Recent Advances in the Photocatalytic Degradation of Phenol over Bi-Based Oxide Catalysts
Abstract
1. Introduction
2. Basics of Photocatalytic Degradation of Pollutants
3. Bi-Based Oxide Photocatalysts in Phenol Degradation
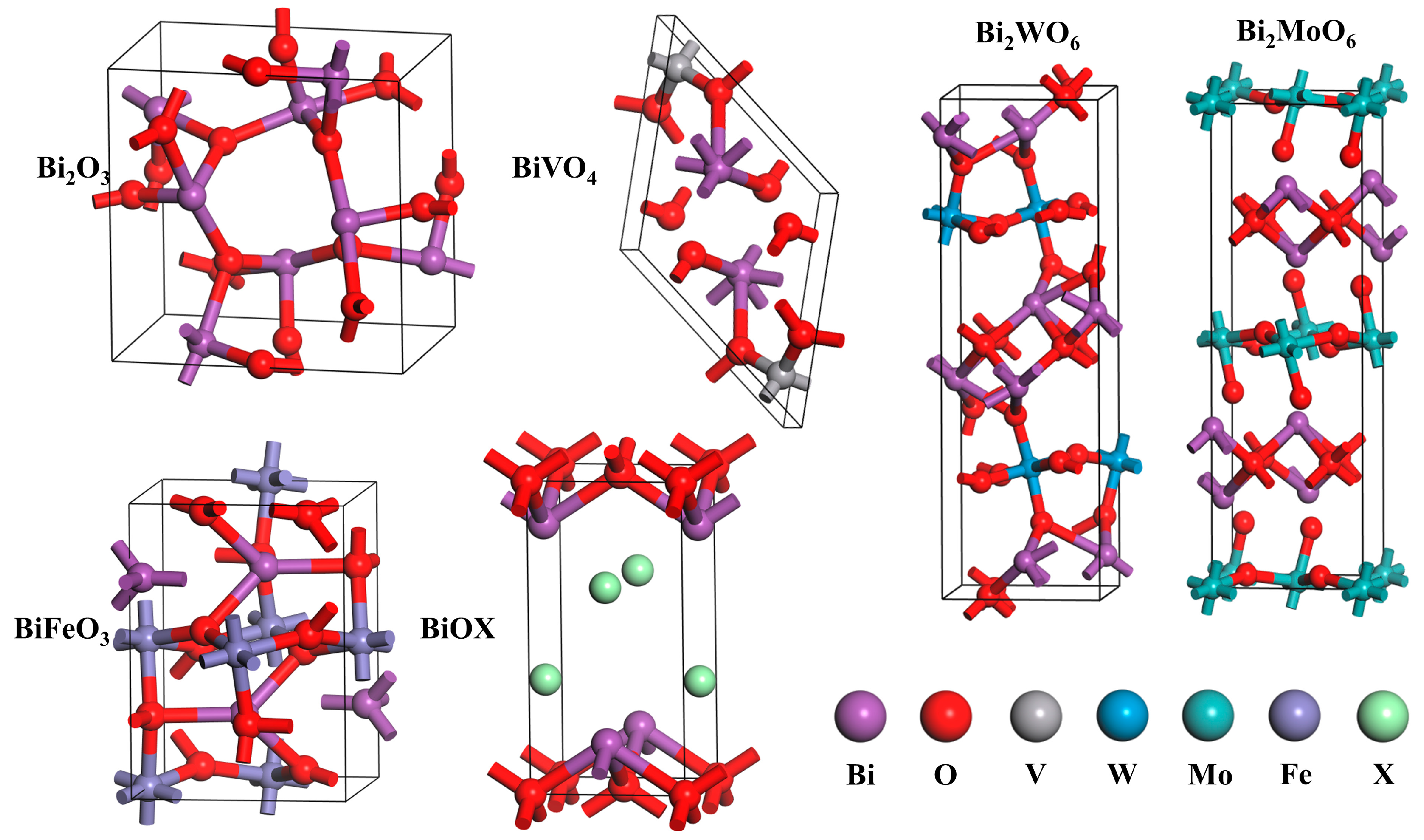
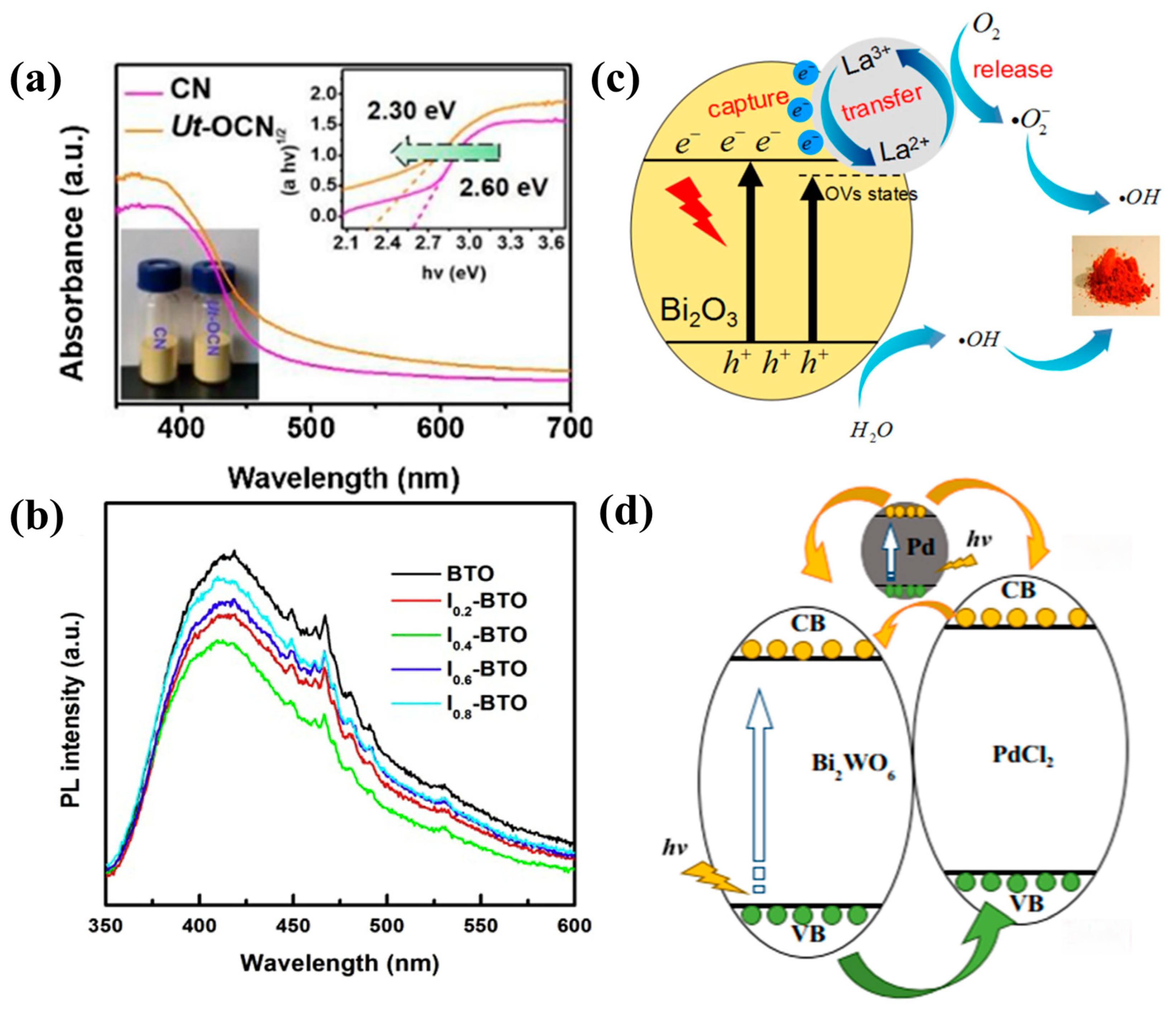
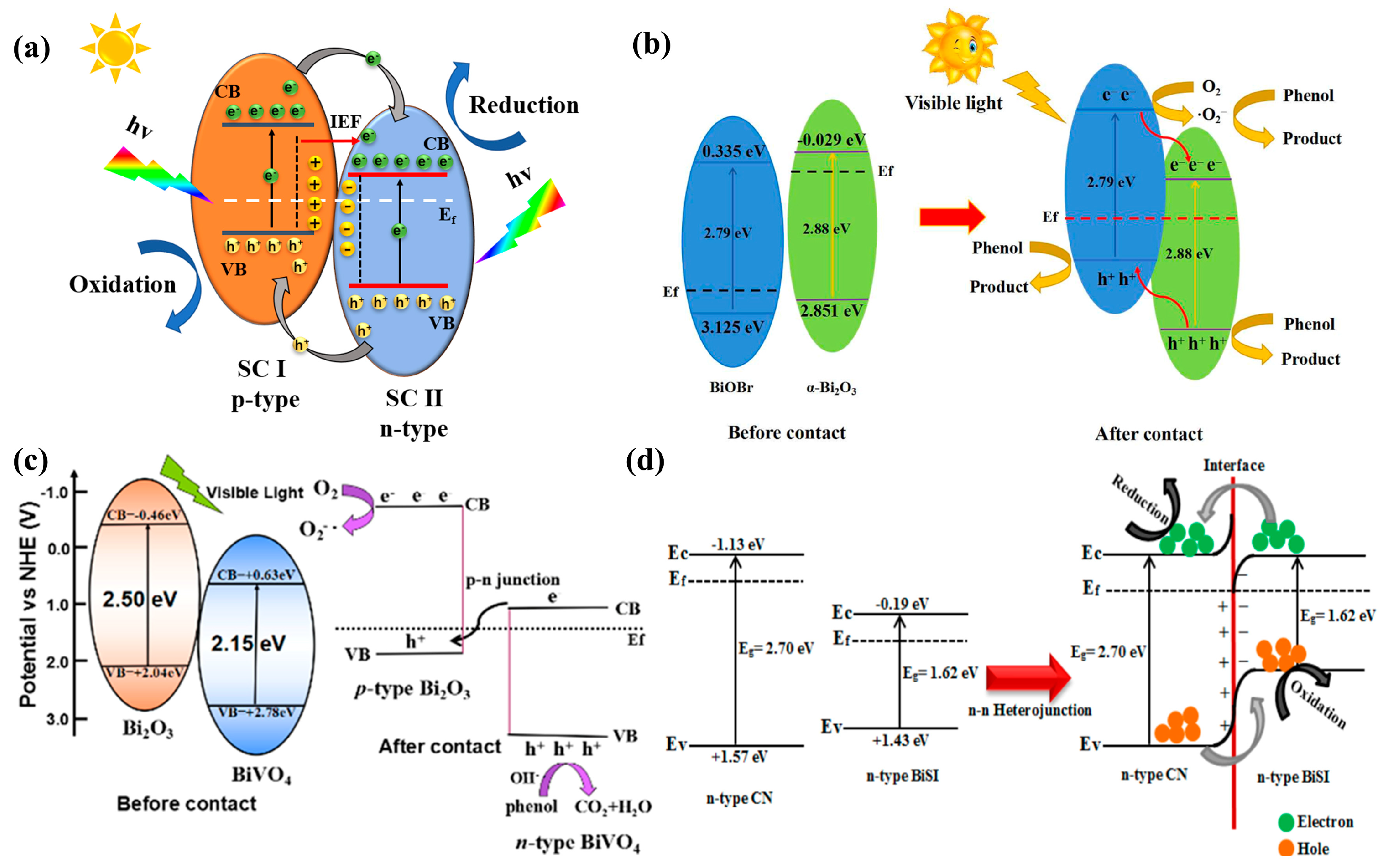
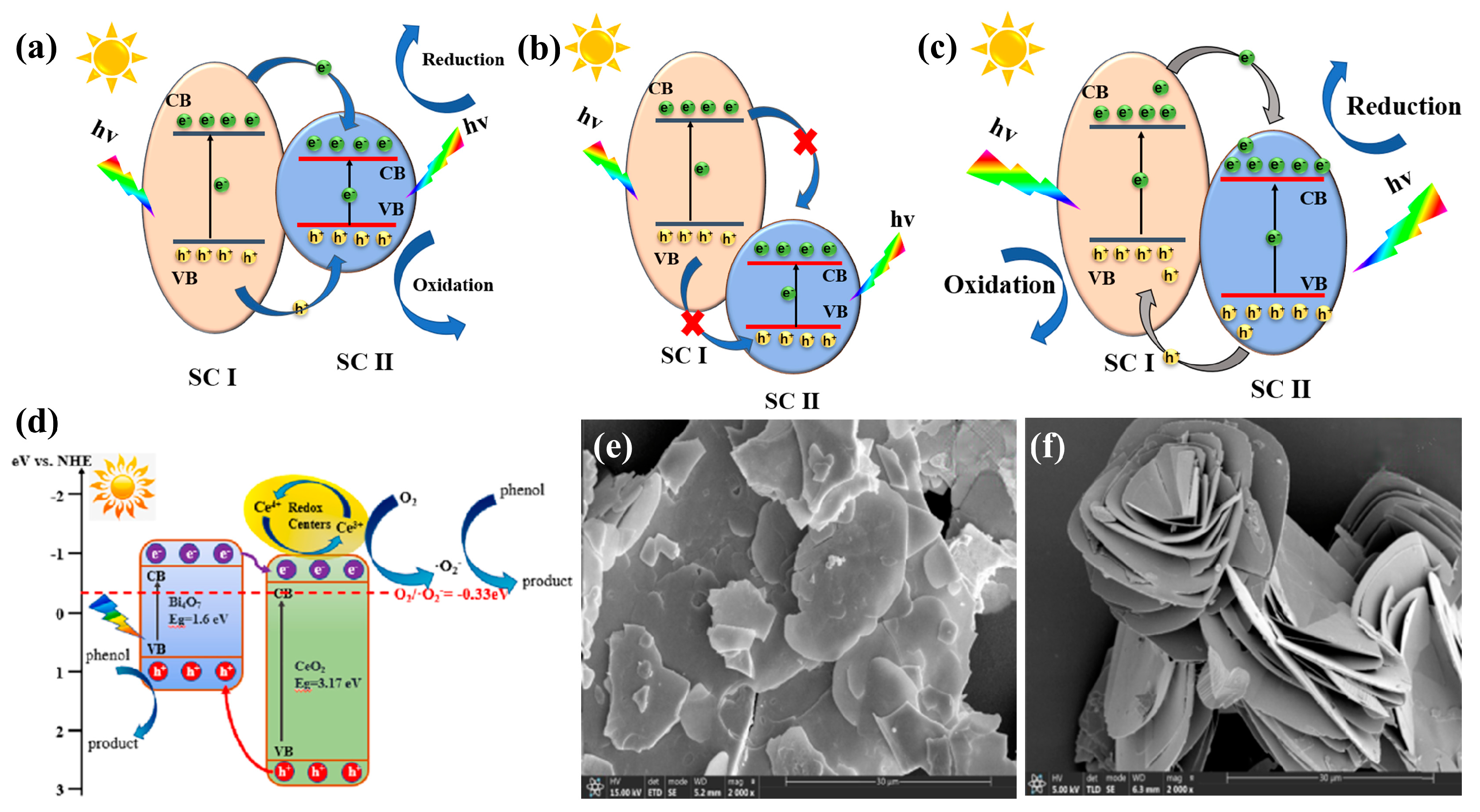
3.1. Bi2O3
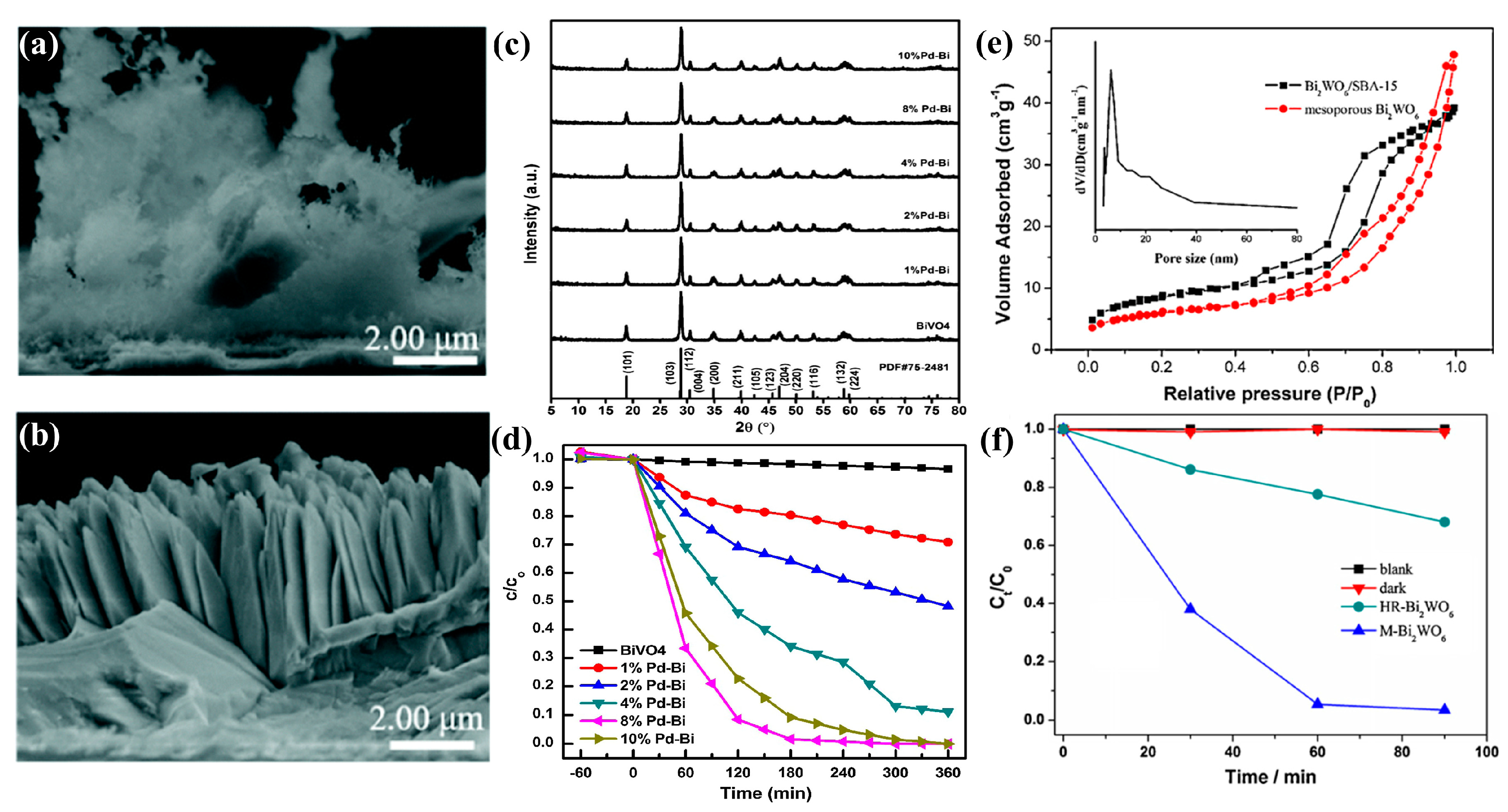
3.2. BiVO4
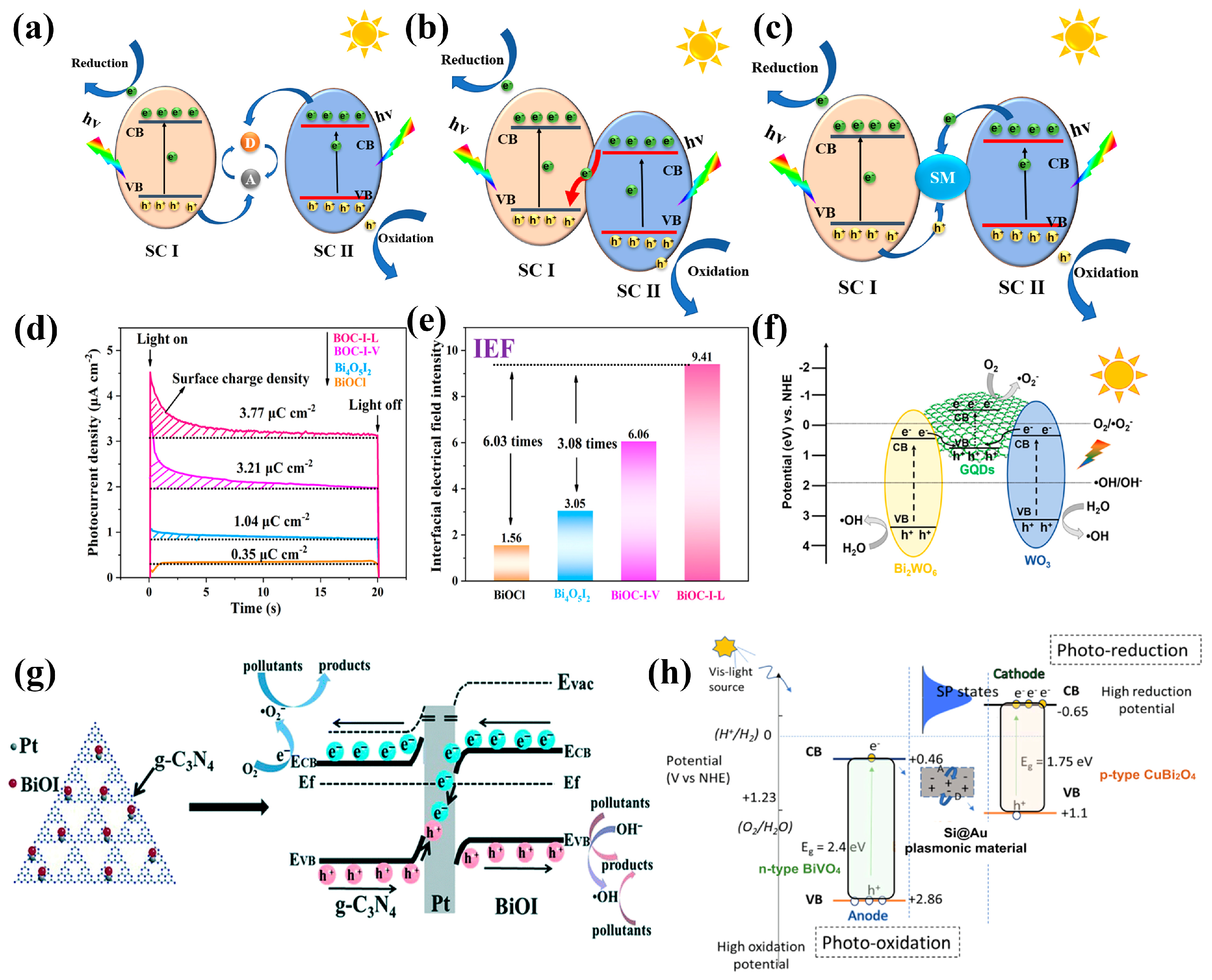
3.3. Bi2WO6
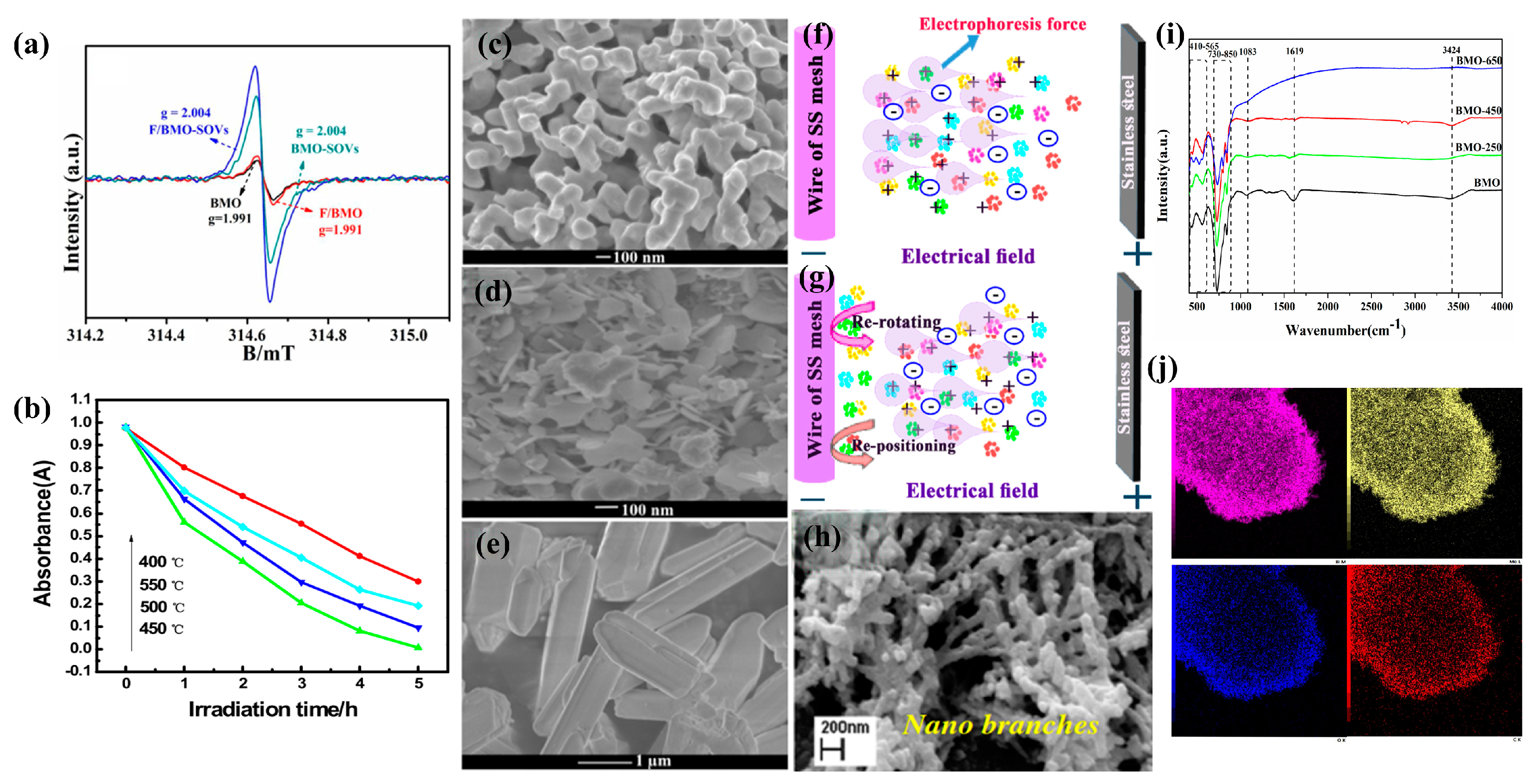
3.4. Bi2MoO6
3.5. BiFeO3
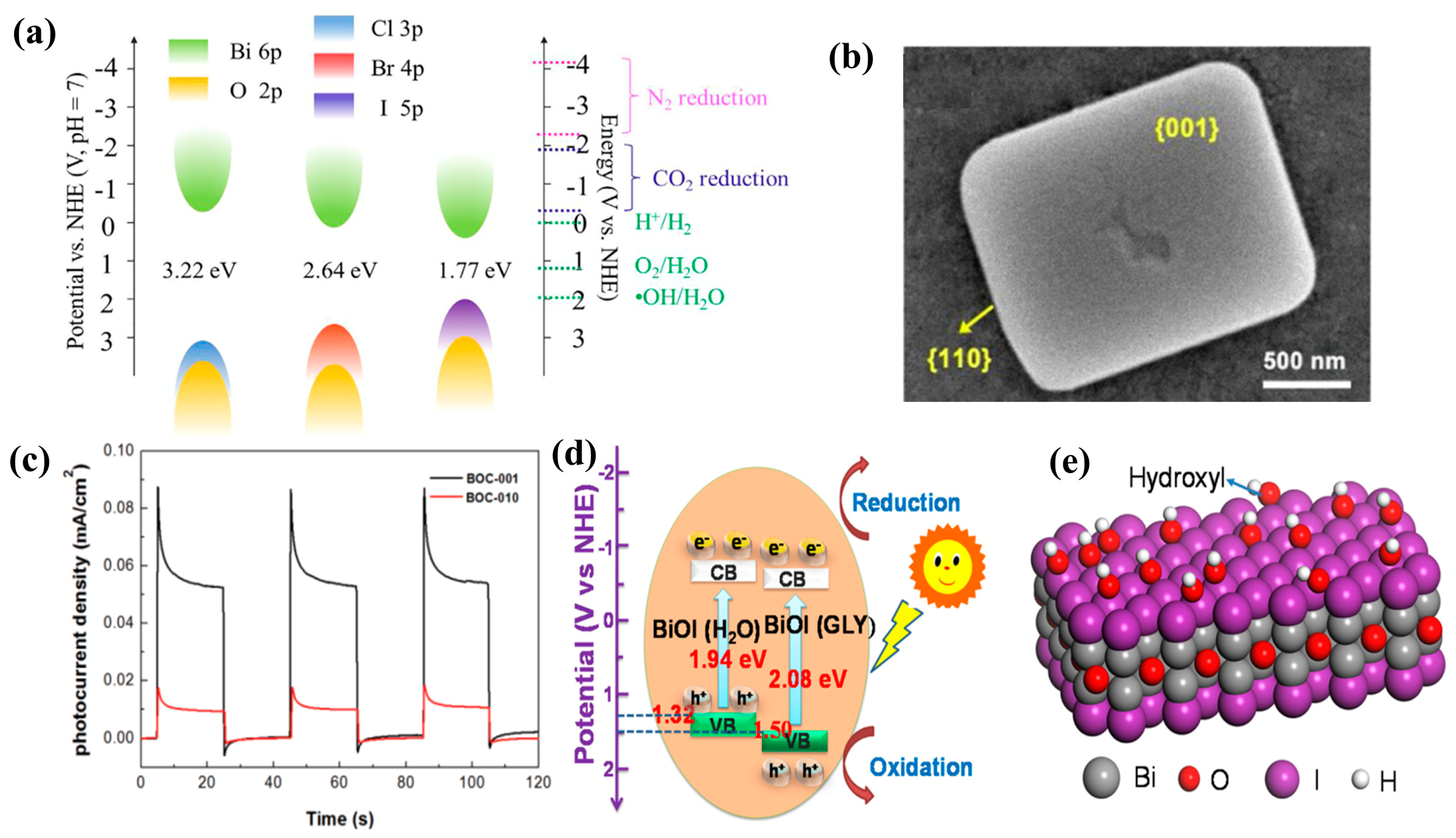
3.6. BiOX
4. Strategies to Boost the Photocatalytic Performance
4.1. Morphology Control
4.2. Defect Engineering
4.2.1. Metal Doping
4.2.2. Non-Metal Doping
5. Types of Semiconductor Heterojunctions
5.1. p-n Junctions, p-p Junctions, and n-n Junctions
5.2. Type I-III
5.3. Z-Scheme
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ranjan, M.; Singh, P.K.; Srivastav, A.L. A review of bismuth-based sorptive materials for the removal of major contaminants from drinking water. Environ. Sci. Pollut. Res. Int. 2020, 27, 17492–17504. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhao, G.; Song, G.; Chen, D.; Chen, H.; Xie, J.; Hayat, T.; Alsaedi, A.; Wang, X. Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium(VI) from aqueous solutions. Chem. Eng. J. 2018, 334, 569–578. [Google Scholar] [CrossRef]
- Guo, C.; Tan, Y.; Yang, S.; Qian, Y. Development of phenols recovery process with novel solvent methyl propyl ketone for extracting dihydric phenols from coal gasification wastewater. J. Clean. Prod. 2018, 198, 1632–1640. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Mukherjee, R.; De, S. Novel carbon-nanoparticle polysulfone hollow fiber mixed matrix ultrafiltration membrane: Adsorptive removal of benzene, phenol and toluene from aqueous solution. Sep. Purif. Technol. 2016, 157, 229–240. [Google Scholar] [CrossRef]
- Li, X.; Chang, J.; Zhang, S.; Xiao, L.; Wu, X.; He, Z. Microcystis@TiO2 Nanoparticles for Photocatalytic Reduction Reactions: Nitrogen Fixation and Hydrogen Evolution. Catalysts 2021, 11, 1443. [Google Scholar] [CrossRef]
- Batra, V.; Kaur, I.; Pathania, D.; Sonu; Chaudhary, V. Efficient dye degradation strategies using green synthesized ZnO-based nanoplatforms: A review. Appl. Surf. Sci. Adv. 2022, 11, 100314. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chou, Y.H. Improved photoelectrode performance of chemical solution-derived Bi2O3 crystals via manipulation of crystal characterization. RSC Adv. 2020, 10, 45042–45058. [Google Scholar] [CrossRef]
- Rashmi; Majid, M.; Sivakumar, S. Tetragonal-zircon BiVO4: A better polymorph for the formation of coherent type-II heterostructures for water splitting applications. Phys. Chem. Chem. Phys. 2023, 25, 27595–27605. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Xu, G.; Chen, S.; Lei, L.; Zhang, M. Ag3PO4/Bi2WO6 hierarchical heterostructures with enhanced visible light photocatalytic activity for the degradation of phenol. Catal. Commun. 2013, 40, 120–124. [Google Scholar] [CrossRef]
