Preparation of Fe2O3/g-C3N4 Photocatalysts and the Degradation Mechanism of NOR in Water under Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalyst
2.2.1. Preparation of g-C3N4
2.2.2. Preparation of Fe2O3/g-C3N4
2.2.3. Preparation of Fe2O3
2.3. Characterization of Photocatalyst
2.4. Photocatalytic Performance Testing
3. Results
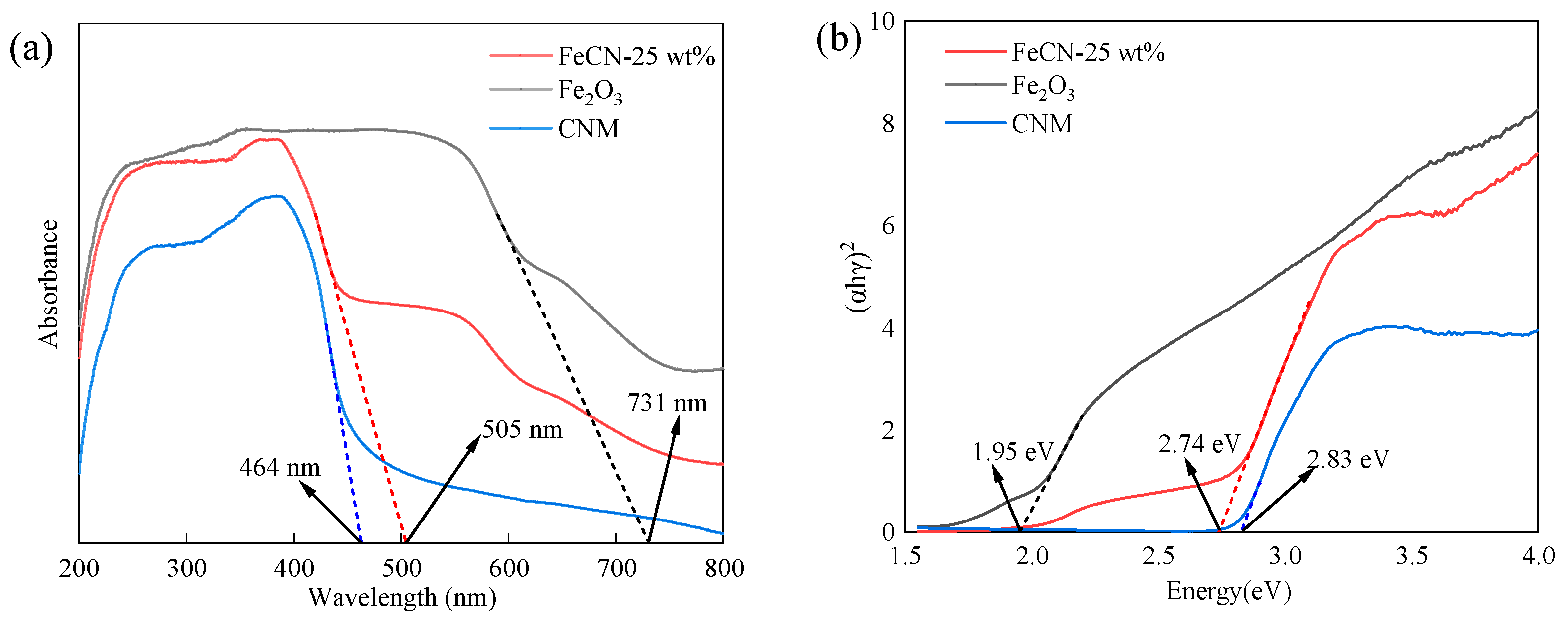
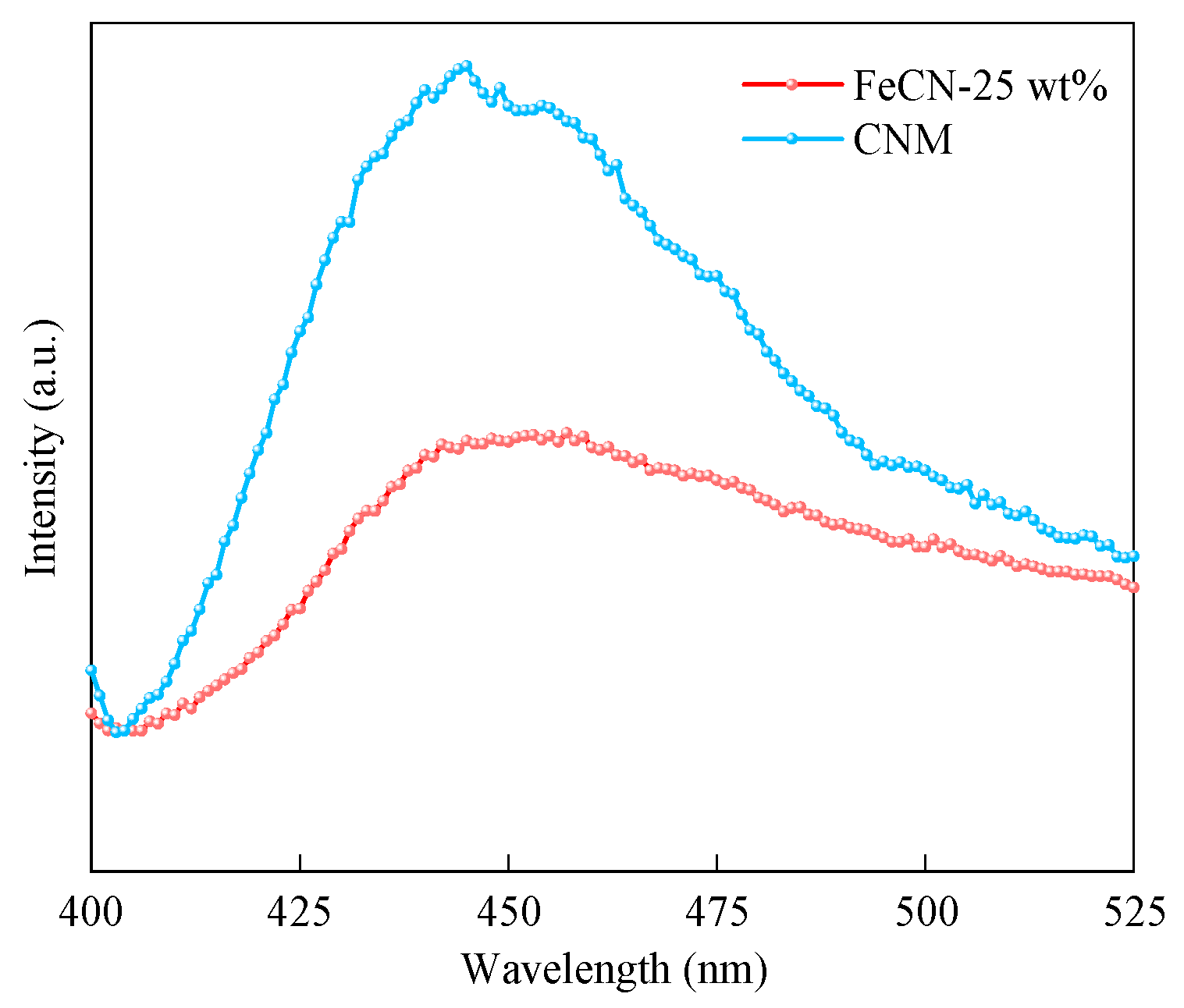
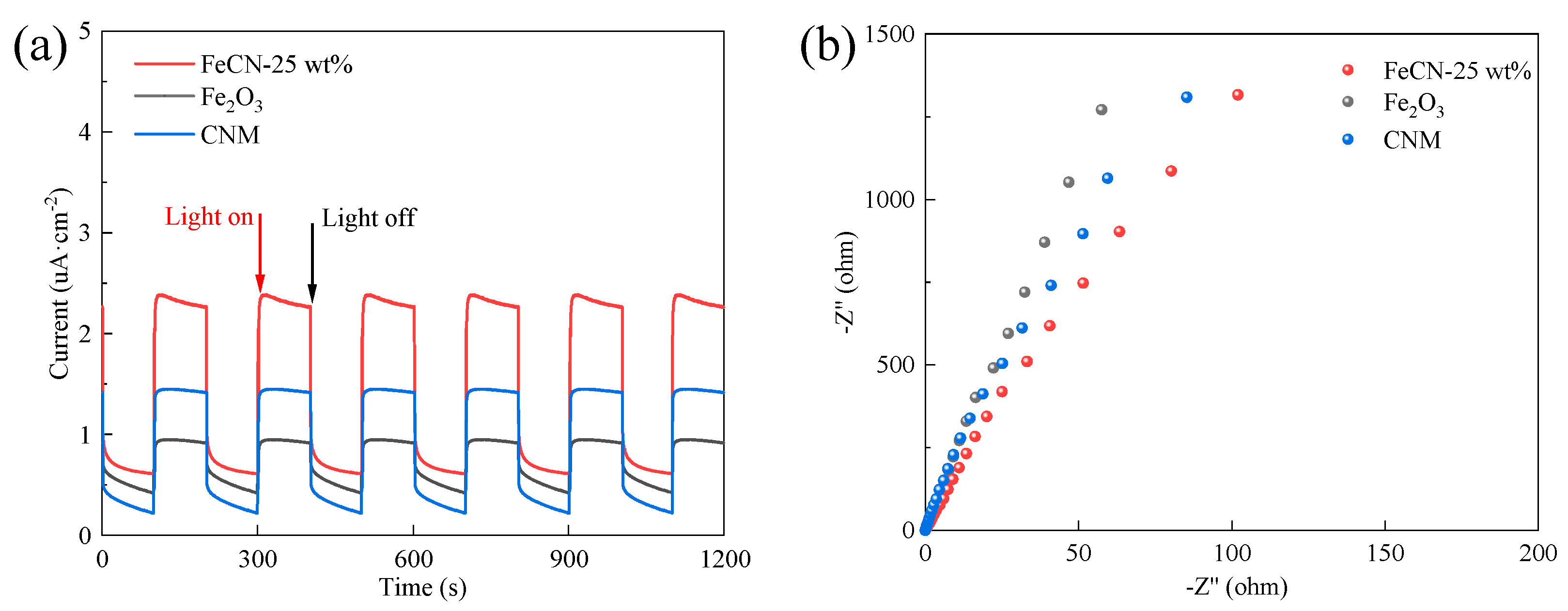
3.1. Characterization of Photocatalysts
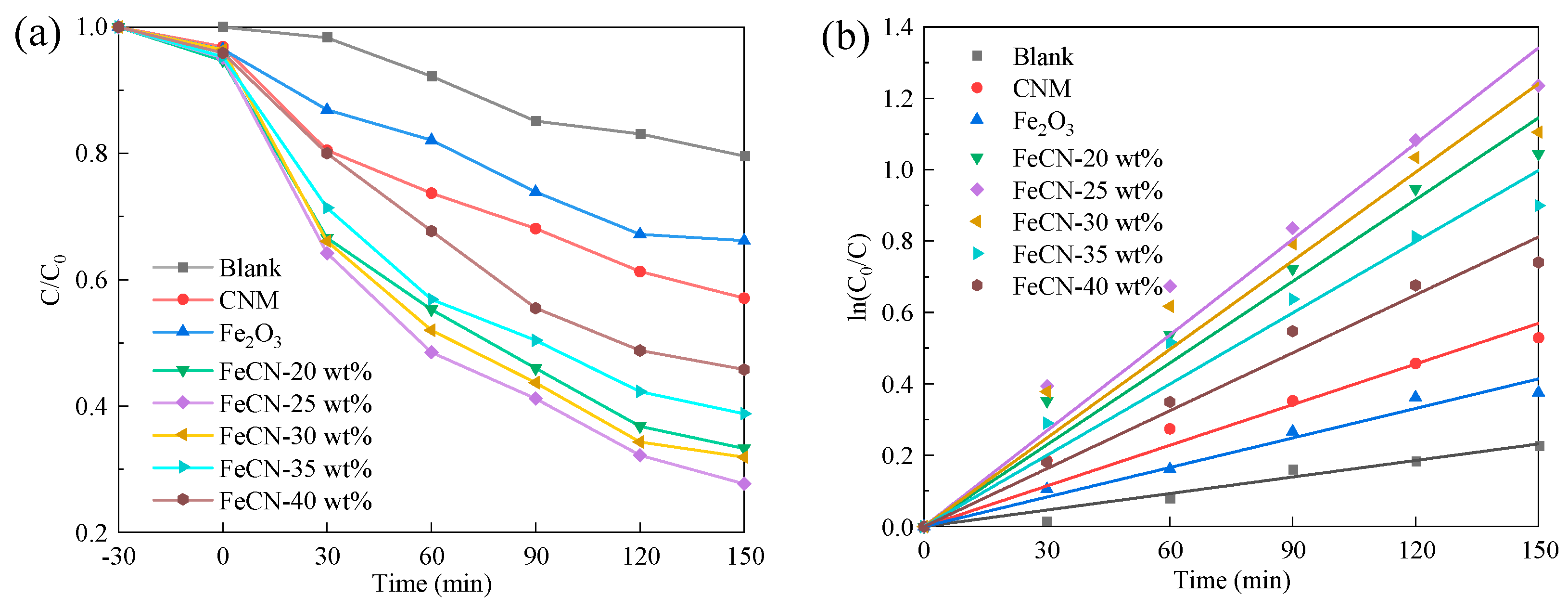
3.2. Photocatalytic Performance
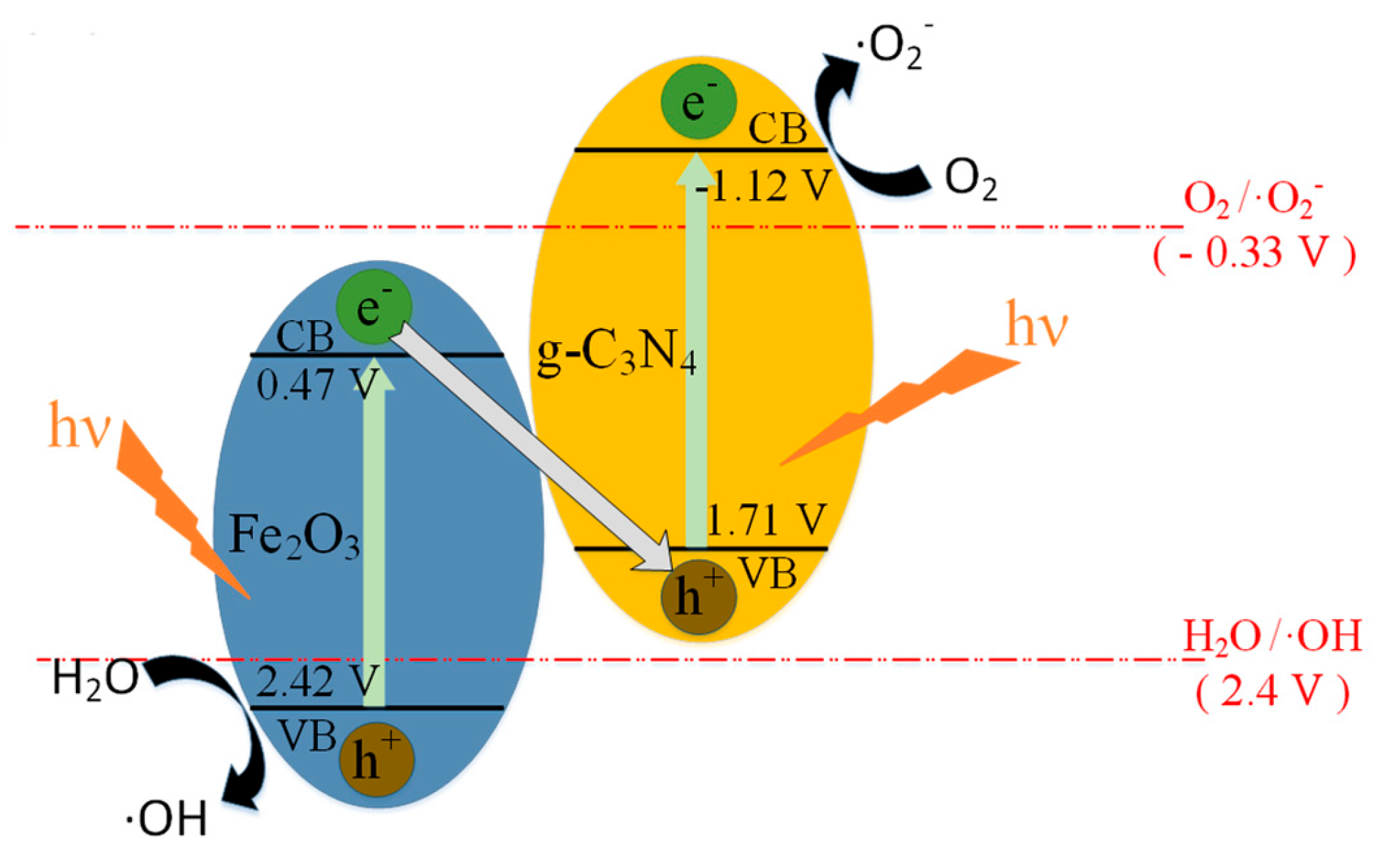
3.3. Photocatalytic Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, Y.; Luan, J. Improved photocatalytic property of peony-like InOOH for degrading norfloxacin. Chem. Eng. J. 2020, 382, 122770. [Google Scholar] [CrossRef]
- Yin, N.; Chen, H.; Yuan, X.; Zhang, M.; Guo, J.; Zhang, Y.; Qiao, L.; Liu, M.; Song, K. Highly efficient photocatalytic degradation of norfloxacin via Bi2Sn2O7/PDIH Z-scheme heterojunction: Influence and mechanism. J. Hazard. Mater. 2022, 436, 129317. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.Y.; Kou, F.Y.; Ouyang, Q.; Chen, J.Y.; Fang, Z.Q. Removal of norfloxacin by surface Fenton system (MnFe2O4/H2O2): Kinetics, mechanism and degradation pathway. Chem. Eng. J. 2018, 351, 747–755. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Prieto, A.; Möder, M.; Rodil, R.; Adrian, L.; Marco-Urrea, E. Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour. Technol. 2011, 10223, 10987–10995. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Zhang, M.; Zhang, B.T.; Yu, Y.G. The electrochemical degradation of ciprofloxacin using a SnO2-Sb/Ti anode: Influencing factors, reaction pathways and energy demand. Chem. Eng. J. 2016, 296, 79–89. [Google Scholar] [CrossRef]
- Zhu, Q.; He, S.; Zhao, X.; Tang, W.; Song, X.; Xue, H.; Yang, L. Photocatalytic degradation of methyl orange by AgCl/ZnO/GO. Res. Environ. Sci. 2020, 33, 969–977. [Google Scholar]
- Akhundi, A.; Badiei, A.; Ghodsi, M.Z.; Habibi-Yangjeh, A.; Muñoz-Batista, M.J.; Luque, R. Graphitic carbon nitride-based photocatalysts: Toward efficient organic transformation for value-added chemicals production. Mol. Catal. 2020, 488, 110902. [Google Scholar] [CrossRef]
