Acid-Etched Fracture Conductivity with In Situ-Generated Acid in Ultra-Deep, High-Temperature Carbonate Reservoirs
Abstract
:1. Introduction
2. Acid-Etched Fracture Conductivity Experiment
2.1. Specimen Preparation
2.2. Experimental Equipment and Procedures
2.3. Experimental Schemes
- (1)
- Fracture types: Due to the natural fracture development in the ultra-deep reservoirs, two types of fractures are expected to form during acid fracturing: (i) primary acid-etched fractures, which extend from the wellbore and have a larger aperture and (ii) secondary acid-etched fractures, which are natural fractures with a small aperture and etched by the leak-off acid during the propagation of the primary fracture. This study simulates these fractures by varying the experimental fracture width and acid concentration at the fracture entrance.
- (2)
- Fracture width in the experiment: The fracture widths of the primary fracture and the secondary fracture are obtained through numerical simulation using a fracture propagation model. The input geological parameters and engineering parameters used in the simulation are the typical values of the Shunbei oil field in the Tarim basin, China [29]. Based on the numerical simulation results of complex fractures, the typical widths of primary and secondary fractures were set at 5 mm and 0.5 mm, respectively.
- (3)
- Experiment temperature: The experimental temperature was set to the typical temperature of 160 °C in the Yijianfang formation. After the experiment temperature reached the target value, it was maintained for 3 h to ensure thorough heating of the acid fluid and rock slabs.
- (4)
