Current Status and Challenges in the Commercial Production of Polyhydroxyalkanoate-Based Bioplastic: A Review
Abstract
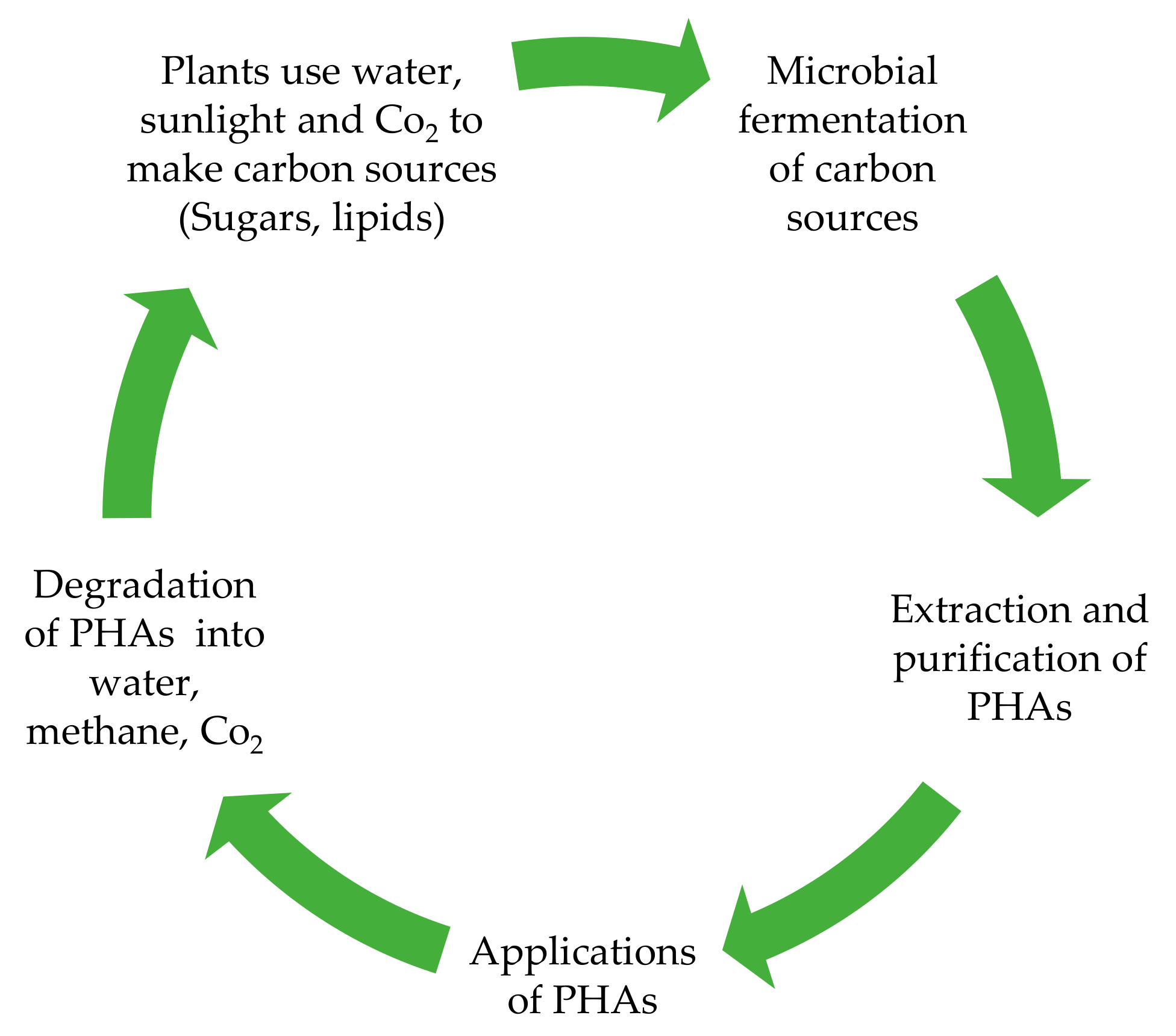
:1. Introduction
2. Production of Biopolymers
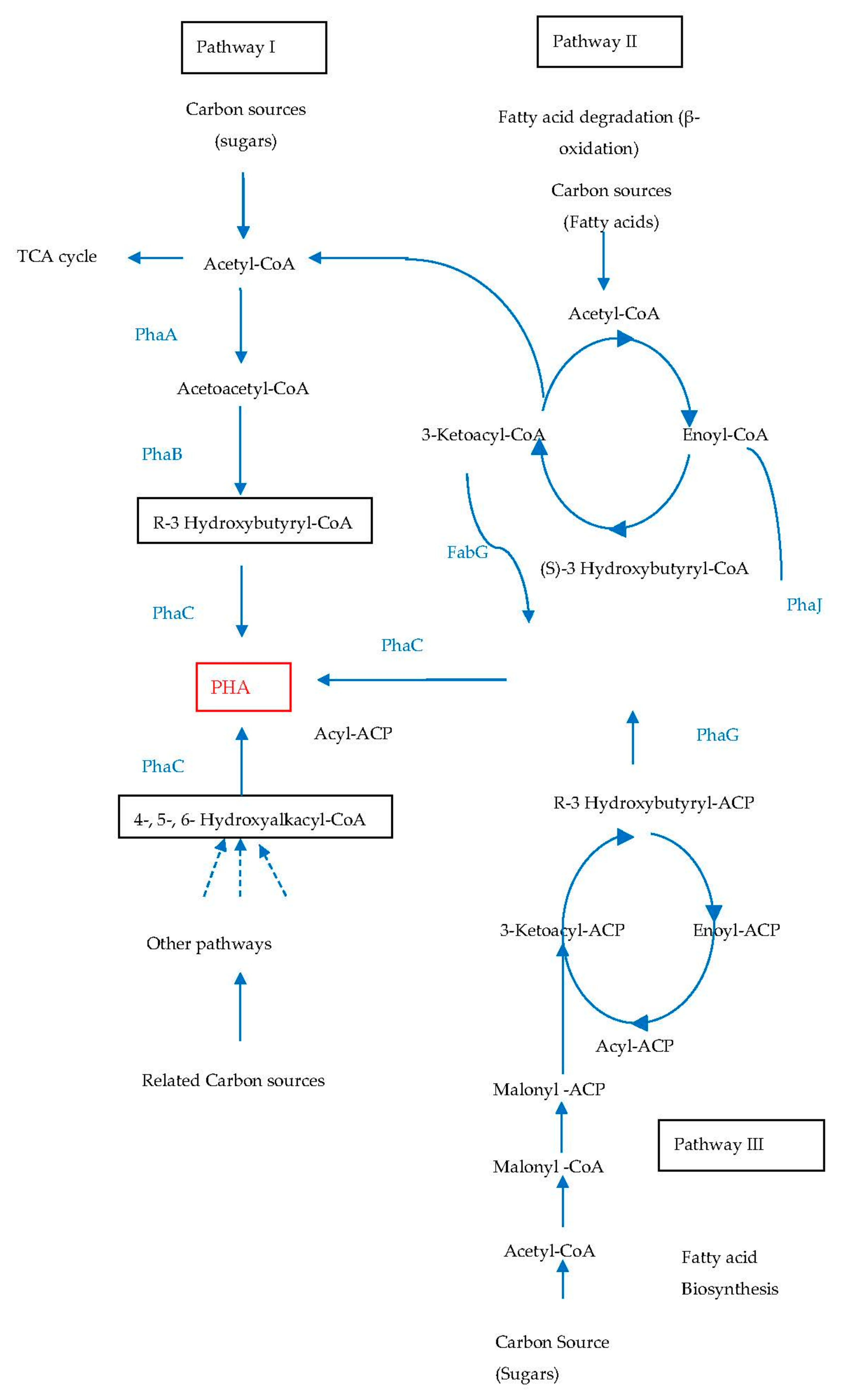
PHA Feedstock
3. Upstream Processing of PHAs
4. Downstream Processing of PHAs
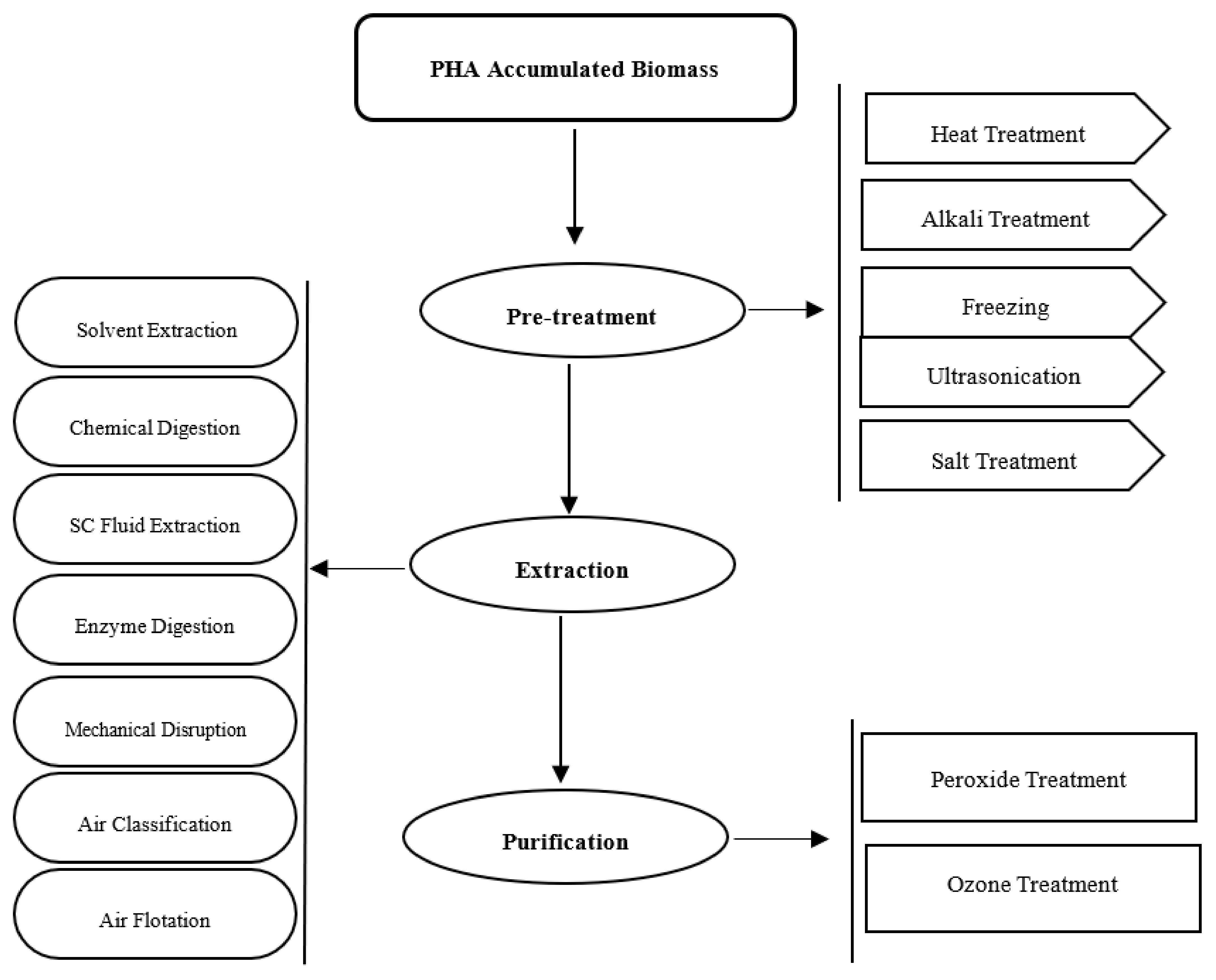
4.1. Pre-Treatment
4.2. Extraction
4.2.1. Solvent Extraction
| Sr. No. | Feedstock | Solvent/Non-Solvent | Bacterial Strain | PHA Compound | Pre-Treatment/Purification Stage | % Recovery | % Purity | Thermal Data | Molecular Weight Data | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | NaOH/Ethanol | Cupriavidunecator | PHA | Post-treatment: Freeze drying | - | - | - | - | [106] |
| 2 | Unknown | Methyl isobutyl ketone/Hexane | Ralstoniaeutropha | PHO | Pre-treatment: Freeze drying | 55 | 99 | - | - | [107] |
| Methyl ethyl ketone/Hexane | 95 | 100 | ||||||||
| Butyl acetate/Hexane | 42 | 100 | ||||||||
| Ethyl acetate/Hexane | 99 | 100 | ||||||||
| 3 | Unknown | Methyl tert-butyl ether/Ethanol | Pseudomonas putida | PHO | Pre-treatment: Freeze drying | 4 | - | - | Mw = 155 kDa; PI = 1.8 | [108] |
| Ethyl acetate/Ethanol | 12 | Mw = 132 kDa; PI = 1.7 | ||||||||
| Acetone/Ethanol | Purification with acetone and activated charcoal | 13 | Mw = 138 kDa; PI = 1.7 | |||||||
| Methylene chloride/Ethanol | 17 | Mw = 156 kDa; PI = 1.9 | ||||||||
| 4 | Unknown | Anisole/Ethanol | Burkholderiasacchari | PHB | Pre-treatment: Freeze drying | 96.7 | 98.3 | - | Mw = 6.8 × 105 Da; PI = 2.34 | [109] |
| Cyclohexanone/Methanol | 93.4 | 98.2 | Mw = 8 × 105 Da; PI = 1.46 | |||||||
| Phenetole/Ethanol | - | - | Mw = 5.6 × 105 Da; PI = 2.24 | |||||||
| 5 | Unknown | DMC/Ethanol | Cupravidusnecator | PHB | Pre-treatment: Freeze drying | - | 95 | TGA: Td = 280 °C | GPC: Mw = 1 MDa; PI = 2.7 | [62] |
| 6 | Unknown | Propylene carbonate/Acetone | Cupriavidusnecator | PHB | Pre-treatment: Thermal | 95 | 84 | DSC: Xc% = 60; Tm = 175 °C; Tg = 4.9 °C | Mw = 740 kDa; PI = 3.1 | [99] |
| 7 | Biodiesel | Chloroform/Ethanol | Pseudomonas citronellolis | PHA | Pre-treatment: Freeze drying and ethanol wash | 26.6 | - | DSC: Xc% = 10.4; Tm = 53.6 °C; Tg = −43.5 °C | Mw = 78 kDa; PI = 2.5 | [84] |
| 8 | Unknown | Ethylene Carbonate/Ethanol | Cupriavidusnecator | PHB | Pre-treatment with NaOCl | 98.6 | 98 | Xc% = 59.2; Tm = 176 °C; Tg = 4.8 °C | - | [110] |
| Methanol/Ethanol | 72.6 | 97 | - | |||||||
| Propanol/Ethanol | 28.49 | 97 | - | |||||||
| Acetic acid/Ethanol | 36.71 | 97 | - | |||||||
| DMSO/Ethanol | 60.6 | 95 | Xc% = 57.3; Tm = 176 °C; Tg = 5.1 °C | |||||||
| DMFO/Ethanol | 30.1 | 97 | - | |||||||
| Hexane/Ethanol | 2.8 | 83 | - | |||||||
| 9 | Unknown | Chloroform/Methanol | Cupriavidusnecator | PHB | Pre-treatment: Freeze drying | - | - | Xc% = 0.52; Tm = 165 °C; Td = 310 °C | Mw = 283 kDa; PI = 2.9; | [111] |
| NaOH | Pre-treatment: Freeze drying; Purification: Ethanol | 78 | 95 | Xc% = 0.6; Tm = 171 °C; Td = 247 °C | Mw = 837 kDa; PI = 1.61 | |||||
| NaOCl | Pre-treatment: Freeze drying; Purification: Ethanol | - | 98 | Xc% = 0.6; Tm = 169 °C; Td = 294 °C | Mw = 249 kDa; PI = 6.82 | |||||
| Dichloromethane/Ethanol | Pre-treatment: Freeze drying and NaOCl digestion; Purification: Ethanol | 90 | 99 | Xc% = 0.6; Tm = 167 °C; Td = 296 °C | Mw = 361 kDa; PI = 2.61 | |||||
| Sulphuric acid | Pre-treatment: Freeze drying; Purification: NaOCl bleaching | - | - | Xc% = 0.84; Tm = 165 °C; Td = 303 °C | Mw = 321 kDa; PI = 7.29 | |||||
| 10 | Unknown | Methylene chloride/Methanol | Pseudomonas putida | PHA | - | - | - | - | Mw = 0.5 MDa | [56] |
| Ethyl acetate/Methanol | ||||||||||
| Acetone/Methanol | ||||||||||
| 11 | Unknown | n-Hexane/Methanol | Pseudomonas putida | PHO | NA | 49 | 100 | - | Mw = 212 kDa; PI = 1.92 | [112] |
| 2-Propanol/Methanol | 12 | 84 | Mw = 321 kDa; PI = 1.26 | |||||||
| Dichloromethane/Methanol | 83 | 100 | - | |||||||
| Ethyl acetate/Methanol | 78 | 99 | - | |||||||
| THF/Methanol | 77 | 100 | - | |||||||
| Acetone/Methanol | 77 | 100 | - | |||||||
| 12 | Green Grass Juice | Chloroform/Ethanol | Wautersiaeutropha | PHB | Pre-treatment: Freeze drying and ethanol wash. | 77.2 | - | Xc% = 64.6; Tm = 180 °C; Tg = 6 °C | Mw = 432 kDa; PI = 4.02 | [113] |
| Silage Juice | 77.3 | - | Xc% = 65.9; Tm = 180 °C; Tg = 6 °C | Mw = 434 kDa; PI = 4.01 | ||||||
| 13 | Whey | Chloroform | Haloferaxmediterranei | PHBV | Pre-treatment: Freeze drying and ethanol wash | 72.8 | - | Tm = 158.9 °C Td = 241 °C; Tg = 6 °C | Mw = 1057 kDa | [114] |