- Fu, F.; Shen, H.; Sun, X.; Xue, W.; Shoneye, A.; Ma, J.; Luo, L.; Wang, D.; Wang, J.; Tang, J. Synergistic effect of surface oxygen vacancies and interfacial charge transfer on Fe(III)/Bi2MoO6 for efficient photocatalysis. Appl. Catal. B 2019, 247, 150–162. [Google Scholar] [CrossRef]
- Gao, P.; Yang, Y.; Yin, Z.; Kang, F.; Fan, W.; Sheng, J.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. A critical review on bismuth oxyhalide based photocatalysis for pharmaceutical active compounds degradation: Modifications, reactive sites, and challenges. J. Hazard. Mater. 2021, 412, 125186. [Google Scholar] [CrossRef]
- Ma, J.; Jin, D.; Li, Y.; Xiao, D.; Jiao, G.; Liu, Q.; Guo, Y.; Xiao, L.; Chen, X.; Li, X.; et al. Photocatalytic conversion of biomass-based monosaccharides to lactic acid by ultrathin porous oxygen doped carbon nitride. Appl. Catal. B 2021, 283, 119520. [Google Scholar] [CrossRef]
- Ghasemi, S.; Rahimnejad, S.; Setayesh, S.R.; Rohani, S.; Gholami, M.R. Transition metal ions effect on the properties and photocatalytic activity of nanocrystalline TiO2 prepared in an ionic liquid. J. Hazard. Mater. 2009, 172, 1573–1578. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, H.; Deng, J.; Liu, Y.; Zhang, L.; Ji, K. Porous F-doped BiVO4: Synthesis and enhanced photocatalytic performance for the degradation of phenol under visible-light illumination. Solid State Sci. 2013, 17, 21–27. [Google Scholar] [CrossRef]
- Mao, M.; Chen, F.; Zheng, C.; Ning, J.; Zhong, Y.; Hu, Y. Facile synthesis of porous Bi2O3-BiVO4 p-n heterojunction composite microrods with highly efficient photocatalytic degradation of phenol. J. Alloys Compd. 2016, 688, 1080–1087. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Li, F.; Yang, W.; Zhao, Y.; Hao, Y.; Liu, S. Novel BiOCl–C3N4 heterojunction photocatalysts: In situ preparation via an ionic-liquid-assisted solvent-thermal route and their visible-light photocatalytic activities. Chem. Eng. J. 2013, 234, 361–371. [Google Scholar] [CrossRef]
- Cao, Y.; El-Shafay, A.S.; Mohammed, A.H.; Almojil, S.F.; Almohana, A.I.; Alali, A.F. Controlling the charge carriers recombination kinetics on the g-C3N4-BiSI n-n heterojunction with efficient photocatalytic activity in N2 fixation and degradation of MB and phenol. Adv. Powder Technol. 2022, 33, 103513. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Dong, W.; Zhang, Q.; Liu, J.; Li, R.; Wang, Y.; Zhang, X.; Fan, C. CeO2 nanoparticles decorated Bi4O7 nanosheets for enhanced photodegradation performance of phenol. Mater. Lett. 2022, 322, 132465. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, C.; Chen, D.; Zhang, J.; Feng, Y.; Xu, K.; Hao, W.; Ding, H.; Lv, G.; Du, Y.; et al. Design of lateral and vertical Bi4O5I2/BiOCl heterojunctions with different charge migration pathway for efficient photoredox activity. Appl. Catal. B 2023, 329, 122554. [Google Scholar] [CrossRef]
