Hybrid Water Disinfection Process Using Electrical Discharges
Abstract
:1. Introduction
- -
- The rupture of the cell wall;
- -
- Changes in cell permeability;
- -
- The modification of the nature of the protoplasm (e.g., disruption of the colloidal structure);
- -
- The induction of abnormal redox processes;
- -
2. Materials and Methods
2.1. Experimental Setup for Electric Discharge Realization
2.2. Measurement of the Frequencies of the Electric Discharge’s Acoustic Radiation
3. Experimental Results
4. Discussion
4.1. Physical Phenomena Accompanying Electric Discharge Cavitation
4.2. Chemical Processes in Electric Discharge Cavitation
- -
- Reactions in the gaseous medium (cavitational pocket);
- -
- Reactions at the interface between the cavitational pocket and the liquid as a result of the interaction of radicals and atoms in the cavity with molecules at the interface;
- -
- Reactions caused by active substances formed in the gas phase entering the surrounding liquid (water) as a result of the collapse of cavitational bubbles;
- -
- Reactions in a liquid medium under the influence of local shock waves caused by collapsing cavitational pockets.
5. Conclusions
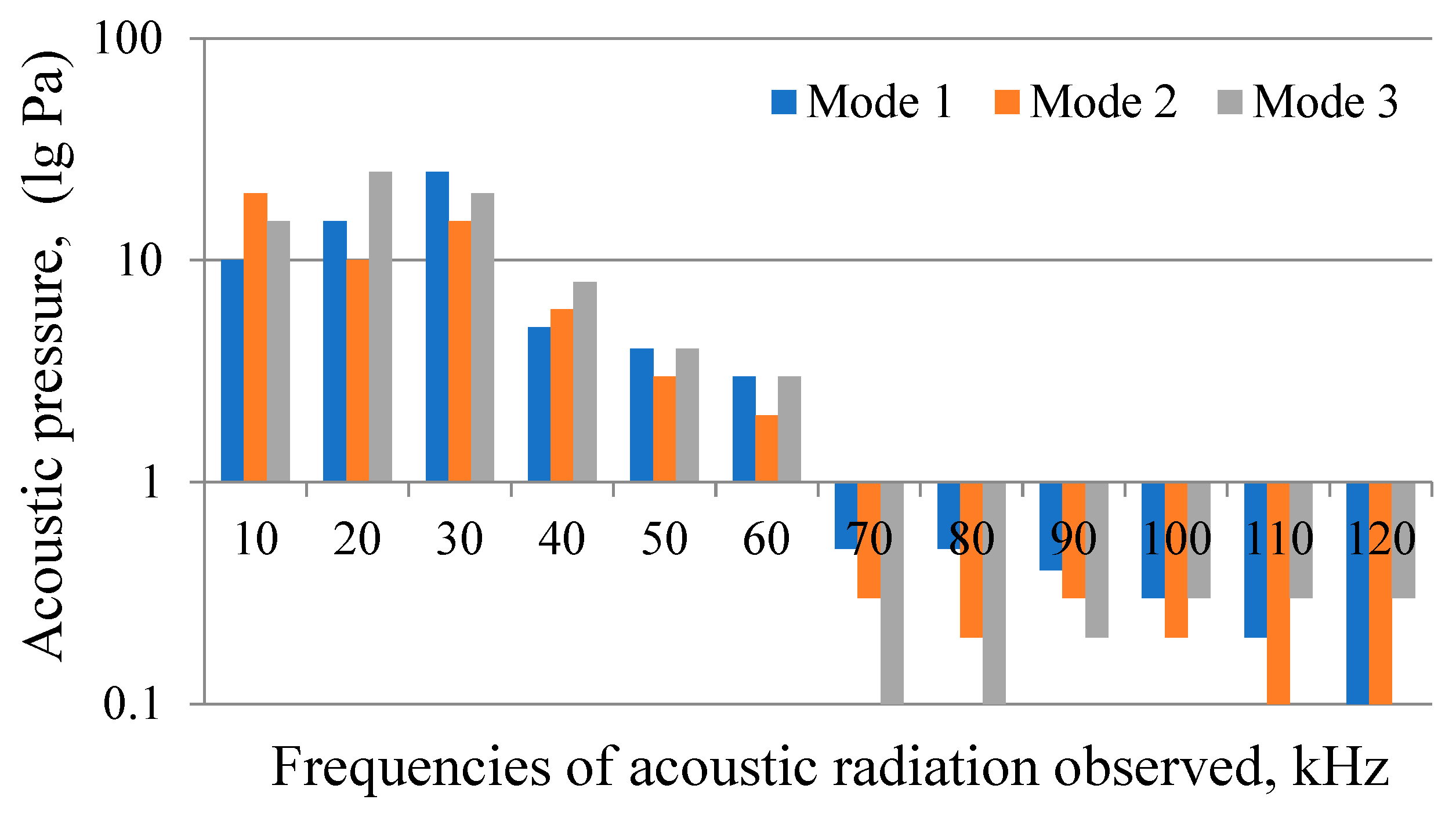
- Previously undefined frequencies of 70–120 kHz that appear in the acoustic radiation spectrum of underwater electric discharge in cavitation mode have been revealed along with confirmed frequencies of 15–65 kHz. Thus, electric discharge in the cavitation mode generates acoustic radiation frequencies corresponding to the resonance conditions of all bubbles initially present in the working medium. Acoustic waves of such a wide frequency range ensure resonant vibration of the bubbles and strong cavitation in the treated water. The phenomena associated with cavitation ensure the inactivation of microbiological objects in the purified water.
