Flow and Heat Transfer of Shale Oil Reservoir during CO2 Enhanced Pyrolysis: A Pore-Scale Modeling
Abstract
1. Introduction
2. Model Description
2.1. Governing Equations
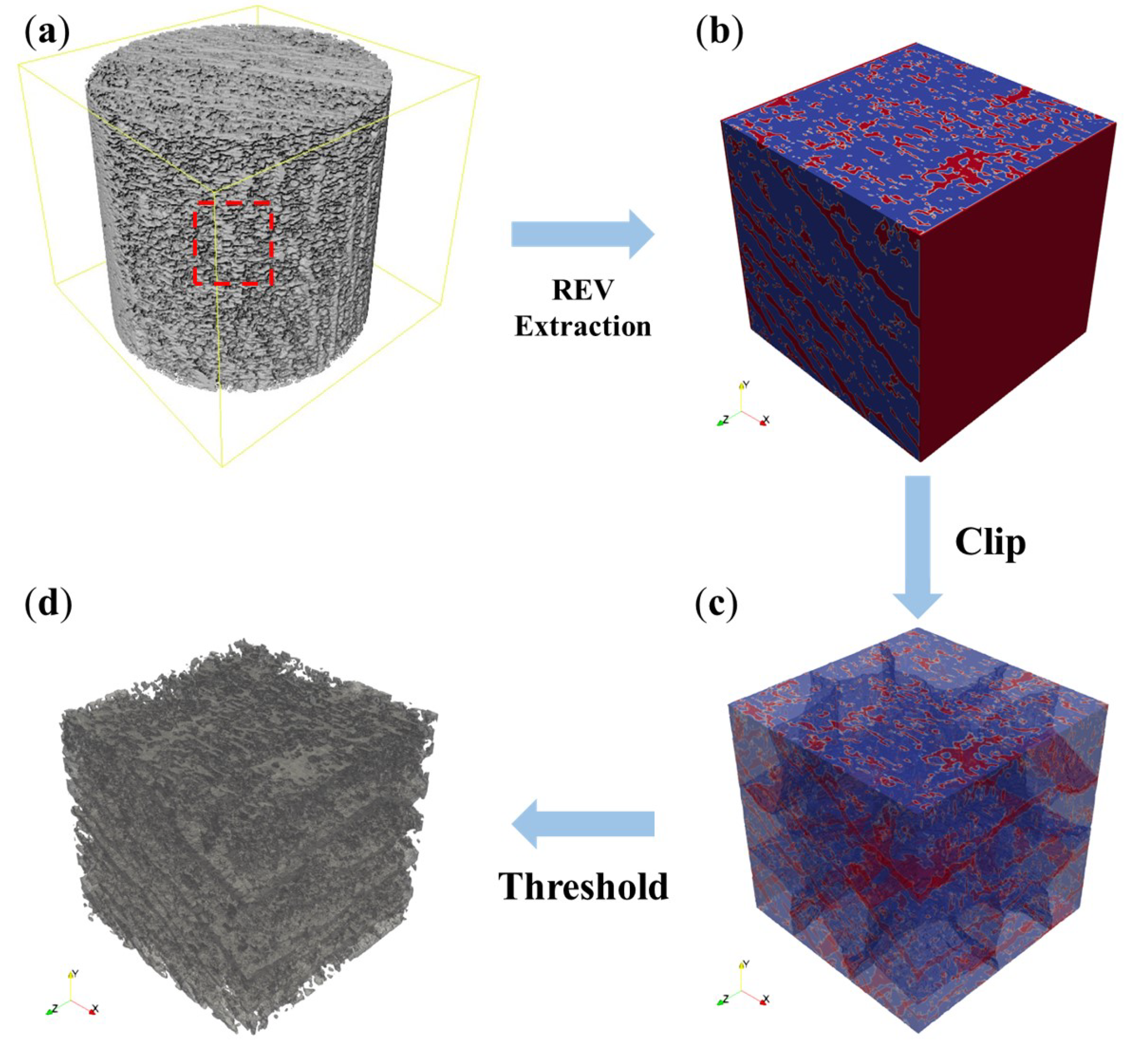
2.2. Oil Shale Model and REV Selection
2.3. Simulation Parameters
