Advancements in Methane Dry Reforming: Investigating Nickel–Zeolite Catalysts Enhanced by Promoter Integration
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
3. Results
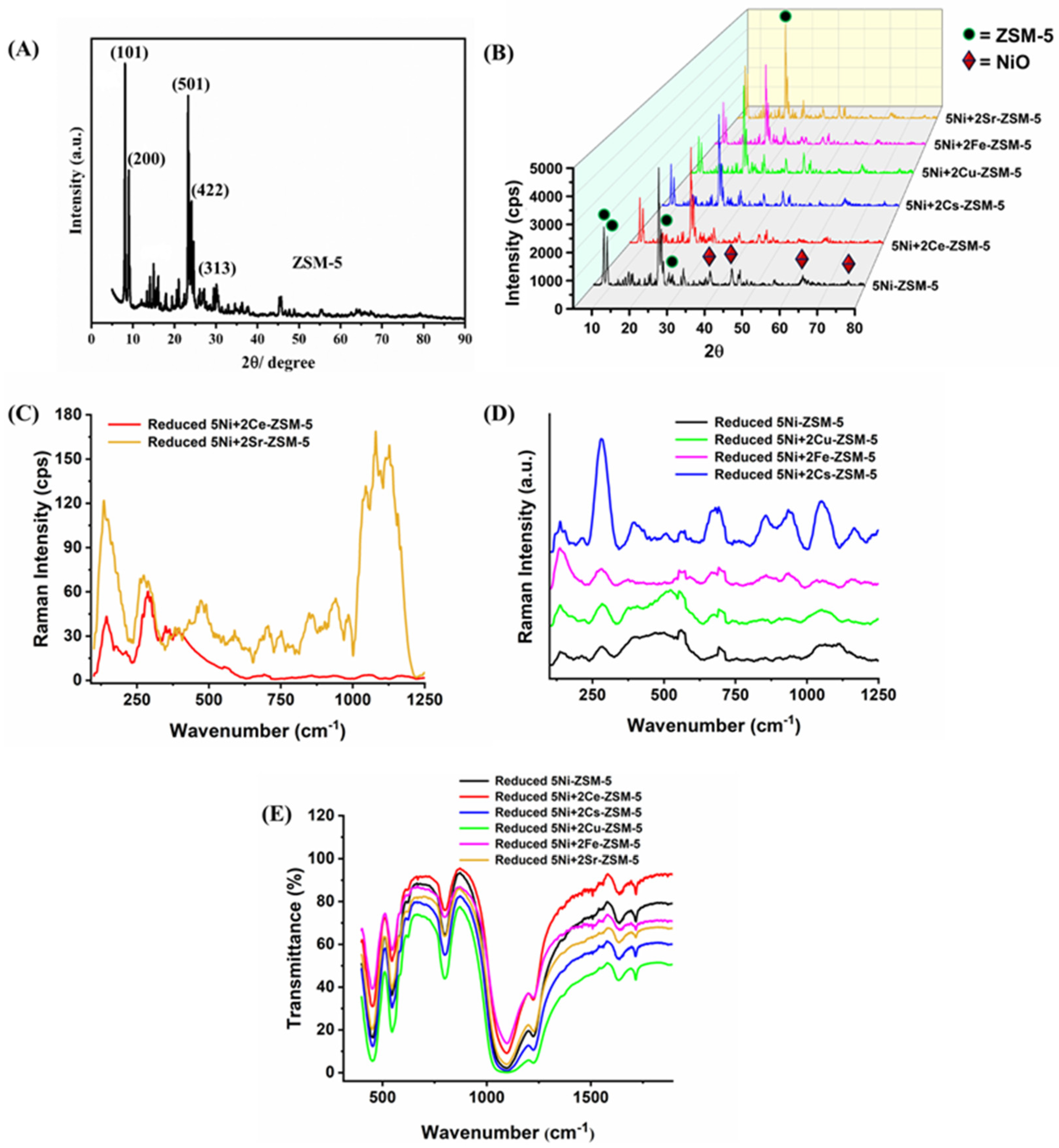
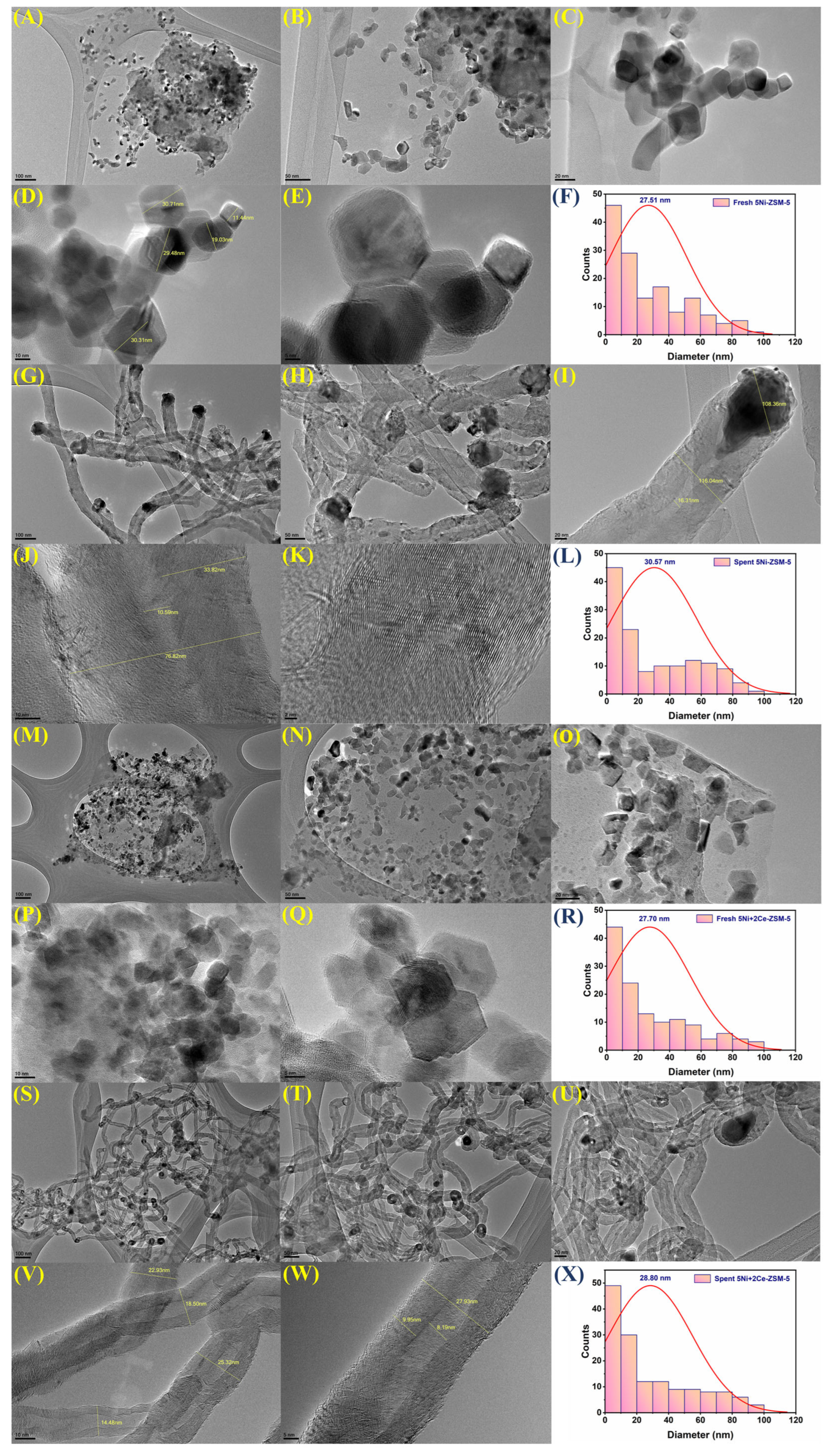
3.1. Characterizations
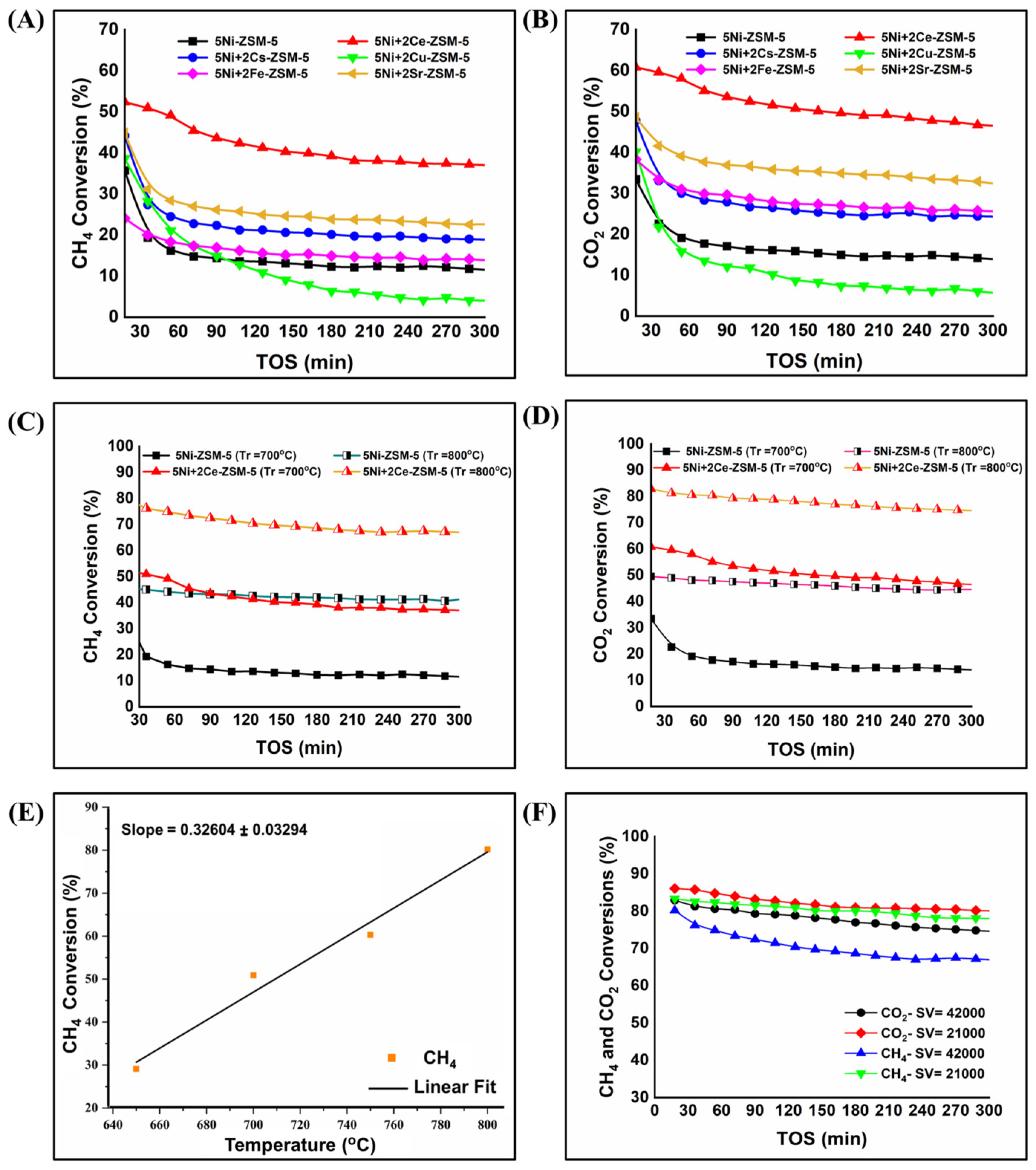
3.2. Catalyst Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Azam, W. Natural Resource Scarcity, Fossil Fuel Energy Consumption, and Total Greenhouse Gas Emissions in Top Emitting Countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Zeng, C.; Stringer, L.C.; Lv, T. The Spatial Spillover Effect of Fossil Fuel Energy Trade on CO2 Emissions. Energy 2021, 223, 120038. [Google Scholar] [CrossRef]
- Durga Devi, A.; Pushpavanam, S.; Singh, N.; Verma, J.; Kaur, M.P.; Roy, S.C. Enhanced Methane Yield by Photoreduction of CO2 at Moderate Temperature and Pressure Using Pt Coated, Graphene Oxide Wrapped TiO2 Nanotubes. Results Eng. 2022, 14, 100441. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Borges, A.V.; Deemer, B.R.; Holgerson, M.A.; Liu, S.; Song, C.; Melack, J.; Raymond, P.A.; Duarte, C.M.; Allen, G.H.; et al. Half of Global Methane Emissions Come from Highly Variable Aquatic Ecosystem Sources. Nat. Geosci. 2021, 14, 225–230. [Google Scholar] [CrossRef]
- Ning, H.; Li, Y.; Zhang, C. Recent Progress in the Integration of CO2 Capture and Utilization. Molecules 2023, 28, 4500. [Google Scholar] [CrossRef] [PubMed]
- Alaedini, A.H.; Tourani, H.K.; Saidi, M. A Review of Waste-to-Hydrogen Conversion Technologies for Solid Oxide Fuel Cell (SOFC) Applications: Aspect of Gasification Process and Catalyst Development. J. Environ. Manag. 2023, 329, 117077. [Google Scholar] [CrossRef]
- Sternberg, A.; Jens, C.M.; Bardow, A. Life Cycle Assessment of CO2-Based C1-Chemicals. Green Chem. 2017, 19, 2244–2259. [Google Scholar] [CrossRef]