- Acid concentration at the secondary fracture entrance: Given the strong uncertainty and randomness in the distribution of natural fractures, the concentration of acid at the entrance to the secondary fractures (intersection of primary and secondary fractures) is difficult to predict. In the experiment, the acid concentration at the entrance to the secondary fractures was set to half of that at the entrance to the primary fractures.
- (5)
- Acid injection rate: The injection rate is determined according to the flow regime similarity [19]. The Reynold’s number of the acid flowing in the fracture in the field condition can be calculated through Equation (2):
- (6)
- Acid–rock contact time (injection time) and injection rate: Based on typical acid–rock contact times during field operations, the acid–rock contact times in the experiment were set to range from 20 to 90 min.
3. Experimental Results and Analysis
3.1. Variation Pattern of Acid-Etched Fracture Conductivity in Primary Fractures with In Situ-Generated Acid
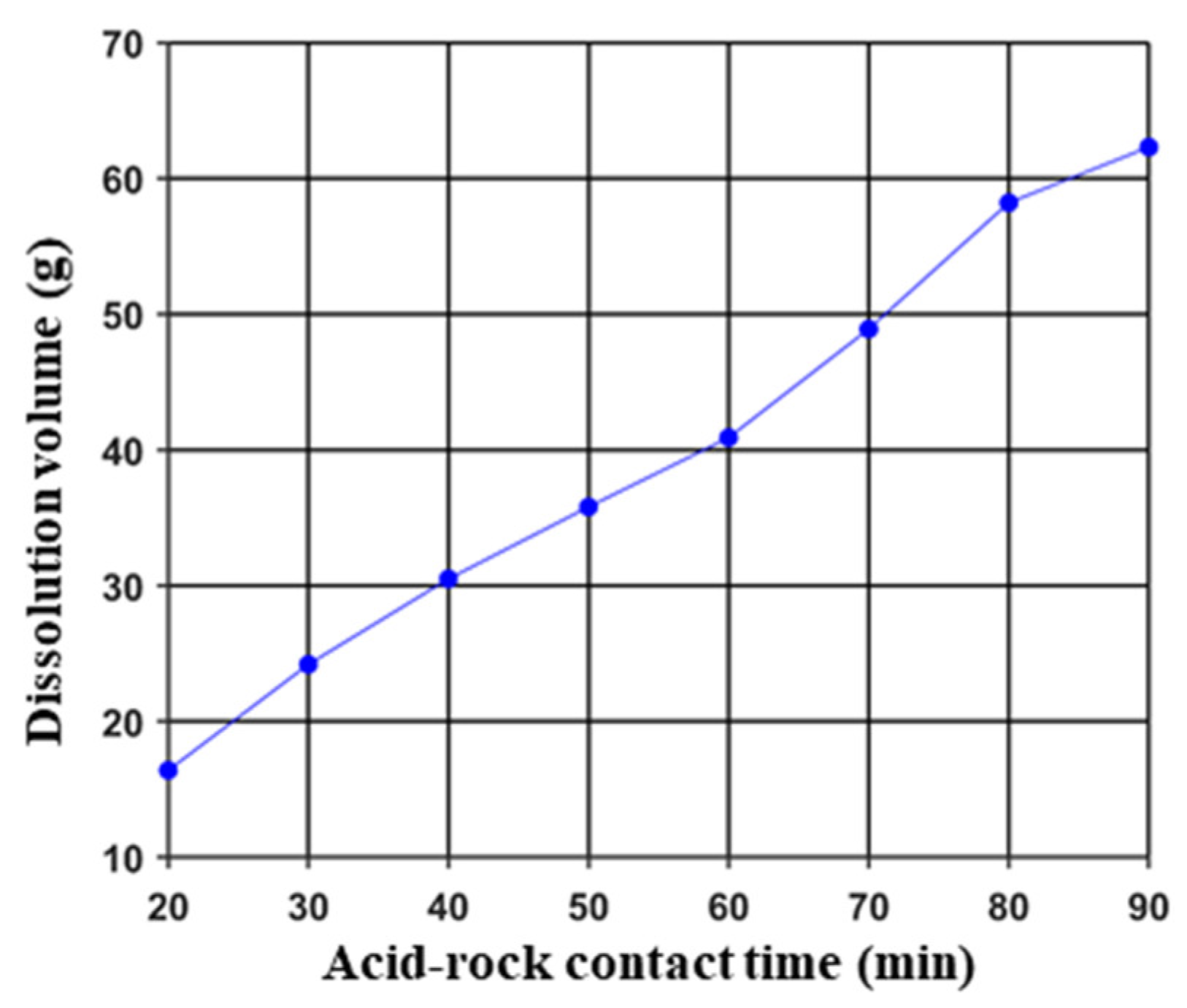
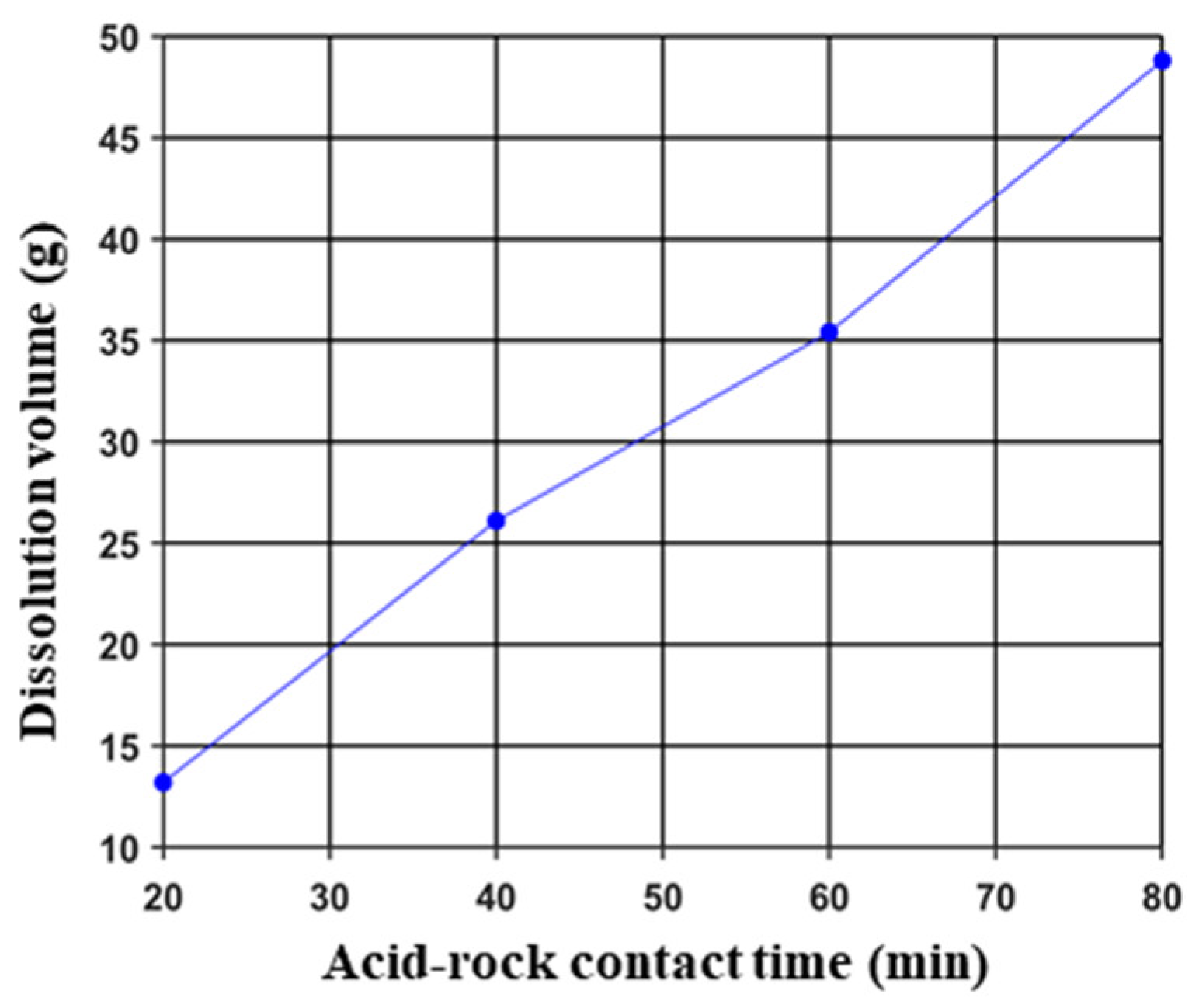
3.1.1. Acid-Etched Surface Morphology and Dissolution Volume
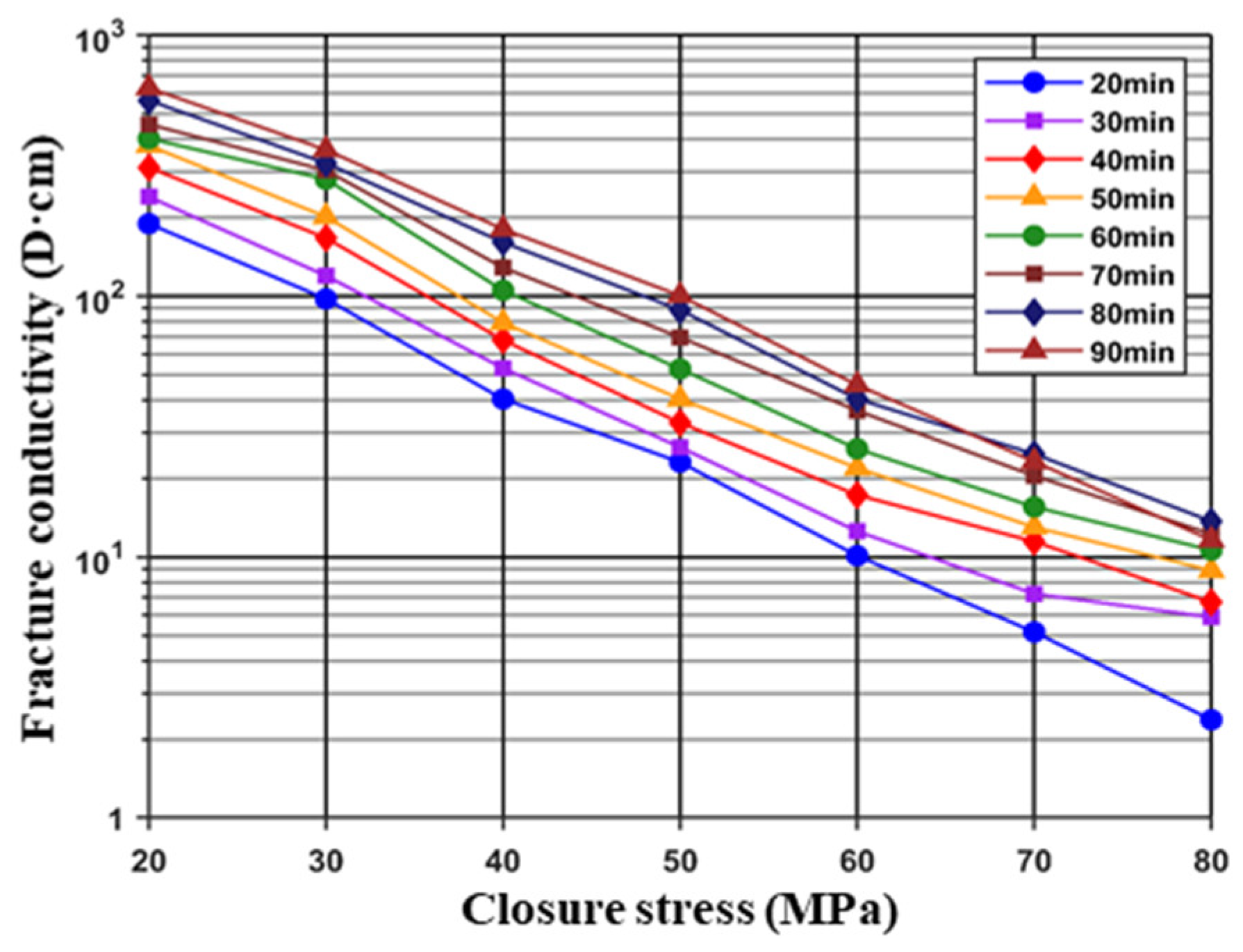
3.1.2. Acid-Etched Fracture Conductivity
3.2. Variation Pattern of Acid-Etched Fracture Conductivity in Secondary Fractures with In Situ-Generated Acid
3.2.1. Acid-Etched Surface Morphology and Dissolution Volume
3.2.2. Acid-Etched Fracture Conductivity
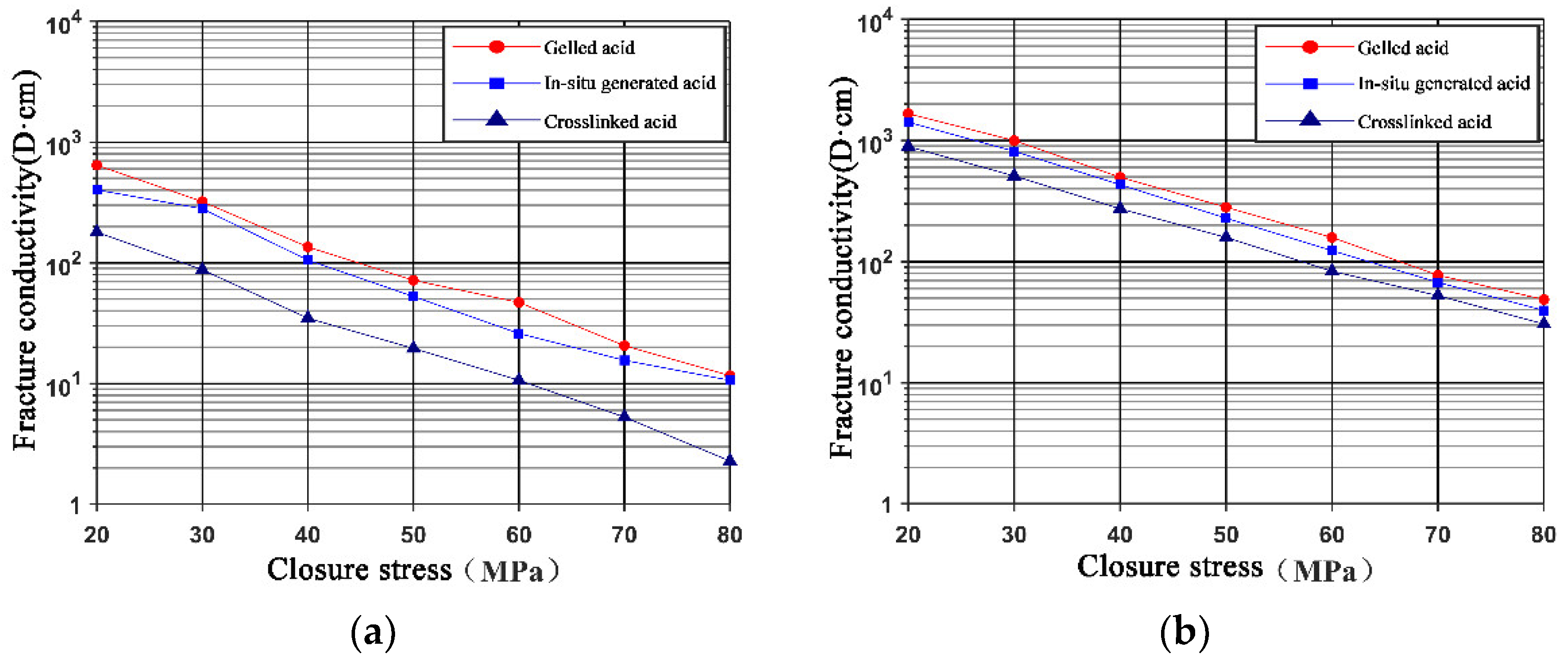
3.3. Comparison of Fracture Conductivity between In Situ-Generated Acid and Conventional Acid Systems
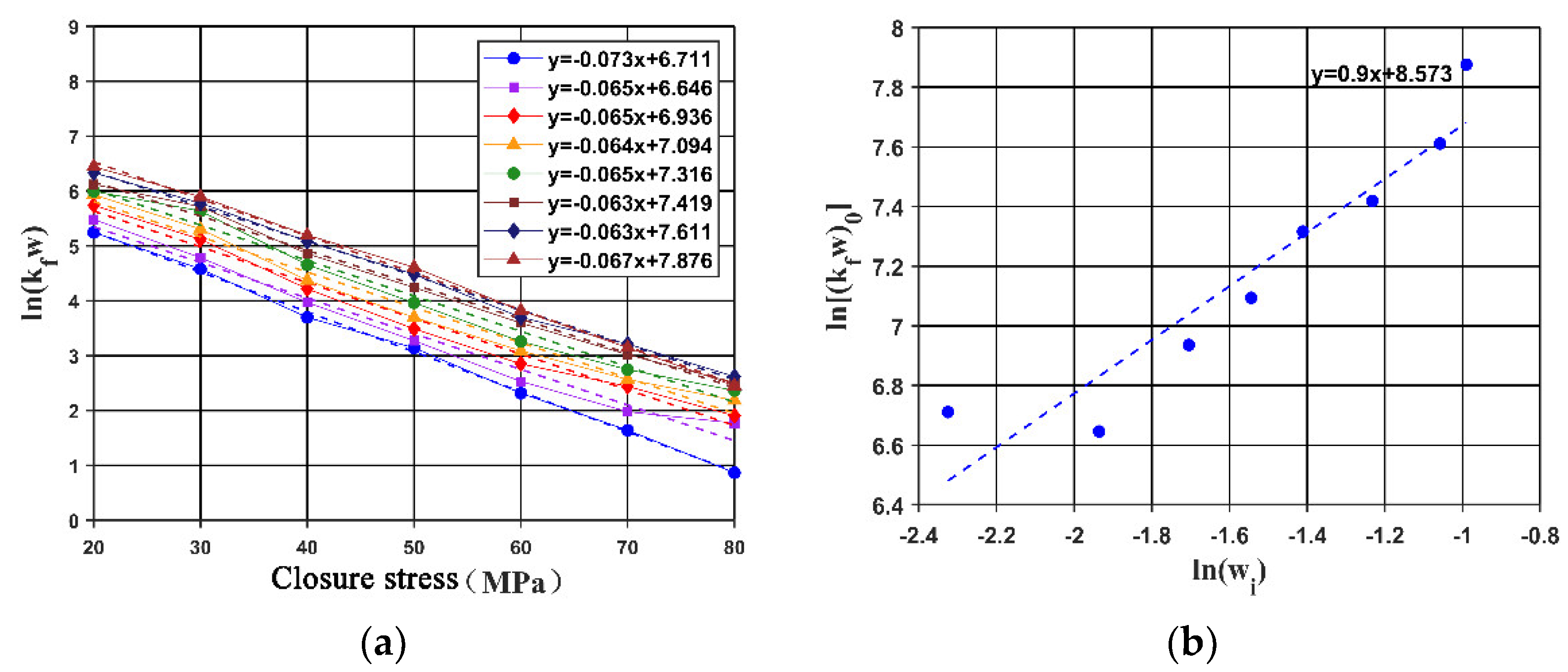
4. Empirical Model of Acid-Etched Fracture Conductivity with In Situ-Generated Acid
5. Conclusions
- (1)
- After in situ-generated acid etching, the surface morphologies of the primary and secondary fractures showed significant differences. The primary fracture surface presented a dense particulate pattern, while the secondary fracture surface exhibited distinct grooves. Due to grooves formed on the secondary fracture surfaces, the secondary fractures exhibited higher conductivity than the primary fractures.
- (2)
- The conductivity of acid-etched fractures increased with the acid–rock contact time. However, when the acid–rock contact time exceeded approximately 70 min, the rate of increase in conductivity slowed down, which suggests that a minimum contact time of 70 min should be achieved during field operation.