3. Results and Discussion
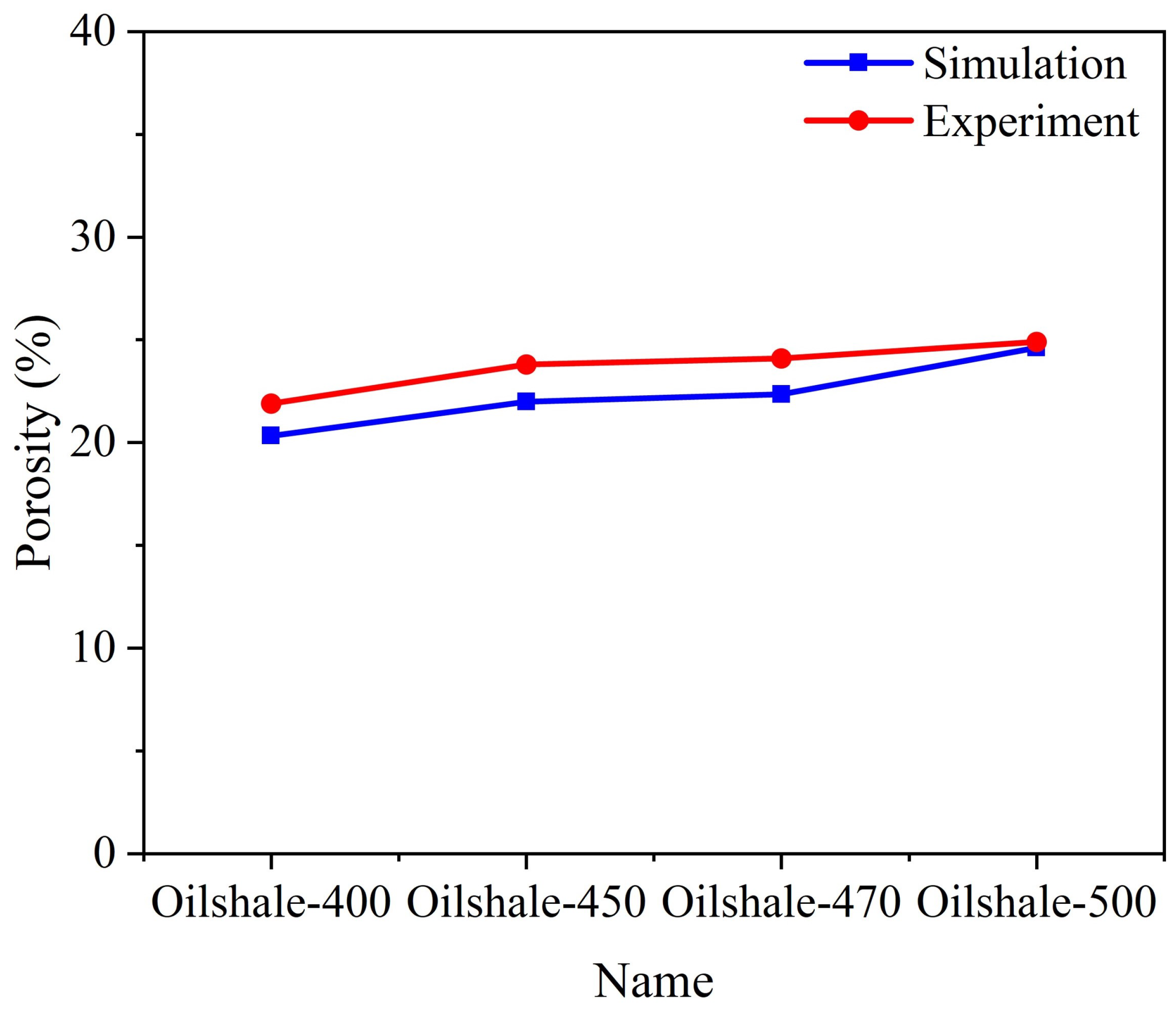
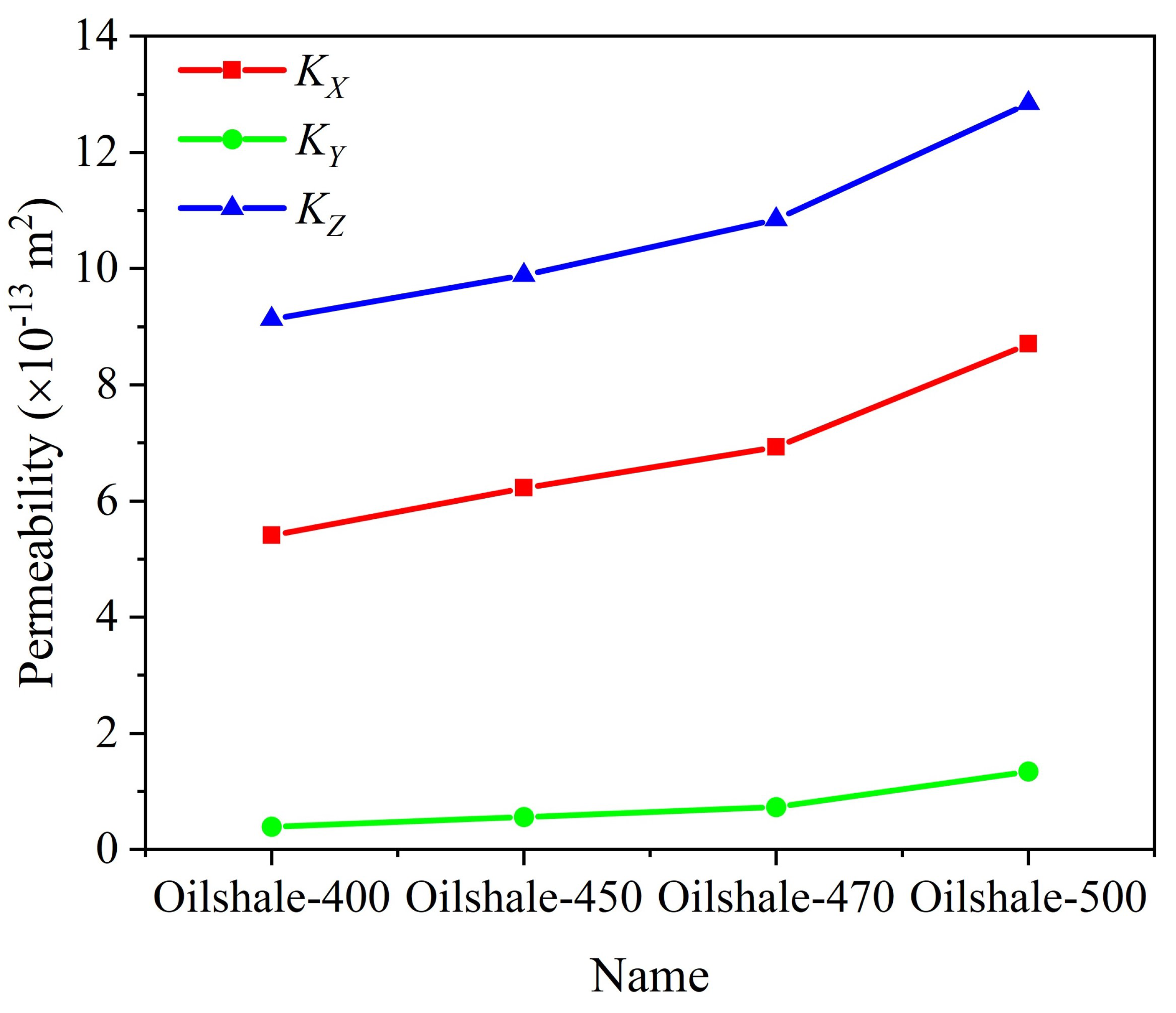
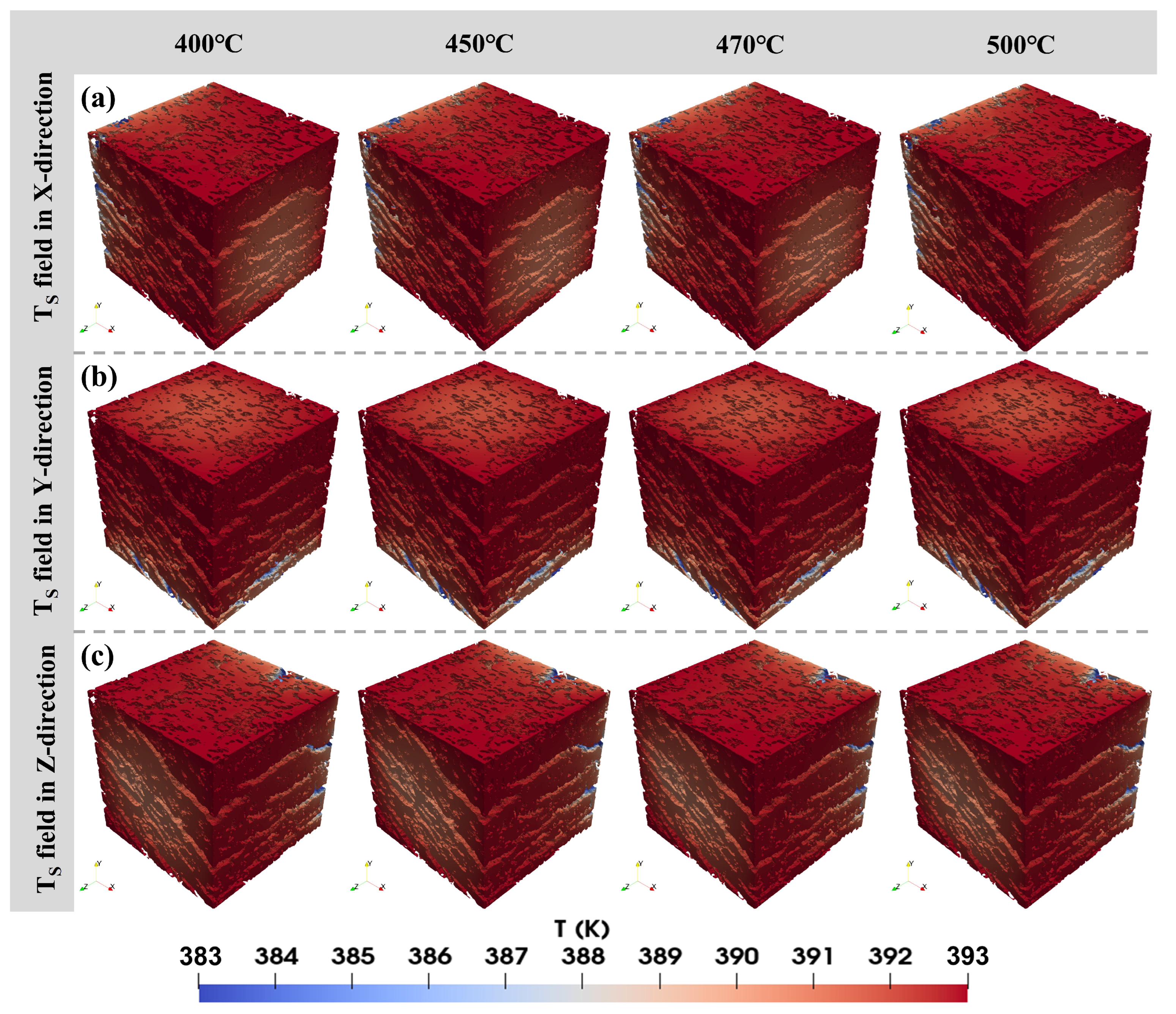
3.1. Influence of Pyrolysis Temperature
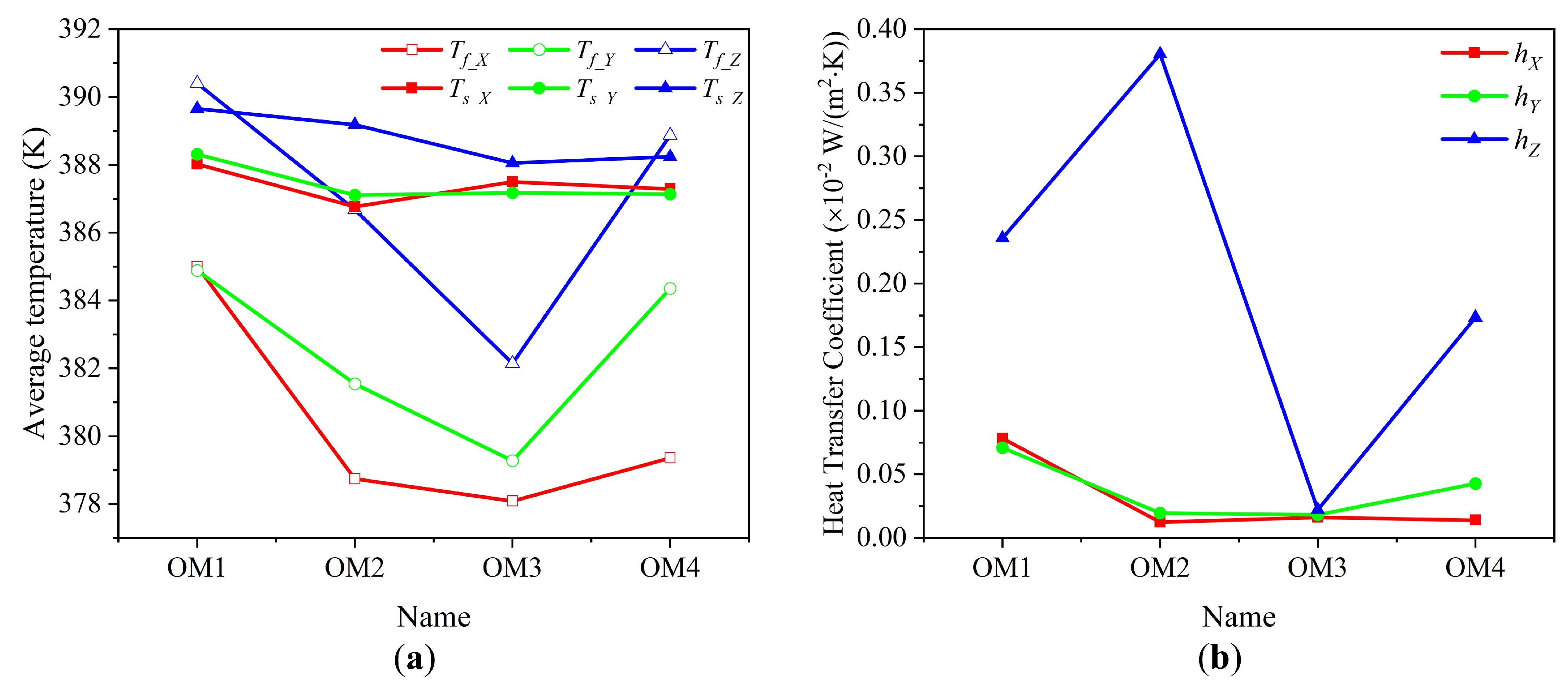
3.2. Influence of Organic Matter Content
4. Conclusions
- The permeability of oil shale samples shows a consistent increase with rising pyrolysis temperatures. While temperature minimally impacts the distribution of velocity and temperature fields, it significantly affects the heat transfer coefficient. Specifically, as temperature increases, the heat transfer coefficient markedly rises perpendicular to the bedding planes but decreases or remains constant parallel to them. This phenomenon is due to changes in the rock’s pore structure from organic matter pyrolysis, which influences fluid flow and heat transfer properties.