- Shi, H.; Shi, L. Preparation and photocatalytic properties of nanocarbon particles/carbon nitride. J. Liaoning Petrochem. Univ. 2021, 41, 41–45. [Google Scholar]
- Yang, W.; Lu, L.; Li, P.; Zhang, J.; Hu, S. Synthesis, modification and application of photocatalytic material graphite phase carbon nitride. J. Petrochem. Univ. 2024, 37, 43–51. [Google Scholar]
- Ho-Kimura, S.; Luo, W. Reinforcement of a BiVO4 anode with an Fe2O3 underlayer for photoelectrochemical water splitting. Sustain. Energy Fuels 2021, 5, 3102–3114. [Google Scholar] [CrossRef]
- Osterloh, F. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, N.; Ali, S.S.; Hou, J.; Aslam, I.; Naeem, H.; Boota, M.; Ul-Hussan, M.; Yin, J.; Wang, X. Diverse morphological study for nonmetal-doped g-C3N4 composites with narrow bandgap for improved photocatalytic activity. Res. Chem. Intermediat. 2022, 48, 2857–2870. [Google Scholar] [CrossRef]
- Adrián, G.; Luis, T.; Ramón, M.F.; Francisco, I.; Elena, M.; Pedro, A.; Rita, S.; Benjamin, S. Defective outermost layers of ceria catalysts determined by electrochemical methods as direct responsible for catalytic activity in total oxidation of a representative VOC. Appl. Surf. Sci. 2024, 653, 159354. [Google Scholar]
