A Study on the Influence of Oxy-Hydrogen Gas Flame on the Combustion Stability of Coal Powder and Nitrogen Oxide Emissions
Abstract
:1. Introduction
2. Numerical Simulation
2.1. Geometric Structure
2.2. Simulation Method
2.3. Model Validation
2.4. Simulation Conditions
3. Results and Discussion
3.1. Temperature Distribution
3.2. Gas Composition Distribution
3.3. NO Emission Characteristics
4. Conclusions
- (1)
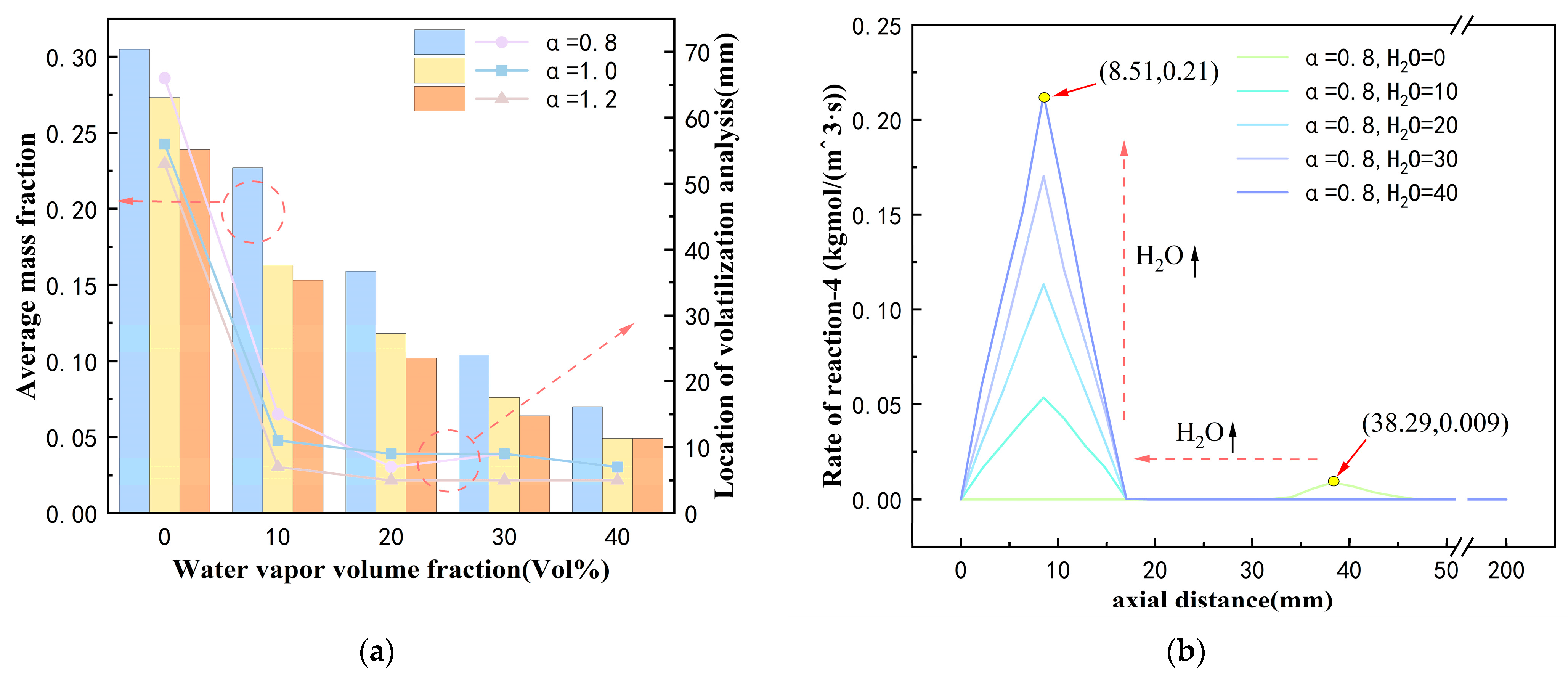
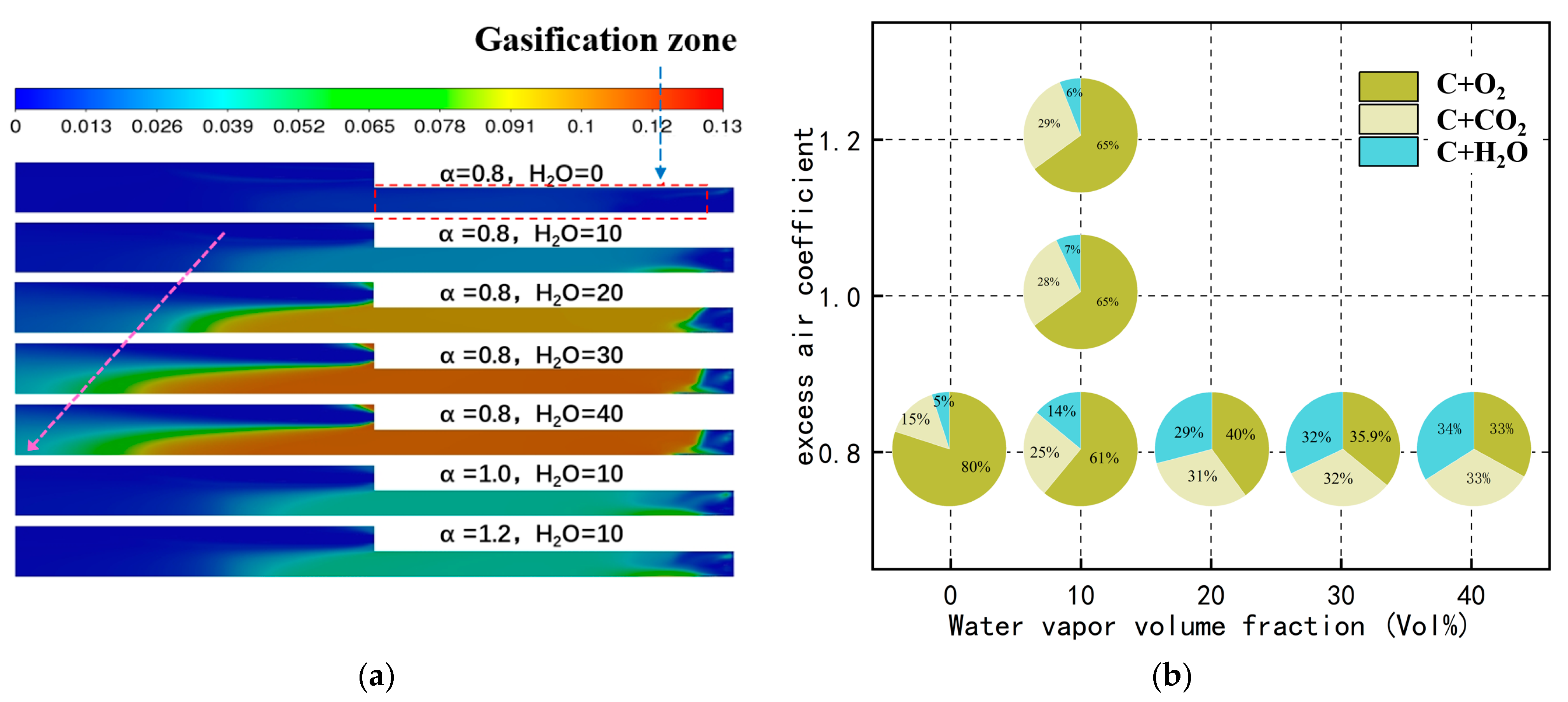
- Without preheating the coal powder flow, the flames and high-temperature steam generated by oxy-hydrogen gas combustion facilitate the rapid release of volatile components from the coal powder. At an oxy-hydrogen gas flow rate of 0.022 kg/h, the combustion chamber temperature rises to 641 K, creating favorable conditions for the rapid release of volatiles. When the excess air coefficient is 0.8, increasing the steam volume fraction from 0% to 40% raises the average temperature in the combustion chamber from 801 K to 1459 K. This substantial temperature increase enhances the peak concentration of volatiles and shifts the release position of the peak concentration from 68 mm to 7 mm;
- (2)
- Under oxy-hydrogen gas ignition, the generation of a large amount of high-temperature steam significantly enhances the gasification reaction between coke and water vapor. With an excess air coefficient of 0.8, increasing the steam volume fraction from 0% to 40% raises the proportion of the reaction between coke and water vapor from 5% to 34%. Additionally, the average mass fraction of the gasification gas increases substantially from 0.006 to 0.12. The high concentration of gasification gas can either directly combust to achieve stable combustion or leverage its reducing properties to reduce NO emissions;