- Organic matter content significantly influences the fluid flow and heat transfer properties of shale samples. Comparing shale samples with varying organic matter contents after thermal treatment shows that differences in organic matter lead to heterogeneity in pore structure, affecting fluid flow and heat transfer characteristics. After pyrolysis, the complete decomposition of organic matter creates large cavities, altering the native pore distribution. If these cavities are interconnected, they enhance fluid flow capacity and improve convective heat transfer. However, if the cavities are isolated, fluids cannot penetrate despite the increased porosity, and heat transfer relies solely on conduction between the fluid and the solid.
- The influence of organic matter content and pyrolysis temperature on fluid flow and heat transfer in shale is primarily due to the effect of organic matter pyrolysis on the native pore structure. The development and connectivity of the pore network are closely linked to the distribution characteristics of the native organic matter, rather than the quantity of organic matter present.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caineng, Z.; Qun, Z.; Hongyan, W.; Qian, S.; Nan, S.; Zhiming, H.; Chenjun, Z.; Yuping, S.; Meizhu, W.; Dexun, L. Theory and Technology of Unconventional Oil and Gas Exploration and Development Helps China Increase Oil and Gas Reserves and Production. Oil Forum 2021, 40, 72. [Google Scholar]
- Wang, J.; Feng, L.; Steve, M.; Tang, X.; Gail, T.E.; Mikael, H. China’s Unconventional Oil: A Review of Its Resources and Outlook for Long-Term Production. Energy 2015, 82, 31–42. [Google Scholar] [CrossRef]
- Zheng, D.; Miska, S.; Ozbayoglu, E.; Zhang, J. Combined Experimental and Well Log Study of Anisotropic Strength of Shale. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 16–18 October 2023. [Google Scholar]
- Zheng, D.; Ozbayoglu, E.; Miska, S.; Zhang, J. Experimental Study of Anisotropic Strength Properties of Shale. In Proceedings of the 57th U.S. Rock Mechanics/Geomechanics Symposium, Atlanta, GA, USA, 25–28 June 2023. [Google Scholar]
- Lei, Y.; Zhijun, J. Global Shale Oil Development and Prospects. China Pet. Explor. 2019, 24, 553. [Google Scholar]
- Hu, S.; Zhao, W.; Hou, L.; Yang, Z.; Zhu, R.; Wu, S.; Bai, B.; Jin, X. Development Potential and Technical Strategy of Continental Shale Oil in China. Pet. Explor. Dev. 2020, 47, 877–887. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, S.; Hou, L.; Yang, T.; Li, X.; Guo, B.; Yang, Z. Types and Resource Potential of Continental Shale Oil in China and Its Boundary with Tight Oil. Pet. Explor. Dev. 2020, 47, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Kang, Z. Evolution of Permeability and Mesostructure of Oil Shale Exposed to High-Temperature Water Vapor. Fuel 2021, 290, 119786. [Google Scholar] [CrossRef]
