Parameter Optimization and Capacitance-Based Monitoring of In Situ Cell Detachment in Microcarrier Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Static Culture in T-Flask
2.2. MC Cell Culture in Bioreactor
2.3. In Situ Cell Detachment in Bioreactor and Subculturing to the Subsequent Bioreactor
2.4. Selection of Critical Parameters for Experimental Design in In Situ Cell Detachment Studies
2.5. Analytical Techniques
2.5.1. Cell Count Determination
2.5.2. Trypsin Activity Assay
2.5.3. Apoptosis and Necrosis Analysis
2.5.4. Online Viable Cell Monitoring Using Capacitance Sensor
2.6. Experimental Design for Model Development of Cell Detachment from MCs
2.7. Model Development of In Situ Cell Detachment from MC
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Agitation Speed on Cell Detachment from MCs and Subsequent Cell Growth
| Trypsin ratio (mL/gMC) | 50 | 90 | ||
| Volume (V) (mL) * | 245 | 329 | ||
| Agitation speed (N) (rpm) | 100 | 200 | 100 | 200 |
| 0.261 | 0.523 | 0.261 | 0.523 | |
| 4429 | 8859 | 4429 | 8859 | |
| 0.0088 | 0.0708 | 0.0066 | 0.052 | |
| 98.5 | 58.5 | 106.0 | 63.0 | |
| Smallest possible eddy size λ min (μm) ** | 68.9–78.3 | 40.9–46.5 | 68.9–78.3 | 40.9–46.5 |
3.2. Effect of Incubation Time on Cell Detachment from MCs and Subsequent Cell Growth
3.3. Effect of Trypsin Volume on Cell Detachment from MCs and Subsequent Cell Growth
3.4. Effect of Washing Steps on Cell Detachment Efficiency and Subsequent Cell Growth
3.5. Monitoring Cell Growth on MCs Using Capacitance Sensor
