Antibacterial Properties of Dandelion Extract-Based PVA/CTS/DAN/CuNP Composite Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of CuNPs
2.3. Preparation of PVA/CTS/DAN/CuNP Gels
2.4. Characterization of PVA/CTS/DAN/CuNP Gels
2.5. Antibacterial Testing
2.5.1. Optimization of Gel Preparation
2.5.2. Optimization of the Amount of CTS as well as CuNPs in PVA/CTS/DAN/CuNP Gels
2.5.3. Stability and Reusability of Antibacterial Gels
2.6. Statistical Analysis
3. Results and Discussion
3.1. Gel Characterization
3.1.1. FT-IR
3.1.2. SEM
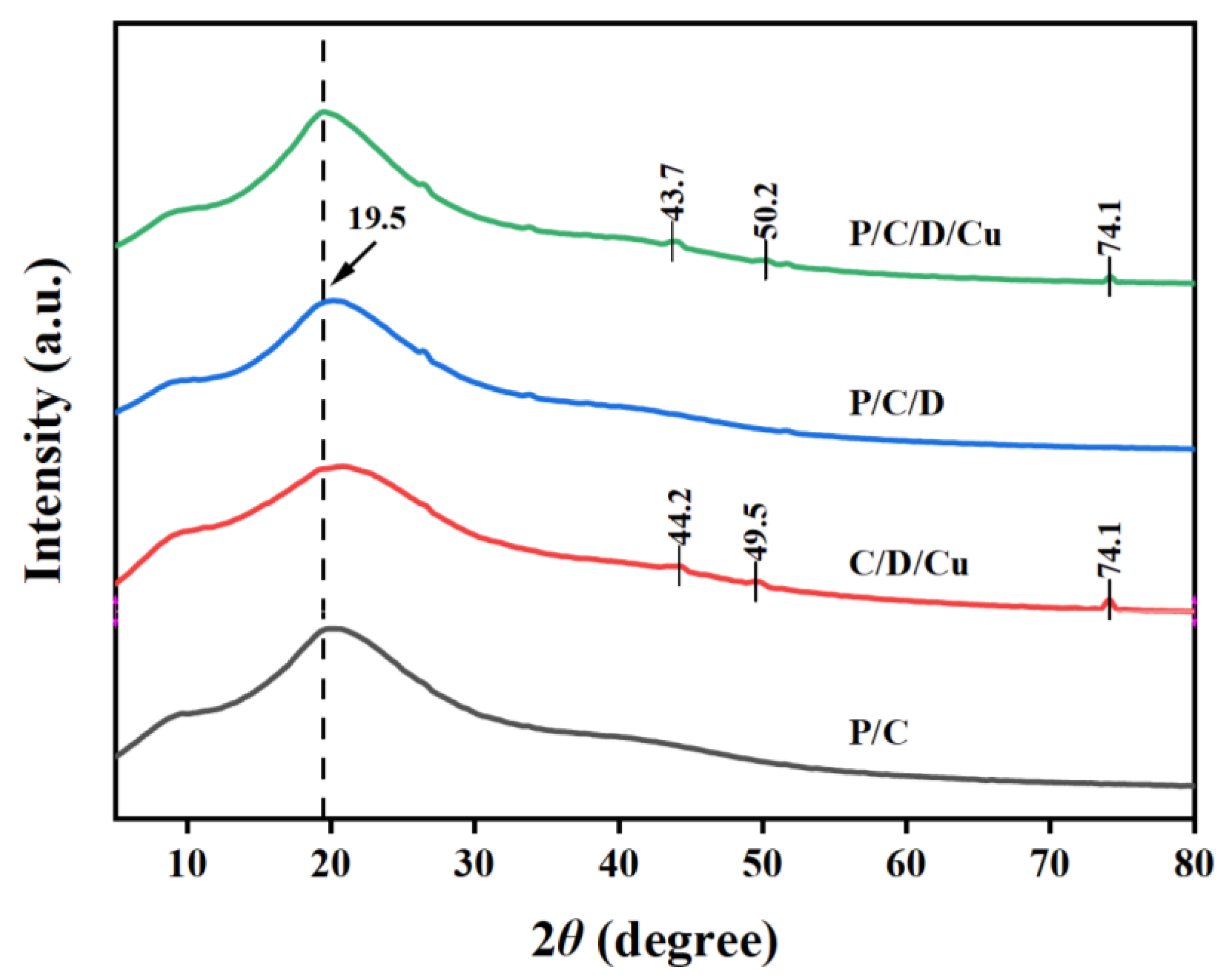
3.1.3. XRD
3.2. Antibacterial Activity
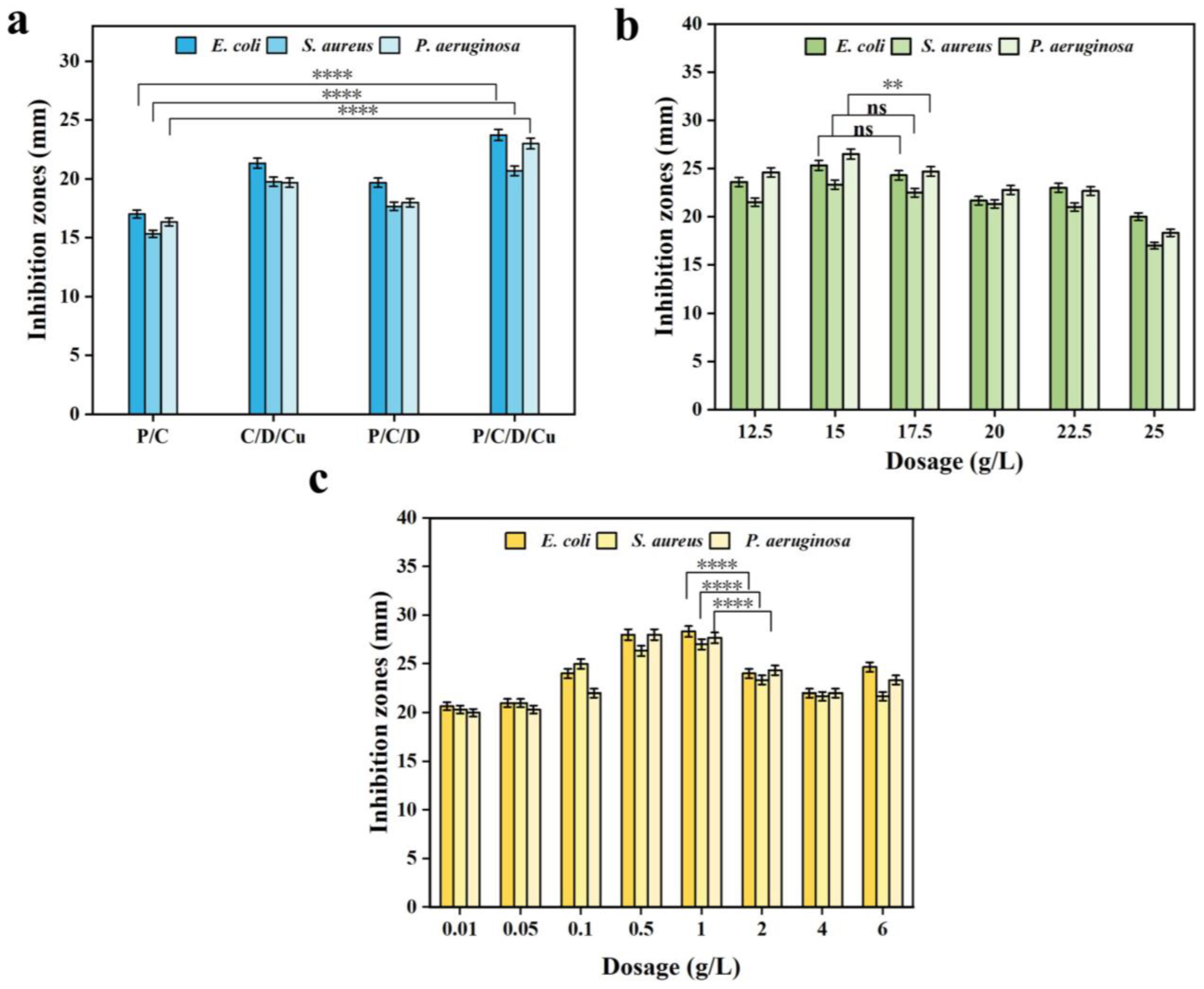
3.2.1. Antibacterial Activity of Composites
3.2.2. Effect of the CTS and CuNP Loading on the Inhibitory Activity of PVA/CTS/DAN/CuNP Gels
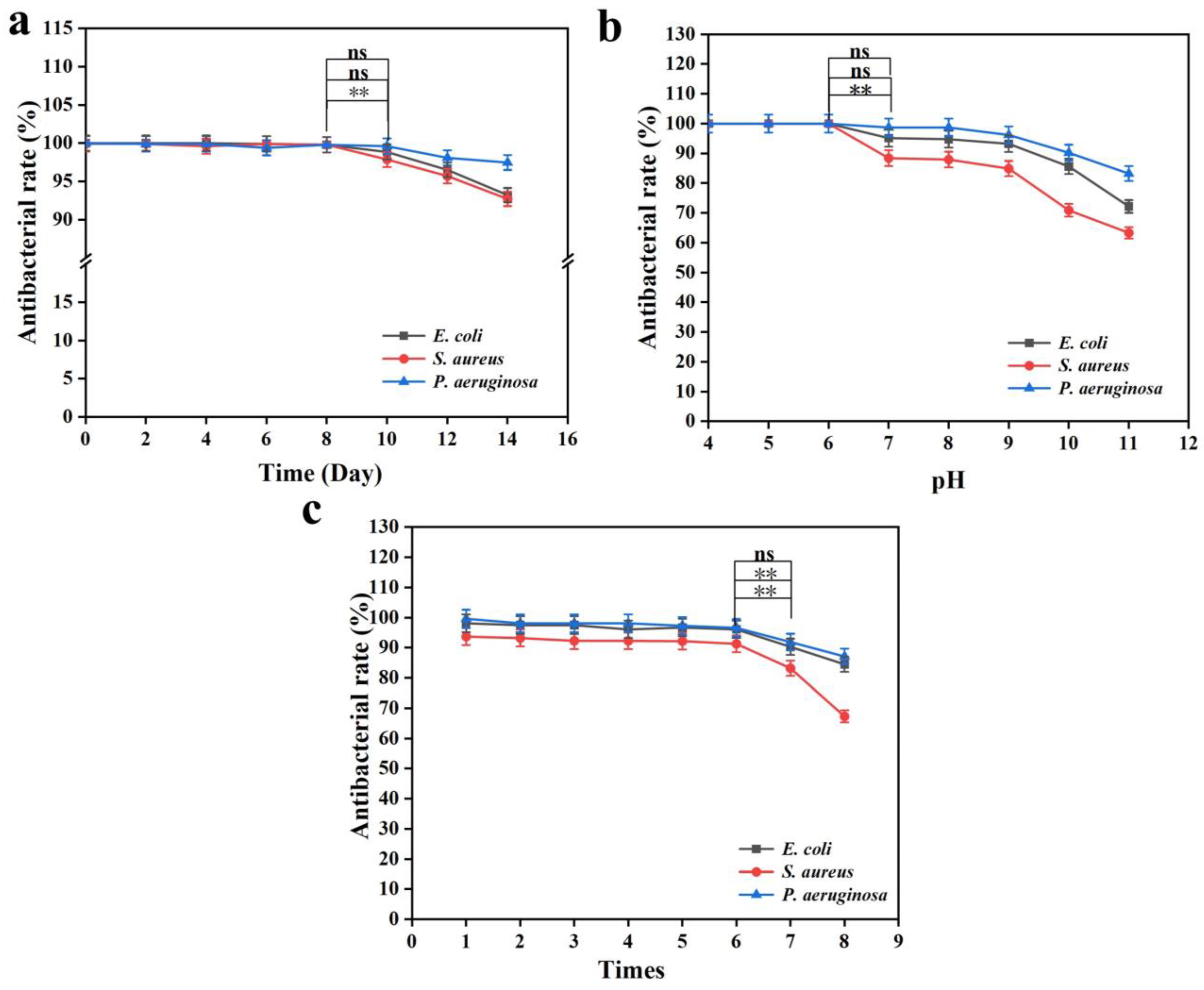
3.3. Reusability and Stability of the Composite Gel
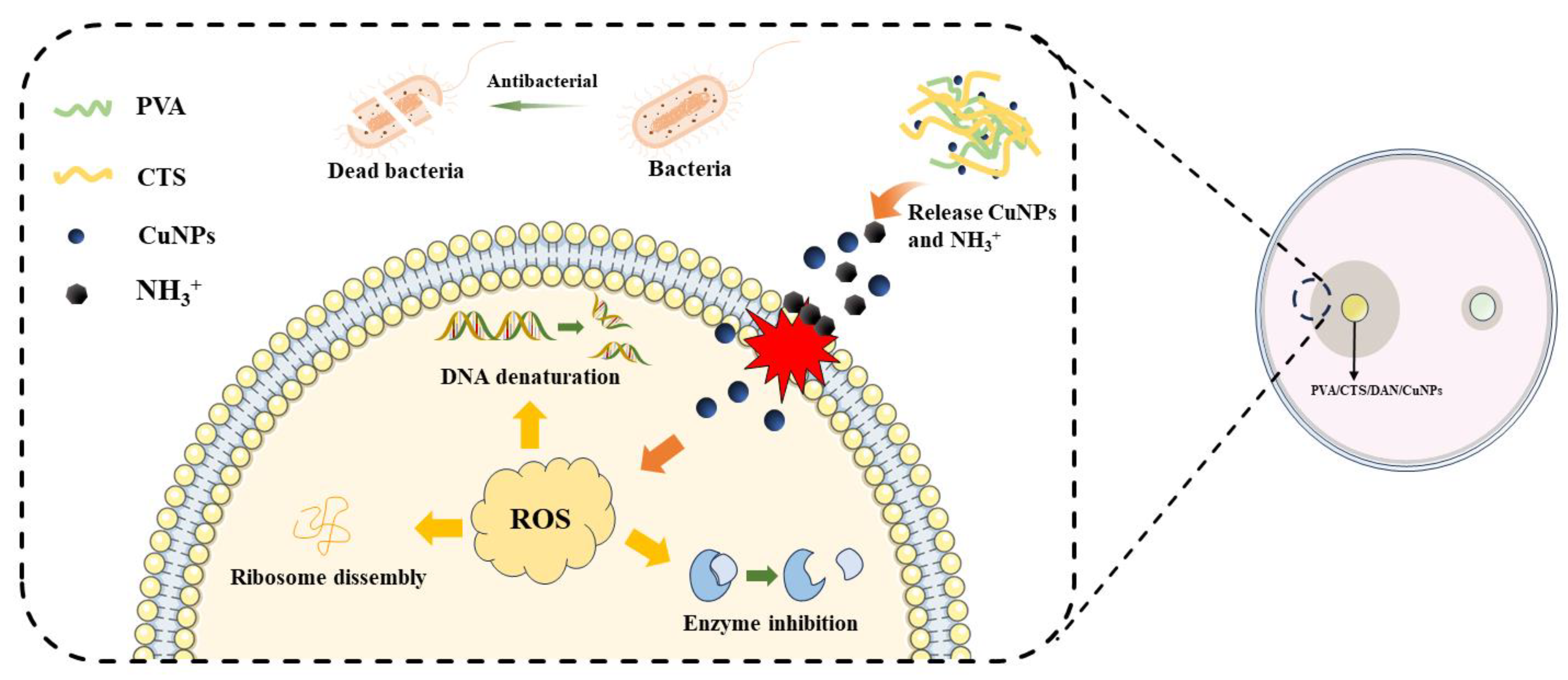
3.4. Proposed Mechanism of Bacterial Inhibition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, N.K. Bioremediation: A green approach for restoration of polluted ecosystems. Environ. Sustain. 2018, 1, 305–307. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Moyo, S.; Makhanya, B.P.; Zwane, P.E. Use of bacterial isolates in the treatment of textile dye wastewater: A review. Heliyon 2022, 8, e09632. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Sahoo, G.; Swain, S.K. Graphene quantum dot decorated magnetic graphene oxide filled polyvinyl alcohol hybrid hydrogel for removal of dye pollutants. J. Mol. Liq. 2020, 302, 112591. [Google Scholar] [CrossRef]
- Gong, T.; Liang, H.; Li, X.; Li, G.; Chen, F.; Wang, H. High-adsorption-capacity and recyclable double-network hydrogel nanofibers for dynamic uranium sorption. Sep. Purif. Technol. 2024, 342, 126963. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Zhu, S.; Yu, M. Green and facile fabrication of nano-ZnO coated cellulose/starch/activated carbon hydrogel for enhanced dyes adsorption and antibacterial activity. Mater. Today Commun. 2022, 33, 104355. [Google Scholar] [CrossRef]