- Yuan, Z.; Zhu, J.; Dong, C.; Wang, L.; Lu, J.; Li, Y.; Ding, J. RSM optimization and kinetics study of α-Fe2O3/g-C3N4@H photocatalyst with S-type heterojunction for esterifying crude castor oil to reduce acidity and synthesize biodiesel. Energy Convers. Manag. 2024, 309, 118449–118465. [Google Scholar] [CrossRef]
- Lu, X.; Xu, K.; Chen, P.; Jia, K.; Liu, S.; Wu, C. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H2 evolution activity. J. Mater. Chem. A 2014, 2, 18924–18928. [Google Scholar] [CrossRef]
- Ye, S.; Qiu, L.G.; Yuan, Y.P.; Zhu, Y.J.; Xia, J.; Zhu, J.F. Facile fabrication of magnetically separable graphitic carbon nitride photocatalysts with enhanced photocatalytic activity under visible light. J. Mater. Chem. A 2013, 1, 3008–3016. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, H.; Deng, J.; Liu, Y.; Wang, Y.; Li, X.; Bai, G.; Gao, B.; Au, C.T. Porous FeOx/BiVO4–δS0.08: Highly efficient photocatalysts for the degradation of Methylene Blue under visible-light illumination. J. Environ. Sci.-China 2013, 25, 2138–2149. [Google Scholar] [CrossRef]