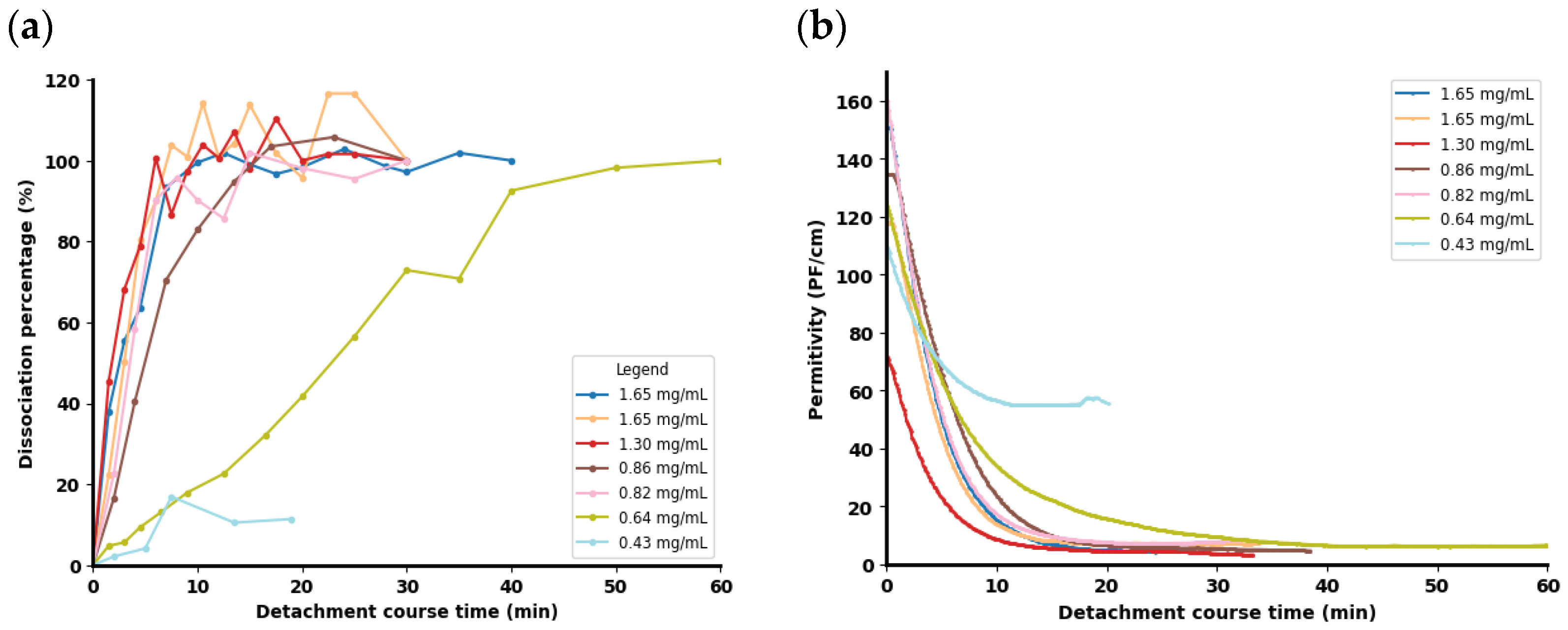
3.6. Monitoring In Situ Cell Detachment by Capacitance Sensor
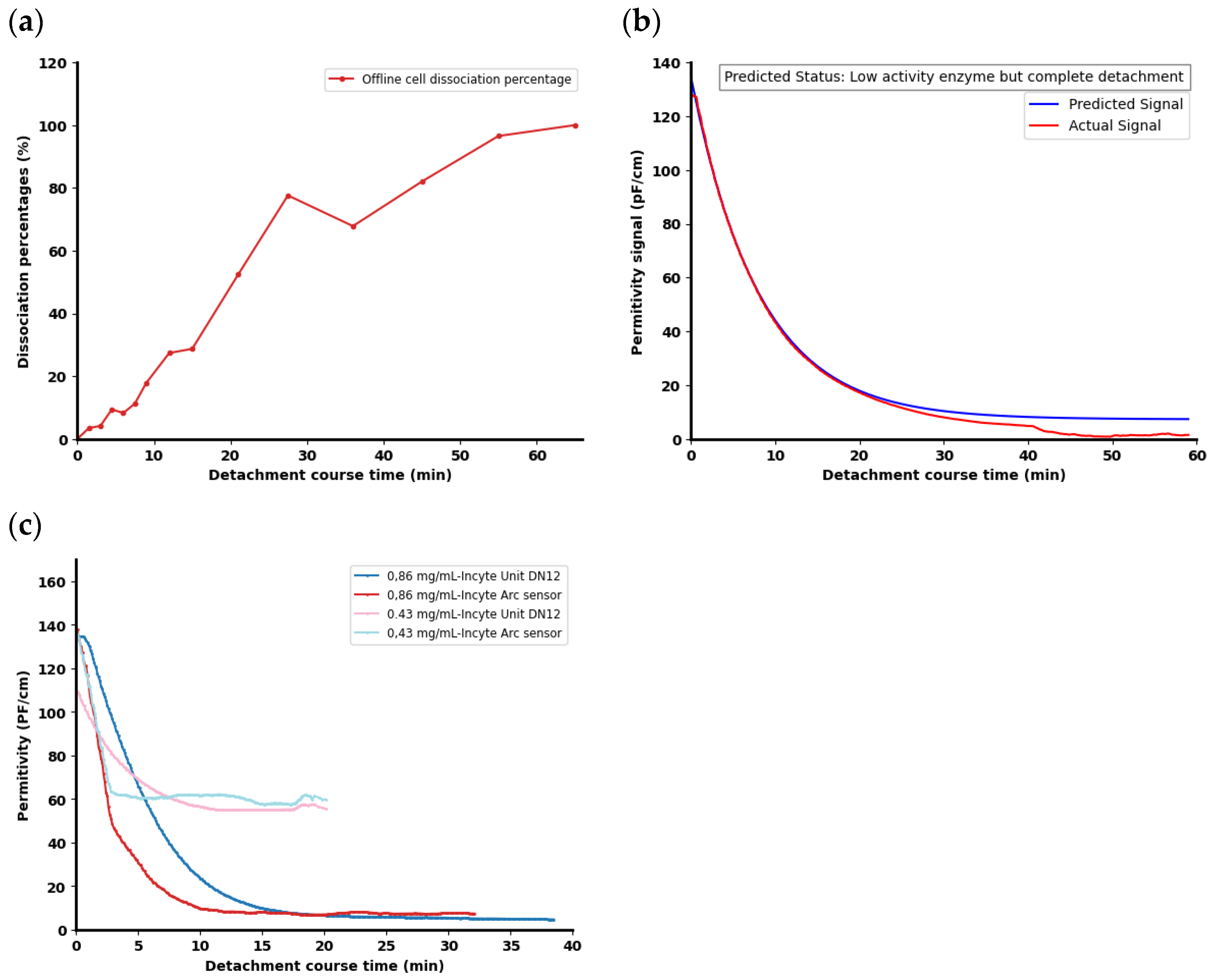
3.7. Decision-Making Tools for In Situ Cell Detachment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Wezel, A.L. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 1967, 216, 64–65. [Google Scholar] [CrossRef]
- Malda, J.; Frondoza, C.G. Microcarriers in the engineering of cartilage and bone. Trends Biotechnol. 2006, 24, 299–304. [Google Scholar] [CrossRef]
- Ton, C.; Stabile, V.; Carey, E.; Maraikar, A.; Whitmer, T.; Marrone, S.; Afanador, N.L.; Zabrodin, I.; Manomohan, G.; Whiteman, M.; et al. Development and scale-up of rVSV-SARS-CoV-2 vaccine process using single use bioreactor. Biotechnol. Rep. 2023, 37, e00782. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Lyu, J.; Li, J.; Li, C.; Zhang, Y.; Guo, Y.; Wang, Y.; Zhang, Y.; Chen, K. Application of bioreactor technology for cell culture-based viral vaccine production: Present status and future prospects. Front. Bioeng. Biotechnol. 2022, 10, 921755. [Google Scholar] [CrossRef] [PubMed]
- Kumraj, G.; Pathak, S.; Shah, S.; Majumder, P.; Jain, J.; Bhati, D.; Hanif, S.; Mukherjee, S.; Ahmed, S. Capacity Building for Vaccine Manufacturing Across Developing Countries: The Way Forward. Hum. Vaccin. Immunother. 2022, 18, 2020529. [Google Scholar] [CrossRef]
- Smeaton, J. Manufacturing COVID-19 Vaccines. Available online: https://post.parliament.uk/manufacturing-covid-19-vaccines (accessed on 14 January 2021).