- Chen, R.; Ren, Z.; Liang, Y.; Zhang, G.; Dittrich, T.; Liu, R.; Liu, Y.; Zhao, Y.; Pang, S.; An, H.; et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature 2022, 610, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Mi, Y.; Yang, Y.; Jiang, Y.; Liu, Y.; Shangguan, W. Conduction band tuning by strengthening s-p hybridization of novel layered oxyhalide Bi4SbO8Cl for efficient visible-light photocatalytic water splitting. Mater. Today Chem. 2022, 26, 101175. [Google Scholar] [CrossRef]
- Liu, Z.; Tai, Y.; Liu, J.; Liu, F.; Han, B.; Fu, W.; Yang, X.; Xie, H.; Liu, Q. A novel mechanism for visible-light degradation of phenol by oxygen vacancy Bi2MoO6 homojunction. Appl. Surf. Sci. 2022, 605, 154671. [Google Scholar] [CrossRef]
- Sarkar, R.; Das, D.; Kumar Das, B.; Mitra, A.; Das, N.S.; Sarkar, S.; Chattopadhyay, K.K. Hollow micro-spherical bismuth oxy-chloride for superior visible light induced dye-sensitized photocatalytic activity and its theoretical insight. Mater. Res. Bull. 2020, 125, 110778. [Google Scholar] [CrossRef]
- Tai, Y.; Han, B.; Liu, Z.; Yang, X.; Fu, W.; Gao, R.; Niu, B.; Liu, X.; Zhang, Y.; Liu, Q. Novel core–shell heterojunction photocatalytic wire mesh for efficient ciprofloxacin degradation under visible light. Sep. Purif. Technol. 2023, 306, 122770. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Rao, J.; Han, Z.; Hu, Y.; Xing, W.; Zhang, C. Doping effect of phosphate in Bi2WO6 and universal improved photocatalytic activity for removing various pollutants in water. Appl. Catal. B 2016, 188, 39–47. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Huang, L.; Wang, C.; Wang, Q.; Zhang, F.; Fan, X.; Xie, M.; Li, H. Promoting LED light driven photocatalytic inactivation of bacteria by novel β-Bi2O3@BiOBr core/shell photocatalyst. J. Alloys Compd. 2020, 816, 152665. [Google Scholar] [CrossRef]
- Li, T.; Quan, S.; Shi, X.; Yang, L.; Liu, C. Fabrication of La-Doped Bi2O3 Nanoparticles with Oxygen Vacancies for Improving Photocatalytic Activity. Catal. Lett. 2019, 150, 640–651. [Google Scholar] [CrossRef]
- Meng, Q.; Luo, M.; Jiang, J.; Wan, X. Construction of BiOBr/α-Bi2O3 p-n heterojunction as a highly efficacious visible-light-induced photocatalyst for phenol removal. Vacuum 2023, 214, 112178. [Google Scholar] [CrossRef]
- Wu, J.; Ding, B.; Qian, X.; Mao, L.; Zheng, H.; Yang, Y.; Zhang, L.; Zheng, S.; Zhang, J. Sunlight driven isotropic beta-Bi2O3 with high charge-carrier mobility for the efficient degradation of bisphenol A and phenol. Dalton Trans. 2022, 51, 8401–8410. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Z.; Zhang, Z. Pd-nanoparticle-decorated peanut-shaped BiVO4 with improved visible light-driven photocatalytic activity comparable to that of TiO2 under UV light. J. Catal. 2017, 356, 53–64. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Xu, J.; Wang, L.; Zhang, Z. Highly efficient photocatalytic oxidation of phenol over ordered mesoporous Bi2WO6. Appl. Catal. B 2011, 106, 559–564. [Google Scholar] [CrossRef]