- Paul, J.; Qamar, A.; Ahankari, S.S.; Thomas, S.; Dufresne, A. Chitosan-based aerogels: A new paradigm of advanced green materials for remediation of contaminated water. Carbohydr. Polym. 2024, 338, 122198. [Google Scholar] [CrossRef]
- Kong, F.; Ge, J.; Zhu, Z.; Chen, C.; Peng, J.; Li, X.; Li, B.; Ma, H. A Conjugated Microporous Polymer/Wood Aerogel with Physical Adsorption, Chemical Degradation and Antibacterial Self-Cleaning Triple Sewage Treatment Functions. Polymers 2023, 15, 3929. [Google Scholar] [CrossRef]
- Liu, H.; Wang, B.; Liu, H.; Zheng, Y.; Li, M.; Tang, K.; Pan, B.; Liu, C.; Luo, J.; Pang, X. Multi-crosslinked robust alginate/polyethyleneimine modified graphene aerogel for efficient organic dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133034. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul Khalil, H.P.S.; Yahya, E.B.; Olaiya, N.G.; Alfatah, T.; Suriani, A.B.; Mohamed, A. Recent trends and future prospects of nanostructured aerogels in water treatment applications. J. Water Process Eng. 2022, 45, 102481. [Google Scholar] [CrossRef]
- Cui, C.; Jia, Y.; Sun, Q.; Yu, M.; Ji, N.; Dai, L.; Wang, Y.; Qin, Y.; Xiong, L.; Sun, Q. Recent advances in the preparation, characterization, and food application of starch-based hydrogels. Carbohydr. Polym. 2022, 291, 119624. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, D.; Yang, V.; Yetter, R.A. Metal-based nanoenergetic materials: Synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar] [CrossRef]
- Kurhade, P.; Kodape, S.; Choudhury, R. Overview on green synthesis of metallic nanoparticles. Chem. Pap. 2021, 75, 5187–5222. [Google Scholar] [CrossRef]