- Badenes, S.M.; Fernandes-Platzgummer, A.; Rodrigues, C.; Diogo, M.M.; Da Silva, C.L.; Cabral, J. Microcarrier Culture Systems for Stem Cell Manufacturing. In Stem Cell Manufacturing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–104. ISBN 9780444632654. [Google Scholar]
- Chen, X.-Y.; Chen, J.-Y.; Tong, X.-M.; Mei, J.-G.; Chen, Y.-F.; Mou, X.-Z. Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol. Lett. 2020, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Niu, Y.; Fu, X.; Lin, Q.; Liang, H.; Liu, L.; Li, N. Large-Scale Microcarrier Culture of Chinese Perch Brain Cell for Viral Vaccine Production in a Stirred Bioreactor. Vaccines 2021, 9, 1003. [Google Scholar] [CrossRef]
- George, M.; Farooq, M.; Dang, T.; Cortes, B.; Liu, J.; Maranga, L. Production of cell culture (MDCK) derived live attenuated influenza vaccine (LAIV) in a fully disposable platform process. Biotechnol. Bioeng. 2010, 106, 906–917. [Google Scholar] [CrossRef]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and detachment strategies in microcarrier-based cell culture technology: A comprehensive review. Mater. Sci. Eng. C 2019, 103, 109782. [Google Scholar] [CrossRef]
- Kiesslich, S.; Kamen, A.A. Vero cell upstream bioprocess development for the production of viral vectors and vaccines. Biotechnol. Adv. 2020, 44, 107608. [Google Scholar] [CrossRef]
- Souza, M.C.d.O.; Da Freire, M.S.; Castilho, L.d.R. Influence of culture conditions on Vero cell propagation on non-porous microcarriers. Braz. Arch. Biol. Technol. 2005, 48, 71–77. [Google Scholar] [CrossRef]
- Mendonça, R.Z.; Prado, J.C.M.; Pereira, C.A. Attachment, spreading and growth of Vero cells on microcarriers for the optimization of large scale cultures. Bioprocess Biosyst. Eng. 1999, 20, 565. [Google Scholar] [CrossRef]
- Maillot, C.; Isla, N.; de Loubiere, C.; Toye, D.; Olmos, E. Impact of microcarrier concentration on mesenchymal stem cell growth and death: Experiments and modeling. Biotechnol. Bioeng. 2022, 119, 3537–3548. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, A.; Schalk, M.; Dürkop, M.; Maurer, M.; Bliem, R.; Kühnel, H. Seed Train Optimization in Microcarrier-Based Cell Culture Post In Situ Cell Detachment through Scale-Down Hybrid Modeling. Bioengineering 2024, 11, 268. [Google Scholar] [CrossRef]
- Sousa, M.; Fenge, C.; Rupprecht, J.; Tappe, A.; Greller, G.; Alves, P.; Carrondo, M.; Roldão, A. Process intensification for Peste des Petites Ruminants Virus vaccine production. Vaccine 2019, 37, 7041–7051. [Google Scholar] [CrossRef] [PubMed]
- Rourou, S.; Riahi, N.; Majoul, S.; Trabelsi, K.; Kallel, H. Development of an in situ detachment protocol of Vero cells grown on Cytodex1 microcarriers under animal component-free conditions in stirred bioreactor. Appl. Biochem. Biotechnol. 2013, 170, 1724–1737. [Google Scholar] [CrossRef]
- Dabros, M.; Dennewald, D.; Currie, D.J.; Lee, M.H.; Todd, R.W.; Marison, I.W.; Stockar, U. von. Cole-Cole, linear and multivariate modeling of capacitance data for on-line monitoring of biomass. Bioprocess Biosyst. Eng. 2009, 32, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Degouys, V.; Cerckel, I.; Garcia, A.; Harfield, J.; Dubois, D.; Fabry, L.; Miller, A.O. Dielectric spectroscopy of mammalian cells. 2. Simultaneous in situ evaluation by aperture impedance pulse spectroscopy and low frequency dielectric spectroscopy of the biomass of HTC cells on Cytodex 3. Cytotechnology 1993, 13, 195–202. [Google Scholar] [CrossRef]