- Uezono, N.; Liu, J.; Pawar, S.A.; Islam, M.M.; Ikeda, S.; Sakurai, T. Crystalline phase control of BiVO4 thin films using RF sputtering. Jpn. J. Appl. Phys. 2023, 62, SK1001. [Google Scholar] [CrossRef]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Serendipitous Assembly of Mixed Phase BiVO4 on B-Doped g-C3N4: An Appropriate p–n Heterojunction for Photocatalytic O2 evolution and Cr(VI) reduction. Inorg. Chem. 2019, 58, 12480–12491. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, H.; Cai, P.; Qiu, R.; Xiong, Y. Simultaneous photocatalytic reduction of Cr(VI) and oxidation of phenol over monoclinic BiVO4 under visible light irradiation. Chemosphere 2006, 63, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Guo, P.; Wang, M.; Ren, F. The effect of pH on the photocatalytic performance of BiVO4 for phenol mine sewage degradation under visible light. Optik 2019, 179, 672–679. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, H.; Zhang, J.; Lin, J.; Yang, S.; Chen, C.; Xi, J.; Kong, Z.; Song, L.; Zeng, J. Novel MoSSe/Bi2WO6 S-scheme heterojunction photocatalysts for significantly improved photoelectrochemical and photocatalytic performance. J. Alloys Compd. 2023, 933, 167784. [Google Scholar] [CrossRef]
- Sattari, M.; Farhadian, M.; Reza Solaimany Nazar, A.; Moghadam, M. Enhancement of Phenol degradation, using of novel Z-scheme Bi2WO6/C3N4/TiO2 composite: Catalyst and operational parameters optimization. J. Photochem. Photobiol. A 2022, 431, 114065. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Li, F.; Yu, C. Eu3+ doped Bi2MoO6 nanosheets fabricated via hydrothermal-calcination route and their superior performance for aqueous volatile phenols removal. J. Taiwan Inst. Chem. Eng. 2021, 125, 276–284. [Google Scholar] [CrossRef]
- Sun, M.; Guo, P.; Wang, M.; Ren, F. The effect of calcination temperature on the photocatalytic performance of Bi2MoO6 for the degradation of phenol under visible light. Optik 2019, 199, 163319. [Google Scholar] [CrossRef]
- Chang Chien, S.-W.; Ng, D.-Q.; Kumar, D.; Lam, S.-M.; Jaffari, Z.H. Investigating the effects of various synthesis routes on morphological, optical, photoelectrochemical and photocatalytic properties of single-phase perovskite BiFeO3. J. Phys. Chem. Solids 2022, 160, 110342. [Google Scholar] [CrossRef]
- Zargazi, M.; Entezari, M.H. A novel synthesis of forest like BiFeO3 thin film: Photo-electrochemical studies and its application as a photocatalyst for phenol degradation. Appl. Surf. Sci. 2019, 483, 793–802. [Google Scholar] [CrossRef]
- Lam, S.-M.; Jaffari, Z.H.; Sin, J.-C.; Zeng, H.; Lin, H.; Li, H.; Mohamed, A.R. Insight into the influence of noble metal decorated on BiFeO3 for 2,4-dichlorophenol and real herbicide wastewater treatment under visible light. Colloids Surf. A 2021, 614, 126138. [Google Scholar] [CrossRef]