- Knyazeva, A.G.; Maslov, A.L.; Martemyanov, S.M. A Two-Phase Model of Shale Pyrolysis. Fuel 2018, 228, 132–139. [Google Scholar] [CrossRef]
- Skala, D.; Kopsen, H.; Sokić, M.; Neumann, H.J.; Jovanović, J. Modelling and Simulation of Oil Shale Pyrolysis. Fuel 1989, 68, 168–173. [Google Scholar] [CrossRef]
- Wang, G.; Yang, D.; Kang, Z.; Zhao, J.; Lv, Y. Numerical Investigation of the in Situ Oil Shale Pyrolysis Process by Superheated Steam Considering the Anisotropy of the Thermal, Hydraulic, and Mechanical Characteristics of Oil Shale. Energy Fuels 2019, 33, 12236–12250. [Google Scholar] [CrossRef]
- Korba, D.; Li, L. Effects of Pore Scale and Conjugate Heat Transfer on Thermal Convection in Porous Media. J. Fluid Mech. 2022, 944, A28. [Google Scholar] [CrossRef]
- Li, X.; Ma, T.; Liu, J.; Zhang, H.; Wang, Q. Pore-Scale Investigation of Gravity Effects on Phase Change Heat Transfer Characteristics Using Lattice Boltzmann Method. Appl. Energy 2018, 222, 92–103. [Google Scholar] [CrossRef]
- Paknahad, R.; Siavashi, M.; Hosseini, M. Pore-Scale Fluid Flow and Conjugate Heat Transfer Study in High Porosity Voronoi Metal Foams Using Multi-Relaxation-Time Regularized Lattice Boltzmann (MRT-RLB) Method. Int. Commun. Heat Mass Transf. 2023, 141, 106607. [Google Scholar] [CrossRef]
- Rabbani, A.; Baychev, T.G.; Ayatollahi, S.; Jivkov, A.P. Evolution of Pore-Scale Morphology of Oil Shale During Pyrolysis: A Quantitative Analysis. Transp. Porous Media 2017, 119, 143–162. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Qu, Z.; Yin, Y.; Kang, Q.; Yu, B.; Tao, W.Q. Modeling of Multi-Scale Transport Phenomena in Shale Gas Production—A Critical Review. Appl. Energy 2020, 262, 114575. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Chen, J. Pore-Scale Conjugate Heat Transfer of Nanofluids within Fibrous Medium with a Double MRT Lattice Boltzmann Model. Int. J. Therm. Sci. 2021, 163, 106804. [Google Scholar] [CrossRef]
- Kopanidis, A.; Theodorakakos, A.; Gavaises, E.; Bouris, D. 3D Numerical Simulation of Flow and Conjugate Heat Transfer through a Pore Scale Model of High Porosity Open Cell Metal Foam. Int. J. Heat Mass Transf. 2010, 53, 2539–2550. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Zhang, Q.; Imani, G.; Zhang, L.; Sun, H.; Zhong, J.; Zhang, K.; Yao, J. Pore-Scale Flow Simulation of CO2 Sequestration in Deep Shale Based on Thermal-Hydro-Mechanical Coupled Model. Phys. Fluids 2024, 36, 022005. [Google Scholar] [CrossRef]
- Meinicke, S.; Dubil, K.; Wetzel, T.; Dietrich, B. Characterization of Heat Transfer in Consolidated, Highly Porous Media Using a Hybrid-Scale CFD Approach. Int. J. Heat Mass Transf. 2020, 149, 119201. [Google Scholar] [CrossRef]