- (3)
- The conductivity of both primary and secondary fractures treated with in situ-generated acid was lower than that treated with gelled acid but higher than with crosslinked acid. A combination of gelled acid and in situ-generated acid is conducive to a deep penetration distance and high fracture conductivity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, F.; Xu, Y.; Zhang, A.; Lv, J. Research and application of autogenic acid deep penetration acid fracturing technology for ultra-deep high temperature carbonate rocks. Drill. Prod. Technol. 2018, 41, 3+35–37. [Google Scholar]
- Liu, Y.; Zhang, Y.; Wang, D.; Xiong, Y. Self-generating hydrochloric acid system for carbonate reservoir. Pet. Sci. Technol. Forum 2015, 34, 165–167+196. [Google Scholar]
- Luo, J.; Cao, C.; Hu, W. Self-generating acid system suitable for Tahe oilfield. Petrochem. Ind. Technol. 2015, 22, 199+207. [Google Scholar]
- Yang, R. The Study of Gelled Self-Generating Acid System in High Temperature Carbonate Reservoir Acidification. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2015. [Google Scholar]
- Fang, Y.; Zhang, Y.; Yang, F.; Hou, F. Performance evaluation of a high temperature in-situ self-generating acid system. Oilfield Chem. 2014, 31, 191–194+198. [Google Scholar]
- Ye, J.; Yang, J.; Geng, S.; Li, Y. Development and evaluation of a new type of self-generated acid fracturing fluid. Petrochem. Ind. Appl. 2019, 38, 8–13. [Google Scholar]
- Li, Q.; Wang, Y.; Wang, F. Effect of thickener and reservoir parameters on the filtration property of CO2 fracturing fluid. Energy Sources 2020, 42, 1705–1715. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Cheng, Y.; Li, Q.; Wang, F.; Wei, J. Numerical simulation of fracture reorientation during hydraulic fracturing in perforated horizontal well in shale reservoirs. Energy Sources 2018, 40, 1807–1813. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Peng, H. Experimental study on the factors affecting the conductivity of dolomite acid etching fractures in eastern sichuan carboniferous. Drill. Prod. Technol. 2003, 3, 3+45–47. [Google Scholar]
- Liu, L.; Fu, Z. Acid-etched fracture conductivity of carbonate reservoir of Tazhong 1 gas field. J. Chengde Pet. Coll. 2010, 12, 1–4+28. [Google Scholar]
- Duan, M. Experimental test on flow conductivity of acid-etching fracture for carbonate rock. Nat. Gas Explor. Dev. 2013, 36, 61–63+74+87. [Google Scholar]
- Li, N.; Zhao, L.; Zhang, Q.; Ren, X.; Liu, P. Study on the conductivity of acid corrosion cracks during acid fracturing. Oil Drill. Prod. Technol. 2008, 6, 59–62+168. [Google Scholar]
- Liu, Y.; Wang, L.; Xiong, Y.; Wu, W.; Zhang, Y. Study on authigenic acid system of high temperature carbonate rocks. Chem. Eng. Oil Gas 2011, 40, 325+367–369. [Google Scholar]