| 14 | Glucose | Chloroform/Ethanol | Cupriavidusnecator | PHB | Pre-treatment: Freeze drying and ethanol wash | 77 | - | Xc% = 68; Tm = 178 °C; Tg = 6 °C | Mw = 665 kDa | [101] |
| 15 | Crude Glycerol | Chloroform | Haloferaxmediterranei | PHBV | Pre-treatment: Freeze drying and ethanol wash | 75.4 | - | Tm = 137.1 °C; Td = 285 °C | Mw = 391 kDa | [115] |
| 16 | Vegetable Oil | Cyclohexanone/Methanol | Cupriavidusnecator | PHB | Pre-treatment: Freeze drying and acetone wash | 99 | 99.5 | - | Mw = 23 kDa | [116] |
| Butyrolactone/Methanol | 45 | 97.2 | - | |||||||
| 17 | Unknown | 0.05M NaOH | Cupriavidusnecator | PHA | Purification with ethanol | 96.9 | 96 | - | Mw = 1.4 × 105 Da | [106] |
| 18 | Unknown | Chloroform/Methanol | Alcaligeneseutrophus | PHB | Pre-treatment with NaOCl | 91 | 97 | Xc% = 65; Tm = 176 °C | Mw = 10 × 105 Da | [60] |
| 19 | Unknown | Sodium hypochlorite | Ralstoniaeutropha | PHB | Pre-treatment: freeze drying; Purification: isopropanol | 91.32 | 96 | - | Mw = 4.6–8.3 × 105 Da | [117] |
| 20 | Sludge | Dimethyl carbonate/Ethanol | Cupriavidusnecator | PHB | Pre-treatment: Freeze drying | 85 | 95 | Td = 280 °C | Mw = 1 MDa | [118] |
| Dichloromethane/Ethanol | 17 | 94 | Td = 290 °C | Mw = 1.1 MDa | ||||||
| NH4Laurate | 102 | 98 | Td = 264 °C | Mw = 0.6 MDa | ||||||
| NH4OH | 70 | 70 | Td = 272 °C | Mw = 0.7 MDa | ||||||
| SDS | 99 | 90 | Td = 271 °C | Mw = 1.2 MDa | ||||||
| 21 | Corn Oil | Acetic acid/Methanol | Burkholderiacepacia | PHB | Pre-treatment with SDS | 89.3 | 90.99 | Tm = 175 °C Td = 280.7 °C | Mw = 8.31 × 105 Da | [119] |
| Chloroform/Methanol | 100 | 95.08 | Tm = 175.3 °C; Td = 291.3 °C | Mw = 8.97 × 105 Da | ||||||
| 22 | Sludge | Butanol | MMC | PHBV | Purification with acetone | 87 | NA | - | - | [40] |
| 23 | Sludge | Butanol | MMC | PHBV | - | - | - | - | Mw = 11 × 105 Da; PI = 2.6 | |
| Chloroform/Hexane | - | Mw = 10.7 × 105 Da; PI = 2.6 | ||||||||
| 24 | Activated sludge | Chloroform | MMC | PHB | - | - | - | - | [120] | |
| Food processing waste | 28.3 | |||||||||
| Jowar grain-based distillery spent water | 34.7 | |||||||||
| Rice grain-based distillery spent water | 36.5 | |||||||||
| 25 | Paper industry waste sludge | Chloroform/Methanol | MMC | PHBV | - | 39.6 | - | - | - | [50] |
| 26 | Paper industry waste sludge | Dimethyl carbonate | MMC | PHB | Purification with chloroform and activated carbon | - | - | - | - | [121] |
| 27 | Sludge | Chloroform | MMC | PHB | Pre-treatment with acetone, ethanol, and NaOCl | 65.84 | - | - | - | [59] |
| 28 | Municipal wastewater sludge | Chloroform/Hexane | MMC | PHA | 58 | - | - | - | [122] | |
| 29 | Sludge | SDS | MMC | PHB | Purification with NaOCl | 44.2 | 99.5 | - | - | [13] |
| 30 | Grain processing waste | Chloroform | MMC | PHB | Purification with hexane | 44 | - | Tm = 100 °C | - | [123] |
| 31 | Municipal wastewater sludge | Chloroform/Methanol | MMC | PHBV | Surfactant pre-treatment | 15 | - | Tm = 152.3 °C; Td = 284 °C | Mw = 3.1 × 105 Da | [124] |
| Acetone/Water | - | 37 | - | Tm = 165.5 °C; Td = 274 °C | Mw = 5.1 × 105 Da | |||||
| 32 | Sludge | SDS | MMC | PHBV | - | 32 | - | Tm = 149.9 °C | Mw = 4.3 × 105 Da | [125] |
| 33 | Sludge | Dimethyl carbonate | MMC | PHA | - | 96 | - | - | - | [126] |
| 34 | Food waste sludge | Chloroform | MMC | PHBV | NaOCl purification | 91 | 100 | Tm,avg = 144 °C; Td = 252–278 °C | Mw = 132 kDa | [122] |
| 35 | Oil mill wastewater | NH4 Laurate | MMC | PHB | NaOCl pre-treatment and NH4OH and ethanol purification | 77 | 100 | Td = 185 °C | Mw = 0.918 × 105 Da | [127] |
| PHBV | 73 | 93 | Td = 205 °C | Mw = 1.438 × 105 Da | ||||||
| 36 | Sludge | Chloroform | MMC | PHB | Pre-treatment with NaOCl | 44 | - | - | - | [1] |
| 37 | Sludge | Chloroform/Methanol | MMC | PHA | Pre-treatment with NaOCl and purification with activated carbon | 7.01 | - | - | - | [67] |
| 38 | Municipal wastewater sludge | Dimethyl carbonate | MMC | PHBV | Purification with 1-Butanol | 30.7 | 98.5 | - | - | [128] |
| Chloroform | 37 | 82.5 | ||||||||
| Dichloromethane | 39 | 86.4 | ||||||||
| 39 | Municipal wastewater sludge | Dichloromethane/Water | MMC | PHBV | Pre-treatment: Acetone wash | 30 | - | Tm = 171 °C | Mw = 3.16 × 106 Da; PI = 1.3 | [43] |
| 40 | Crude Glycerol | Chloroform/Petroleum ether | MMC | PHB | Pre-treatment: Freeze-drying and acetone wash | - | - | Xc% = 66; Tm = 171 °C; Tg = 4.9 °C | Mw = 8.34–19.5 × 104 Da | [107] |
4.2.2. Other Methods
4.3. Purification
4.4. Life Cycle Assessment of PHA Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tiseo, I. Annual Production of Plastics Worldwide from 1950 to 2022. 2022. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 22 September 2022).