- (3)
- The high-temperature steam generated by oxy-hydrogen gas combustion facilitates the gasification reactions during coal powder combustion, resulting in the production of a substantial amount of highly reducing gasification gas. This reducing gas can directly reduce NO to N2, thereby lowering nitrogen oxide emissions. With an excess air coefficient of 0.8, increasing the water vapor volume fraction from 0% to 40% reduces NO emissions from 132 ppm to 86 ppm. Furthermore, as the water vapor volume fraction increases, the rate of reduction in NO emissions decreases from 15.38% to 5.49%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arjun, T.; Atul, K.; Muraleedharan, A.P.; Walton, P.A.; Bijinraj, P.; Raj, A.A. A review on analysis of HHO gas in IC engines. Mater. Today Proc. 2019, 11, 1117–1129. [Google Scholar] [CrossRef]
- Gad, M.S.; El-Fakharany, M.K.; Elsharkawy, E.A. Effect of HHO gas enrichment on performance and emissions of a diesel engine fueled by biodiesel blend with kerosene additive. Fuel 2020, 280, 118632. [Google Scholar] [CrossRef]
- Kenanoğlu, R.; Baltacioğlu, E. An experimental investigation on hydroxy (HHO) enriched ammonia as alternative fuel in gas turbine. Int. J. Hydrogen Energy 2021, 46, 29638–29648. [Google Scholar] [CrossRef]