- It is experimentally established that the intensity of the high-frequency part of the spectrum of acoustic radiation of an electric discharge in water can be adjusted by changing the length of the uninsulated end of the electrode–anode protruding into the discharge gap and the inductance of the discharge circuit.
- High-frequency acoustic waves generated by a special mode of electrical discharge in water and the resulting powerful volumetric cavitation are the driving force for a significant reduction in disinfectants quantity, temperature, and treatment time for water disinfection. The primary effect of electric discharge cavitation is the generation of a large number of chemically active particles and the ensuing intensification of oxidation processes. This replaces most of the active chlorine required for disinfection with active particles formed from the water medium itself; these active particles are effective but are less hazardous to the environment. Other factors of electrical discharge in the cavitation mode are of secondary importance: local shock waves, acoustic flows and turbulence, flotation effects, and rectified diffusion.
- The similarity of the effect of ultrasound and electrical discharge in cavitation mode on water systems is demonstrated experimentally. Underwater electrical discharge as a source of cavitation does not have such intrinsic limitations as the erosion resistance of the ultrasonic emitters. At the same time, cavitation does not interfere with any stage of the discharge. In addition, both the intensity and the total power of cavitation processes induced by underwater electrical discharges are always higher compared to ultrasonic treatment due to the wider range of acoustic frequencies generated. As a result, the possibilities for industrial application of water disinfection by electrical discharge in the cavitation mode are much wider than in the case of ultrasonic treatment. This is crucial for the practical use of underwater electrical discharge not only to accelerate oxidation, but also to intensify other chemical engineering processes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bezsonov, Y.; Mitryasova, O.; Smyrnov, V.; Smyrnova, S. Influence of the South-Ukraine electric power producing complex on the ecological condition of the Southern Bug River. East.-Eur. J. Enterp. Technol. 2017, 4, 20–28. [Google Scholar] [CrossRef]
- Bernatska, N.; Dzhumelia, E.; Dyakiv, V.; Mitryasova, O.; Salamon, I. Web-Based Information and Analytical Monitoring System Tools–Online Visualization and Analysis of Surface Water Quality of Mining and Chemical Enterprises. Ecol. Eng. Environ. Technol. 2023, 24, 99–108. [Google Scholar] [CrossRef]
- Mitryasova, O.; Pohrebennyk, V.; Salamon, I.; Oleksiuk, A.; Mats, A. Temporal Patterns of Quality Surface Water Changes. J. Ecol. Eng. 2021, 22, 283–295. [Google Scholar]
- Mitryasova, O.; Pohrebennyk, V. Hydrochemical Indicators of Water System Analysis as Factors of the Environmental Quality State. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems. Studies in Systems, Decision, and Control; Królczyk, G., Wzorek, M., Król, A., Kochan, O., Su, J., Kacprzyk, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 198, pp. 91–104. [Google Scholar] [CrossRef]
- Smyrnov, V.; Mitryasova, O.; Salamon, I.; Smyrnova, S.; Chvyr, V.; Mats, A. The Distribution of Heavy Metals Mobile Forms in the Industrial Urban Agglomeration Soil. J. Ecol. Eng. 2013, 24, 317–327. [Google Scholar]
- Ponnusami, A.B.; Sinha, S.; Ashokan, H.; Paul, M.V.; Hariharan, S.P.; Arun, J.; Gopinath, K.P.; Le, Q.H.; Pugazhendhi, A. Advanced oxidation process (AOP) combined biological process for wastewater treatment: A review on advancements, feasibility and practicability of combined techniques. Environ. Res. 2023, 237, 116944. [Google Scholar] [CrossRef]