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of Bioplastics: Routes and Benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Veluru, S.; Seeram, R. Biotechnological Approaches: Degradation and Valorization of Waste Plastic to Promote the Circular Economy. Circ. Econ. 2024, 3, 100077. [Google Scholar] [CrossRef]
- Yu, S.; Su, W.; Wu, D.; Yao, Z.; Liu, J.; Tang, J.; Wu, W. Thermal Treatment of Flame Retardant Plastics: A Case Study on a Waste TV Plastic Shell Sample. Sci. Total Environ. 2019, 675, 651–657. [Google Scholar] [CrossRef]
- Liu, K.; Tan, Q.; Yu, J.; Wang, M. A Global Perspective on E-Waste Recycling. Circ. Econ. 2023, 2, 100028. [Google Scholar] [CrossRef]
- Kanzariya, R.; Gautam, A.; Parikh, S.; Shah, M.; Gautam, S. Formation of Polyhydroxyalkanoates Using Agro and Industrial Waste as a Substrate—A Review. Biotechnol. Genet. Eng. Rev. 2023, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Cho, S.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Nair, S.; Kim, D.; Shin, H.; et al. Developing Microbial Co-Culture System for Enhanced Polyhydroxyalkanoates (PHA) Production Using Acid Pretreated Lignocellulosic Biomass. Polymers 2022, 4, 726. [Google Scholar] [CrossRef]
- Zulfiqar Ali Raza, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Zytner, P.; Kumar, D.; Elsayed, A.; Mohanty, A.; Ramarao, B.V.; Misra, M. A Review on Polyhydroxyalkanoate (PHA) Production through the Use of Lignocellulosic Biomass. RSC Sustain. 2023, 1, 2120–2134. [Google Scholar] [CrossRef]
- Liang, X.; Cha, D.K.; Xie, Q. Properties, Production, and Modification of Polyhydroxyalkanoates. Resour. Conserv. Recycl. Adv. 2024, 21, 200206. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wang, X.; Zhang, Y.; Zhu, T.; Peng, L.; Xu, Y.; Chen, X.; Wang, D.; Ni, B.J.; et al. Towards Scaling-up Implementation of Polyhydroxyalkanoate (PHA) Production from Activated Sludge: Progress and Challenges. J. Clean. Prod. 2024, 447, 141542. [Google Scholar] [CrossRef]
- Pesante, G.; Frison, N. Bioresource Technology Reports Recovery of Bio-Based Products from PHA-Rich Biomass Obtained from Biowaste: A Review. Bioresour. Technol. Rep. 2023, 21, 101345. [Google Scholar]
- Yadav, B.; Talan, A.; Tyagi, R.D.; Drogui, P. Concomitant Production of Value-Added Products with Polyhydroxyalkanoate (PHA) Synthesis: A Review. Bioresour. Technol. 2021, 337, 125419. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, P.; Mhaisalkar, V.; Chakrabarti, T. Study on Poly-Hydroxyalkanoate (PHA) Production in Pilot Scale Continuous Mode Wastewater Treatment System. Bioresour. Technol. 2010, 101, 2896–2899. [Google Scholar] [CrossRef] [PubMed]
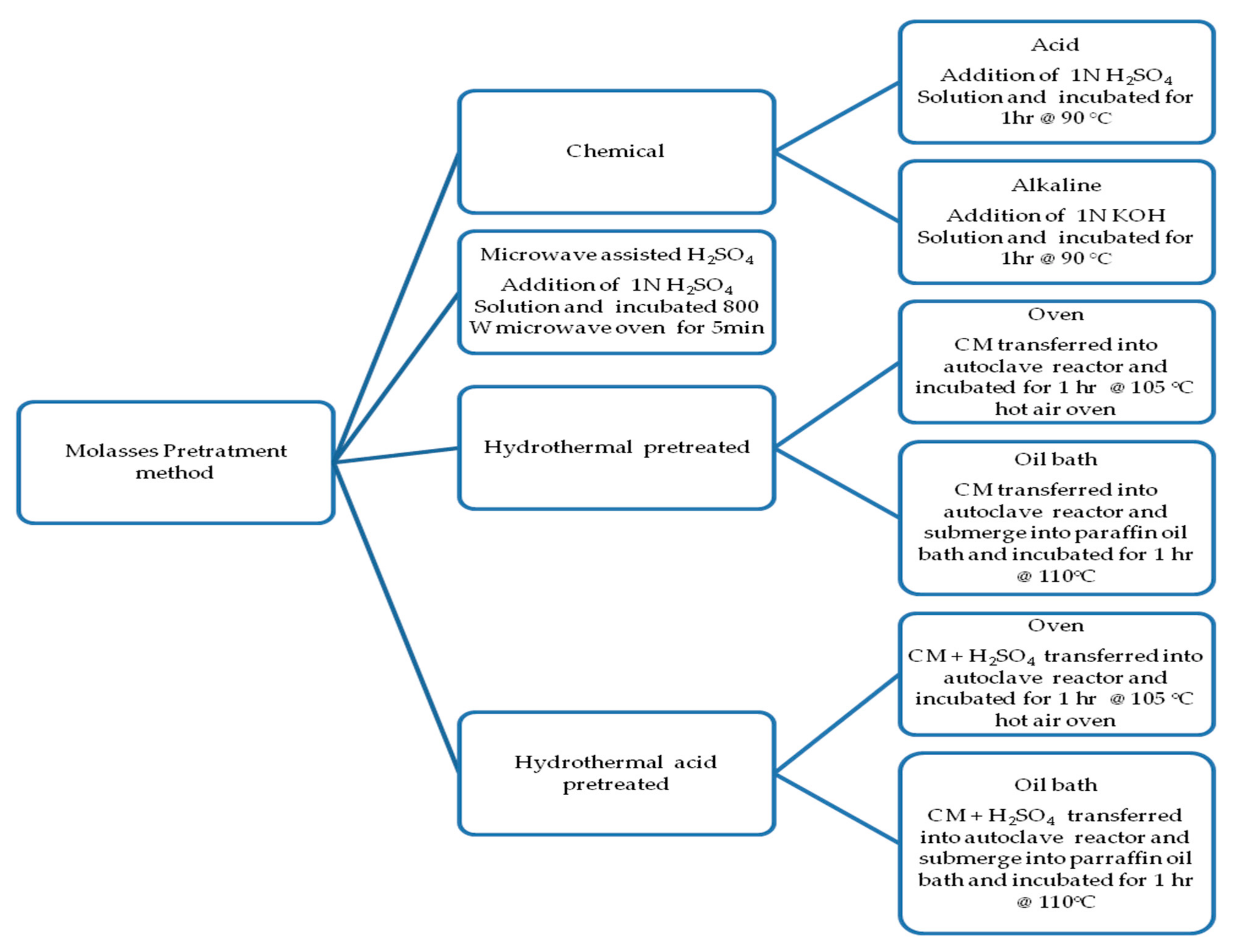
- Sen, K.Y.; Hussin, M.H.; Baidurah, S. Biosynthesis of Poly(3-Hydroxybutyrate) (PHB) by Cupriavidus necator from Various Pretreated Molasses as Carbon Source. Biocatal. Agric. Biotechnol. 2018, 17, 51–59. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yoon, J.J.; Kim, H.J.; Hong, J.W.; Gi Hong, Y.; Song, H.S.; Moon, Y.M.; Jeon, J.M.; Kim, Y.G.; Yang, Y.H. Engineering of Artificial Microbial Consortia of Ralstonia eutropha and Bacillus subtilis for Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Copolymer Production from Sugarcane Sugar without Precursor Feeding. Bioresour. Technol. 2018, 257, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.D.; Srivastava, S.K.; Singh, R.P. Statistical Optimization of Physical Process Variables for Bio-Plastic (PHB) Production By. Biomass Bioenergy 2013, 55, 243–250. [Google Scholar] [CrossRef]
- Kanzariya, R.; Gautam, A.; Parikh, S.; Gautam, S. Thermally Stable P(3HB) Synthesis from Cane Molasses by Co-Culture of Alcaligenes Sp. NCIM 5085 and Bacillus subtilis. Waste Biomass Valorization 2024, 15, 3535–3552. [Google Scholar] [CrossRef]
- Andler, R.; Pino, V.; Moya, F.; Soto, E.; Valdés, C.; Andreeßen, C.; Pino, V.; Moya, F.; Soto, E.; Valdés, C.; et al. Synthesis of Poly-3-Hydroxybutyrate (PHB) by Bacillus cereus Using Grape Residues as Sole Carbon Source. Int. J. Biobased Plast. 2021, 3, 98–111. [Google Scholar] [CrossRef]
- Andreeben, B.; Lange, A.B.; Robenek, H.; Steinbuchel, A. Conversion of Glycerol to Poly (3-Hydroxypropionate) in Recombinant Escherichia Coli. Appl. Environ. Microbiol. 2010, 76, 622–626. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 11, 1157. [Google Scholar] [CrossRef]
- Kulpreecha, S.; Boonruangthavorn, A.; Meksiriporn, B.; Thongchul, N. Inexpensive Fed-Batch Cultivation for High Poly (3-Hydroxybutyrate) Production by a New Isolate of Bacillus megaterium. J. Biosci. Bioeng. 2009, 107, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Bormann, E.J.; Roth, M. The Production of Polyhydroxybutyrate by Methylobacterium rhodesianum and Ralstonia eutropha in Media Containing Glycerol and Casein Hydrolysates. Biotechnol. Lett. 1999, 21, 1059–1063. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, X.; Gong, L.; Li, P.; Dai, C.; Shao, W. High Poly (Hydroxybutyrate) Production by Pseudomonas fluorescens A2a5 from Inexpensive Substrates. Enzyme Microb. Technol. 2008, 42, 167–172. [Google Scholar] [CrossRef]
- Ciesielski, S.; Pisutpaisal, N.; Mozejko, J. Plant Oils as Promising Substrates for Polyhydroxyalkanoates Production. Clean. Prod. 2014, 106, 408–421. [Google Scholar] [CrossRef]
- Colombo, B.; Favini, F.; Scaglia, B.; Sciarria, T.P.; D’Imporzano, G.; Pognani, M.; Alekseeva, A.; Eisele, G.; Cosentino, C.; Adani, F. Enhanced Polyhydroxyalkanoate (PHA) Production from the Organic Fraction of Municipal Solid Waste by Using Mixed Microbial Culture. Biotechnol. Biofuels 2017, 10, 201. [Google Scholar] [CrossRef]
- Lopez-Vazquez, C.M.; Song, Y.I.; Hooijmans, C.M.; Brdjanovic, D.; Moussa, M.S.; Gijzen, H.J.; Van Loosdrecht, M.C.M. Temperature Effects on the Aerobic Metabolism of Glycogen-Accumulating Organisms. Biotechnol. Bioeng. 2008, 101, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vazquez, C.M.; Hooijmans, C.M.; Brdjanovic, D.; Gijzen, H.J.; van Loosdrecht, M.C.M. Temperature Effects on Glycogen Accumulating Organisms. Water Res. 2009, 43, 2852–2864. [Google Scholar] [CrossRef]
- Fiorese, M.L.; Freitas, F.; Pais, J.; Ramos, A.M.; De Aragão, G.M.F.; Reis, M.A.M. Recovery of Polyhydroxybutyrate (PHB) from Cupriavidus necator Biomass by Solvent Extraction with 1,2-Propylene Carbonate. Eng. Life Sci. 2009, 9, 454–461. [Google Scholar] [CrossRef]
- Vogli, L.; Macrelli, S.; Marazza, D.; Galletti, P.; Torri, C.; Samorì, C.; Righi, S. Life Cycle Assessment and Energy Balance of a Novel Polyhydroxyalkanoates Production Process with Mixed Microbial Cultures Fed on Pyrolytic Products of Wastewater Treatment Sludge. Energies 2020, 13, 2706. [Google Scholar] [CrossRef]
- Jia, Q.; Xiong, H.; Wang, H.; Shi, H.; Sheng, X.; Sun, R.; Chen, G. Production of Polyhydroxyalkanoates (PHA) by Bacterial Consortium from Excess Sludge Fermentation Liquid at Laboratory and Pilot Scales. Bioresour. Technol. 2014, 171, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Arcos-Hernandez, M.V.; Pratt, S.; Laycock, B.; Johansson, P.; Werker, A.; Lant, P.A. Waste Activated Sludge as Biomass for Production of Commercial-Grade Polyhydroxyalkanoate (PHA). Waste Biomass Valorization 2013, 4, 117–127. [Google Scholar] [CrossRef]