- Abass, H.H.; Al-Mulhem, A.A.; Alqam, M.H.; Khan, M.R. Acid fracturing or proppant fracturing in carbonate formation? A rock mechanics view. In Proceedings of the SPE Annual Technical Conference and Exhibition? San Antonio, TX, USA, 24–27 September 2006; p. 102590-MS. [Google Scholar]
- Wang, M.; Wu, Y.; Sun, H.; Chen, P.; Jia, X. Influence of acid on the conductivity of acid corrosion fracture. Spec. Oil Gas Reserv. 2019, 26, 153–158. [Google Scholar]
- Nasr-El-Din, H.A.; Al-Driweesh, S.M.; Metcalf, A.S.; Chesson, J.B. Fracture Acidizing: What Role Does Formation Softening Play in Production Response? Soc. Pet. Eng. 2008, 23, 103344-PA. [Google Scholar] [CrossRef]
- Li, L.; Zhang, R.; Li, P.; Yang, Z. An experimental study on the RD self-generating acid performance in acid-fracturing. Appl. Chem. Ind. 2014, 43, 9–12. [Google Scholar]
- Hao, W.; Yin, X.; Huang, W.; Wu, X.; Che, X. Experimental study on evaluation of authigenic acid system. Petrochem. Ind. Appl. 2020, 39, 96–101. [Google Scholar]
- Gou, B.; Guan, C.; Li, X. Acid-etching fracture morphology and conductivity for alternate stages of self-generating acid and gelled acid during acid-fracturing. J. Pet. Sci. Eng. 2021, 200, 108358. [Google Scholar] [CrossRef]
- Kamali, A.; Pournik, M. Fracture closure and conductivity decline modeling–Application in unpropped and acid etched fractures. J. Unconv. Oil Gas Resour. 2016, 14, 44–55. [Google Scholar] [CrossRef]
- Nierode, D.E.; Kruk, K.F. An evaluation of acid fluid loss additives, retarded acids, and acidized fracture conductivity. In Proceedings of the SPE Annual Technical Conference and Exhibition? Las Vegas, NV, USA, 30 September–3 October 1973; p. 4549. [Google Scholar]
- Walsh, J.B. Effect of pore pressure and confining pressure on fracture permeability. Int. J. Rock Mech. Min. Sci. 1981, 18, 429–435. [Google Scholar] [CrossRef]
- Mou, J. Modeling Acid Transport and Non-Uniform Etching in a Stochastic Domain in Acid Fracturing. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2009. [Google Scholar]
- Pan, B.; Chen, Y.; Yang, D.; Liu, X.; Li, H. Study of authigenic acid for Chuandongbei high temperature carbonate reservoir. Guangdong Chem. Ind. 2017, 41, 45–46. [Google Scholar]
- Qu, X.; Ti, Y.; Cui, H.; Wang, C.; Yang, X. Preparation and performance evaluation of a polybasic organic authigenic acid. Contemp. Chem. Ind. 2023, 52, 1895–1898+1953. [Google Scholar]
- Gao, Y.; Lian, S.; Shi, Y.; Yang, X.; Zhou, F.; Xiong, C.; Li, F.; Han, X.; Zhang, N. A new acid fracturing fluid system for high temperature deep well carbonate reservoir. In Proceedings of the SPE Asia Pacific Hydraulic Fracturing Conference, Beijing, China, 24–26 August 2016; p. 181823-MS. [Google Scholar]