- Cao, P.; Adegbite, S.; Wu, T. Thermodynamic Equilibrium Analysis of CO2 Reforming of Methane: Elimination of Carbon Deposition and Adjustment of H2/CO Ratio. Energy Procedia 2017, 105, 1864–1869. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; No, M.L.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Dry Reforming of Methane over Sub-Stoichiometric NiAl2O4-Mediated Ni/Al2O3 Catalysts. Fuel 2024, 358, 130166. [Google Scholar] [CrossRef]
- Velisoju, V.K.; Virpurwala, Q.J.S.; Attada, Y.; Bai, X.; Davaasuren, B.; Ben Hassine, M.; Yao, X.; Lezcano, G.; Kulkarni, S.R.; Castano, P. Overcoming the Kinetic and Deactivation Limitations of Ni Catalyst by Alloying It with Zn for the Dry Reforming of Methane. J. CO2 Util. 2023, 75, 102573. [Google Scholar] [CrossRef]
- Bamatraf, N.A.; Alreshaidan, S.B.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Abahussain, A.A.M.; Alotibi, M.F.; Bagabas, A.A.; Al-Fatesh, A.S. Different Supported Ni Catalysts for Dry Reforming of Methane: Effect of Calcination Temperature. J. King Saud Univ.-Sci. 2023, 35, 102958. [Google Scholar] [CrossRef]
- Jamsaz, A.; Pham-Ngoc, N.; Wang, M.; Jeong, D.H.; Oh, E.S.; Shin, E.W. Synergistic Effect of Macroporosity and Crystallinity on Catalyst Deactivation Behavior over Macroporous Ni/CexZr1-XO2–Al2O3 for Dry Reforming of Methane. Chem. Eng. J. 2023, 476, 146821. [Google Scholar] [CrossRef]
- de la Cruz-Flores, V.G.; Martinez-Hernandez, A.; Gracia-Pinilla, M.A. Deactivation of Ni-SiO2 Catalysts That Are Synthetized via a Modified Direct Synthesis Method during the Dry Reforming of Methane. Appl. Catal. A Gen. 2020, 594, 117455. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Alipour, Z.; Babu Borugadda, V.; Wang, H.; Dalai, A.K. Syngas Production through Dry Reforming: A Review on Catalysts and Their Materials, Preparation Methods and Reactor Type. Chem. Eng. J. 2023, 452, 139416. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Wang, S.; Li, X. Hydrogen Production via Steam Reforming of Acetic Acid over Biochar-Supported Nickel Catalysts. Int. J. Hydrogen Energy 2018, 43, 18160–18168. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Wang, S. Effect of La2O3 Replacement on Γ-Al2O3 Supported Nickel Catalysts for Acetic Acid Steam Reforming. Int. J. Hydrogen Energy 2017, 42, 20540–20548. [Google Scholar] [CrossRef]
- Kaengsilalai, A.; Luengnaruemitchai, A.; Jitkarnka, S.; Wongkasemjit, S. Potential of Ni Supported on KH Zeolite Catalysts for Carbon Dioxide Reforming of Methane. J. Power Sources 2007, 165, 347–352. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, L.; Tian, H.; Chen, K.; Yang, B.; Cao, W.; Zhang, Q.; Xu, J. N2 RF-Plasma-Assisted Preparation of Small Size Ni–Ce Catalysts for Dry Reforming of Methane with High Activity and Great Coke-Resistant Performance. Vacuum 2024, 220, 112784. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Al-Fatesh, A.S.; Singh, S.K.; Almutairi, G.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Frusteri, L.; Labhasetwar, N.K. Cs Promoted Ni/ZrO2-Al2O3 Catalysts for Dry Reforming of Methane: Promotional Effects of Cs for Enhanced Catalytic Activity and Stability. Arab. J. Chem. 2024, 17, 105564. [Google Scholar] [CrossRef]
- Nguyen, H.H.T.; Pham, C.Q.; Phuong, P.T.T.; Kim Hoang Pham, L.; Tuong Vi Tran, T.; Trinh, T.H.; Nguyen, Q.A.; Anh Nguyen, T.; Nguyen, T.M.; Vo, D.V.N. Enhanced Hydrogen Production and Carbon-Resistance in the Dry Reforming of Methane over M−Ni/KIT-6 Catalysts (M = Fe or Cu): Role of the Promoters. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Alrashed, M.M.; El-Salamony, R.A.; Roushdy, M.H.; Alwan, S.M.; Osman, A.I.; Bayazed, M.; Fakeeha, A.H.; Ibrahim, A.A.; Kumar, R. Tailoring Strontium-Promoted Alumina-Zirconia Supported Ni-Catalysts for Enhanced CO2 Utilization via Dry Reforming of Methane: Sr Loading Effects and Process Optimization. J. CO2 Util. 2023, 75, 102578. [Google Scholar] [CrossRef]