- Ezeuko, A.S.; Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. The effectiveness of silver nanoparticles as a clean-up material for water polluted with bacteria DNA conveying antibiotics resistance genes: Effect of different molar concentrations and competing ions. OpenNano 2022, 7, 100060. [Google Scholar] [CrossRef]
- Dong, C.; Feng, W.; Xu, W.; Yu, L.; Xiang, H.; Chen, Y.; Zhou, J. The Coppery Age: Copper (Cu)—Involved Nanotheranostics. Adv. Sci. 2020, 7, 2001549. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Ahmad Khawaja, A.; Javed, M.; Mahmood, S.; Iqbal, S.; Nadeem, S.; Jahangir, M.; Ahmad, M.; Bahadur, A.; Alshalwi, M. Highly synergistic antibacterial activity of copper (II)-based nano metal–organic framework. Inorg. Chem. Commun. 2024, 159, 111802. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Guo, Z.; Zhang, H.; Wang, J.; Jia, P.; Bu, T.; Liu, Y.; Li, L.; Wang, L. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int. J. Biol. Macromol. 2021, 167, 10–22. [Google Scholar] [CrossRef]
- Rafi, R.; Zulfiqar, S.; Asad, M.; Zeeshan, R.; Zehra, M.; Khalid, H.; Akhtar, N.; Yar, M. Smart wound dressings based on carbon doped copper nanoparticles for selective bacterial detection and eradication for efficient wound healing application. Mater. Today Commun. 2023, 35, 105914. [Google Scholar] [CrossRef]
- Chand Mali, S.; Dhaka, A.; Sharma, S.; Trivedi, R. Review on biogenic synthesis of copper nanoparticles and its potential applications. Inorg. Chem. Commun. 2023, 149, 110448. [Google Scholar] [CrossRef]
- Cuong, H.N.; Pansambal, S.; Ghotekar, S.; Oza, R.; Thanh Hai, N.T.; Viet, N.M.; Nguyen, V.-H. New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: A review. Environ. Res. 2022, 203, 111858. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kaushik, D.; Kumar, A.; Gupta, P.; Proestos, C.; Oz, E.; Orhan, E.; Kaur, J.; Khan, M.R.; Elobeid, T.; et al. Green synthesis of copper nanoparticles from Nigella sativa seed extract and evaluation of their antibacterial and antiobesity activity. Int. J. Food Sci. Technol. 2023, 58, 2883–2892. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kwon, D.Y.; Kang, E.S.; Kang, S.; Park, S. Gastroprotective actions of Taraxacum coreanum Nakai water extracts in ethanol-induced rat models of acute and chronic gastritis. J. Ethnopharmacol. 2017, 208, 84–93. [Google Scholar] [CrossRef]
