch cultures in a stirred tank bioreactor. Biotechnol. Biotechnol. Equip. 2011, 25, 2468–2476. [Google Scholar] [CrossRef]
- Li, D.; Cong, W.; Cai, Z.; Shi, D.; Ouyang, F. Effect of Iron Stress, Light Stress, and Nitrogen Source on Physiological Aspects of Marine Red Tide Alga. J. Plant Nutr. 2004, 27, 29–41. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Choochote, W.; Paiboonsin, K.; Ruangpan, S.; Pharuang, A. Effects of Urea and Light Intensity on the Growth of Chlorella sp. In Proceedings of the the 8th International Symposium on Biocontrol and Biotechnology, Pattaya, Thailand, 4–6 October 2010; pp. 127–134. [Google Scholar]
- Yeh, K.L.; Chang, J.S.; Chen, W.M. Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng. Life Sci. 2010, 10, 201–208. [Google Scholar] [CrossRef]
- Lee, C.G.; Palsson, B.Ø. High-density algal photobioreactors using light-emitting diodes. Biotechnol. Bioeng. 1994, 44, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Binnal, P.; Babu, P.N. Optimization of environmental factors affecting tertiary treatment of municipal wastewater by Chlorella protothecoides in a lab scale photobioreactor. J. Water Process Eng. 2017, 17, 290–298. [Google Scholar] [CrossRef]
- Kamla, Y.; Bouzit, M.; Ameur, H.; Arab, M.I.; Hadjeb, A. Effect of the inclination of baffles on the power consumption and fluid flows in a vessel stirred by a Rushton turbine. Chin. J. Mech. Eng. 2017, 30, 1008–1016. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, S.; Yan, C.; Wu, X.; Wen, S.; Cong, W. Installation of flow deflectors and wing baffles to reduce dead zone and enhance flashing light effect in an open raceway pond. Bioresour. Technol. 2015, 198, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Guo, W.; Song, Y.; Kumar, S.; Ali, K.A.; Zhou, J. Enhancing vorticity magnitude of turbulent flow to promote photochemical efficiency and trichome helix pitch of Arthrospira platensis in a raceway pond with conic baffles. Bioresour. Technol. 2018, 269, 1–8. [Google Scholar] [CrossRef]
- Mohammed, K.; Ahammad, S.Z.; Sallis, P.J.; Mota, C.R. Energy-efficient stirred-tank photobioreactors for simultaneous carbon capture and municipal wastewater treatment. Water Sci. Technol. 2014, 69, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.; Ahammad, Z.S.; Sallis, P.J.; Mota, C.R. Optimisation of red light-emitting diodes irradiance for illuminating mixed microalgal culture to treat municipal wastewater. WIT Trans. Ecol. Environ. 2013, 178, 263–270. [Google Scholar]
- Cerón-García, M.C.; Macías-Sánchez, M.D.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. A Process for Biodiesel Production Involving the Heterotrophic Fermentation of Chlorella protothecoides with Glycerol as the Carbon Source. Appl. Energy 2013, 103, 341–349. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Senge, M.; Senger, H. Response of the photosynthetic apparatus during adaptation of Chlorella and Ankistrodesmus to irradiance changes. J. Plant Physiol. 1990, 136, 675–679. [Google Scholar] [CrossRef]
- Aleya, L.; Dauta, A.; Reynolds, C.S. Endogenous regulation of the growth-rate responses of a spring-dwelling strain of the freshwater alga, Chlorella minutissima, to light and temperature. Eur. J. Protistol. 2011, 47, 239–244. [Google Scholar] [CrossRef] [PubMed]
- González-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Dataset to assess the shadow effect of an outdoor microalgae culture. Data Brief 2019, 25, 104143. [Google Scholar] [CrossRef]
- Wang, S.K.; Stiles, A.R.; Guo, C.; Liu, C.Z. Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Huang, J.; Wan, M.; Jiang, J.; Zhang, A.; Zhang, D. Evaluating the effects of geometry and arrangement parameter of flat panel photobioreactor on microalgae biomass production and economic performance in China. Algal Res. 2021, 57, 102343. [Google Scholar] [CrossRef]