- Owgi, A.H.K.; Jalil, A.A.; Aziz, M.A.A.; Alhassan, M.; Hambali, H.U.; Nabgan, W.; Saravanan, R.; Hatta, A.H. Effect of Promoters (Ce, Sr, Cs, and Sm) on the Activity and Coke Formation of FSA Support Ni in the Dry Reforming of Methane. Fuel 2023, 340, 127592. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-Based Catalysts for Reforming of Methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Khan, W.U.; Khan, M.R.; Busquets, R.; Ahmad, N. Contribution of Oxide Supports in Nickel-Based Catalytic Elimination of Greenhouse Gases and Generation of Syngas. Energies 2021, 14, 7324. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E. Ni/Y-Zeolite Catalysts for Carbon Dioxide Reforming of Methane. Adv. Mater. Res. 2012, 550–553, 325–328. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Diao, X.; Liang, J.; Wu, C.; Sun, Y. Effective Catalytic Steam Reforming of Naphthalene over Ni-Modified ZSM-5 via One-Pot Hydrothermal Synthesis. Waste Manag. 2022, 147, 1–9. [Google Scholar] [CrossRef]
- Bizkarra, K.; Barrio, V.L.; Gartzia-Rivero, L.; Bañuelos, J.; López-Arbeloa, I.; Cambra, J.F. Hydrogen Production from a Model Bio-Oil/Bio-Glycerol Mixture through Steam Reforming Using Zeolite L Supported Catalysts. Int. J. Hydrogen Energy 2019, 44, 1492–1504. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface Area and Pore Texture of Catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Protim Mudoi, M.; Singh, V. Pore Size Estimation of Indian Coal through Low-Pressure N2 Adsorption. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhai, K.; Xue, W.; Wang, H.; Wu, X.; Zhai, S. Raman Spectra of Sillimanite, Andalusite, and Kyanite at Various Temperatures. Phys. Chem. Miner. 2020, 47, 23. [Google Scholar] [CrossRef]