- Hemann-Krauss, C.; Koller, M.; Obruca, S.; Pernicova, I.; Braunegg, G. Archaeal Production of Polyhydroxyalkanoate (PHA) Co- and Terpolyesters from Biodiesel Industry-Derived By-Products Carmen. Polyhydroxyalkanoates Biosynth. Chem. Struct. Appl. 2013, 2013, 129268. [Google Scholar]
- Chen, G.-Q. Industrial Production of PHA. Plast. Bact. Nat. Funct. Appl. 2010, 14, 121–132. [Google Scholar] [CrossRef]
- Kapritchkoff, F.M.; Viotti, A.P.; Alli, R.C.P.; Zuccolo, M.; Pradella, J.G.C.; Maiorano, A.E.; Miranda, E.A.; Bonomi, A. Enzymatic Recovery and Purification of Polyhydroxybutyrate Produced by Ralstonia eutropha. J. Biotechnol. 2006, 122, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Chang, Y.K.; Kim, B.S.; Chang, H.N. Optimization of Microbial Poly(3-hydroxybutyrate) Recover Using Dispersions of Sodium Hypochlorite Solution and Chloroform. Biotechnol. Bioeng. 1994, 44, 256–261. [Google Scholar] [CrossRef]
- Heinrich, D.; Madkour, M.H.; Al-Ghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. Large Scale Extraction of Poly(3-Hydroxybutyrate) from Ralstonia eutropha H16 Using Sodium Hypochlorite. AMB Express 2012, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Anton, B.; Tomas, K.; Simon, F.M.; Monica, A. A Process for Polyhydroxyalkanoate (PHA) Production from Municipal Wastewater Treatment with Biological Carbon and Nitrogen Removal Demonstrated at Pilot-Scale. New Biotechnol. 2016, 35, 42–53. [Google Scholar] [CrossRef]
- Follonier, S.; Riesen, R.; Zinn, M. Pilot-Scale Production of Functionalized Mcl-PHA from Grape Pomace Supplemented with Fatty Acids. Chem. Biochem. Eng. Q. 2015, 29, 113–121. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.W.; Wei, Y.H.; Wu, H.S.; Wang, S.S. Isolation and Purification of Bacterial Poly(3-Hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of High Quality Polyhydroxyalkanoate Co- And Terpolyesters for Potential Medical Application by the Archaeon Haloferax Mediterranei. Macromol. Symp. 2007, 253, 33–39. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Hermann, C.; Horvat, P.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P.; Braunegg, G. Biotechnological Production of Poly(3-Hydroxybutyrate) with Wautersia eutropha by Application of Green Grass Juice and Silage Juice as Additional Complex Substrates. Biocatal. Biotransform. 2005, 23, 329–337. [Google Scholar] [CrossRef]
- Huong, K.H.; Azuraini, M.J.; Aziz, N.A.; Amirul, A.A.A. Pilot Scale Production of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Biopolymers with High Molecular Weight and Elastomeric Properties. J. Biosci. Bioeng. 2017, 124, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Anterrieu, S.; Quadri, L.; Geurkink, B.; Dinkla, I.; Bengtsson, S.; Arcos-Hernandez, M.; Alexandersson, T.; Morgan-Sagastume, F.; Karlsson, A.; Hjort, M.; et al. Integration of Biopolymer Production with Process Water Treatment at a Sugar Factory. New Biotechnol. 2014, 31, 308–323. [Google Scholar] [CrossRef]
- Lam, W.; Wang, Y.; Chan, P.L.; Chan, S.W.; Tsang, Y.F.; Chua, H.; Yu, P.H.F. Production of Polyhydroxyalkanoates (PHA) Using Sludge from Different Wastewater Treatment Processes and the Potential for Medical and Pharmaceutical Applications. Environ. Technol. 2017, 38, 1779–1791. [Google Scholar] [CrossRef]
- Montano Herrera, L. Composition of Mixed Culture PHA Biopolymers and Implications for Downstream Processing. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2015. [Google Scholar] [CrossRef]
- Hu, S.; McDonald, A.G.; Coats, E.R. Characterization of Polyhydroxybutyrate Biosynthesized from Crude Glycerol Waste Using Mixed Microbial Consortia. J. Appl. Polym. Sci. 2013, 129, 1314–1321. [Google Scholar] [CrossRef]
- Furrer, P.; Panke, S.; Zinn, M. Efficient Recovery of Low Endotoxin Medium-Chain-Length Poly([R]-3-Hydroxyalkanoate) from Bacterial Biomass. J. Microbiol. Methods 2007, 69, 206–213. [Google Scholar] [CrossRef]
- Catherine, M.C.; Guwy, A.; Massanet-Nicolau, J. Effect of Acetate Concentration, Temperature, PH and Nutrient Concentration on Polyhydroxyalkanoates (PHA) Production by Glycogen Accumulating Organisms. Bioresour. Technol. Reports 2022, 20, 101226. [Google Scholar] [CrossRef]
- López-Abelairas, M.; García-Torreiro, M.; Lú-Chau, T.; Lema, J.M.; Steinbüchel, A. Comparison of Several Methods for the Separation of Poly(3-Hydroxybutyrate) from Cupriavidus necator H16 Cultures. Biochem. Eng. J. 2015, 93, 250–259. [Google Scholar] [CrossRef]
- Larriba, O.; Rovira-Cal, E.; Juznic-Zonta, Z.; Guisasola, A.; Baeza, J.A. Evaluation of the Integration of P Recovery, Polyhydroxyalkanoate Production and Short Cut Nitrogen Removal in a Mainstream Wastewater Treatment Process. Water Res. 2020, 172, 115474. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; De Wever, H. Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock. Appl. Sci. 2018, 8, 1416. [Google Scholar] [CrossRef]
- Ntaikou, I.; Valencia Peroni, C.; Kourmentza, C.; Ilieva, V.I.; Morelli, A.; Chiellini, E.; Lyberatos, G. Microbial Bio-Based Plastics from Olive-Mill Wastewater: Generation and Properties of Polyhydroxyalkanoates from Mixed Cultures in a Two-Stage Pilot Scale System. J. Biotechnol. 2014, 188, 138–147. [Google Scholar] [CrossRef]
- Lorini, L.; Martinelli, A.; Pavan, P.; Majone, M.; Valentino, F. Downstream Processing and Characterization of Polyhydroxyalkanoates (PHAs) Produced by Mixed Microbial Culture (MMC) and Organic Urban Waste as Substrate. Biomass Convers. Biorefinery 2021, 11, 693–703. [Google Scholar] [CrossRef]
- Amulya, K.; Jukuri, S.; Venkata Mohan, S. Sustainable Multistage Process for Enhanced Productivity of Bioplastics from Waste Remediation through Aerobic Dynamic Feeding Strategy: Process Integration for up-Scaling. Bioresour. Technol. 2015, 188, 231–239. [Google Scholar] [CrossRef]
- Valentino, F.; Lorini, L.; Gottardo, M.; Pavan, P.; Majone, M. Effect of the Temperature in a Mixed Culture Pilot Scale Aerobic Process for Food Waste and Sewage Sludge Conversion into Polyhydroxyalkanoates. J. Biotechnol. 2020, 323, 54–61. [Google Scholar] [CrossRef]
- Ayub, N.D.; Tribelli, P.M.; López, N.I. Polyhydroxyalkanoates Are Essential for Maintenance of Redox State in the Antarctic Bacterium Pseudomonas Sp. 14-3 during Low Temperature Adaptation. Extremophiles 2009, 13, 59–66. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated Production of Polyhydroxyalkanoates (PHAs) with Municipal Wastewater and Sludge Treatment at Pilot Scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef]
- Valentino, F.; Moretto, G.; Lorini, L.; Bolzonella, D.; Pavan, P.; Majone, M. Pilot-Scale Polyhydroxyalkanoate Production from Combined Treatment of Organic Fraction of Municipal Solid Waste and Sewage Sludge. Ind. Eng. Chem. Res. 2019, 58, 12149–12158. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Bengtsson, S.; De Grazia, G.; Alexandersson, T.; Quadri, L.; Johansson, P.; Magnusson, P.; Werker, A. Mixed-Culture Polyhydroxyalkanoate (PHA) Production Integrated into a Food-Industry Effluent Biological Treatment: A Pilot-Scale Evaluation. J. Environ. Chem. Eng. 2020, 8, 104469. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the Production, Structural Characteristics and Potential Applications of Bioplastics Derived from Polyhydroxyalkanoates. Int. J. Biol. Macromol. 2017, 107, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of Polyhydroxyalkanoates from Single and Mixed Microbial Cultures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 624021. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y. Polyhydroxyalkanoates Production from Synthetic Waste Using Pseudomonas pseudoflava: Polyhydroxyalkanoate Synthase Enzyme Activity Analysis from P. pseudoflava and P. palleronii. Bioresour. Technol. 2017, 234, 99–105. [Google Scholar] [CrossRef]