- Yin, J.; Liao, G.; Zhou, J.; Huang, C.; Ling, Y.; Lu, P.; Li, L. High performance of magnetic BiFeO3 nanoparticle-mediated photocatalytic ozonation for wastewater decontamination. Sep. Purif. Technol. 2016, 168, 134–140. [Google Scholar] [CrossRef]
- Sun, X.; Xu, T.; Xian, T.; Yi, Z.; Liu, G.; Dai, J.; Yang, H. Insight on the enhanced piezo-photocatalytic mechanism of In2O3/BiFeO3 heterojunctions for degradation of tetracycline hydrochloride. Appl. Surf. Sci. 2023, 640, 158408. [Google Scholar] [CrossRef]
- Mansingh, S.; Sultana, S.; Acharya, R.; Ghosh, M.K.; Parida, K.M. Efficient Photon Conversion via Double Charge Dynamics CeO2-BiFeO3 p-n Heterojunction Photocatalyst Promising toward N2 Fixation and Phenol-Cr(VI) Detoxification. Inorg. Chem. 2020, 59, 3856–3873. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Li, H.; Guo, S.; Dai, S. Bismuth oxyhalide layered materials for energy and environmental applications. Nano Energy 2017, 41, 172–192. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Liang, J.; Yuan, X.; Jiang, L.; Yu, H.; Sun, H.; Zhu, Z.; Ye, S.; Tang, N.; et al. Fabrication and regulation of vacancy-mediated bismuth oxyhalide towards photocatalytic application: Development status and tendency. Coord. Chem. Rev. 2021, 443, 214033. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, Q.; Bao, S.; Lu, Y.; Guan, Z.; Zhang, J.; Tian, B. Z-scheme heterostructure BiOCl-Ag-AgBr with enhanced sunlight-driven photocatalytic activity in simultaneous removal of Cr6+ and phenol contaminants. Catal. Today 2021, 376, 151–161. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhou, C.; Huo, D.; Zhang, R.; Wang, L. Hydroxyl-regulated BiOI nanosheets with a highly positive valence band maximum for improved visible-light photocatalytic performance. Appl. Catal. B 2020, 268, 118390. [Google Scholar] [CrossRef]
- Wen, R.; Yang, L.; Wu, S.; Zhou, D.; Jiang, B. Tuning surface sites to boost photocatalytic degradation of phenol and ciprofloxacin. Chin. Chem. Lett. 2023, 34, 107204. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L. Bi2WO6 Quantum Dots Decorated Reduced Graphene Oxide: Improved Charge Separation and Enhanced Photoconversion Efficiency. J. Phys. Chem. C 2013, 117, 9113–9120. [Google Scholar] [CrossRef]
- Ruan, X.; Wen, X.; Liang, D.; Hu, Y. Hierarchically peony-analogous bismuth tungstate with oxygen vacancies for enhanced photocatalytic degradation of phenolic compounds. J. Clean. Prod. 2021, 324, 129287. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Yang, R.; Shao, M. Facile synthesis of Bi decorated 2D and 3D BiOBr micro-nanostructures with enhanced photocatalytic activity. Micro Nano Lett. 2018, 13, 1040–1045. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X. Room temperature synthesis of Bi4O5I2 and Bi5O7I ultrathin nanosheets with a high visible light photocatalytic performance. Dalton Trans. 2016, 45, 7720–7727. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.; Liu, C.; Xie, T. Preparation and photocatalytic activity of porous Bi5O7I nanosheets. Appl. Surf. Sci. 2014, 319, 265–271. [Google Scholar] [CrossRef]