- Miecznikowski, A.; Hanuza, J. Infrared and Raman Studies of ZSM-5 and Silicalite-1 at Room, Liquid Nitrogen and Helium Temperatures. Zeolites 1987, 7, 249–254. [Google Scholar] [CrossRef]
- McMillan, P.; Piriou, B. The Structures and Vibrational Spectra of Crystals and Glasses in the Silica-Alumina System. J. Non. Cryst. Solids 1982, 53, 279–298. [Google Scholar] [CrossRef]
- Ghule, A.V.; Ghule, K.; Punde, T.; Liu, J.Y.; Tzing, S.H.; Chang, J.Y.; Chang, H.; Ling, Y.C. In Situ Monitoring of NiO-Al2O3 Nanoparticles Synthesis by Thermo-Raman Spectroscopy. Mater. Chem. Phys. 2010, 119, 86–92. [Google Scholar] [CrossRef]
- Vafaeian, Y.; Haghighi, M.; Aghamohammadi, S. Ultrasound Assisted Dispersion of Different Amount of Ni over ZSM-5 Used as Nanostructured Catalyst for Hydrogen Production via CO2 Reforming of Methane. Energy Convers. Manag. 2013, 76, 1093–1103. [Google Scholar] [CrossRef]
- Acharya, K.; Al-Fatesh, A.S.; Almutairi, G.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Siddiqui, M.R.H.; Kumar, R. The Role of Strontium as an Economic Promoter Over WO3 + ZrO2 Supported Ni Catalyst for H2 Production through Dry Reforming of Methane. Catal. Lett. 2023, 154, 2023–2035. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Sun, J.; Gao, W.; Cui, Y. Effect of Metal Additives on the Catalytic Performance of Ni/Al2O3 Catalyst in Thermocatalytic Decomposition of Methane. Int. J. Hydrogen Energy 2019, 44, 7205–7215. [Google Scholar] [CrossRef]
- Puigdollers, A.R.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493. [Google Scholar] [CrossRef]