- Meinicke, S.; Dietrich, B.; Wetzel, T. Pore-scale numerical analysis of hydrodynamics and conjugate heat transfer in solid sponges. In International Heat Transfer Conference Digital Library; Begel House Inc.: Danbury, CT, USA, 2018. [Google Scholar]
- Meinicke, S.; Wetzel, T.; Dietrich, B. Scale-Resolved CFD Modelling of Single-Phase Hydrodynamics and Conjugate Heat Transfer in Solid Sponges. Int. J. Heat Mass Transf. 2017, 108, 1207–1219. [Google Scholar] [CrossRef]
- Hugo, J.M.; Topin, F.; Tadrist, L.; Brun, E. From Pore Scale Numerical Simulation of Conjugate Heat Transfer in Cellular Material to Effectives Transport Properties of Real Structures. In Proceedings of the 2010 14th International Heat Transfer Conference, Washington, DC, USA, 8–13 August 2010; American Society of Mechanical Engineers Digital Collection: New York, NY, USA, 2011; pp. 931–936. [Google Scholar]
- Li, J.; Zhang, P.; Lu, S.; Chen, C.; Xue, H.; Wang, S.; Li, W. Scale-Dependent Nature of Porosity and Pore Size Distribution in Lacustrine Shales: An Investigation by BIB-SEM and X-Ray CT Methods. J. Earth Sci. 2019, 30, 823–833. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H. Pore-Scale Study on Flow and Heat Transfer in 3D Reconstructed Porous Media Using Micro-Tomography Images. Appl. Therm. Eng. 2016, 100, 602–610. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, T.; Lu, Y.; Wang, B.; Zeng, G.; Zhang, X. The Study on Solving Large Pore Heat Transfer Simulation in Malan Loess Based on Volume Averaging Method Combined with CT Scan Images. Sustainability 2023, 15, 12389. [Google Scholar] [CrossRef]
- Sun, M.; Hu, C.; Zha, L.; Xie, Z.; Yang, L.; Tang, D.; Song, Y.; Zhao, J. Pore-Scale Simulation of Forced Convection Heat Transfer under Turbulent Conditions in Open-Cell Metal Foam. Chem. Eng. J. 2020, 389, 124427. [Google Scholar] [CrossRef]
- Tembely, M.; AlSumaiti, A.M.; Jouini, M.S.; Rahimov, K. The Effect of Heat Transfer and Polymer Concentration on Non-Newtonian Fluid from Pore-Scale Simulation of Rock X-Ray Micro-CT. Polymers 2017, 9, 509. [Google Scholar] [CrossRef]
- Tiwari, P.; Deo, M.; Lin, C.L.; Miller, J.D. Characterization of Oil Shale Pore Structure before and after Pyrolysis by Using X-Ray Micro CT. Fuel 2013, 107, 547–554. [Google Scholar] [CrossRef]
- Maes, J.; Menke, H.P. Geochemfoam: Direct modelling of flow and heat transfer in micro-ct images of porous media. Heat Mass Transf. 2022, 58, 1937–1947. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Singh, K.; Bijeljic, B.; Blunt, M.J. Dynamic imaging of oil shale pyrolysis using synchrotron X-ray microtomography. Geophys. Res. Lett. 2016, 43, 6799–6807. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Bijeljic, B.; Blunt, M.J. Microstructural imaging and characterization of oil shale before and after pyrolysis. Fuel 2017, 197, 562–574. [Google Scholar] [CrossRef]
