Effects of Autogenous Gas Tungsten Arc Welding (GTAW) on Corrosion Resistance of Stainless Steel 316L
Abstract
:1. Introduction
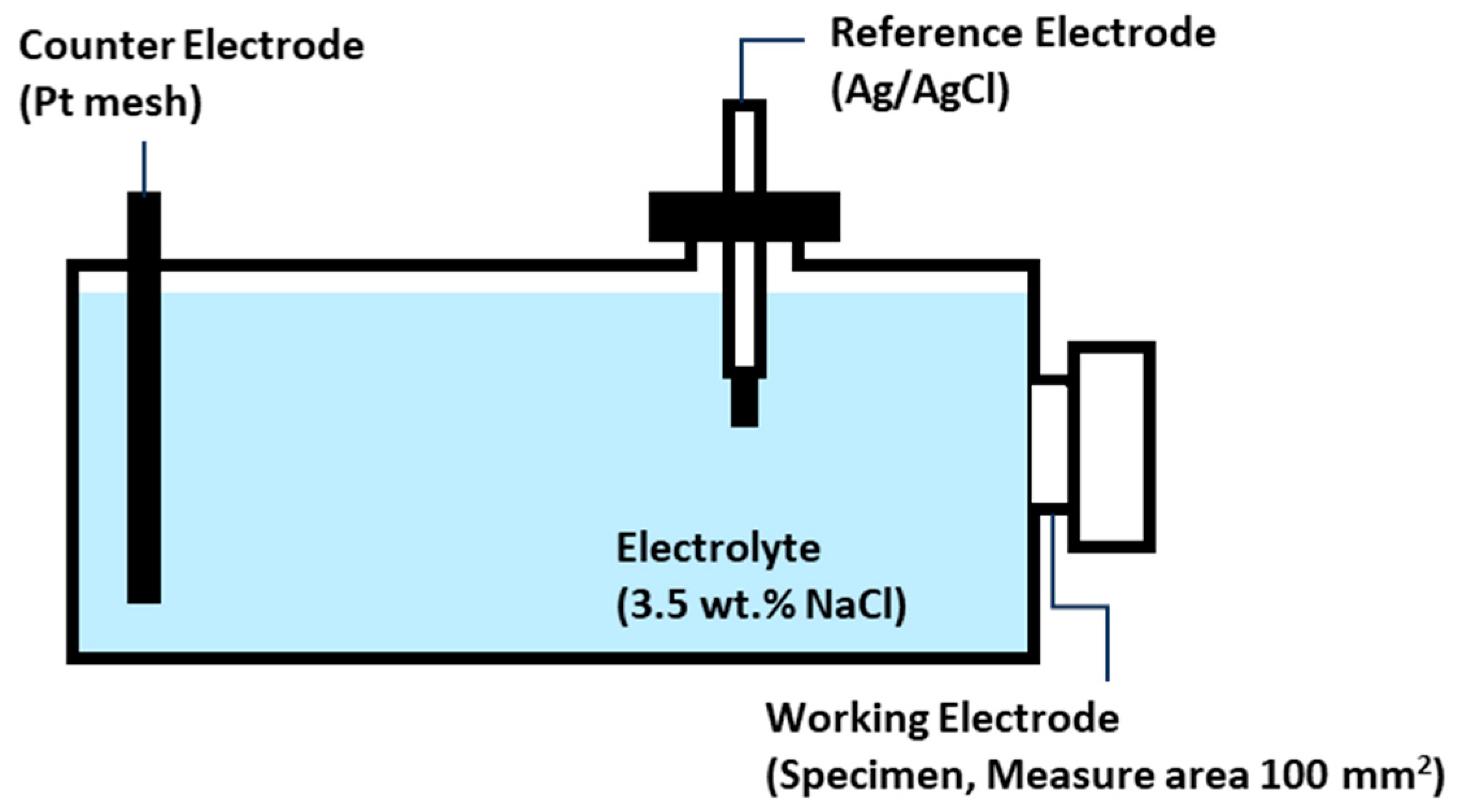
2. Experimental Procedure
2.1. GTAW Process
2.2. Characterization
3. Results and Discussion
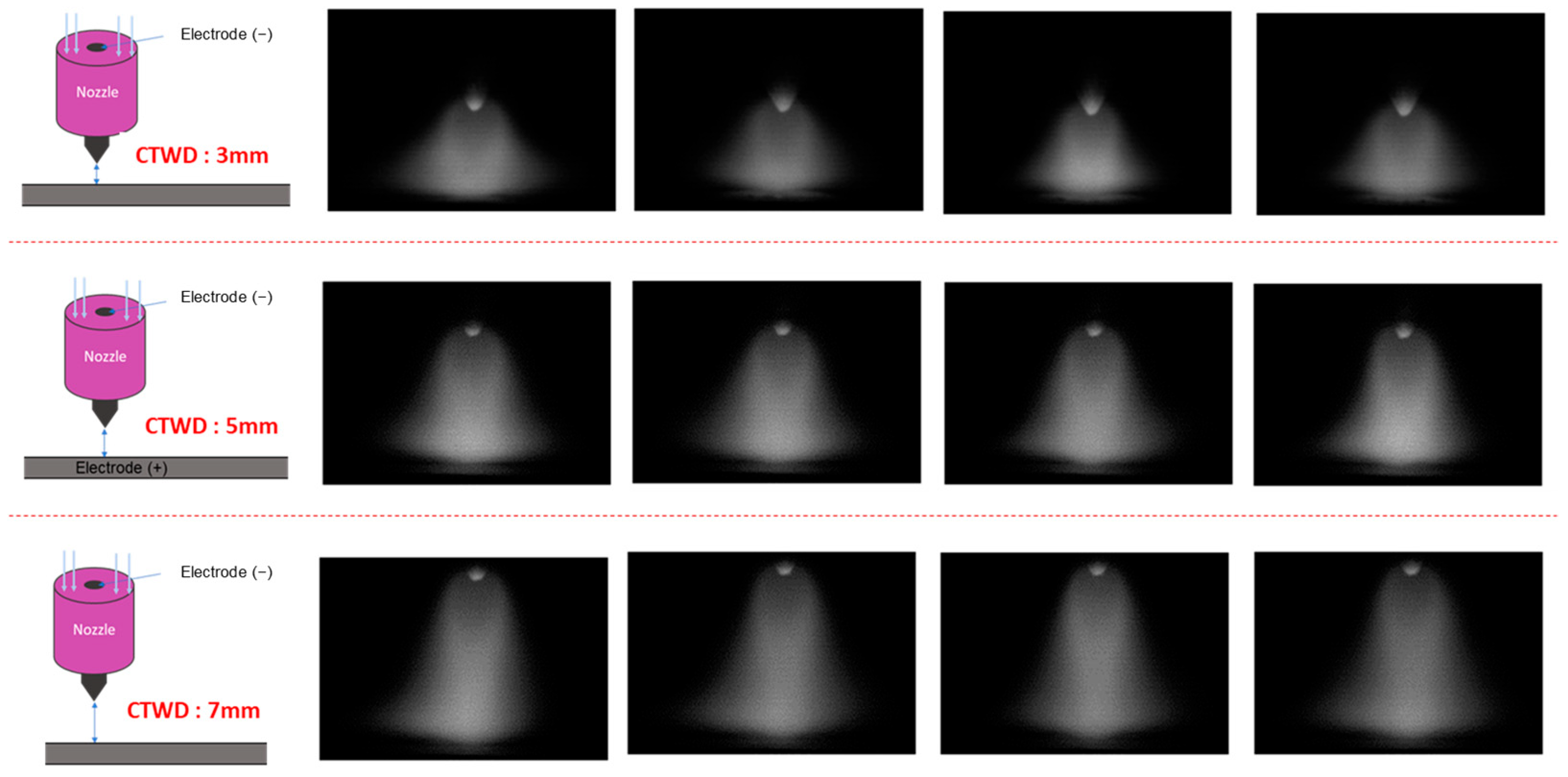
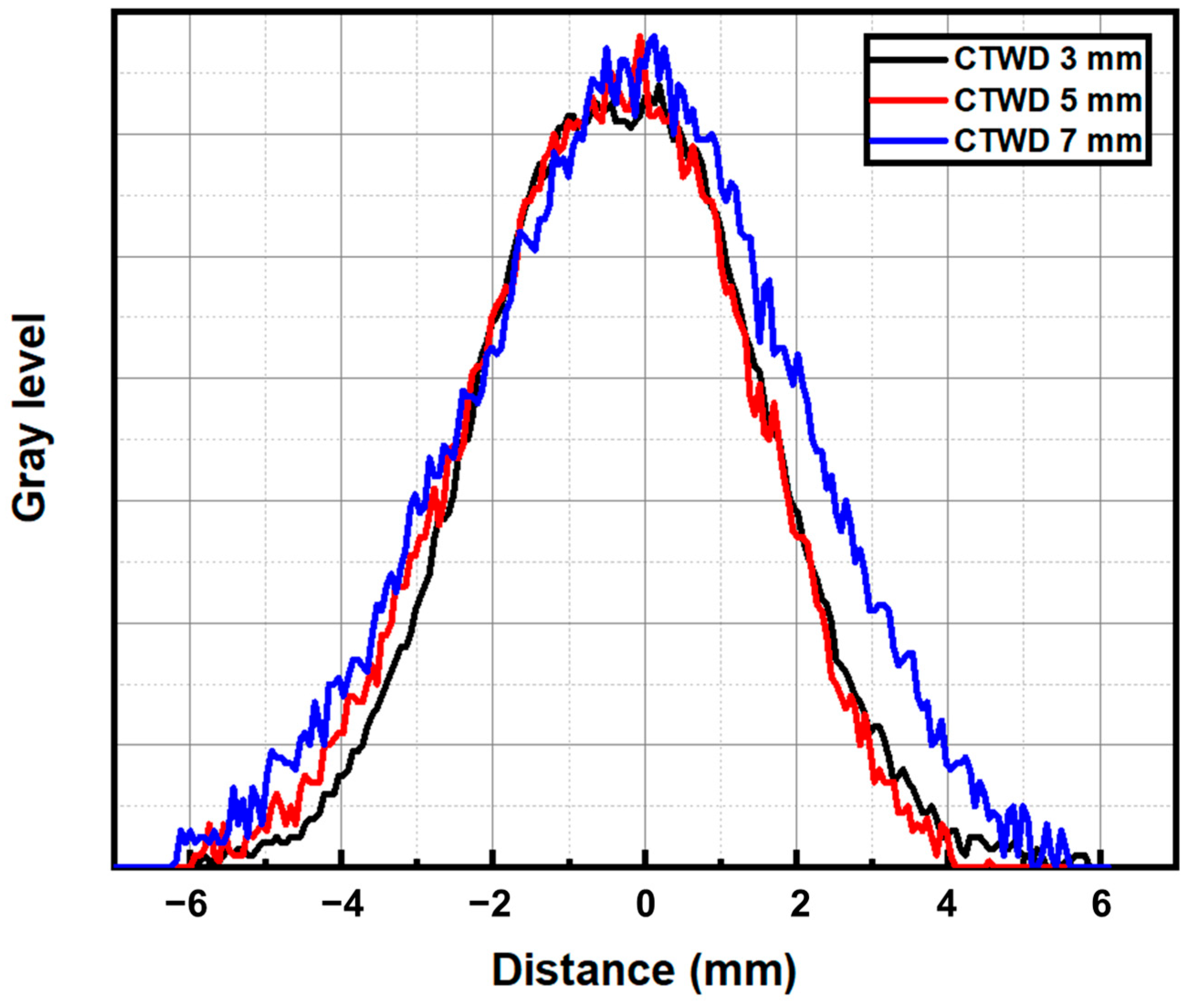
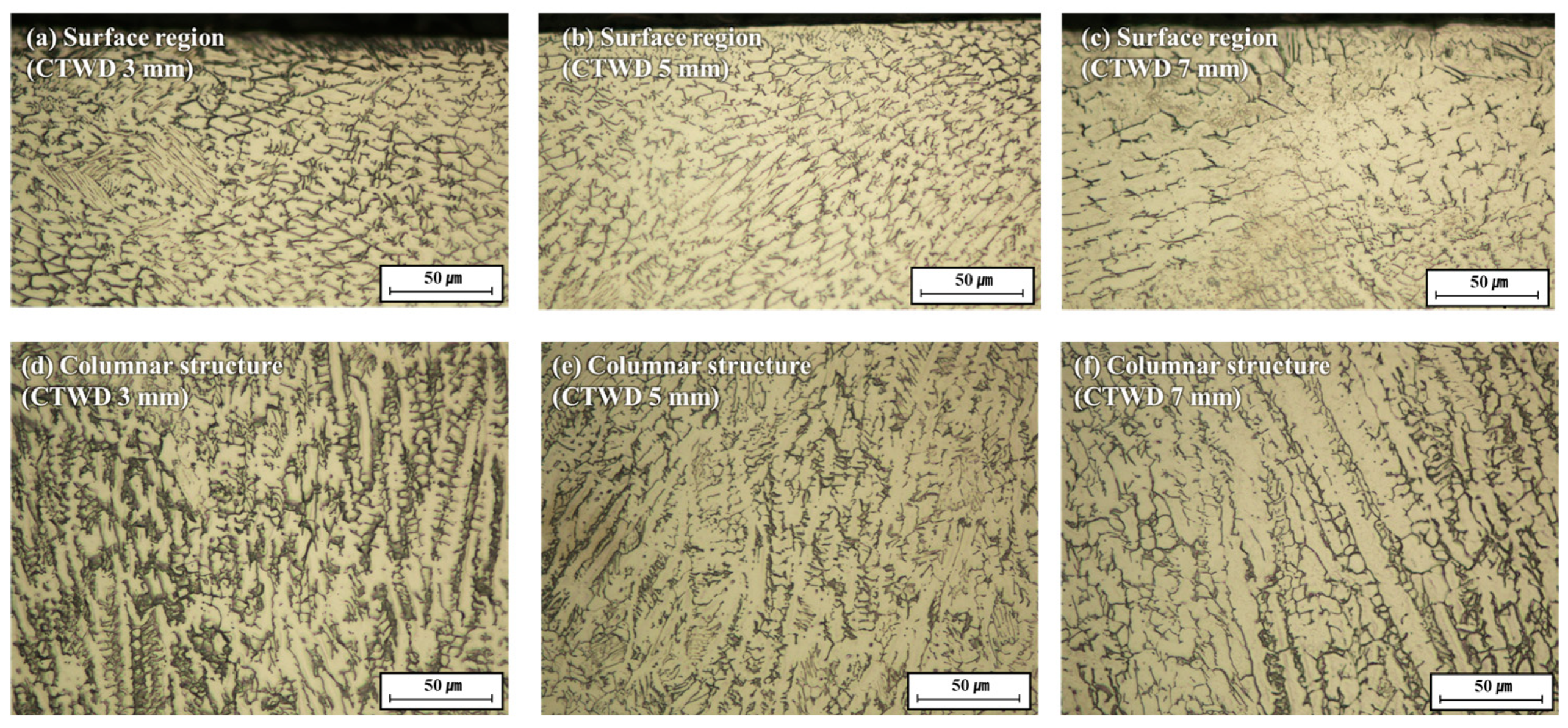
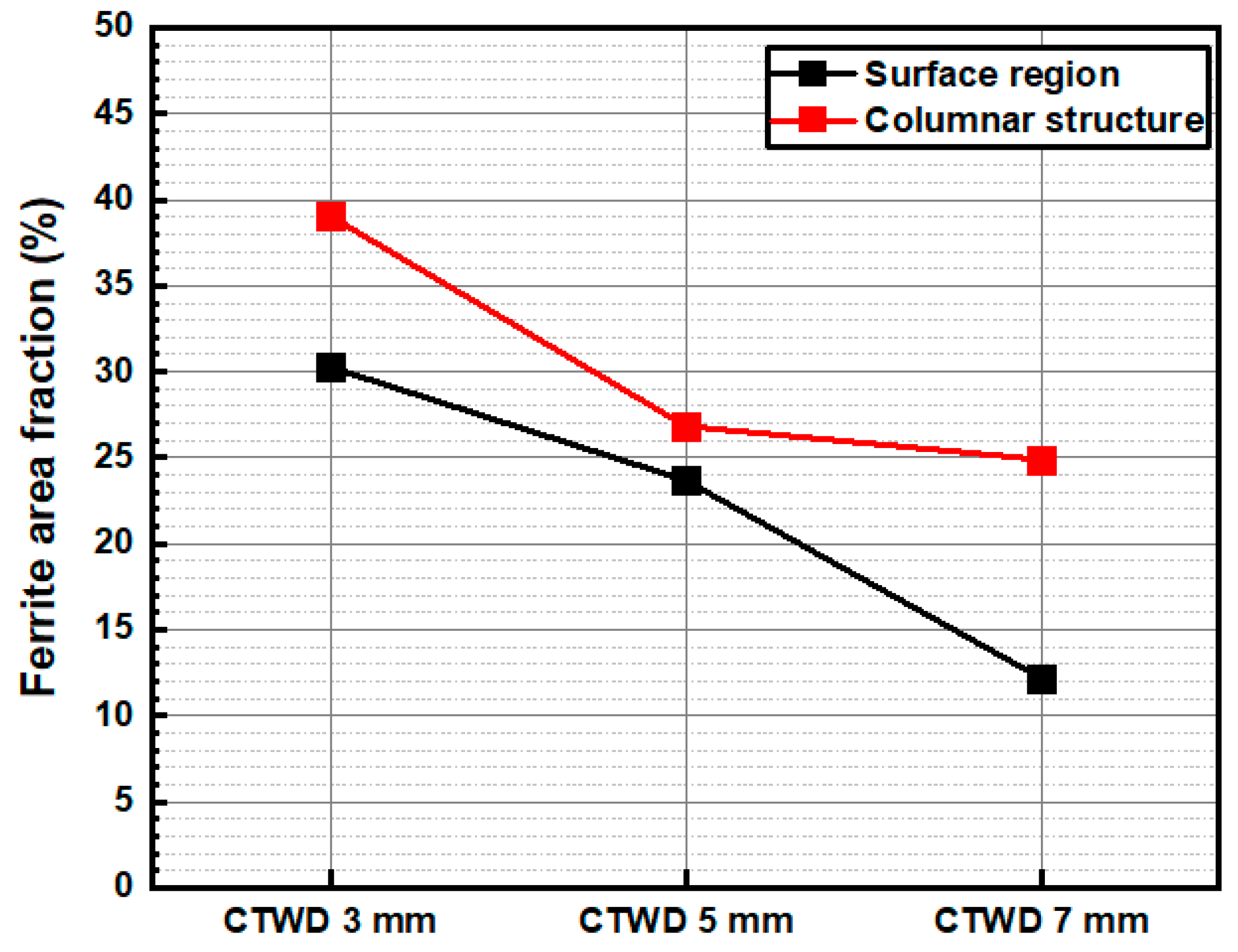
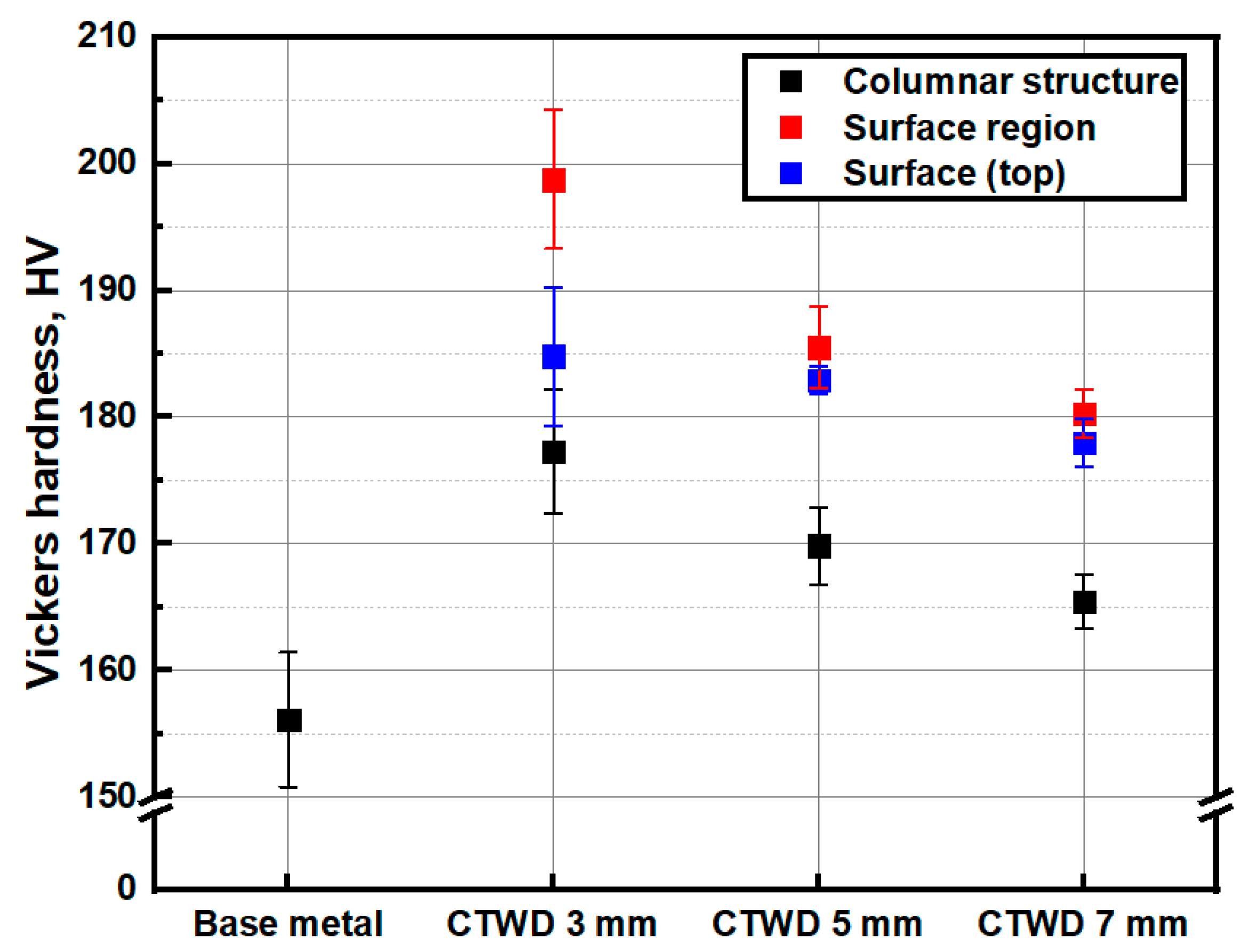
3.1. Arc Plasma Distribution and Microstructure of Molten Pool
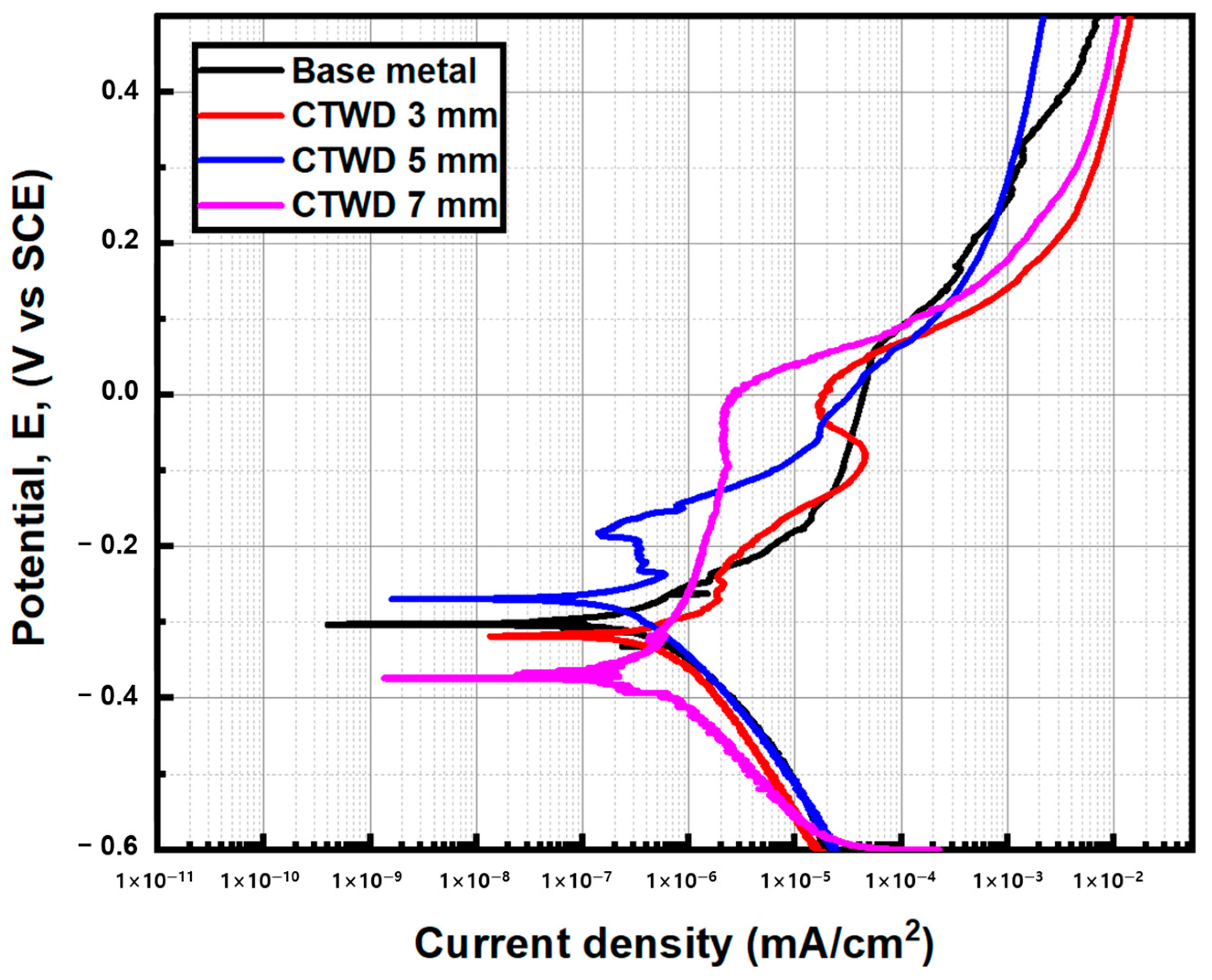
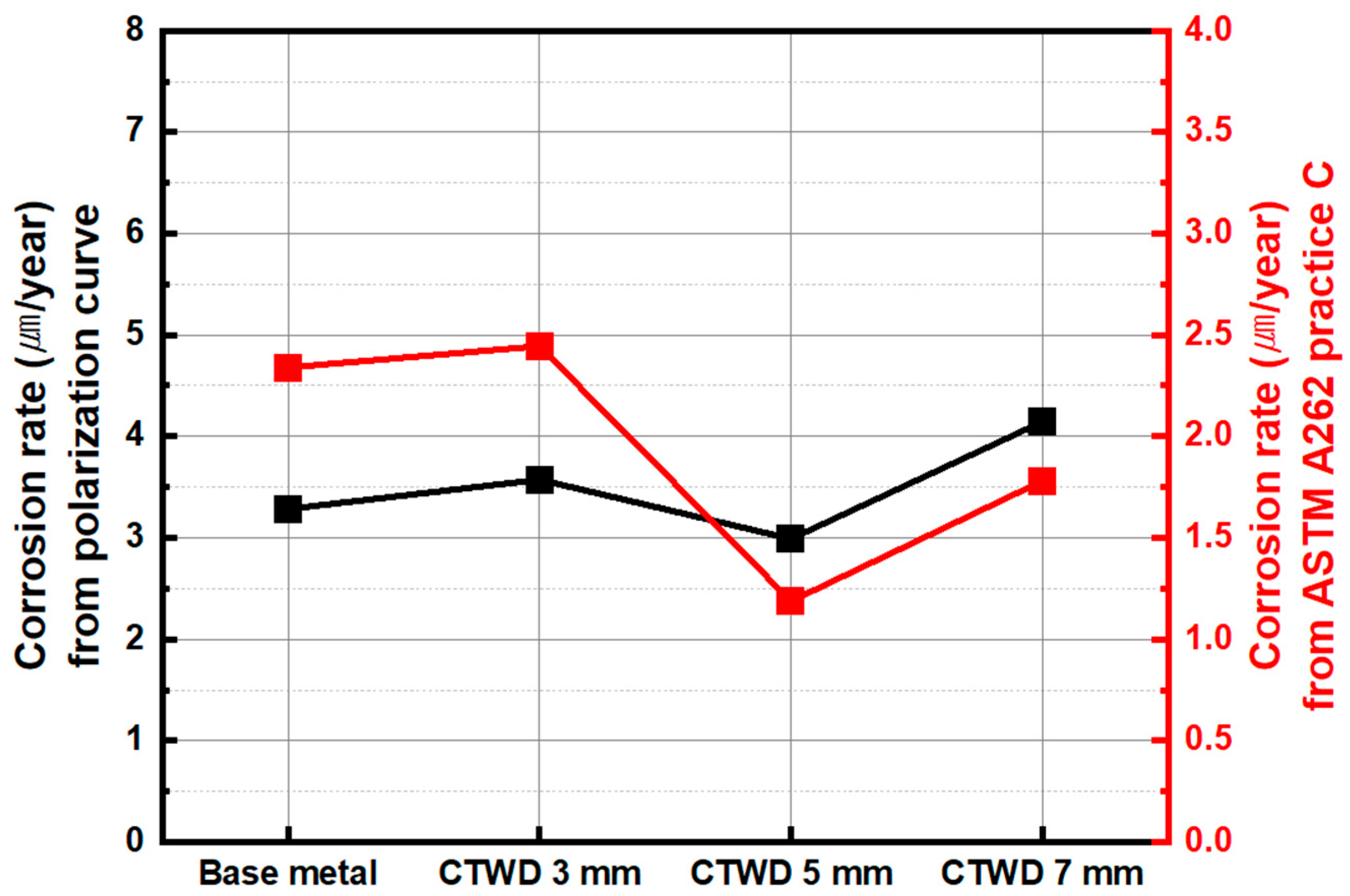
3.2. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Wang, Y.; Wang, H.; Xin, C.; Wang, X. Effects of long-term thermal aging on the stress corrosion cracking behavior of cast austenitic stainless steels in simulated PWR primary water. J. Nucl. Mater. 2016, 469, 262–268. [Google Scholar] [CrossRef]