- Zou, N.; Richmond, A. Effect of light-path length in outdoor flat plate reactors on output rate of cell mass and of EPA in Nannochloropsis sp. J. Biotechnol. 1999, 70, 351–356. [Google Scholar] [CrossRef]
- Gonzalez-Camejo, J.; Aparicio, S.; Jimenez-Benitez, A.; Paches, M.; Ruano, M.V.; Borras, L.; Barat, R.; Seco, A. Improving membrane photobioreactor performance by reducing light path: Operating conditions and key performance indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef] [PubMed]
- Bechet, Q.; Shilton, A.; Guieysse, B. Modeling the effects of light and temperature on algae growth: State of the art and critical assessment for productivity prediction during outdoor cultivation. Biotechnol. Adv. 2013, 31, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, J.C.; Yada, H.; Masui, H.; Tanaka, H. A novel internally illuminated stirred tank photobioreactor for large-scale cultivation of photosynthetic cells. J. Ferment. Bioeng. 1996, 82, 61–67. [Google Scholar] [CrossRef]
- Sánchez-Contreras, M.I.; Morales-Arrieta, S.; Okoye, P.U.; Guillén-Garcés, R.A.; Sebastian, P.J.; Arias, D.M. Recycling industrial wastewater for improved carbohydrate-rich biomass production in a semi-continuous photobioreactor: Effect of hydraulic retention time. J. Environ. Manag. 2021, 284, 112065. [Google Scholar] [CrossRef] [PubMed]
- Deniz, I. Scaling-up of Haematococcus pluvialis production in stirred tank photobioreactor. Bioresour. Technol. 2020, 310, 123434. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Maximizing biomass and lipid production in heterotrophic culture of Chlorella vulgaris: Techno-economic assessment. Recent Pat. Food Nutr. Agric. 2019, 10, 115–123. [Google Scholar] [CrossRef] [PubMed]



| Condition 1 | Condition 2 | Condition 3 | Condition 4 | |||
|---|---|---|---|---|---|---|
| Ankistrodesmus | Productivity (g/L/d) | Cactus | 0.59 ± 0.062 Aa | 0.69 ± 0.045 Ba | 0.97 ± 0.081 Ca | 0.66 ± 0.063 Da |
| Cylindrical | 0.41 ± 0.019 Ab | 0.42 ± 0.060 Bb | 0.49 ± 0.004 Cb | 0.23 ± 0.005 Db | ||
| Specific growth rate (d−1) | Cactus | 0.07 ± 0.009 Aa | 0.17 ±0.009 Ba | 0.17 ± 0.010 Ca | 0.15 ± 0.010 Da | |
| Cylindrical | 0.03 ± 0.002 Ab | 0.13 ± 0.017 Bb | 0.14 ± 0.013 Cb | 0.05 ± 0.003 Db | ||
| Energy efficiency (g/d/kWh) | Cactus | 0.31 ± 0.033 Aa | 0.18 ± 0.012 Ba | 0.13 ± 0.010 Ca | 0.04 ± 0.004 Da | |
| Cylindrical | 0.22 ± 0.010 Ab | 0.11 ± 0.016 Bb | 0.06 ± 0.003 Cb | 0.02 ± 0.000 Db |
| Condition 1 | Condition 2 | Condition 3 | Condition 4 | Condition 5 | |||
|---|---|---|---|---|---|---|---|
| Chlorella | Productivity (g/L/d) | Cactus | 0.43 ± 0.007 Aa | 0.49 ± 0.018 Ba | 0.52 ± 0.008 Ca | 1 ± 0.032 Da | 0.24 ± 0.002 Ea |
| Cylindrical | 0.32 ± 0.007 Ab | 0.29 ± 0.007 Bb | 0.28 ± 0.003 Cb | 0.74 ± 0.043 Db | 0.19 ± 0.012 Eb | ||
| Specific growth rate (d−1) | Cactus | 0.09 ± 0.007 Aa | 0.14 ± 0.002 Ba | 0.14 ± 0.005 Ca | 0.23 ± 0.008 Da | 0.08 ±0.016 Ea | |
| Cylindrical | 0.05 ± 0.013 Ab | 0.11 ± 0.002 Bb | 0.11 ± 0.001 Cb | 0.21 ± 0.004 Db | 0.03 ± 0.004 Eb | ||
| Energy efficiency (g/d/kWh) | Cactus | 0.23 ± 0.003 Aa | 0.13 ± 0.004 Ba | 0.07 ± 0.001 Ca | 0.07 ± 0.002 Da | 0.01 ± 0.000 Ea | |
| Cylindrical | 0.17 ± 0.003 Ab | 0.08 ± 0.002 Bb | 0.04 ± 0.000 Cb | 0.05 ± 0.002 Db | 0.01 ± 0.000 Eb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijazi, R.M.; Mounsef, J.R.; Kanaan, H.Y. Comparison of Light Intensity Effect on Microalgal Growth in Cactus-like and Cylindrical Photo Bioreactors. Processes 2024, 12, 1664. https://doi.org/10.3390/pr12081664
Hijazi RM, Mounsef JR, Kanaan HY. Comparison of Light Intensity Effect on Microalgal Growth in Cactus-like and Cylindrical Photo Bioreactors. Processes. 2024; 12(8):1664. https://doi.org/10.3390/pr12081664
Chicago/Turabian StyleHijazi, Rayane Mustafa, Jihane Rahbani Mounsef, and Hadi Youssef Kanaan. 2024. "Comparison of Light Intensity Effect on Microalgal Growth in Cactus-like and Cylindrical Photo Bioreactors" Processes 12, no. 8: 1664. https://doi.org/10.3390/pr12081664