- Ismail, M.I.M. Green synthesis and characterizations of copper nanoparticles. Mater. Chem. Phys. 2020, 240, 122283. [Google Scholar] [CrossRef]
- Gazil, O.; Gancheva, T.; Bilodeau-Calame, M.; Favis, B.D.; Virgilio, N. Controlling the distribution of nanoparticles in hydrogels via interfacial synthesis. Nanoscale Adv. 2020, 2, 5263–5270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; You, S.; Mao, R.; Xiang, Y.; Cai, E.; Deng, H.; Shen, J.; Qi, X. Architecting polyelectrolyte hydrogels with Cu-assisted polydopamine nanoparticles for photothermal antibacterial therapy. Mater. Today Bio 2022, 15, 100264. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, H.; Wang, X.; Dong, S.; Guo, L.; Zhang, S.; Yang, X.; Liu, C.; Jiang, X.; Kan, M.; et al. Advances in preparation and application of antibacterial hydrogels. J. NanoBiotechnol. 2023, 21, 300. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion mechanism and application progress of hydrogels. Eur. Polym. J. 2022, 173, 111277. [Google Scholar] [CrossRef]
- Sung, B.; Kim, M.H.; Abelmann, L. Magnetic microgels and nanogels: Physical mechanisms and biomedical applications. Bioeng. Transl. Med. 2020, 6, e10190. [Google Scholar] [CrossRef]
- Chen, Q.; He, Y.; Li, Q.; Yang, K.; Sun, L.; Xu, H.; Wang, R. Intelligent design and medical applications of antimicrobial hydrogels. Colloid. Interface Sci. Commun. 2023, 53, 100696. [Google Scholar] [CrossRef]