- Amulya, K.; Reddy, M.V.; Mohan, S.V. Bioresource Technology Acidogenic Spent Wash Valorization through Polyhydroxyalkanoate (PHA) Synthesis Coupled with Fermentative Biohydrogen Production. Bioresour. Technol. 2014, 158, 336–342. [Google Scholar] [CrossRef]
- Khardenavis, A.A.; Kumar, M.S.; Mudliar, S.N.; Chakrabarti, T. Biotechnological Conversion of Agro-Industrial Wastewaters into Biodegradable Plastic, Poly b-Hydroxybutyrate. Bioresour. Technol. 2007, 98, 3579–3584. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing Microbial Polyhydroxyalkanoate (PHA) Biopolyesters in a Sustainable Manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Gottardo, M.; Micolucci, F.; Pavan, P.; Bolzonella, D.; Rossetti, S.; Majone, M. Organic Fraction of Municipal Solid Waste Recovery by Conversion into Added-Value Polyhydroxyalkanoates and Biogas. ACS Sustain. Chem. Eng. 2018, 6, 16375–16385. [Google Scholar] [CrossRef]
- Atlić, A.; Koller, M.; Scherzer, D.; Kutschera, C.; Grillo-Fernandes, E.; Horvat, P.; Chiellini, E.; Braunegg, G. Continuous Production of Poly([R]-3-Hydroxybutyrate) by Cupriavidus necator in a Multistage Bioreactor Cascade. Appl. Microbiol. Biotechnol. 2011, 91, 295–304. [Google Scholar] [CrossRef]
- Valentino, F.; Lorini, L.; Pavan, P.; Bolzonella, D.; Majone, M. Organic Fraction of Municipal Solid Waste Conversion into Polyhydroxyalkanoates (PHA) in a Pilot Scale Anaerobic/Aerobic Process. Chem. Eng. Trans. 2019, 74, 265–270. [Google Scholar] [CrossRef]
- Moretto, G.; Russo, I.; Bolzonella, D.; Pavan, P.; Majone, M.; Valentino, F. An Urban Biorefinery for Food Waste and Biological Sludge Conversion into Polyhydroxyalkanoates and Biogas. Water Res. 2020, 170, 115371. [Google Scholar] [CrossRef]
- Patel, M.; Gapes, D.J.; Newman, R.H.; Dare, P.H. Physico-Chemical Properties of Polyhydroxyalkanoate Produced by Mixed-Culture Nitrogen-Fixing Bacteria. Appl. Microbiol. Biotechnol. 2009, 82, 545–555. [Google Scholar] [CrossRef]
- Mulders, M.; Tamis, J.; Abbas, B.; Sousa, J.; Dijkman, H.; Rozendal, R.; Kleerebezem, R. Pilot-Scale Polyhydroxyalkanoate Production from Organic Waste: Process Characteristics at High PH and High Ammonium Concentration. J. Environ. Eng. 2020, 146, 04020049. [Google Scholar] [CrossRef]
- Wisuthiphaet, N.; Chanprateep, S. Optimisation of the Use of Products from the Cane Sugar Industry for Poly (3-Hydroxybutyrate) Production by Azohydromonas Lata DSM 1123 in Fed-Batch Cultivation. Process Biochem. 2016, 51, 352–361. [Google Scholar] [CrossRef]
- Gouda, M.K.; Swellam, A.E.; Omar, S.H. Production of PHB by a Bacillus megaterium Strain Using Sugarcane Molasses and Corn Steep Liquor as Sole Carbon and Nitrogen Sources. Microbiol. Res. 2001, 156, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Santimano, M.; Prabhu, N.N.; Garg, S. PHA Production Using Low Cost Agro Industrie Waste by Bacillus Sp. Strain COl1/A6. Res. J. Microbiol. 2009, 4, 89–96. [Google Scholar] [CrossRef]
- Akaraonye, E.; Moreno, C.; Knowles, C.J.; Keshavarz, T.; Roy, I. Poly (3-Hydroxybutyrate) Production by Bacillus cereus SPV Using Sugarcane Molasses as the Main Carbon Source. Biotechnilogy 2012, 7, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, W.N.; Jamil, N.; Ali, I.; Ayaz, M.H.; Hasnain, S. Screening for Polyhydroxyalkanoate (PHA)-Producing Bacterial Strains and Comparison of PHA Production from Various Inexpensive Carbon Sources. Ann. Microbiol. 2011, 61, 623–629. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Yadav, A.; Jha, A.; Srivastava, S.K. Utilizing of Sugar Refinery Waste (Cane Molasses) for Production of Bio-Plastic Under Submerged Fermentation Process. Polym. Environ. 2012, 20, 446–453. [Google Scholar] [CrossRef]
- Basnett, P. Biosynthesis of Polyhydroxyalkanoates, Their Novel Blends and Composites for Biomedical Applications. Ph.D. Thesis, University of Westminster, London, UK, 2014. [Google Scholar]
- Harada, K.; Kobayashi, S.; Oshima, K.; Yoshida, S.; Tsuge, T.; Sato, S. Engineering of Aeromonas caviae Polyhydroxyalkanoate Synthase Through Site-Directed Mutagenesis for Enhanced Polymerization of the 3-Hydroxyhexanoate Unit. Front. Bioeng. Biotechnol. 2021, 9, 627082. [Google Scholar] [CrossRef]
- Riedel, S.L.; Jahns, S.; Koenig, S.; Bock, M.C.E.; Brigham, C.J.; Bader, J.; Stahl, U. Polyhydroxyalkanoates Production with Ralstonia eutropha from Low Quality Waste Animal Fats. J. Biotechnol. 2015, 214, 119–127. [Google Scholar] [CrossRef]
- Rao, U.; Sridhar, R.; Sehgal, P.K. Biosynthesis and Biocompatibility of Produced by Cupriavidus necator from Spent Palm Oil. Biochem. Eng. J. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Wong, Y.; Brigham, C.J.; Rha, C.; Sinskey, A.J.; Sudesh, K. Bioresource Technology Biosynthesis and Characterization of Polyhydroxyalkanoate Containing High 3-Hydroxyhexanoate Monomer Fraction from Crude Palm Kernel Oil by Recombinant Cupriavidus necator. Bioresour. Technol. 2012, 121, 320–327. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Schiller, M.; Kwiecień, M.; Adamus, G.; Kowalczuk, M.; Strohmeier, K.; Schober, S.; et al. Biodegradable Latexes from Animal-Derived Waste: Biosynthesis and Characterization of Mcl-PHA Accumulated by Ps. citronellolis. React. Funct. Polym. 2013, 73, 1391–1398. [Google Scholar] [CrossRef]
- Mo, J.; Ciesielski, S. Saponified Waste Palm Oil as an Attractive Renewable Resource for Mcl-Polyhydroxyalkanoate Synthesis. J. Biosci. Bioeng. 2013, 116, 485–492. [Google Scholar] [CrossRef]
- Chlororaphis, P.; Sharma, P.K.; Munir, R.I.; de Kievit, T.; Levin, D.B. Synthesis of Polyhydroxyalkanoates (PHAs) from Vegetable Oils and Free Fatty Acids by Wild-1 Type and Mutant Strains of Pseudomonas chlororaphis. Can. J. Microbiol. 2017, 63, 1009–1024. [Google Scholar]
- Cavalheiro, M.B.T.; Raposo, R.S.; Almeida, M.C.; Cesário, M.T.; Sevrin, C.; Grandfils, C.; Fonseca, M.M. Bioresource Technology Effect of Cultivation Parameters on the Production of Poly (3-Hydroxybutyrate-Co-4-Hydroxybutyrate) and Poly (3-Hydroxybutyrate-4-Hydroxybutyrate-3-Hydroxyvalerate) by Cupriavidus necator Using Waste Glycerol. Bioresour. Technol. 2012, 111, 391–397. [Google Scholar] [CrossRef]
- Pérez Rivero, C.; Sun, C.; Theodoropoulos, C.; Webb, C. Building a Predictive Model for PHB Production from Glycerol. Biochem. Eng. J. 2016, 116, 113–121. [Google Scholar] [CrossRef]
- Gahlawat, G.; Soni, S.K. Valorization of Waste Glycerol for the Production of Poly (3-Hydroxybutyrate) and Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Copolymer by Cupriavidus necator and Extraction in a Sustainable Manner. Bioresour. Technol. 2017, 243, 492–501. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Polyhydroxyalkanoate Production and Degradation Patterns in Bacillus Species. Indian J. Microbiol. 2017, 57, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, S.; Zinn, M.; Egli, T.; Thöny-Meyer, L.; Ren, Q. Improved Productivity of Poly (4-Hydroxybutyrate) (P4HB) in Recombinant Escherichia coli Using Glycerol as the Growth Substrate with Fed-Batch Culture. Microb. Cell Fact. 2014, 13, 131. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Foglia, T.A. Bacterial Poly (Hydroxyalkanoate) Polymer Production from the Biodiesel Co-Product Stream. J. Polym. Environ. 2004, 12, 105–112. [Google Scholar] [CrossRef]