- Nuthalapati, S.; Kee, K.; Pedapati, S.R.; Jumbri, K. A review of chloride induced stress corrosion cracking characterization in austenitic stainless steels using acoustic emission technique. Nucl. Eng. Technol. 2024, 56, 688–706. [Google Scholar] [CrossRef]
- Ji, C.; Huang, H.; Sun, J.; Chen, P. Experiment and simulation research on bonding mechanism of bimetallic clad pipes fabricated by solid-liquid cast-rolling bonding (SLCRB) process. J. Manuf. Process. 2018, 34, 593–602. [Google Scholar] [CrossRef]
- Klenam, D.; McBagonluri, F.; Bamisaye, O.; Asumadu, T.; Ankah, N.; Bodunrin, M.; Andrews, A.; Soboyejo, W. Corrosion resistant materials in high-pressure high-temperature oil wells: An overview and potential application of complex concentrated alloys. Eng. Fail. Anal. 2024, 157, 107920. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Wang, W.; Jin, L.; Xiao, H. Corrosion Behavior of AISI 316L Stainless Steel Used as Inner Lining of Bimetallic Pipe in a Seawater Environment. Materials 2021, 14, 1539. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Lee, S.-J.; Chong, S.-O. Electrochemical characteristics under cavitation-erosion for STS 316L in seawater. Mater. Res. Bull. 2014, 58, 244–247. [Google Scholar] [CrossRef]
- Vignal, V.; Voltz, C.; Thiébaut, S.; Demésy, M.; Heintz, O.; Guerraz, S. Pitting Corrosion of Type 316L Stainless Steel Elaborated by the Selective Laser Melting Method: Influence of Microstructure. J. Mater. Eng. Perform. 2021, 30, 5050–5058. [Google Scholar] [CrossRef]