- Gu, X.; Cheng, M.; Zhang, X.; Zeng, Y. The pollutant discharge improvement by introducing HHO gas into biomass boiler. Int. J. Hydrogen Energy 2021, 46, 23292–23300. [Google Scholar] [CrossRef]
- Demir, U.; Çelebi, S.; Özer, S. Experimental investigation of the effect of fuel oil, graphene and HHO gas addition to diesel fuel on engine performance and exhaust emissions in a diesel engine. Int. J. Hydrogen Energy 2024, 52, 1434–1446. [Google Scholar] [CrossRef]
- Liu, Y.F. Experimental Study on Effect of Oxy-Hydrogen Gas HHO Supply on Performance of Heavy-Duty Diesel Engine. Master’s Thesis, Jilin University, Changchun, China, 2019. [Google Scholar]
- Wang, X. Experimental Study on the Influence of Oxy-Hydrogen Gas Supply on Gasoline Engine Performance. Master’s thesis, Jilin University, Changchun, China, 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Zhu, Y.; Kang, X.; Hou, B.; Zhou, H. Combustion stability and emission characteristics of a pulverized coal furnace operating at ultra-low loads assisted by clean gas (HHO) from water electrolysis. Fuel 2024, 370, 131811. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Yu, X.; Guo, Z.; Li, M.; Wang, T. Effect of brown gas (HHO) addition on combustion and emission in gasoline engine with exhaust gas recirculation (EGR) and gasoline direct injection. J. Clean. Prod. 2022, 360, 132078. [Google Scholar] [CrossRef]
- Pratt, D.T.; Smoot, L.; Pratt, D. Pulverized Coal Combustion and Gasification; Springer: Berlin, Germany, 1979; Volume 1007, p. 978-1. Available online: https://link.springer.com/book/10.1007/978-1-4757-1696-2 (accessed on 16 August 2024).