- Zver, M.; Dobnik, D.; Zaplotnik, R.; Mozetič, M.; Filipić, A.; Primc, G. Non-thermal plasma inactivation of viruses in water solutions. J. Water Process Eng. 2023, 53, 103839. [Google Scholar] [CrossRef]
- Olsińska, U. Characteristics of bromate formation prevention methods in water intended for human consumption. Environ. Prot. 2017, 39, 17–26. [Google Scholar]
- Huang, Y.; Zhang, H.; Zamyadi, A.; Andrews, S.; Hofmann, R. Predicted impact of aeration on toxicity from trihalomethanes and other disinfection byproducts. J.—Am. Water Work. Assoc. 2017, 109, 13–21. [Google Scholar] [CrossRef]
- Selbes, M.; Brown, J.; Lauderdale, C.; Karanfil, T. Removal of selected C- and N-DBP precursors in biologically active filters. J. —Am. Water Work. Assoc. 2017, 109, E73–E84. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.; Ma, J.; Wang, J.; Wang, L.; Guo, M. Study on the mechanism of cerium oxide catalytic ozonation for controlling the formation of bromate in drinking water. Desalination Water Treat. 2016, 57, 15533–15546. [Google Scholar] [CrossRef]
- WHO. Bromine as a Drinking-water Disinfectant; WHO: Geneva, Switzerland, 2018.
- Dymaczewski, Z.; Jeż-Walkowiak, J.; Michałkiewicz, M.; Sozański, M.M. The importance of the disinfection process in ensuring the microbiological safety of water intended for human consumption. Environ. Prot. 2019, 41, 1. [Google Scholar]
- Fisher, I.; Kastl, G.; Sathasivan, A. New model of chlorine-wall reaction for simulating chlorine concentration in drinking water distribution systems. Water Res. 2017, 125, 427–437. [Google Scholar] [CrossRef]
- Gelete, G.; Gokcekus, H.; Ozsahin, D.U.; Uzun, B.; Gichamo, T. Evaluating disinfection techniques of water treatment. Desalination Water Treat. 2020, 177, 408–415. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas. 2017, 24, 055501. [Google Scholar] [CrossRef]
- Malyushevskaya, A.; Koszelnik, P.; Yushchishina, A.; Mitryasova, O.; Mats, A.; Gruca-Rokosz, R. Eco-Friendly Principles on the Extraction of Humic Acids Intensification from Biosubstrates. J. Ecol. Eng. 2023, 24, 317–327. [Google Scholar] [CrossRef]
- Malyushevskaya, A.P. Properties of starch size treated by an electric discharge in the mode of nonlinear volume cavitation. Surf. Eng. Appl. Electrochem. 2011, 47, 555–557. [Google Scholar] [CrossRef]
- Malyushevskaya, A.P.; Malyushevskii, P.P. A novel method to control electrical-discharge nonlinear bulk cavitation. Surf. Eng. Appl. Electrochem. 2007, 43, 59–64. [Google Scholar] [CrossRef]
- Song, K.; Liu, Y.; Umar, A.; Ma, H.; Wang, H. Ultrasonic cavitation: Tackling organic pollutants in wastewater. Chemosphere 2024, 350, 141024. [Google Scholar] [CrossRef]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on discharge Plasma for water treatment: Mechanism, reactor geometries, active species and combined processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- Malyushevskaya, A.P.; Koszelnik, P.; Yushchishina, A.; Mitryasova, O.; Mats, A.; Gruca-Rokosz, R. Synergy Effect during Water Treatment by Electric Discharge and Chlorination. Environments 2023, 10, 93. [Google Scholar] [CrossRef]
- Akdoğan, E.; Şirin, H.T. Plasma surface modification strategies for the preparation of antibacterial biomaterials: A review of the recent literature. Mater. Sci. Eng. C 2021, 131, 112474. [Google Scholar] [CrossRef]
- Wu, U.; Nyborg, W.L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008, 60, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, W.; Mettin, R. 3-Acoustic Cavitation: Bubble Dynamics in High-Power Ultrasonic Fields; Gallego-Juárez, J.A., Graff, K.F., Ultrasonics, P., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 37–78. [Google Scholar] [CrossRef]