- Singh, G.; Kumari, A.; Mittal, A. Cost Effective Production of Poly-b-Hydroxybutyrate by Bacillus subtilis NG05 Using Sugar Industry Waste Water. J. Polym. Environ. 2013, 21, 441–449. [Google Scholar] [CrossRef]
- Munir, S.; Jamil, N. Characterization of Polyhydroxyalkanoates Produced by Contaminated Soil Bacteria Using Wastewater and Glucose as Carbon Sources. Trop. J. Pharm. Res. 2015, 14, 1605–1611. [Google Scholar] [CrossRef]
- Kourmentza, C.; Ntaikou, I.; Lyberatos, G.; Kornaros, M. Polyhydroxyalkanoates from Pseudomonas Sp. Using Synthetic and Olive Mill Wastewater under Limiting Conditions Author. Int. J. Biol. Macromol. 2014, 74, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Khardenavis, A.A.; Vaidya, A.N.; Kumar, M.S.; Chakrabarti, T. Utilization of Molasses Spentwash for Production of Bioplastics by Waste Activated Sludge. Waste Manag. 2009, 29, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Alfano, S.; Lorini, L.; Majone, M.; Sciubba, F.; Valentino, F.; Martinelli, A. Ethylic Esters as Green Solvents for the Extraction of Intracellular Polyhydroxyalkanoates Produced by Mixed Microbial Culture. Polymers 2021, 13, 2789. [Google Scholar] [CrossRef]
- Conca, V.; da Ros, C.; Valentino, F.; Eusebi, A.L.; Frison, N.; Fatone, F. Long-Term Validation of Polyhydroxyalkanoates Production Potential from the Sidestream of Municipal Wastewater Treatment Plant at Pilot Scale. Chem. Eng. J. 2020, 390, 124627. [Google Scholar] [CrossRef]
- Pardo-Planas, O.; Atiyeh, H.K.; Phillips, J.R.; Aichele, C.P.; Mohammad, S. Process Simulation of Ethanol Production from Biomass Gasification and Syngas Fermentation. Bioresour. Technol. 2017, 245, 925–932. [Google Scholar] [CrossRef]
- Anis, S.N.S.; Md Iqbal, N.; Kumar, S.; Amirul, A.A. Effect of Different Recovery Strategies of P(3HB-Co-3HHx) Copolymer from Cupriavidus necator Recombinant Harboring the PHA Synthase of Chromobacterium Sp. USM2. Sep. Purif. Technol. 2013, 102, 111–117. [Google Scholar] [CrossRef]
- Tamer, I.M.; Moo-Young, M. Optimization of Poly(β-Hydroxybutyric Acid) Recovery from Alcaligenes latus: Combined Mechanical and Chemical Treatments. Bioprocess Eng. 1998, 19, 459–468. [Google Scholar] [CrossRef]
- Kosseva, M.R.; Rusbandi, E. Trends in the Biomanufacture of Polyhydroxyalkanoates with Focus on Downstream Processing. Int. J. Biol. Macromol. 2018, 107, 762–778. [Google Scholar] [CrossRef]
- Gahlawat, G. Polyhydroxyalkanoates Biopolymers Production Strategies; Springer International Publishing: Cham, Switzerland, 2020; ISBN 9781119438922. [Google Scholar]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Suárez-Ojeda, M.E. Bioplastic Recovery from Wastewater: A New Protocol for Polyhydroxyalkanoates (PHA) Extraction from Mixed Microbial Cultures. Bioresour. Technol. 2019, 282, 361–369. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of Polyhydroxyalkanoates (PHAs) from Wastewater: A Review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Doi, Y. Kinetics and Mechanism of Synthesis and Degradation of Poly(3-Hydroxybutyrate) in Alcaligenes eutrophus. Macromolecules 1992, 25, 2324–2329. [Google Scholar] [CrossRef]
- Neves, A.; Müller, J. Use of Enzymes in Extraction of Polyhydroxyalkanoates Produced by Cupriavidus necator. Biotechnol. Prog. 2012, 28, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Rosengart, A.; Cesário, M.T.; de Almeida, M.C.M.D.; Raposo, R.S.; Espert, A.; de Apodaca, E.D.; da Fonseca, M.M.R. Efficient P(3HB) Extraction from Burkholderia sacchari Cells Using Non-Chlorinated Solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Silva, P.T.S. Production of Bacterial Reserve Compounds from Industrial Wastewaters. Master’s Thesis, Universidade do Minho, Braga, Portugal, 2013. [Google Scholar]
- Riedel, S.L.; Brigham, C.J.; Budde, C.F.; Bader, J.; Rha, C.; Stahl, U.; Sinskey, A.J. Recovery of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Ralstonia eutropha Cultures with Non-Halogenated Solvents. Biotechnol. Bioeng. 2013, 110, 461–470. [Google Scholar] [CrossRef] [PubMed]
- de Souza Reis, G.A.; Michels, M.H.A.; Fajardo, G.L.; Lamot, I.; de Best, J.H. Optimization of Green Extraction and Purification of PHA Produced by Mixed Microbial Cultures from Sludge. Water 2020, 12, 1185. [Google Scholar] [CrossRef]
- Mudliar, S.N.; Vaidya, A.N.; Suresh Kumar, M.; Dahikar, S.; Chakrabarti, T. Techno-Economic Evaluation of PHB Production from Activated Sludge. Clean Technol. Environ. Policy 2008, 10, 255–262. [Google Scholar] [CrossRef]
- Sethupathy, A.; Sivashanmugam, P. Amelioration of Methane Production Efficiency of Paper Industry Waste Sludge through Hydrolytic Enzymes Assisted with Poly3hydroxybutyrate. Energy 2021, 214, 119083. [Google Scholar] [CrossRef]
- Tamis, J.; Lužkov, K.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Enrichment of Plasticicumulans Acidivorans at Pilot-Scale for PHA Production on Industrial Wastewater. J. Biotechnol. 2014, 192, 161–169. [Google Scholar] [CrossRef]
- Tamis, J.; Mulders, M.; Dijkman, H.; Rozendal, R.; van Loosdrecht, M.C.M.; Kleerebezem, R. Pilot-Scale Polyhydroxyalkanoate Production from Paper Mill Wastewater: Process Characteristics and Identification of Bottlenecks for Full-Scale Implementation. J. Environ. Eng. 2018, 144, 04018107. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Valentino, F.; Hjort, M.; Cirne, D.; Karabegovic, L.; Gerardin, F.; Johansson, P.; Karlsson, A.; Magnusson, P.; Alexandersson, T.; et al. Polyhydroxyalkanoate (PHA) Production from Sludge and Municipal Wastewater Treatment. Water Sci. Technol. 2014, 69, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Johnston, B.; Townrow, D.E.; Radecka, I.; Koller, M.; Chaber, P.; Adamus, G.; Kowalczuk, M. Biomass Extraction Using Non-Chlorinated Solvents for Biocompatibility Improvement of Polyhydroxyalkanoates. Polymers 2018, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Lorini, L.; Martinelli, A.; Capuani, G.; Frison, N.; Reis, M.; Sommer Ferreira, B.; Villano, M.; Majone, M.; Valentino, F. Characterization of Polyhydroxyalkanoates Produced at Pilot Scale from Different Organic Wastes. Front. Bioeng. Biotechnol. 2021, 9, 628719. [Google Scholar] [CrossRef] [PubMed]
- Khardenavis, A.; Guha, P.K.; Kumar, M.S.; Mudliar, S.N.; Chakrabarti, T. Activated Sludge Is a Potential Source for Production of Biodegradable Plastics from Wastewater. Environ. Technol. 2005, 26, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Madkour, H.M.; Heinrich, D.; Alghamdi, M.A.; Shabbaj, I.I.; Steinbu, A. PHA Recovery from Biomass. Macromolecules 2013, 14, 2963–2972. [Google Scholar] [CrossRef]
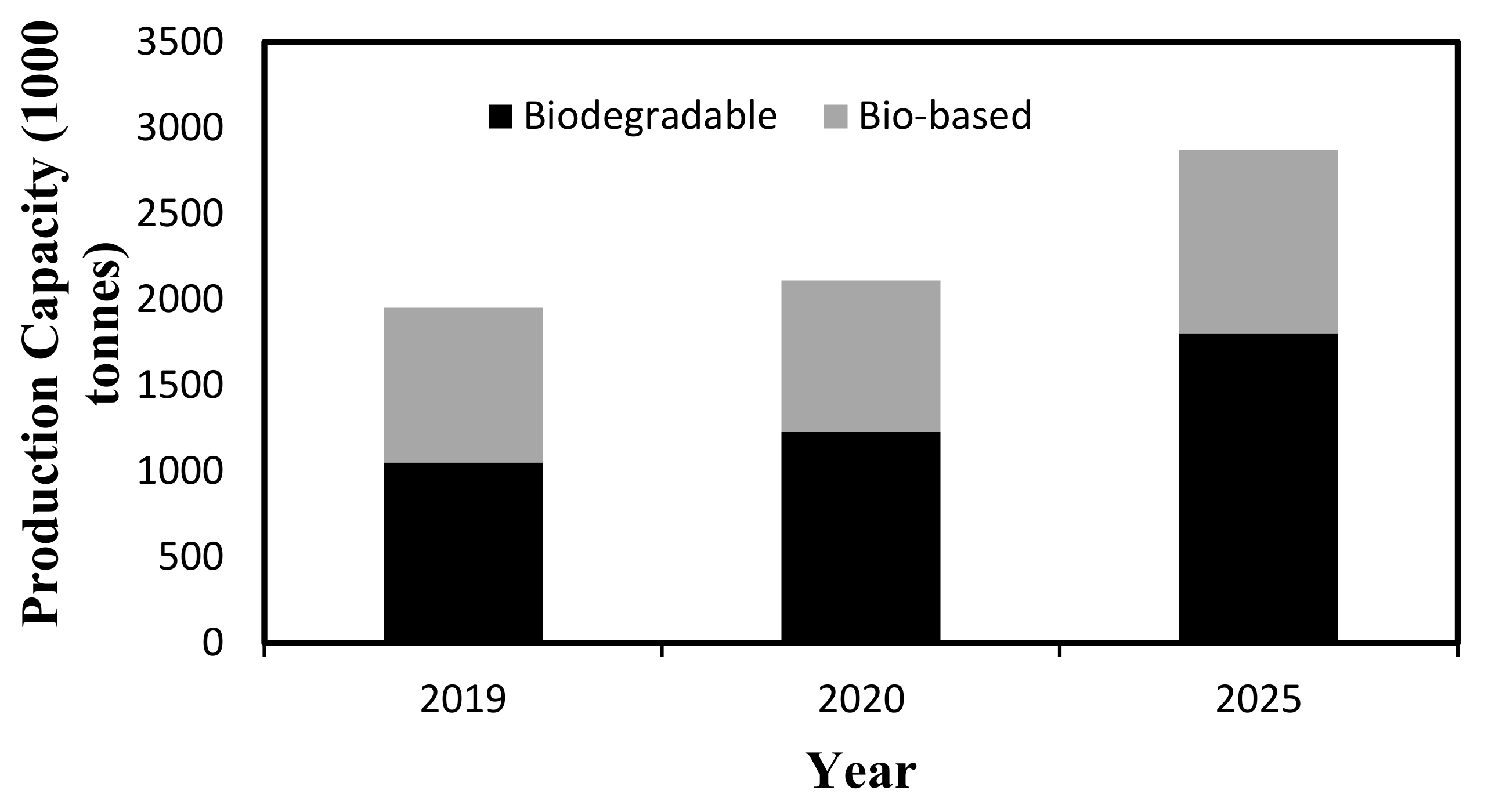
- ECTC. Biodegradable Plastic and World Biopolymers Market 2019–2020. Available online: https://ect-center.com/blog/biopolymers-market-2019 (accessed on 22 September 2022).