- Ren, Z.; Ernst, F. Stress–Corrosion Cracking of AISI 316L Stainless Steel in Seawater Environments: Effect of Surface Machining. Metals 2020, 10, 1324. [Google Scholar] [CrossRef]
- Cai, B.; Liu, Y.; Tian, X.; Wang, F.; Li, H.; Ji, R. An experimental study of crevice corrosion behaviour of 316L stainless steel in artificial seawater. Corros. Sci. 2010, 52, 3235–3242. [Google Scholar] [CrossRef]
- Li, S.-X.; He, Y.-N.; Yu, S.-R.; Zhang, P.-Y. Evaluation of the effect of grain size on chromium carbide precipitation and intergranular corrosion of 316L stainless steel. Corros. Sci. 2013, 66, 211–216. [Google Scholar] [CrossRef]
- Malhotra, D.; Shahi, A.S.; Gupta, K. Effect of GTAW remelting on the corrosion performance of AISI 316L cladding. Mater. Corros. 2021, 72, 141–153. [Google Scholar] [CrossRef]
- Brytan, Z.; Bonek, M.; Dobrzański, L.A.; Ugues, D.; Grande, M.A. The Laser Surface Remelting of Austenitic Stainless Steel. Mater. Sci. Forum 2010, 654–656, 2511–2514. [Google Scholar] [CrossRef]
- Kwok, C.; Lo, K.; Chan, W.; Cheng, F.; Man, H. Effect of laser surface melting on intergranular corrosion behaviour of aged austenitic and duplex stainless steels. Corros. Sci. 2011, 53, 1581–1591. [Google Scholar] [CrossRef]
- Panmongkol, P.; Phung-On, I. Effect of backing gas mixtures on corrosion properties of stainless steel grade 304 weld metal by autogenous GTAW. J. Mater. Res. Technol. 2021, 11, 1559–1570. [Google Scholar] [CrossRef]
- Ishida, T. Formation of stainless steel layer on mild steel by welding arc cladding. J. Mater. Sci. 1991, 26, 6431–6435. [Google Scholar] [CrossRef]
- Khara, B.; Mandal, N.D.; Sarkar, A.; Sarkar, M.; Chakrabarti, B.; Das, S. Weld cladding with austenitic stainless steel for imparting corrosion resistance. Indian Weld. J. 2016, 49, 74. [Google Scholar] [CrossRef]