- Zhang, R.; Dai, H.; Du, Y.; Zhang, L.; Deng, J.; Xia, Y.; Zhao, Z.; Meng, X.; Liu, Y. P123-PMMA dual-templating generation and unique physicochemical properties of three-dimensionally ordered macroporous iron oxides with nanovoids in the crystalline walls. Inorg. Chem. 2011, 50, 2534–2544. [Google Scholar] [CrossRef]
- Fang, S.; Yang, X.; Kangle, L.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Cui, Y.; Briscoe, J.; Wang, Y.; Tarakina, N.V.; Dunn, S. Enhanced photocatalytic activity of heterostructured ferroelectric BaTiO3/α-Fe2O3 and the significance of interface morphology control. ACS Appl. Mater. Interfaces 2017, 9, 24518–24526. [Google Scholar] [CrossRef]
- Kumaravel, S.; Avula, B.; Chandrasatheesh, C.; Niyitanga, T.; Saranya, R.; Hasan, I.; Abisheik, T.; Rai, R.S.; Pandiyan, V.; Balu, K. Rational construction of MOF derived α-Fe2O3/g-C3N4 composite for effective photocatalytic degradation of organic pollutants and electrocatalytic oxygen evolution reaction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 310, 123972–123986. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.M.; Zhang, W.D.; Wan, M.X.; Fu, S.Y. Novel electromagnetic functionalized γ-Fe2O3/polypyrrole composite nanostructures with high conductivity. J. Polym. Sci. Part A Pol. Chem. 2009, 47, 4446–4453. [Google Scholar] [CrossRef]