- Zhu, F.; She, X.; Huang, H.; Zhang, Z.; Ji, J.; Li, L. Fabrication of multifunctional polypyrrole hydrogel enhanced by polyvinyl alcohol. Polym. Bull. 2023, 81, 4107–4121. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, S.; Zuo, J.; Liang, S.; Yang, J.; Wang, J.; Wei, D. Progress in preparation and properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2023, 242, 124915. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, J.; Zeng, G.; Xu, H.; Lv, Y.; Liang, X.; Jin, L.; Jiang, X. Chitosan-crosslinked polyvinyl alcohol anti-swelling hydrogel designed to prevent abdominal wall adhesion. Mater. Today Bio 2024, 24, 100931. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, W.; Chen, G.; Li, X.; Wang, Y.; Xiong, J.; Wei, L. Preparation and Characterization of Polyvinyl Alcohol—Chitosan/Cerium Hydrogel with Significant Antibacterial Activity. Starch Stärke 2021, 73, 2000253. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Shi, X.; Zhang, R.; He, Y.; Zhang, H.; Wang, W. Application of polyvinyl alcohol/chitosan copolymer hydrogels in biomedicine: A review. Int. J. Biol. Macromol. 2023, 242, 125192. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Li, J.; Huo, T.; Niu, Y.; Yu, L. Novel double cross-linked gels of soybean protein isolates and soluble dietary fiber from soybean coats with their functionalities. Food Hydrocoll. 2021, 113, 106474. [Google Scholar] [CrossRef]
- Garnica-Palafox, I.M.; Sánchez-Arévalo, F.M. Influence of natural and synthetic crosslinking reagents on the structural and mechanical properties of chitosan-based hybrid hydrogels. Carbohydr. Polym. 2016, 151, 1073–1081. [Google Scholar] [CrossRef]
- Kaur, R.; Goyal, D.; Agnihotri, S. Chitosan/PVA silver nanocomposite for butachlor removal: Fabrication, characterization, adsorption mechanism and isotherms. Carbohydr. Polym. 2021, 262, 117906. [Google Scholar] [CrossRef]
- Na, X.; Zou, B.; Zheng, X.; Du, M.; Zhu, B.; Wu, C. Synergistic antimicrobial hybrid bio-surface formed by self-assembled BSA nanoarchitectures with chitosan oligosaccharide. Biomacromolecules 2023, 24, 4093–4102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, T.; Liu, Y.; Shang, F.; Chen, B.; Hu, Y.; Wang, S.; Guo, Z. Sodium alginate–soy protein isolate–chitosan–capsaicin–nanosilver multifunctional antibacterial composite gel. Processes 2024, 12, 662. [Google Scholar] [CrossRef]