- Malyushevskaya, A.P.; Malyushevskij, P.P.; Levda, V.I. Electroexplosive nonlinear, volumetric cavitation in technological reactors. Part I (Electrodischarge generation of a gas phase—Nucleus of cavitation). Electron. Process. Mater. 2004, 40, 46–53. [Google Scholar]
- Malyushevskaya, A.P.; Kataev, N.M.; Malyushevsky, P.P. Ustanovlenye akustycheskykh kharakterystyk podvodnykh elektrycheskykh razriadov y sredy. Elektronnaia obrabotka materyalov [Establishing the acoustic characteristics of underwater electrical discharges in environments]. Electron. Mater. Process. 1999, 6, 33–36. [Google Scholar]
- Islam, M.H.; Pollet, B.G. Chapter 15—Acoustic cavitation and sonochemistry in industry: State of the art. In Energy Aspects of Acoustic Cavitation and Sonochemistry; Hamdaoui, O., Kerboua, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 265–279. [Google Scholar] [CrossRef]
- Vargel, C. Chapter C.11—Erosion and cavitation. In Corrosion of Aluminium, 2nd ed.; Vargel, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–288. [Google Scholar] [CrossRef]
- Grigoriev, B.A.; GerasimovA, A.; Alexandrov, I.S.; Nemzer, B.V. Chapter 5—Fundamental equations of state of individual substances. In Thermophysical Properties of Individual Hydrocarbons of Petroleum and Natural Gases; Grigoriev, B.A., Gerasimov, A.A., Alexandrov, I.S., Nemzer, B.V., Eds.; Gulf Professional Publishing: Oxford, UK, 2022; pp. 335–395. [Google Scholar] [CrossRef]
- Zhou, Y. Turbulence theories and statistical closure approaches. Phys. Rep. 2021, 935, 1–117. [Google Scholar] [CrossRef]
- Bulatovic, S.M. 6—Summary of the Theoretical Aspects of Flotation. In Handbook of Flotation Reagents; Bulatovic, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 87–124. [Google Scholar] [CrossRef]
- Crum, L.A. Acoustic cavitation series: Part five rectified diffusion. Ultrasonics 1984, 22, 215–223. [Google Scholar] [CrossRef]
- Frommelt, T.; Schneider, M.; Wixforth, A. 8—Acoustic Methods for Manipulating Droplets. In Micro and Nano Technologies; Berthier, J., Ed.; William Andrew Publishing: Norwich, NY, USA, 2008; pp. 353–388. [Google Scholar] [CrossRef]
- Blake, W.K. Chapter 1—Hydrodynamically Induced Cavitation and Bubble Noise. In Mechanics of Flow-Induced Sound and Vibration, 2nd ed.; Blake, W.K., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–80. [Google Scholar] [CrossRef]
- Hegedűs, F.; Kalmár, C.; Turányi, T.; Gy, I.; Papp, Z. Chapter 4—Sonochemical reactions, when, where and how: Modelling approach. In Energy Aspects of Acoustic Cavitation and Sonochemistry; Hamdaoui, O., Kerboua, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–77. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitation in Biotechnology. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 957–965. [Google Scholar] [CrossRef]
- Ming, L.; Zhi, N.; Chunhua, S. Numerical simulation of cavitation bubble collapse within a droplet. Comput. Fluids 2007, 152, 157–163. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. A review on the current techniques and technologies of organic pollutants removal from water/wastewater. Mater. Today Proc. 2020, 31, A158–A165. [Google Scholar] [CrossRef]
- Agarkoti, C.; Gogate, P.R.; Pandit, A.B. Coupling of acoustic/hydrodynamic cavitation with ozone (O3), hydrogen peroxide (H2O2), magnesium oxide (MgO) and manganese dioxide (MnO2) for the effective treatment of CETP effluent. Sep. Purif. Technol. 2022, 284, 120281. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Effect of carbon tetrachloride (CCl4) sonochemistry on the size of active bubbles for the production of reactive oxygen and chlorine species in acoustic cavitation field. Chem. Eng. J. 2021, 426, 130251. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, X.; Shao, Z.; Xiang, K.; Huang, W.; Tian, H.; Huang, Y. Investigation of singlet oxygen and superoxide radical produced from vortex-based hydrodynamic cavitation: Mechanism and its relation to cavitation intensity. Sci. Total Environ. 2024, 929, 172761. [Google Scholar] [CrossRef] [PubMed]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T.F. Dissolved gas and ultrasonic cavitation—A review. Ultrason. Sonochemistry 2013, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yushchyshyna, A.N.; Maliushevskii, P.P.; Smalko, A.A.; Petrichenko, L.A.; Tyhonenko, S.M. Iodometric determination of electric discharge volume cavitation. Electron. Process. Mater. 2002, 2, 76–80. [Google Scholar]
- Gągol, M.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs). Ultrason. Sonochemistry 2019, 58, 104610. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Lan, Y.; Nie, S.; Qin, T.; Nie, S.; Zhou, J. Synergistic effect of hydrodynamic cavitation characteristics of self-excited oscillation cavity for degradation of dye wastewater. J. Clean. Prod. 2022, 380, 135116. [Google Scholar] [CrossRef]
- Kudo, K.; Ito, H.; Ihara, S.; Terato, H. Quantitative analysis of oxidative DNA damage induced by high-voltage pulsed discharge with cavitation. J. Electrost. 2015, 73, 131–139. [Google Scholar] [CrossRef]
- Fuciarelli, A.F.; Sisk, E.C.; Thomas, R.M.; Miller, D.L. Induction of base damage in DNA solutions by ultrasonic cavitation. Free Radic. Biol. Med. 1995, 18, 231–238. [Google Scholar] [CrossRef]
- Kamal, H.; Ali, A.; Manickam, S.; Le, C.F. Impact of cavitation on the structure and functional quality of extracted protein from food sources—An overview. Food Chem. 2023, 407, 135071. [Google Scholar] [CrossRef]

| Mode No. | Length of the Uninsulated End of the Anode, mm | Discharge Circuit Inductance, μH | Current Pulse Period, μs | Duration of Current First Half-Wave, μs | Duration of the Leading Edge of the Current Pulse, μs | Current Pulse Amplitude, kA |
|---|---|---|---|---|---|---|
| 1 | 4 | 1.2 | 5.4 | 2.7 | 1.35 | 16 |
| 2 | 10 | 1.2 | 6.5 | 3.25 | 1.62 | 12 |
| 3 | 7 | 2.6 | 9.0 | 4.5 | 1.8 | 14 |
| Specific Energy of Discharge Treatment (Cavitation Mode), kJ/dm3 | E. coli Concentration after Treatment, (lg CFU/dm3) | ||||
|---|---|---|---|---|---|
| Additional Chemical Agent | |||||
| Cl, 1 mg/dm3 | Cl, 0.8 mg/dm3 | Cl, 0.6 mg/dm3 | Cl, 0.4 mg/dm3 | Cl, 0.2 mg/dm3 | |
| 5 | 4 | 4.3 | 4.6 | 5 | 5.2 |
| 15 | 1 | 2.5 | 3 | 3.2 | 3.4 |
| 25 | 0 | 0 | 0 | 0 | 0.5 |
| Initial Bubble Radius, R0, m | Maximum Bubble Radius, Rmax, m | Minimum Bubble Radius Rmin, m | Gas Content, δ | Pressure Developed When the Bubble Collapses pmax, Pa | Ratio of the Sound Speed in the Medium to the Velocity of the Leading Edge of the Microcavitation Wave |
|---|---|---|---|---|---|
| 1·10−3 | 2.9·10−3 | 5.8·10−5 | 4.8·10−2 | 7.4·103 | 4.5 |
| 5·10−4 | 2.6·10−3 | 2.5·10−5 | 1.0·10−2 | 1.9·104 | 6.2 |
| 1·10−4 | 2.3·10−3 | 0.9·10−6 | 2.1·10−4 | 2.8·107 | 13.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyushevskaya, A.P.; Koszelnik, P.; Mitryasova, O.; Yushchishina, A.; Mats, A.; Papciak, D.; Zdeb, M.M. Hybrid Water Disinfection Process Using Electrical Discharges. Processes 2024, 12, 1846. https://doi.org/10.3390/pr12091846
Malyushevskaya AP, Koszelnik P, Mitryasova O, Yushchishina A, Mats A, Papciak D, Zdeb MM. Hybrid Water Disinfection Process Using Electrical Discharges. Processes. 2024; 12(9):1846. https://doi.org/10.3390/pr12091846
Chicago/Turabian StyleMalyushevskaya, Antonina P., Piotr Koszelnik, Olena Mitryasova, Anna Yushchishina, Andrii Mats, Dorota Papciak, and Monika Magdalena Zdeb. 2024. "Hybrid Water Disinfection Process Using Electrical Discharges" Processes 12, no. 9: 1846. https://doi.org/10.3390/pr12091846