- Kumar, A.; Chopkar, M.K.; Verma, J.; Taiwade, R.V.; Tandon, V.; Shukla, S. Effect of filler and autogenous TIG welding on microstructure, mechanical properties and corrosion resistance of nitronic 50 stainless steel. Mater. Res. Express 2018, 6, 026557. [Google Scholar] [CrossRef]
- Pujari, K.; Patil, D.; Mewundi, G. Selection of GTAW process parameter and optimizing the weld pool geometry for AA 7075-T6 Aluminium alloy. Mater. Today Proc. 2018, 5, 25045–25055. [Google Scholar] [CrossRef]
- Streicher, M.A. Corrosion Tests and Standards—Application and Interpretation, 2nd ed.; Baboian, R., Ed.; ASTM: West Conshohocken, PA, USA, 2005; p. 257. [Google Scholar]
- Baghel, A.; Sharma, C.; Upadhyay, V.; Singh, R. Optimization of process parameters for autogenous TIG welding of austenitic stainless-steel SS-304. Int. J. Interact. Des. Manuf. 2023, 1–15. [Google Scholar] [CrossRef]
- Baskoro, A.S.; Widyianto, A.; Prasetyo, E.; Kiswanto, G. The Taguchi and Response Surface Method for Optimizing Orbital Pipe Welding Parameters in Pulsed Current Gas Tungsten Arc Welding (PC-GTAW) for SS316L. Trans. Indian Inst. Met. 2024, 77, 1607–1620. [Google Scholar] [CrossRef]
- Moghaddam, M.A.; Kolahan, F. Modeling and optimization of A-GTAW process using back propagation neural network and heuristic algorithms. Int. J. Press. Vessels Pip. 2021, 194, 104531. [Google Scholar] [CrossRef]
- ASTM A262; Stand Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels. American Society for Testing and Materials (ASTM International): West Conshohocken, PA, USA, 2014.