- Du, X.; Huang, J.; Zhang, J.; Yan, Y.; Wu, C.; Hu, Y.; Yan, C.; Lei, T.; Chen, W.; Fan, C.; et al. Modulating Electronic Structures of Inorganic Nanomaterials for Efficient Electrocatalytic Water Splitting. Angew. Chem. Int. Ed. Engl. 2019, 58, 4484–4502. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, N.; Zheng, L.; Zhang, L.; Bian, Z.; Wang, H. Interface engineering of p-p Z-scheme BiOBr/Bi12O17Br2 for sulfamethoxazole photocatalytic degradation. Chemosphere 2022, 307, 135666. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Wang, L.; Zhou, Y.; Jin, S.; Jin, H.; Li, J. Breaking through the interfacial energy barrier limitations of type-I heterojunctions via ferroelectric polarization engineering: A case study of Bi5Ti3FeO15/BiOCl. Inorg. Chem. Front. 2023, 10, 3112–3120. [Google Scholar] [CrossRef]
- Long, Y.; Li, L.; Zhou, L.; Zhang, S.; Wang, L.; Zheng, Z.; Wu, S.; Hei, Y.; Jiang, F. Fabrication of the AgI/BiOI/BiPO4 multi-heterojunction with high photocatalytic activity. Mater. Res. Bull. 2020, 126, 110787. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Huang, D.; Zeng, G.; Xu, P.; Zhou, C.; Lai, C.; Wang, H.; Cheng, M.; Wang, W. Multiply structural optimized strategies for bismuth oxyhalide photocatalysis and their environmental application. Chem. Eng. J. 2019, 374, 1025–1045. [Google Scholar] [CrossRef]
- Cheng, T.; Ma, Q.; Gao, H.; Meng, S.; Lu, Z.; Wang, S.; Yi, Z.; Wu, X.; Liu, G.; Wang, X.; et al. Enhanced photocatalytic activity, mechanism and potential application of Idoped-Bi4Ti3O12 photocatalysts. Mater. Today Chem. 2022, 23, 100750. [Google Scholar] [CrossRef]
- Meng, X.; Qin, H.; Zhang, Z. New insight into the enhanced visible light-driven photocatalytic activity of Pd/PdCl2-doped Bi2WO6 photocatalysts. J. Colloid Interface Sci. 2018, 513, 877–890. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bi2MoO6 co-modified by reduced graphene oxide and palladium (Pd2+and Pd0) with enhanced photocatalytic decomposition of phenol. Appl. Catal. B 2017, 209, 383–393. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Meng, X.; Li, Z.; Chen, J.; Xie, H.; Zhang, Z. Enhanced visible light-induced photocatalytic activity of surface-modified BiOBr with Pd nanoparticles. Appl. Surf. Sci. 2018, 433, 76–87. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, L.; Fang, W.X.; Ren, J.; Li, Y.Y.; Yao, H.C.; Wang, J.S.; Li, Z.J. Enhanced Photoreduction CO2 Activity over Direct Z-Scheme alpha-Fe2O3/Cu2O Heterostructures under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 8631–8639. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, L.; Tai, Y.; Deng, J.; Wu, Q.; Zhao, Y.; Xie, H.; Liu, Q. Synergistic Effects of Sulfur Vacancies and Internal Electric Fields in FeS/MoS2 Heterojunctions: A New Approach to Photocatalytic Chromium Removal. Chemosphere 2024, 364, 143021. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Liu, Z.; Cheng, X.; Li, C.; Ren, P.; Guo, K.; Yue, H.; Xie, B.; Li, T.; Wang, Z.; et al. Boosting piezo-photocatalytic activity of BiVO4/BiFeO3 heterojunctions through built-in polarization field tailoring carrier transfer performances. Chem. Eng. J. 2023, 464, 142617. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.T.; Yuan, Z.Y. Enabling Internal Electric Fields to Enhance Energy and Environmental Catalysis. Adv. Energy Mater. 2023, 13, 2203720. [Google Scholar] [CrossRef]