- Howard, J.B.; Williams, G.C.; Fine, D.H. Kinetics of carbon monoxide oxidation in postflame gases. Symp. (Int.) Combust. 1973, 14, 975–986. [Google Scholar] [CrossRef]
- Field, M.A. Measurements of the effect of rank on combustion rates of pulverized coal. Combust. Flame 1970, 14, 237–248. [Google Scholar] [CrossRef]
- Hautman, D.J.; Dryer, F.L.; Schug, K.P.; Glassman, I. A multiple-step overall kinetic mechanism for the oxidation of hydrocarbons. Combust. Sci. Technol. 1981, 25, 219–235. [Google Scholar] [CrossRef]
- Jones, W.P.; Lindstedt, R.P. Global reaction schemes for hydrocarbon combustion. Combust. Flame 1988, 73, 233–249. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, H.; Xu, Y.; Yang, S.; Pan, H.; Xiao, W.; Fu, Y.; Xue, Z. Experimental investigation on oxy-hydrogen gas flame injecting coal powder gasification and combustion. Processes 2024, 12, 692. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Zha, X.; Gao, G.; Li, X.; Wu, F.; Luo, C.; Zhang, L. Internal association between combustion behavior and NOx emissions of pulverized coal MILD-oxy combustion affected by adding H2O. Energy 2023, 263, 125878. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Wu, J.; Wang, Z.; Wang, M.; Song, Z. Effect of H2O on char-nitrogen conversion during char-O2/H2O combustion under high-temperature entrained flow conditions. Combust. Flame 2019, 207, 391–405. [Google Scholar] [CrossRef]




| Project | Equation | Chemical Reaction | A | Ea/(kJ/mol) | Cite |
|---|---|---|---|---|---|
| Carbon surface reaction | (1) | C(s) + 0.5O2 → CO | 2.3 | 9.23E+07 | [10] |
| (2) | C(s) + CO2 → 2CO | 4.4 | 1.62E+08 | [10] | |
| (3) | C(s) + H2O → CO+H2 | 1.33 | 1.47E+08 | [10] | |
| Volume combustion reaction | (4) | Vol + 1.471O2 → 1.38CO + 2.069H2O + 0.041N2 | 2.119E+11 | 2.027E+08 | Fluent |
| (5) | CO + 0.5O2 → CO2 | 1.3E+11 | 1.26E+08 | [11] | |
| (6) | H2 + 0.5O2 → H2O | 3.9E+17 | 1.7E+08 | [12] | |
| (7) | CO + H2O → CO2 + H2 | 2.75E+09 | 8.4E+07 | [13] | |
| (8) | CO2 + H2 → CO + H2O | 6.81E+10 | 1.14E+08 | [14] |
| Industrial Analysis/(%) | Elemental Analysis/(%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Vad | Aad | FCad | Cdaf | Hdaf | Ndaf | Odaf | Sdaf |
| 1.10 | 31.69 | 11.77 | 55.44 | 85.77 | 5.43 | 1.38 | 5.22 | 2.21 |
| Condition | Excess Air Coefficient | Water Vapor Volume Fraction/% | Mass Flow Rate of Primary Air/(kg/h) | Mass Flow Rate of Secondary Air/(kg/h) |
|---|---|---|---|---|
| 1 | 0.8 | 0 | 0.034 | 0.136 |
| 2 | 0.8 | 10 | 0.034 | 0.136 |
| 3 | 0.8 | 20 | 0.034 | 0.136 |
| 4 | 0.8 | 30 | 0.034 | 0.136 |
| 5 | 0.8 | 40 | 0.034 | 0.136 |
| 6 | 1.0 | 0 | 0.042 | 0.169 |
| 7 | 1.0 | 10 | 0.042 | 0.169 |
| 8 | 1.0 | 20 | 0.042 | 0.169 |
| 9 | 1.0 | 30 | 0.042 | 0.169 |
| 10 | 1.0 | 40 | 0.042 | 0.169 |
| 11 | 1.2 | 0 | 0.051 | 0.203 |
| 12 | 1.2 | 10 | 0.051 | 0.203 |
| 13 | 1.2 | 20 | 0.051 | 0.203 |
| 14 | 1.2 | 30 | 0.051 | 0.203 |
| 15 | 1.2 | 40 | 0.051 | 0.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Cui, J.; Pan, H.; Zhao, H.; Yang, S.; Xue, Z.; Fu, Y.; Xu, Y. A Study on the Influence of Oxy-Hydrogen Gas Flame on the Combustion Stability of Coal Powder and Nitrogen Oxide Emissions. Processes 2024, 12, 1777. https://doi.org/10.3390/pr12081777
Xiao W, Cui J, Pan H, Zhao H, Yang S, Xue Z, Fu Y, Xu Y. A Study on the Influence of Oxy-Hydrogen Gas Flame on the Combustion Stability of Coal Powder and Nitrogen Oxide Emissions. Processes. 2024; 12(8):1777. https://doi.org/10.3390/pr12081777
Chicago/Turabian StyleXiao, Wenke, Jie Cui, Honggang Pan, Honglei Zhao, Shuo Yang, Zhijia Xue, Yudong Fu, and Youning Xu. 2024. "A Study on the Influence of Oxy-Hydrogen Gas Flame on the Combustion Stability of Coal Powder and Nitrogen Oxide Emissions" Processes 12, no. 8: 1777. https://doi.org/10.3390/pr12081777