- Cho, D.; Lee, S.; Na, S. Characterization of welding arc and weld pool formation in vacuum gas hollow tungsten arc welding. J. Mater. Process. Technol. 2012, 213, 143–152. [Google Scholar] [CrossRef]
- Guo, S.; Xu, J.; Gu, J.; Peng, Y.; Zhou, Q.; Wang, K. Effect of cellular structure on the mechanical properties of 316L stainless steel fabricated by EBF3. J. Mater. Res. Technol. 2023, 25, 5469–5482. [Google Scholar] [CrossRef]
- Morozova, I.; Kehm, C.; Obrosov, A.; Yang, Y.; Miah, K.U.M.; Uludintceva, E.; Fritzsche, S.; Weiß, S.; Michailov, V. On the Heat Treatment of Selective-Laser-Melted 316L. J. Mater. Eng. Perform. 2023, 32, 4295–4305. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, S.-H.; Moon, H.-S.; Kim, M.-H.; Cho, D.-W. Effect of process parameters on root pass welding and analysis of microstructure in V-groove pulsed gas metal arc welding for mild steel. Int. J. Adv. Manuf. Technol. 2020, 109, 1969–1985. [Google Scholar] [CrossRef]
- Kiran, D.V.; Cho, D.-W.; Lee, H.-K.; Kang, C.Y.; Na, S.-J. A study on the quality of two-wire tandem submerged arc welds under iso-heat input conditions. Int. J. Adv. Manuf. Technol. 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Kovitya, P.; Cram, L.E. A two-dimensional model of gas-tungsten welding arcs. Weld. J. 1986, 65, 34–39. [Google Scholar]
- Nestor, O.H. Heat intensity and current density distributions at the anode of high current, inert gas arcs. J. Appl. Phys. 1962, 33, 1638–1648. [Google Scholar] [CrossRef]
- Kujanp, V.; Suutala, N.; Takalo, T.; Moisio, T. Correlation Between Solidification Cracking and Microstructure in Austenitic and Austenitic-Ferritic Stainless Steel. Weld. Res. Int. 1979, 9, 55–76. [Google Scholar]
- Brooks, J.A.; Goods, S.H.; Robino, C.V. Weld properties of AISI 303 free-machining stainless steel. Weld. J. 2003, 82, 84-S. [Google Scholar]
- Kotecki, D.J.; Siewert, T.A. WRC-1992 Constitution Diagram for Stainless Steel Weld Metals: A Modification of the WRC-1988 Diagram. Weld. J. 1992, 71, 171s–178s. [Google Scholar]



| Element | Fe | Cr | Ni | Mo | C | Mn | P | S | Si | Cu | N | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition (wt.%) | Bal. | 18.2 | 11.5 | 2.50 | 0.0180 | 1.84 | 0.020 | 0.0160 | 0.690 | 0.160 | 0.0675 | 0.032 |
| Parameters | Value |
|---|---|
| Set current | 135 A |
| Shielding gas | Argon 100%, 20 L/min |
| Welding speed | 5 mm/s |
| Tungsten electrode | 60-degree tip angle, 3.2 mm diameter |
| Contact tip to work distance | 3, 5, and 7 mm |
| Cases | Voltage (V) | Current (A) | Calculated Heat Input (J/mm) |
|---|---|---|---|
| CTWD 3 mm | 115.9 | 14.00 | 19,771 |
| CTWD 5 mm | 135.2 | 16.34 | 26,510 |
| CTWD 7 mm | 146.9 | 17.70 | 31,202 |
| CTWD 3 mm | CTWD 5 mm | CTWD 7 mm | |
|---|---|---|---|
| Bead width (mm) | 6.68 | 7.79 | 4.91 |
| Maximum penetration depth (mm) | 1.14 | 1.04 | 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, I.; Jeong, G.-H.; Kim, S.-K.; Kim, Y.-H.; Murphy, A.B.; Park, T.-K.; Kim, D.; Park, H.; Cho, D.-W. Effects of Autogenous Gas Tungsten Arc Welding (GTAW) on Corrosion Resistance of Stainless Steel 316L. Processes 2024, 12, 1757. https://doi.org/10.3390/pr12081757
Song I, Jeong G-H, Kim S-K, Kim Y-H, Murphy AB, Park T-K, Kim D, Park H, Cho D-W. Effects of Autogenous Gas Tungsten Arc Welding (GTAW) on Corrosion Resistance of Stainless Steel 316L. Processes. 2024; 12(8):1757. https://doi.org/10.3390/pr12081757
Chicago/Turabian StyleSong, Inyoung, Gwang-Ho Jeong, Sang-Kyo Kim, Yun-Hwan Kim, Anthony B. Murphy, Tae-Kook Park, Ducklae Kim, Hyunwoo Park, and Dae-Won Cho. 2024. "Effects of Autogenous Gas Tungsten Arc Welding (GTAW) on Corrosion Resistance of Stainless Steel 316L" Processes 12, no. 8: 1757. https://doi.org/10.3390/pr12081757