- Yang, D.; Fan, B.; He, Y.-C. UV-blocking, antibacterial, corrosion resistance, antioxidant, and fruit packaging ability of lignin-rich alkaline black liquor composite film. Int. J. Biol. Macromol. 2024, 275, 133344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, T.; Liu, Y.; Shang, F.; Chen, B.; Hu, Y.; Wang, S.; Guo, Z. Supramolecular hydrogel of poly(vinyl alcohol)/chitosan: A dual cross-link design. Adv. Polym. Technol. 2017, 37, 2186–2194. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, P.; Zhong, L.; Zhang, R.; Hou, X.; Ren, X.; Wang, J.; Chu, X.; Lu, Y.; Zhou, Z. Chitosan-polyvinyl alcohol-diatomite hydrogel removes methylene blue from water. Int. J. Biol. Macromol. 2024, 254, 127886. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhao, Z.; Shi, X.; Zhang, R.; He, Y.; Zhang, H.; Wang, W. Magnesium-Doped Nano-Hydroxyapatite/Polyvinyl Alcohol/Chitosan Composite Hydrogel: Preparation and Characterization. Int. J. Nanomed. 2024, 19, 651–671. [Google Scholar] [CrossRef]
- Cao, J.; He, G.; Ning, X.; Chen, X.; Fan, L.; Yang, M.; Yin, Y.; Cai, W. Preparation and properties of O-chitosan quaternary ammonium salt/polyvinyl alcohol/graphene oxide dual self-healing hydrogel. Carbohydr. Polym. 2022, 287, 119318. [Google Scholar] [CrossRef]
- Xu, J.; Song, W.; Wu, N.; Tong, J.; Ren, L. Preparation and characterization of chitosan/polyvinyl porous alcohol aerogel microspheres with stable physicochemical properties. Int. J. Biol. Macromol. 2021, 187, 614–623. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Miriam, L.R.J.; Kumar, R.P.A.; Jose, P.J.M.; Kings, A.J. Amine functionalised graphene embedded polyvinyl alcohol (PVA) and PVA-chitosan hydrogel composites. Int. J. Biol. Macromol. 2024, 267, 131497. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Y.; Javeed, A.; Chen, F.; Li, J.; Guan, Y.; Chen, B.; Han, B. Preparation and properties of polyvinyl alcohol/chitosan-based hydrogel with dual pH/NH3 sensor for naked-eye monitoring of seafood freshness. Int. J. Biol. Macromol. 2024, 263, 130440. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kong, N.; Hou, Z.; Men, L.; Yang, P.; Wang, Z. Sponge-like porous polyvinyl alcohol/chitosan-based hydrogel with integrated cushioning, pH-indicating and antibacterial functions. Int. J. Biol. Macromol. 2024, 272, 132904. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, M.S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chen, K.; Qiu, H. Preparation and properties of antibacterial composite hydrogels based on polyvinyl alcohol, chitosan, and nano-metal oxide. Cellulose 2024, 31, 3607–3622. [Google Scholar] [CrossRef]
- Xu, M.; Luo, H.; Rong, H.; Wu, S.; Zheng, Z.; Chen, B. Calcium alginate gels-functionalized polyurethane foam decorated with silver nanoparticles as an antibacterial agent for point-of-use water disinfection. Int. J. Biol. Macromol. 2023, 231, 123289. [Google Scholar] [CrossRef]
- Jiang, L.a.; Tian, S.; Xie, Y.; Lv, X.; Sun, S. High Strength, Conductivity, and Bacteriostasis of the P(AM-co-AA)/Chitosan Quaternary Ammonium Salt Composite Hydrogel through Ionic Crosslinking and Hydrogen Bonding. Langmuir 2023, 39, 8698–8709. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, J.; Lai, J.; Zheng, X.; Zhang, L.-M. Multifunctional chitosan-based gel sponge with efficient antibacterial, hemostasis and strong adhesion. Int. J. Biol. Macromol. 2024, 256, 128505. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, W.; Liu, H.; Yuan, T.; Yang, Y.; Zhou, W.; Hu, Y.; Yang, Z. Preparation of 3D Printed Chitosan/Polyvinyl Alcohol Double Network Hydrogel Scaffolds. Macromol. Biosci. 2021, 21, 2000398. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sánchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302. [Google Scholar] [CrossRef]