- Chen, L.; He, J.; Liu, Y.; Chen, P.; Au, C.-T.; Yin, S.-F. Recent advances in bismuth-containing photocatalysts with heterojunctions. Chin. J. Catal. 2016, 37, 780–791. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, W.; Yu, J.C.; Liu, H.; Wei, L. Design and fabrication of heterojunction photocatalysts for energy conversion and pollutant degradation. Chin. J. Catal. 2014, 35, 1609–1618. [Google Scholar] [CrossRef]
- Lin, J.; He, J.; Hu, J.; Dong, J.; Liu, A.; Yang, Y.; Tang, L.; Li, L.; Zhou, Y.; Zou, Z. In situ construction of a 2D/2D heterostructured ZnIn2S4/Bi2MoO6 Z-scheme system for boosting the photoreduction activity of Cr(vi). Catal. Sci. Technol. 2021, 11, 3885–3893. [Google Scholar] [CrossRef]
- Ji, Q.; Xu, Z.; Xiang, W.; Wu, Y.; Cheng, X.; Xu, C.; Qi, C.; He, H.; Hu, J.; Yang, S.; et al. Enhancing the performance of pollution degradation through secondary self-assembled composite supramolecular heterojunction photocatalyst BiOCl/PDI under visible light irradiation. Chemosphere 2020, 253, 126751. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Jiang, W.; Yao, W.; Yang, H.; Zhu, Y. Self-assembled perylene diimide based supramolecular heterojunction with Bi2WO6 for efficient visible-light-driven photocatalysis. Appl. Catal. B 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Liao, H.; Li, Z.; Luo, L.; Zhong, J.; Li, J. Water hyacinth powder -assisted preparation of defects-rich and flower-like BiOI/Bi5O7I heterojunctions with excellent visible light photocatalytic activity. Surf. Interfaces 2021, 27, 101470. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2013, 24, 2421–2440. [Google Scholar] [CrossRef]
- Özçelik, V.O.; Azadani, J.G.; Yang, C.; Koester, S.J.; Low, T. Band alignment of two-dimensional semiconductors for designing heterostructures with momentum space matching. Phys. Rev. B 2016, 94, 35125. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, X.; Chen, Y.; Guo, S.; Wu, S.; Song, C.; Huang, S.; Huang, X.; Tai, X.; Lin, T.; et al. HgCdTe/black phosphorus van der Waals heterojunction for high-performance polarization-sensitive midwave infrared photodetector. Sci. Adv. 2022, 8, eabn1811. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Fang, H.; Guo, X.; Rui, Z. Plasmonic Metal Bridge Leading Type III Heterojunctions to Robust Type B Photothermocatalysts. Ind. Eng. Chem. Res. 2021, 60, 8420–8429. [Google Scholar] [CrossRef]
- Xiao, Y.; Tan, S.; Wang, D.; Wu, J.; Jia, T.; Liu, Q.; Qi, Y.; Qi, X.; He, P.; Zhou, M. CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Appl. Surf. Sci. 2020, 530, 147116. [Google Scholar] [CrossRef]