- Sharma, S.; Hu, Z.; Zhang, P.; McFarland, E.W.; Metiu, H. CO2 methanation on Ru-doped ceria. J. Catal. 2011, 278, 297–309. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Effect of Ce on 5 wt.% Ni/ZSM-5 Catalysts in the CO2 Reforming of CH4 Reaction. Int. J. Hydrogen Energy 2014, 39, 15482–15496. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M. V X-Ray Diffraction Study of Nickel Oxide Reduction by Hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Rasi, N.M.; Karcz, A.; Ponnurangam, S.; Mahinpey, N. Insight into MgO-Supported NiO Reactivity from Atomic-Scale Electronegativity for Oxygen Carrier Design and Catalyst Production Applications. Catal. Today 2022, 404, 244–252. [Google Scholar] [CrossRef]
- Zhou, R.-X.; Yu, T.-M.; Jiang, X.-Y.; Chen, F.; Zheng, X.-M. Temperature-Programmed Reduction and Temperature-Programmed Desorption Studies of CuO/ZrO2 Catalysts. Appl. Surf. Sci. 1999, 148, 263–270. [Google Scholar] [CrossRef]
- Luo, M.F.; Fang, P.; He, M.; Xie, Y.L. In Situ XRD, Raman, and TPR Studies of CuO/Al2O3 Catalysts for CO Oxidation. J. Mol. Catal. A Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S.; Abasaeed, A.E.; Khan, W.U. Methane Decomposition over Iron Catalyst for Hydrogen Production. Int. J. Hydrogen Energy 2015, 40, 7593–7600. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction Behavior of Iron Oxides in Hydrogen and Carbon Monoxide Atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Patel, R.; Al-Fatesh, A.S.; Fakeeha, A.H.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Al-Zahrani, S.A.; Abasaeed, A.E.; Srivastava, V.K.; Kumar, R. Impact of Ceria over WO3–ZrO2 Supported Ni Catalyst towards Hydrogen Production through Dry Reforming of Methane. Int. J. Hydrogen Energy 2021, 46, 25015–25028. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Zhang, X.; Tu, B.; Ou, D.; Cheng, M. Carbonates Formed during BSCF Preparation and Their Effects on Performance of SOFCs with BSCF Cathode. Int. J. Hydrogen Energy 2012, 37, 19036–19044. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.; Guo, X.; Wang, L.; Xie, M. Methane Activation without Using Oxidants over Mo/HZSM-5 Zeolite Catalysts. Catal. Lett. 1994, 30, 135–149. [Google Scholar] [CrossRef]