- Liu, J.; Yu, P.; Li, Y.; Wan, C.; Du, D. Numerical simulation on convective heat transfer characteristics in porous media based on the digital rock technology. Int. J. Heat Mass Transf. 2022, 196, 123323. [Google Scholar] [CrossRef]
- Xie, F.; Zhu, C.; Tang, B. An experimental study on anisotropy of thermal conductivity in shale. Energy Geosci. 2023, 4, 100195. [Google Scholar] [CrossRef]
- Cheng, C.; Lin, H.; Jiang, Y.; Feng, L.; Xia, Y.; Mu, C. Thermal conductivity of gas-bearing shale of the Longmaxi Formation in the southern Sichuan. Pertol. Geol. 2019, 41, 289–294. [Google Scholar]
- Chen, C.; Zhu, C.; Tang, B.; Chen, T. Progress in the study of the influencing factors of rock thermal conductivity. Prog. Geophys. 2020, 35, 2047–2057. [Google Scholar]
- Liu, Z.; Ma, H.; Wang, Z.; Guo, Y.; Li, W.; Hou, Z. Experimental study on the thermophysical properties of Jimsar oil shale. Oil Shale 2023, 40, 194–211. [Google Scholar] [CrossRef]

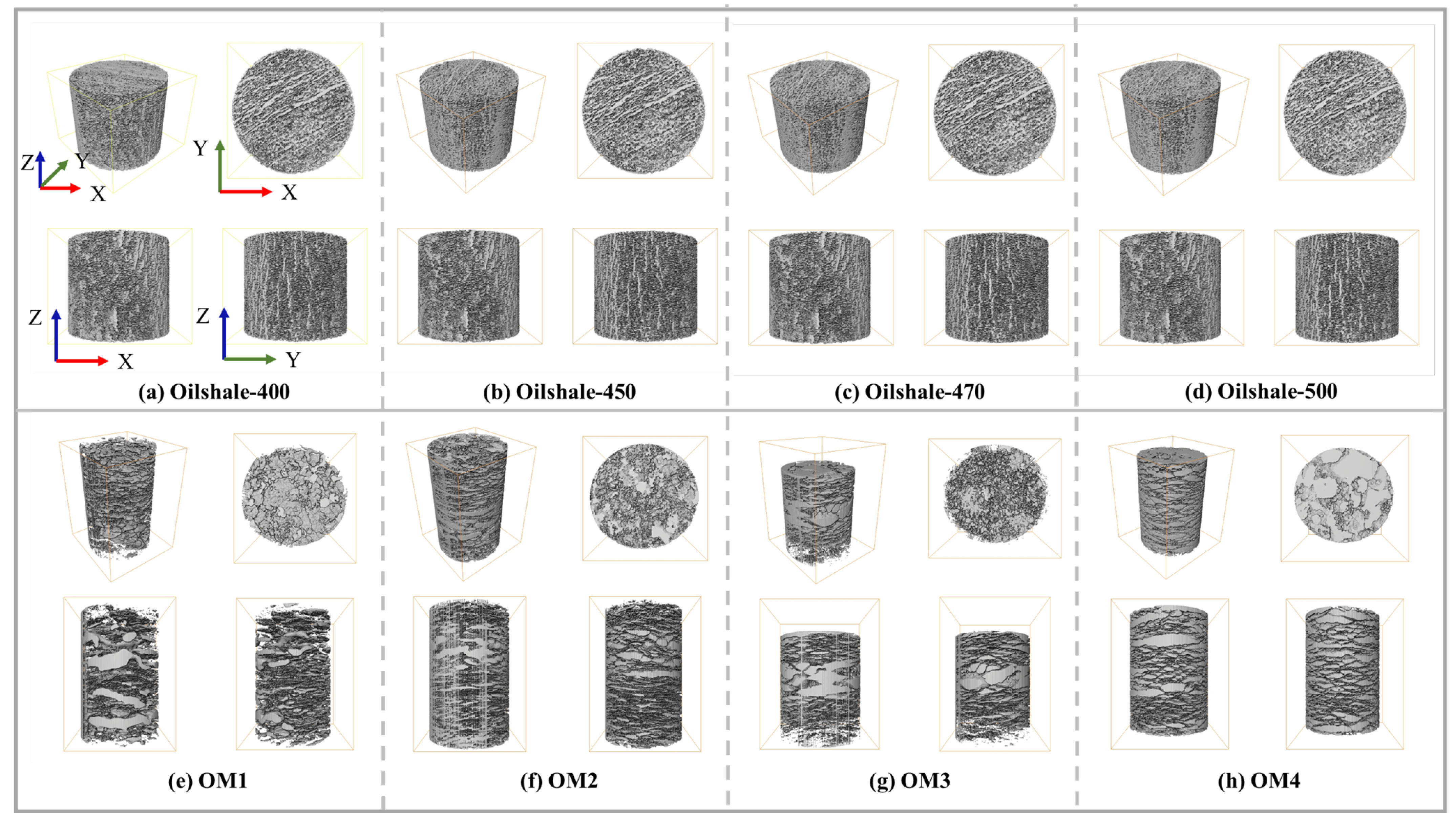








| Name | Width | Height | Number of Images | Porosity |
|---|---|---|---|---|
| Oilshale-400/450/470/500 | 1280 | 1280 | 1020 | 21.9%/23.8%/24.1%/24.9% |
| OM1 | 1311 | 1388 | 1790 | 12.1% |
| OM2 | 1302 | 1295 | 1790 | 19.1% |
| OM3 | 1426 | 1285 | 1790 | 23.0% |
| OM4 | 1412 | 1400 | 1789 | 29.3% |
| Properties | Oil Shale | |
|---|---|---|
| Density (kg/) | 290.81 | 2675.00 |
| Dynamic viscosity (/s) | ||
| Specific heat capacity (kJ/g/K) | 3.05 | 1.36 |
| Thermal conductivity (W/m/K) | 0.04 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Weng, D.; Cai, B.; Zhang, Y.; Zhang, Y.; Wang, B.; Wang, H. Flow and Heat Transfer of Shale Oil Reservoir during CO2 Enhanced Pyrolysis: A Pore-Scale Modeling. Processes 2024, 12, 1694. https://doi.org/10.3390/pr12081694
Shi Y, Weng D, Cai B, Zhang Y, Zhang Y, Wang B, Wang H. Flow and Heat Transfer of Shale Oil Reservoir during CO2 Enhanced Pyrolysis: A Pore-Scale Modeling. Processes. 2024; 12(8):1694. https://doi.org/10.3390/pr12081694
Chicago/Turabian StyleShi, Yang, Dingwei Weng, Bo Cai, Yunpeng Zhang, Yaochen Zhang, Bin Wang, and Haizhu Wang. 2024. "Flow and Heat Transfer of Shale Oil Reservoir during CO2 Enhanced Pyrolysis: A Pore-Scale Modeling" Processes 12, no. 8: 1694. https://doi.org/10.3390/pr12081694
APA StyleShi, Y., Weng, D., Cai, B., Zhang, Y., Zhang, Y., Wang, B., & Wang, H. (2024). Flow and Heat Transfer of Shale Oil Reservoir during CO2 Enhanced Pyrolysis: A Pore-Scale Modeling. Processes, 12(8), 1694. https://doi.org/10.3390/pr12081694