- Tyagi, R.D.; Yan, S.; Bala Subramanian, S.; Surampalli, R.Y. Bioplastics from Waste Activated Sludge-Batch Process. Pract. Period. Hazardous Toxic Radioact. Waste Manag. 2008, 12, 239–248. [Google Scholar] [CrossRef]
- Wampfler, B.; Ramsauer, T.; Rezzonico, S.; Hischier, R.; Köhling, R.; Thöny-Meyer, L.; Zinn, M. Isolation and Purification of Medium Chain Length Poly(3-Hydroxyalkanoates) (Mcl-PHA) for Medical Applications Using Nonchlorinated Solvents. Biomacromolecules 2010, 11, 2716–2723. [Google Scholar] [CrossRef]
- Wen, Q.; Ji, Y.; Chen, Z.; Lee, D.J. Use of Sodium Chloride to Rapidly Restore Polyhydroxyalkanoates Production from Filamentous Bulking without Polyhydroxyalkanoates Productivity Impairment. Bioresour. Technol. 2020, 313, 123663. [Google Scholar] [CrossRef]
- Werker, A.; Bengtsson, S.; Korving, L.; Hjort, M.; Anterrieu, S.; Alexandersson, T.; Johansson, P.; Karlsson, A.; Karabegovic, L.; Magnusson, P.; et al. Consistent Production of High Quality PHA Using Activated Sludge Harvested from Full Scale Municipal Wastewater Treatment—PHARIO. Water Sci. Technol. 2018, 78, 2256–2269. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hassan, M.A.; Phang, L.Y.; Shirai, Y.; Che Man, H.; Ariffin, H.; Amirul, A.A.; Syairah, S.N. Efficient Polyhydroxyalkanoate Recovery from Recombinant Cupriavidus necator by Using Low Concentration of NaOH. Environ. Eng. Sci. 2012, 29, 783–789. [Google Scholar] [CrossRef]
- Dai, Y.; Yuan, Z.; Jack, K.; Keller, J. Production of Targeted Poly(3-Hydroxyalkanoates) Copolymers by Glycogen Accumulating Organisms Using Acetate as Sole Carbon Source. J. Biotechnol. 2007, 129, 489–497. [Google Scholar] [CrossRef]
- Gautam, S.; Gautam, A. Biopolymer—A Beginning towards Back to Nature. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kitakyushu City, Japan, 10–13 April 2018; pp. 369–375. [Google Scholar]
- Munir, S.; Jamil, N. Polyhydroxyalkanoate (PHA) Production in Open Mixed Cultures Using Waste Activated Sludge as Biomass. Arch. Microbiol. 2020, 202, 1907–1913. [Google Scholar] [CrossRef]
- Lu, M.S.; Lai, L.L.; Tsai, Y.P. Comparison of Optimum Conditions When Extracting Phas from Different Waste Sludge Sources. J. Environ. Eng. Landsc. Manag. 2018, 26, 190–194. [Google Scholar] [CrossRef]
- Ryu, H.W.; Hahn, S.K.; Chang, Y.K.; Chang, H.N. Production of Poly (3-Hydroxybutyrate) by High Cell Density Fed-Batch Culture of Alcaligenes eutrophus with Phosphate Limitation. Biotechnol. Bioeng. 1997, 55, 28–32. [Google Scholar] [CrossRef]
- Ghatnekar, M.S.; Pai, J.S.; Ganesh, M. Production and Recovery of Poly-3-Hydroxy-Butyrate from Methylobacterium Sp V49. J. Chem. Technol. Biotechnol. 2002, 77, 444–448. [Google Scholar] [CrossRef]
- Anis, S.N.S.; Iqbal, N.M.; Kumar, S.; Al-Ashraf, A. Increased Recovery and Improved Purity of PHA from Recombinant Cupriavidus necator. Bioengineered 2013, 4, 115–118. [Google Scholar] [CrossRef]
- Martínez, V.; García, P.; García, J.L.; Prieto, M.A. Controlled Autolysis Facilitates the Polyhydroxyalkanoate Recovery in Pseudomonas putida KT2440. Microb. Biotechnol. 2011, 4, 533–547. [Google Scholar] [CrossRef]
- Hejazi, P.; Vasheghani-Farahani, E.; Yamini, Y. Supercritical Fluid Disruption of Ralstonia eutropha for Poly(β-Hydroxybutyrate) Recovery. Biotechnol. Prog. 2003, 19, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Aramvash, A.; Moazzeni Zavareh, F.; Gholami Banadkuki, N. Comparison of Different Solvents for Extraction of Polyhydroxybutyrate from Cupriavidus necator. Eng. Life Sci. 2018, 18, 20–28. [Google Scholar] [CrossRef]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) Production: A Feasible Economic Option for the Treatment of Sewage Sludge in Municipalwastewater Treatment Plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]


| Sr. No. | Company Name | Country | Year | PHA Product | Commercial Name | Development Stage |
| 1 | Genecis | Canada | - | PHBV | - | Research |
| 2 | Danimer Scientific | Georgia | 2007 | mcl-PHA | Nodax™ | Commercial |
| 3 | Tainan Biological Materials Co. Ltd. | China | 2000 | PHB, PHBV | ENMAT | Commercial |
| 4 | Tianjin Green Bio Material Co. | China | 2003 | P (3, 4HB) | Sogreen® | Commercial |
| 5 | Metabolix | Massachusetts | 1992 | PHB | Yield10 Bioscience-MIREL | Commercial |
| 6 | Shenzhen Ecomann Biotechnology Co Ltd. | China | 2008 | PHBV, PHA+ Other Polymer Blends | Ecomann | Commercial |
| 7 | Kaneka | Japan | 1948 | - | AONILEX | Commercial |
| 8 | RWDC Industries | Singapore | - | Solon | Commercial | |
| 9 | Newlight Technologies, LLC | US | 2007 | PHB | AirCarbon | Commercial |
| 10 | Biomer | Germany | 1994 | PHB | Biomer® | Commercial |
| 11 | Bioplastech | Ireland | - | mcl-PHA | - | Research |
| 12 | BASF | US | - | Ecoflex | Commercial | |
| 13 | Tepha Inc. | Massachusetts | 1998 | P4HB, P(3HB-co-4HB) | TephaFLEX® | Commercial |
| 14 | Poly Ferm | Canada | 2015 | mcl-PHA | VersaMerTM | Commercial |
| 15 | PHB Industrial S.A. | Brazil | 2000 | PHB, PHBV | BIOCYCLE® | Commercial |
| 16 | Full Cycle Bioplastics | US | - | PHBV | - | Commercial |
| 17 | Cardia Bioplastics | Australia | - | - | CardiaCompostables | Commercial |
| 18 | Blue PHA | China | - | - | - | Commercial |
| 19 | Mango Materials | US | - | PHB | YOPP PHA | Pilot |
| 20 | SIRIM Bioplastics | Malaysia | 2011 | Several types | - | Research |
| 21 | GoPHA | Netherlands | - | Several types | - | Research |
| 22 | PHA Builder | China | - | Several types | - | Research |
| 23 | COFCO | China | - | PHB | COFCO Biochemicals | Commercial |
| 24 | NAFIGATE Corporation | Czech | 2015 | PHB | - | Research |
| 25 | Helian Polymers | Netherlands | - | PHB, PHBV | NPX BioBalls | Commercial |
| 26 | Terra VerdaeBioWorksInc | Canada | 2009 | - | - | Pilot |
| 27 | Rodenburg Biopolymer | Netherlands | 2000 | - | Optinyl® | Pilot |
| 28 | Biomatera | Canada | 1998 | PHA Resins | Biomatera | Commercial |
| 29 | Mosanto | Japan | 1996 | P(3HB-CO-3HV) | Biopol | - |
| 30 | Zeneca | UK | 1970 | P(3HB-CO-3HV) | Biopol | - |
| Sr. No. | Feed | Culture | Equipment | PHA Accumulation/Recovery | Period of Operation | Reference |
|---|---|---|---|---|---|---|
| 1 | Food waste and sewage sludge | MMC | CSTR (380 L), coaxial centrifuge, SBR (100 L), FBR (70 L) | 48% g PHA/g VSS | - | [29] |
| 2 | Activated sludge | MMC | SBR-1(2 L), SBR-2(2 L) | 51–63% | - | [30] |
| 3 | Wastewater from food industry | E. coli, S. terrae, A. ichthiosmia, P. putida, B. pumilus, P. huttiensis, B. cereus, Y. frederiksenii. | SBR (6 L), centrifuge, membrane filter (ceramic) | Varied according to strain | - | [31] |
| 4 | Activated sludge | Bacterial consortium (S-150) | Fermentation reactor (4000 L), membrane filter (ceramic), bioreactor (70 L) | 59.47% of dry cell weight | - | [32] |
| 5 | Municipal solid waste and sewage sludge | MMC | SBR (120 L), anaerobic CSTR (380 L), filter press (SS) | 46% | 2 months | [33] |
| 6 | Activated sludge | MMC | SBR, fill-up reactor, decanter, bow sieve, dryer, centrifuge, oven | 17–22% | 32 days | [34] |
| 7 | Activated sludge | Cupriavidus sp. | Bioreactor (30 L), fermentation reactor (50 L), centrifuge | 28–63% | - | [35] |
| 8 | Food industry effluent | MMC | SBR (460 L), mixing tank (500 L), bowl–scroll centrifuge, accumulation reactor (400 L) | 0.40 to 0.70 gPHA/gVSS | 1 year | [36] |
| 9 | Activated sludge | MMC | Fermentation reactor (1000 L), bowl–scroll centrifuge, micro-filter (60 μm mesh size), holding tank (500 L), SBR (500 L), accumulation reactor (550 L), settling tank (120 L), oven (Binder FP240) | 0.39 gPHA/gVSS | 22 months | [37] |
| 10 | Activated sludge | - | SBR-1 (500 L), SBR-2 (500 L), accumulation reactor (500 L), settling tank (120 L), biomass-thickening units, filter bed drum centrifuge, oven | 49% | 225 days | [38] |
| 11 | Cellulosic primary sludge | - | Rotating belt dynamic filter, fermentation unit (2600 L), ultrafiltration unit, nitration SBR (1100 L), selection SBR (2800 L), accumulation reactor (1000 L) | 55% of PHA (VSS basis) | 600 days | [29] |
| 12 | Food waste | MMC | Percolation biocell reactor (100 L), percolate tank, centrifuge, ammonia stripper, SBR (800 mL), accumulation glass reactor (300 mL) | 223 ± 28 g kg−1 of feed | 30 days | [39] |
| 13 | Activated sludge | MMC | Batch fermentation reactor (1200 L), FBR (500 L), solvent extractor (10 L) | 50% | 10 months | [40] |
| 14 | Activated sludge | MMC | Fermentation CSTR (200 L), enrichment SBR (400 L), FBR (180 L) | - | - | [41] |
| 15 | Municipal solid waste and sewage sludge | MMC | Fermentation reactor (380 L), SBR (100 L), FBR (70–90 L) | 40–50% | - | [42] |
| Cellulosic primary sludge | - | 44.0–13.0 and 56.0–87.0 w/w, | ||||