- Singh, N.; Paknikar, K.M.; Rajwade, J. Gene expression is influenced due to ‘nano’ and ‘ionic’ copper in pre-formed Pseudomonas aeruginosa biofilms. Environ. Res. 2019, 175, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int. J. Biol. Macromol. 2020, 143, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Targhi, A.A.; Moammeri, A.; Jamshidifar, E.; Abbaspour, K.; Sadeghi, S.; Lamakani, L.; Akbarzadeh, I. Synergistic effect of curcumin-Cu and curcumin-Ag nanoparticle loaded niosome: Enhanced antibacterial and anti-biofilm activities. BioOrg. Chem. 2021, 115, 105116. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y.C. Antibacterial, antioxidant, Cr (VI) adsorption and dye adsorption effects of biochar-based silver nanoparticles-sodium alginate-tannic acid composite gel beads. Int. J. Biol. Macromol. 2024, 271, 132453. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, U.T.; Velidandi, A.; Rao, G.N. Nano-sized copper particles: Chemical synthesis, characterization, and their size and surface charge dependent antibacterial potential. Results Chem. 2024, 7, 101258. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Ren, F.; Cao, H.; Yuan, M.; Ye, T.; Xu, F. Application of antimicrobial properties of copper. Appl. Organomet. Chem. 2024, 38, 7506. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Zhang, T.; He, Y. Antibacterial Properties of Dandelion Extract-Based PVA/CTS/DAN/CuNP Composite Gel. Processes 2024, 12, 1809. https://doi.org/10.3390/pr12091809
Huang M, Zhang T, He Y. Antibacterial Properties of Dandelion Extract-Based PVA/CTS/DAN/CuNP Composite Gel. Processes. 2024; 12(9):1809. https://doi.org/10.3390/pr12091809
Chicago/Turabian StyleHuang, Meizi, Tingting Zhang, and Yucai He. 2024. "Antibacterial Properties of Dandelion Extract-Based PVA/CTS/DAN/CuNP Composite Gel" Processes 12, no. 9: 1809. https://doi.org/10.3390/pr12091809