- Kusmierek, E. A CeO2 Semiconductor as a Photocatalytic and Photoelectrocatalytic Material for the Remediation of Pollutants in Industrial Wastewater: A Review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Zhou, W.; Jiang, H.; Liu, H.; Chen, X.; Tian, G. WO3/BiVO4/BiOCl porous nanosheet composites from a biomass template for photocatalytic organic pollutant degradation. J. Alloys Compd. 2019, 802, 76–85. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, Y.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Direct Dual Z-Scheme Bi2WO6/GQDs/WO3 Inverse Opals for Enhanced Photocatalytic Activities under Visible Light. ACS Sustain. Chem. Eng. 2020, 8, 7921–7927. [Google Scholar] [CrossRef]
- Jiang, J.; Song, Y.; Wang, X.; Li, T.; Li, M.; Lin, Y.; Xie, T.; Dong, S. Enhancing aqueous pollutant photodegradation via a Fermi level matched Z-scheme BiOI/Pt/g-C3N4 photocatalyst: Unobstructed photogenerated charge behavior and degradation pathway exploration. Catal. Sci. Technol. 2020, 10, 3324–3333. [Google Scholar] [CrossRef]
- Toe, E.D.; Kurniawan, W.; Andrews, E.M.; Nakasaki, K.; Hinode, H.; Aziz, M. All-solid-state Z-scheme plasmonic Si@Au nanoparticles on CuBi2O4/BiVO4 for efficient photocatalytic activity. Adv. Powder Technol. 2021, 32, 4330–4342. [Google Scholar] [CrossRef]
- Duan, J.; Liu, M.; Song, X.; Wang, W.; Zhang, Z.; Li, C. Enhanced photocatalytic degradation of organic pollutants using carbon nanotube mediated CuO and Bi2WO6 sandwich flaky structures. Nanotechnology 2020, 31, 425202. [Google Scholar] [CrossRef]
- Raizada, P.; Thakur, P.; Sudhaik, A.; Singh, P.; Thakur, V.K.; Hosseini-Bandegharaei, A. Fabrication of dual Z-scheme photocatalyst via coupling of BiOBr/Ag/AgCl heterojunction with P and S co-doped g-C3N4 for efficient phenol degradation. Arab. J. Chem. 2020, 13, 4538–4552. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Xu, H.; Wang, H.; Da, Z.; Huang, S.; Ji, H.; Li, H. In situ oxidation synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl/g-C3N4 and its activity. Ceram. Int. 2014, 40, 9293–9301. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Qian, M.; Cheng, X.; Liu, Z. Recent Advances in the Photocatalytic Degradation of Phenol over Bi-Based Oxide Catalysts. Processes 2024, 12, 1799. https://doi.org/10.3390/pr12091799
Liu Z, Qian M, Cheng X, Liu Z. Recent Advances in the Photocatalytic Degradation of Phenol over Bi-Based Oxide Catalysts. Processes. 2024; 12(9):1799. https://doi.org/10.3390/pr12091799
Chicago/Turabian StyleLiu, Zhangpei, Maosheng Qian, Xiaomeng Cheng, and Zhiming Liu. 2024. "Recent Advances in the Photocatalytic Degradation of Phenol over Bi-Based Oxide Catalysts" Processes 12, no. 9: 1799. https://doi.org/10.3390/pr12091799
APA StyleLiu, Z., Qian, M., Cheng, X., & Liu, Z. (2024). Recent Advances in the Photocatalytic Degradation of Phenol over Bi-Based Oxide Catalysts. Processes, 12(9), 1799. https://doi.org/10.3390/pr12091799