| Fruit waste | Upflow sludge reactor (60 L), SBR (100 L), stirring tank reactor (60 L) | 70% of dry cell weight | ||||
| 16 | Biomass | MMC | SBR (1000 L), filter (polyamine monofilament cloth), solvent extractor | 18–30% | - | [43] |
| 17 | Dairy wastewater | - | Anaerobic acidogenic reactor, upflow sludge reactor, PHA synthesis reactor, clarifier, centrifuge | 43% of dry weight | - | [38] |
| 18 | Sugar processing wastewater | MMC | Two SBRs (4 L), accumulation reactor (500 L), FBR (400 L) | 60% g-PHA/g-VSS | 24 weeks | [44] |
| 19 | Municipal wastewater and sludge | MMC | Fermentation reactor (1000 L), bowl–scroll centrifuge, drum filter (mesh of 130–600 μm), feeding tank (500 L), SBR (500 L), accumulation reactor (500 L), sludge-thickening units, oven | 34% (g PHA/g VSS) | - | [33] |
| 20 | Wastewater from chocolate factory | Plasticicumulansacidivorans | Upflow sludge reactor (60 L), anaerobic tank (1500 L), enrichment reactor (200 L), FBR (200 L), centrifuge | 0.76 gPHA/gVSS | - | [45] |
| 21 | Paper industry wastewater | Plasticicumulansacidivorans | Upflow sludge reactor (60 L), anaerobic tank (1500 L), enrichment reactor (170 L), FBR (200 L), centrifuge | 0.70–0.80 g PHA/g VSS | 42 days | [2] |
| 22 | Municipal solid waste | - | Fermentation CSTR (200 L), filter bag (5.0 μm porosity), SBR (140 L), PHA synthesis reactor | 55% | - | [46] |
| 23 | Municipal solid waste | MMC | Fermentation CSTR (200 L), SBR-1 (100 L), SBR-2 (50 L), accumulation reactor | 49% (g PHA/g VSS) | - | [28] |
| 24 | Municipal solid waste | - | Fermentation reactor (380 L), coaxial centrifuge (5.0 μm porosity, ultrafiltration membrane (0.2 μm porosity), CSTR (230 L), selection reactor (100 L), FBR (80–120 L) | 7.6% | - | [47] |
| 25 | Activated sludge | - | SBR-1 (2500 L), nitrification SBR-2 (2500 L), precipitation reactor (150 L), buffer tank (2500 L) | 6.9–9.2% (gPHA/gTSS) | 439 days | [48] |
| 26 | Municipal solid waste | P. acidivorans | Settling tank (1 m3), buffer vessel (1500 L), enrichment reactor (180 L), SBR, accumulation reactor (180 L), centrifuge | 77 ± 18% PHA | 757 days | [49] |
| 27 | Food waste | - | Fermentation reactor (380 L), coaxial centrifuge (5.0 μm porosity, ultrafiltration membrane (0.2 μm porosity), CSTR (230 L), selection reactor (100 L), FBR (80–120 L) | 7.6% | - | [47] |
| 28 | Pulp–paper industry wastewater | - | 2 bioreactors(15 L) | 39.6% dry sludge | - | [50] |
| 29 | Oil mill wastewater | MMC | SBR (16 L), FBR (2 L), solvent extraction | 74 ± 8% | 350 days | [51] |
| 30 | Activated sludge | - | Primary settler, activated sludge unit, secondary settler, thickening units, sludge digester, heat exchangers, buffer tanks, enrichment SBR, accumulation SBR, centrifuge | - | - | [52] |
| 31 | Olive oil mill wastewater | MMC and Pseudomonas sp. | Feed Tank (50 L), acidification reactor (20 L), fermentation SBR (50 L), aerobic reactor | 25% | - | [53] |
| 32 | Wine grape waste | Pseudomonasputida | Fermentation bioreactor (300 L), marine propeller tank, filter, autoclave, orbital shaker, batch centrifuge, extraction reactor | 41% | - | [54] |
| 33 | Food waste | - | Anaerobic bioreactor (34 L), enrichment reactor, PHA production reactor, SBR reactor | 23.7% | - | [55] |
| C-Source | N-Source | Bacterial Strain | PHA | Fermentation Mode | Operating Conditions | PHA Yield | π (g L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CM | NH4Cl, (NH4)2SO4 NH4NO3, urea | Alcaligenes sp. NCIM5085 | PHB | Batch | Temp. = 30 °C; pH = 6.54; agitation speed = 3.13 Hz; incubation time = 48 h | 8.8 ± 0.4 g L−1 | 0.19 | [17] |
| CM and WS | (NH4)2SO4 | A. latus DSM1123 | PHB | Fed batch | Temp. = 30 °C; pH = 7; agitation speed = 200–600 rpm; incubation time = 72 h; C/N ratio = 4–200 | 16.9 g L−1 L with 60-Brix syrup @ 500 rpm and 200 C/N | 0.234 | [73] |
| CM | (NH4)2SO4 | A. eutrophus | PHB | Batch | Temp. = 30 ± 1 °C; pH = 7; agitation speed = 250 ± 10 rpm; incubation time = 84 h | 0.78 g L−1 | 0.013 ± 0.022 | [15] |
| CM | NH4Cl | A. eutrophus H16 and 5119, B. subtilis: R. eutrophus 5119 | PHBV | Batch | Temp. = 30 °C; pH = 7; agitation speed = 160 rpm; incubation time = 72 h | 2.30 g L−1 | - | [16] |
| CM | NH4Cl, CSL, (NH4)2SO4 NH4NO3 (NH4)3PO4 | B. megaterium | PHB | Batch | Temp. = 30 °C; pH = 7; agitation speed = 130 rpm; incubation time = 48 h | 46.2% per mg CDM | - | [74] |
| CM | bacillus sp. Strain COl1/A6 | - | Shaken flask | Temp. = 30 °C; agitation speed = 170 rpm; incubation time = 48 h | 54% CDW | - | [75] | |
| CM | urea | B. megaterium BA-019 | PHB | Batch and fed batch | Temp. = 30 °C; pH = 7: agitation speed = 200 rpm; incubation time = 36 h; C/N = 10–100 | 42%DCW @25 C/N | 1.27 | [22] |
| CM | (NH4)3PO4 | B. cereus SPV | PHB | Shaken flask and fed batch | Temp. = 30 °C; pH = 6.8: agitation speed = 200 rpm; incubation time = 60 h | 6.63 g L−1 | - | [76] |
| SCL | monosodium glutamate | P. fluorescens A2a5 | PHB | batch | Temp. = 25 °C; pH = 7; incubation time = 144 h | 22 g L−1 | 0.23 | [24] |
| CM and CO | P. Putida | PHA | Shaken flask | Temp. = 20–55 °C; pH = 3–9: agitation speed = 200 rpm; incubation time = 78 h | 35.63% CDW @ 37 °C and 7pH | [77] | ||
| CM | urea | P. aeruginosa | PHB | Batch | Temp. = 37 °C; pH = 7.0 ± 0.5: agitation speed = 150 rpm; incubation time = 72 h | 5.60 g L−1 | 0.12 | [78] |
| CM | P. mendocina | P(3HO)) | Batch | Temp. = 30 °C; pH = 6.8: agitation speed = 200 rpm; inoculum concentration = 10 %vv−1; incubation time = 48 h | 43.2% CDW | [79] | ||
| PKO | (NH4)2SO4 | Engineering Aeromonas caviae (PhaCAc) | 14.9 mol %P(3HB-co-3HHx) | Batch | Temp. = 30 °C; pH = 7; incubation time = 72 h | 14.1 ± 0.4 g L−1 | [80] | |
| WFO, WAF, IWAF | Urea, NH4Cl | A. eutrophus H16 and recombinant strain of A. eutrophus | PHB, P(3HB-co-3HHx) | Batch and fed batch | Temp. = 30 °C; pH = 6.8; agitation speed = 300–1200 rpm; incubation time = 72 h | 82% DCW PHB, 72% DCW P(3HB-co-3HHx) | 0.4 | [81] |
| OO | - | A. caviae | P(3HB-co-3HHx) | Batch | Temp. = 30 °C; pH = 7; incubation time = 24 h | - | - | [82] |
| CO, PKO, CPO, PO SBO, CO and POO | - | Recombinant strain of A. eutrophus | P(3HB-co-70% 3HHx) | Batch | Temp. = 30 °C; agitation speed = 200 rpm; incubation time = 48 h | 1.3 g L−1 | - | [83] |
| WPO | (NH4)2SO4 | Pseudomonas Sp.Gl01 | mcl-PHA | Fed batch | Temp. = 30 °C; pH = 7; agitation speed = 220 rpm; incubation time = 48 h | 1.6 g L−1 | 0.0907 | [25] |
| Tallow-based biodiesel | - | P. citronellolis | mcl-PHA | Batch | Temp. = 30 °C; pH = 7; incubation time = 72 h | 26% DCW | 0.036 | [84] |
| WRO | - | Pseudomonas Sp.Gl01 | mcl-PHA | Fed batch | Temp. = 30 °C; pH = 7; incubation time = 48 h | 2.0 g L−1 | 0.0374 | [85] |
| WFO | P. chlororaphis PA23 | mcl-PHA (3HO,3HD,3HDD) | Batch | Temp. = 30 °C; pH = 7; incubation time = 48 h; shaking | 32.5% CDW | [86] | ||
| CG, GBL and PA | (NH4)2SO4 | A. eutrophus DSM 545 | P(3HB-4HB), P(3HB-4HB-3HV) | Fed batch | 36.9% DCW | 0.25 | [87] | |
| CG | (NH4)2SO4, yeast extract | C. necator DSM 545 | PHB | Fed batch | 70% CDW | 0.05 | [88] | |
| CG | (NH4)2SO4 | C. necator DSM 545 | PHB, PHBV | Shaken flask | 5.26 | 0.12–0.15 | [89] | |
| Sources, glucose, CG | NH4Cl | B. cereus and B. thuringiensis | PHAs | Shaken flask | 2725 mgL−1 | [90] | ||
| CG | recombinant E. coil | P(4HB) | Fed batch | 15 g L−1 | 0.207 | [91] | ||
| Biodiesel waste with glycerol | P. oleovorans NRRL B-14682 | PHB | Shaken flask | 13–27% DCW | [92] | |||
| SIWW | B. subtilis NG05 | PHB | batch | 0.5 g L−1 | [93] | |||
| Dairy wastewater effluents | C necator DSM 13513 | PHB | Batch and fed batch | 1.34% | [62] | |||
| Bioindustrial WW | P. aeruginosa B. subtilis | P (3HBco-3HV) | Shaken flask | 45%DCW | [94] | |||
| Olive mill wastewater | Pseudomonas sp., MMC | PHBV | Batch and 3-stage fermentation | 64%DCW | [95] | |||
| Molasses spent wash | activated sludge | PHB | Shaken flask | 31% DCW | 0.022 | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, S.; Gautam, A.; Pawaday, J.; Kanzariya, R.K.; Yao, Z. Current Status and Challenges in the Commercial Production of Polyhydroxyalkanoate-Based Bioplastic: A Review. Processes 2024, 12, 1720. https://doi.org/10.3390/pr12081720
Gautam S, Gautam A, Pawaday J, Kanzariya RK, Yao Z. Current Status and Challenges in the Commercial Production of Polyhydroxyalkanoate-Based Bioplastic: A Review. Processes. 2024; 12(8):1720. https://doi.org/10.3390/pr12081720
Chicago/Turabian StyleGautam, Shina, Alok Gautam, Juily Pawaday, Rekha Karshanbhai Kanzariya, and Zhitong Yao. 2024. "Current Status and Challenges in the Commercial Production of Polyhydroxyalkanoate-Based Bioplastic: A Review" Processes 12, no. 8: 1720. https://doi.org/10.3390/pr12081720