- Babar, S.; Gavade, N.; Shinde, H.; Mahajan, P.; Lee, K.H.; Mane, N.; Deshmukh, A.; Garadkar, K.; Bhuse, V. Evolution of waste iron rust into magnetically separable g-C3N4-Fe2O3 photocatalyst: An efficient and economical waste management approach. ACS Appl. Nano Mater. 2018, 1, 4682–4694. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Lu, G.; Gu, G.; Li, M.; Han, X.; Wang, X.; Li, W. Preparation of Fe2O3/g-C3N4 Photocatalysts and the Degradation Mechanism of NOR in Water under Visible Light Irradiation. Processes 2024, 12, 1748. https://doi.org/10.3390/pr12081748
Li Z, Lu G, Gu G, Li M, Han X, Wang X, Li W. Preparation of Fe2O3/g-C3N4 Photocatalysts and the Degradation Mechanism of NOR in Water under Visible Light Irradiation. Processes. 2024; 12(8):1748. https://doi.org/10.3390/pr12081748
Chicago/Turabian StyleLi, Zheng, Guang Lu, Guizhou Gu, Min Li, Xinyue Han, Xin Wang, and Wei Li. 2024. "Preparation of Fe2O3/g-C3N4 Photocatalysts and the Degradation Mechanism of NOR in Water under Visible Light Irradiation" Processes 12, no. 8: 1748. https://doi.org/10.3390/pr12081748