- Ashok, J.; Kumar, S.N.; Venugopal, A.; Kumari, V.D.; Subrahmanyam, M. COX-Free H2 Production via Catalytic Decomposition of CH4 over Ni Supported on Zeolite Catalysts. J. Power Sources 2007, 164, 809–814. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Kumar, R.; Sofiu, M.L.; Frusteri, F.; Ibrahim, A.A.; Srivastava, V.K.; Kasim, S.O.; Fakeeha, A.H.; Abasaeed, A.E.; Osman, A.I. Optimizing Acido-Basic Profile of Support in Ni Supported La2O3+ Al2O3 Catalyst for Dry Reforming of Methane. Int. J. Hydrogen Energy 2021, 46, 14225–14235. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Sehested, J.; Nørskov, J.K. Hydrogen and Synthesis Gas by Steam- and CO2 Reforming. Adv. Catal. 2002, 47, 65–139. [Google Scholar] [CrossRef]
- Chava, R.; Seriyala, A.K.; Varma, D.B.A.; Yeluvu, K.; Roy, B.; Appari, S. Investigation of Ba Doping in A-Site Deficient Perovskite Ni-Exsolved Catalysts for Biogas Dry Reforming. Int. J. Hydrogen Energy 2023, 48, 27652–27670. [Google Scholar] [CrossRef]
- Cong, C.; Yu, T.; Saito, R.; Dresselhaus, G.F.; Dresselhaus, M.S. Second-Order Overtone and Combination Raman Modes of Graphene Layers in the Range of 1690–2150 cm−1. ACS Nano 2011, 5, 1600–1605. [Google Scholar] [CrossRef]
- Patel, N.; Al-Fatesh, A.S.; Bamatraf, N.A.; Osman, A.I.; Alreshaidan, S.B.; Fakeeha, A.H.; Wazeer, I.; Kumar, R. 5Ni/MgO and 5Ni/MgO + MOx (M = Zr, Ti, Al) Catalyst for Hydrogen Production via Dry Reforming of Methane: Promotor-Free, Cost-Effective, and Handy Catalyst System. Catal. Lett. 2024, 154, 3441–3456. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. In Situ Auto-Gasification of Coke Deposits over a Novel Ni-Ce/W-Zr Catalyst by Sequential Generation of Oxygen Vacancies for Remarkably Stable Syngas Production via CO2-Reforming of Methane. Appl. Catal. B Environ. 