- Jia, G.; Jiang, X.; Li, Y. Research and application of self-generating acid fracturing technology in Tahe Oilfield. Complex Hydrocarb. Reserv. 2017, 10, 73–75. [Google Scholar]
- Xia, X.; Mou, J.; Zhang, S.; Li, Y. Study on the conductivity of acid etched fractures in mixed-lithologic reservoir of Daniudi gas field. Fault-Block Oil Gas Field 2024, 5, 125–133. [Google Scholar]
- Guo, T.; Lv, M.; Luo, P.; Yang, X.; Che, M.; Weng, D.; Qu, Z.; Liu, Y. Assessment and optimization of fracture-karst cave connectivity in horizontal well hydraulic fracturing of carbonate reservoirs. Rock Mech. Bull. 2024, 3, 100112. [Google Scholar] [CrossRef]
- Mou, J.; Zhu, D.; Hill, A.D. Acid-etched channels in heterogeneous carbonates—A newly discovered mechanism for creating acid-fracture conductivity. SPE J. 2009, 15, SPE-119619-PA. [Google Scholar]
- Malagon, C.; Pournik, M.; Hill, A.D. The texture of acidized fracture surfaces—Implications for acid fracture conductivity. In Proceedings of the Presented at the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2006; p. SPE-102167-MS. [Google Scholar]
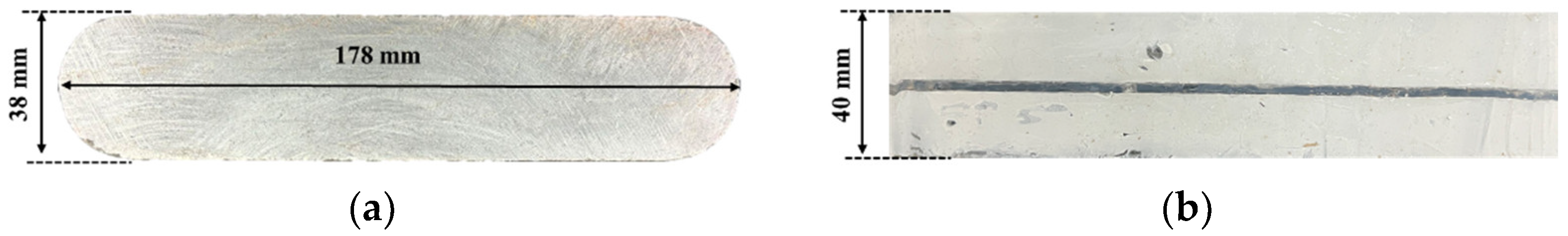
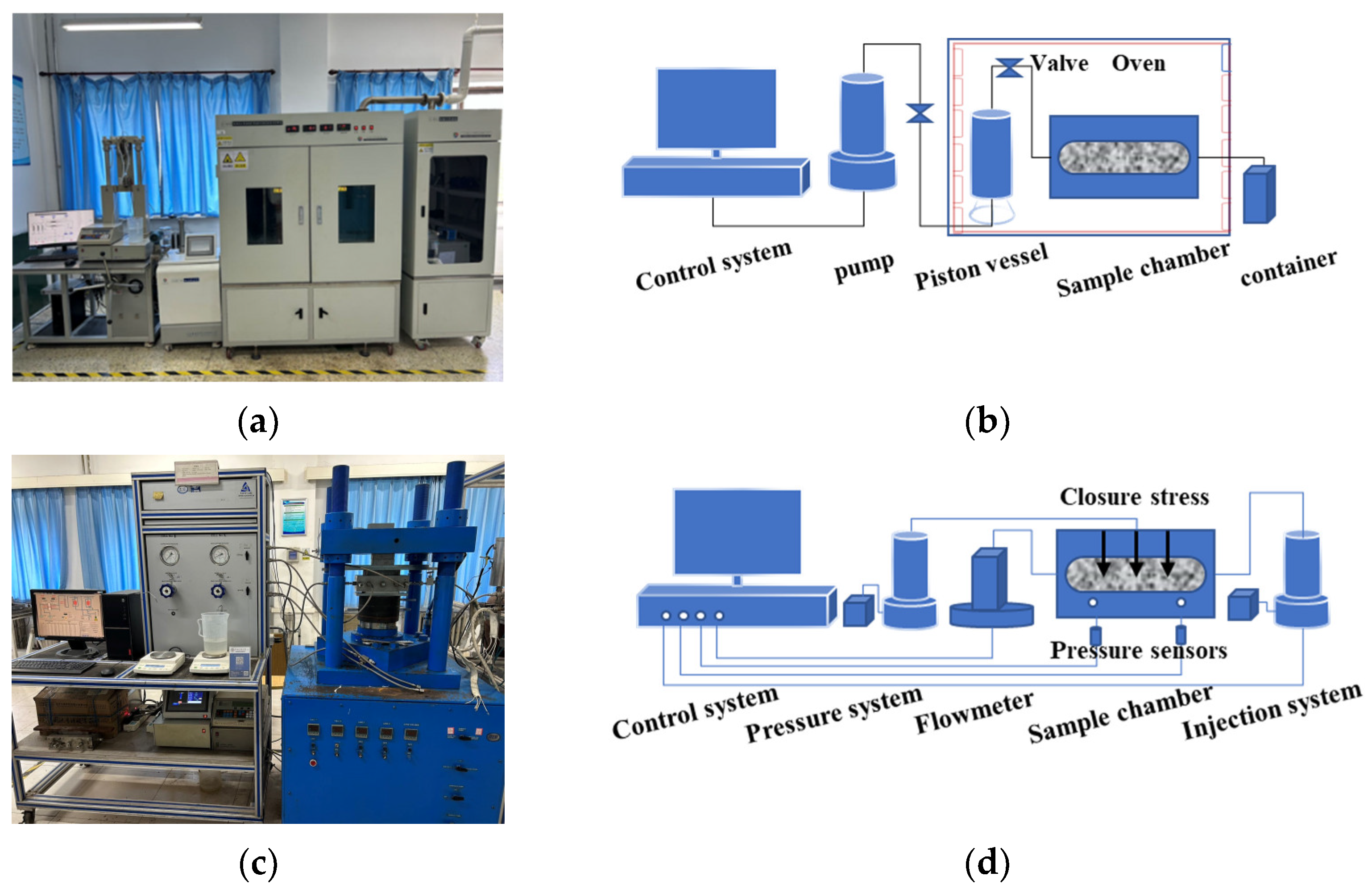




| Sample Size (mm) | Permeability (mD) | Density (g/cm3) | Mineralogy (%) | |||
|---|---|---|---|---|---|---|
| Calcite | Dolomite | Quartz | Feldspar | |||
| 178 × 38 × 20 | 0.01 | 2.710 | 95.3 | 1.6 | 2.5 | 0.6 |
| No. | Acid Type | Injection Time (min) | Acid Concentration (%) | Fracture Type |
|---|---|---|---|---|
| 1# | in situ-generated acid | 20 | 20 | primary fractures |
| 2# | 30 | |||
| 3# | 40 | |||
| 4# | 50 | |||
| 5# | 60 | |||
| 6# | 70 | |||
| 7# | 80 | |||
| 8# | 90 | |||
| 9# | 20 | 10 | secondary fractures | |
| 10# | 40 | |||
| 11# | 60 | |||
| 12# | 80 | |||
| 13# | gelled acid | 60 | 20 | primary fractures |
| 14# | 10 | secondary fractures | ||
| 15# | crosslinked acid | 20 | primary fractures | |
| 16# | 10 | secondary fractures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Pu, H.; Li, J.; Wang, J.; Chen, X.; Mou, J.; Gao, B. Acid-Etched Fracture Conductivity with In Situ-Generated Acid in Ultra-Deep, High-Temperature Carbonate Reservoirs. Processes 2024, 12, 1792. https://doi.org/10.3390/pr12091792
Jia H, Pu H, Li J, Wang J, Chen X, Mou J, Gao B. Acid-Etched Fracture Conductivity with In Situ-Generated Acid in Ultra-Deep, High-Temperature Carbonate Reservoirs. Processes. 2024; 12(9):1792. https://doi.org/10.3390/pr12091792
Chicago/Turabian StyleJia, Haizheng, Hongyuan Pu, Jianmin Li, Junchao Wang, Xi Chen, Jianye Mou, and Budong Gao. 2024. "Acid-Etched Fracture Conductivity with In Situ-Generated Acid in Ultra-Deep, High-Temperature Carbonate Reservoirs" Processes 12, no. 9: 1792. https://doi.org/10.3390/pr12091792