2021, 280, 119445. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. CeO2 Promoted Ni-MgO-Al2O3 Nanocatalysts for Carbon Dioxide Reforming of Methane. J. CO2 Util. 2018, 24, 128–138. [Google Scholar] [CrossRef]
- Peymani, M.; Alavi, S.M.; Rezaei, M. Preparation of Highly Active and Stable Nanostructured Ni/CeO2 Catalysts for Syngas Production by Partial Oxidation of Methane. Int. J. Hydrogen Energy 2016, 41, 6316–6325. [Google Scholar] [CrossRef]
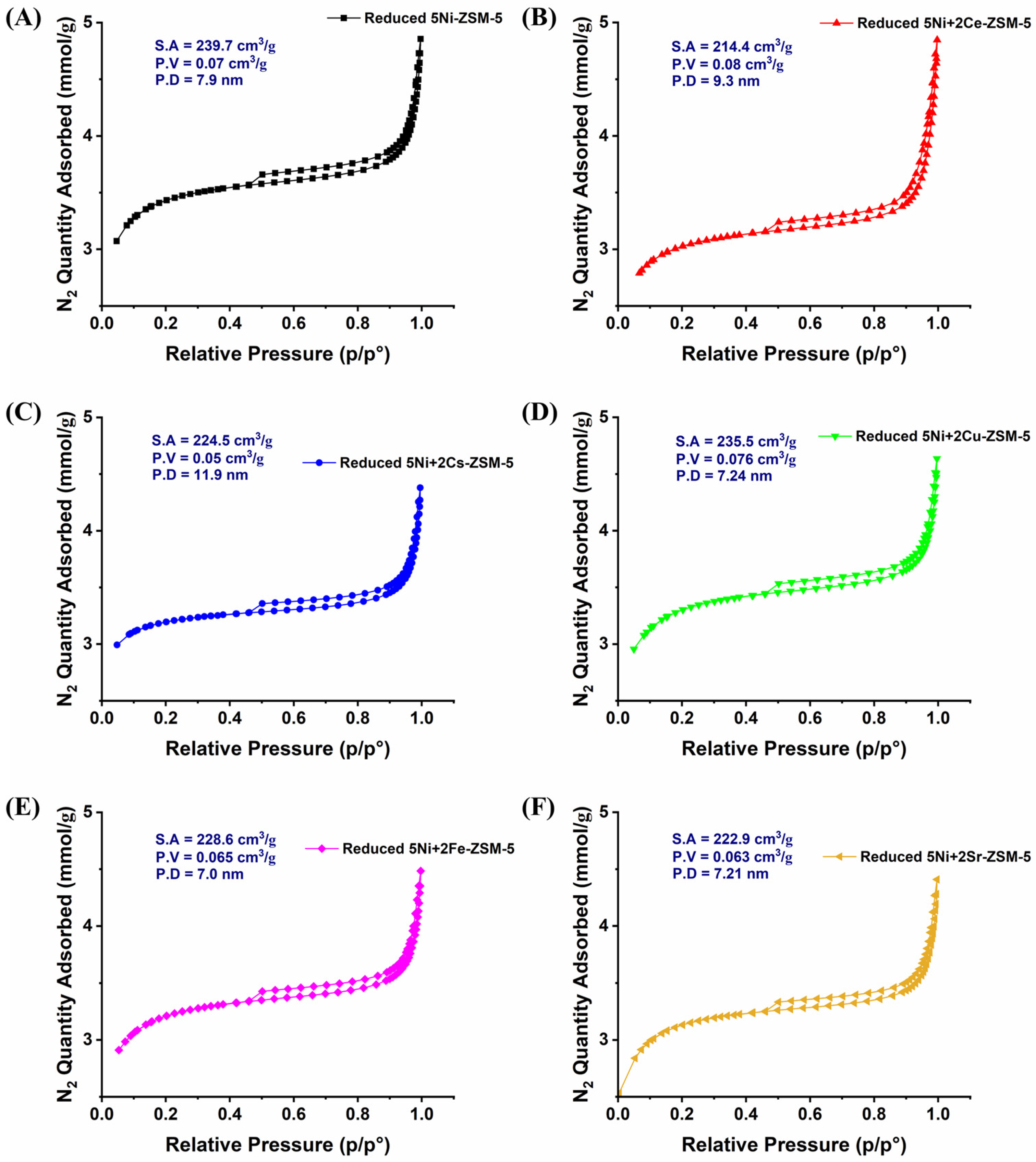



| Samples | BET-Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) |
|---|---|---|---|
| 5Ni-ZSM-5 | 235 | 0.088 | 54.2 |
| 5Ni+2Ce-ZSM-5 | 226 | 0.099 | 62.5 |
| 5Ni+2Cs-ZSM-5 | 222 | 0.071 | 71.5 |
| 5Ni+2Cu-ZSM-5 | 217 | 0.087 | 56.8 |
| 5Ni+2Fe-ZSM-5 | 264 | 0.103 | 52.3 |
| 5Ni+2Sr-ZSM-5 | 241 | 0.092 | 56.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakeeha, A.H.; Ibrahim, A.A.; Osman, A.I.; Abasaeed, A.E.; Alanazi, Y.M.; Almubaddel, F.S.; Al-Fatesh, A.S. Advancements in Methane Dry Reforming: Investigating Nickel–Zeolite Catalysts Enhanced by Promoter Integration. Processes 2024, 12, 1826. https://doi.org/10.3390/pr12091826
Fakeeha AH, Ibrahim AA, Osman AI, Abasaeed AE, Alanazi YM, Almubaddel FS, Al-Fatesh AS. Advancements in Methane Dry Reforming: Investigating Nickel–Zeolite Catalysts Enhanced by Promoter Integration. Processes. 2024; 12(9):1826. https://doi.org/10.3390/pr12091826
Chicago/Turabian StyleFakeeha, Anis H., Ahmed A. Ibrahim, Ahmed I. Osman, Ahmed E. Abasaeed, Yousef M. Alanazi, Fahad S. Almubaddel, and Ahmed S. Al-Fatesh. 2024. "Advancements in Methane Dry Reforming: Investigating Nickel–Zeolite Catalysts Enhanced by Promoter Integration" Processes 12, no. 9: 1826. https://doi.org/10.3390/pr12091826