- El Wajgali, A.; Esteban, G.; Fournier, F.; Pinton, H.; Marc, A. Impact of microcarrier coverage on using permittivity for on-line monitoring high adherent Vero cell densities in perfusion bioreactors. Biochem. Eng. J. 2013, 70, 173–179. [Google Scholar] [CrossRef]
- Justice, C.; Leber, J.; Freimark, D.; Pino Grace, P.; Kraume, M.; Czermak, P. Online- and offline- monitoring of stem cell expansion on microcarrier. Cytotechnology 2011, 63, 325–335. [Google Scholar] [CrossRef]
- Sion, C.; Ghannoum, D.; Ebel, B.; Gallo, F.; Isla, N.; de Guedon, E.; Chevalot, I.; Olmos, E. A new perfusion mode of culture for WJ-MSCs expansion in a stirred and online monitored bioreactor. Biotechnol. Bioeng. 2021, 118, 4453–4464. [Google Scholar] [CrossRef] [PubMed]
- Juanola, S.; Garcia, L.; Mouriño, M.; Cheeseman, S.; Urniza, A.; Cheeseman, S.; Scholz, J.; Boulais, A. Control and Scale-Up of A Microcarrier-Based Viral Vaccine Process Control and Scale-Up of A Microcarrier-Based Viral Vaccine Process Using BioPAT® ViaMass for Inline Viable Cell Density Measurement. Available online: https://www.sartorius.com/download/457214/5/appl-note-biopat-viamass-zoetis-2568350-000-e-data.pdf (accessed on 21 January 2020).
- Petiot, E.; Ansorge, S.; Rosa-Calatrava, M.; Kamen, A. Critical phases of viral production processes monitored by capacitance. J. Biotechnol. 2017, 242, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.J.; Cicalese, S.M.; Davis, H.B.; Dogdas, B.; Shah, T.; Culp, T.; van Hoang, M. Cell confluency analysis on microcarriers by micro-flow imaging. Cytotechnology 2016, 68, 2469–2478. [Google Scholar] [CrossRef]
- Odeleye, A.O.O.; Castillo-Avila, S.; Boon, M.; Martin, H.; Coopman, K. Development of an optical system for the non-invasive tracking of stem cell growth on microcarriers. Biotechnol. Bioeng. 2017, 114, 2032–2042. [Google Scholar] [CrossRef]
- Anton, F.; Burzlaff, A.; Kasper, C.; Brückerhoff, T.; Scheper, T. Preliminary Study towards the Use of In-situ Microscopy for the Online Analysis of Microcarrier Cultivations. Eng. Life Sci. 2007, 7, 91–96. [Google Scholar] [CrossRef]
- Benavides, O.R.; Gibbs, H.C.; White, B.P.; Kaunas, R.; Gregory, C.A.; Walsh, A.J.; Maitland, K.C. Volumetric imaging of human mesenchymal stem cells (hMSCs) for non-destructive quantification of 3D cell culture growth. PLoS ONE 2023, 18, e0282298. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Onodera, T.; Homan, K.; Liang, X.; Matsuoka, M.; Miyazaki, T.; Yoshiaki, H.; Saito, M.; Iwasaki, N. Optical coherence tomography evaluation of the spatiotemporal effects of 3D bone marrow stromal cell culture using a bioreactor. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Bournonville, S.; de Geris, L.; Kerckhofs, G. Micro computed tomography with and without contrast enhancement for the characterization of microcarriers in dry and wet state. Sci. Rep. 2021, 11, 2819. [Google Scholar] [CrossRef]
- Schnitzler, A.C.; Verma, A.; Kehoe, D.E.; Jing, D.; Murrell, J.R.; Der, K.A.; Aysola, M.; Rapiejko, P.J.; Punreddy, S.; Rook, M.S. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochem. Eng. J. 2016, 108, 3–13. [Google Scholar] [CrossRef]
- Merck. Trypsin Assay Procedure. Available online: https://www.sigmaaldrich.com/AT/de/technical-documents/protocol/protein-biology/enzyme-activity-assays/enzymatic-assay-of-trypsin (accessed on 28 August 2024).
- United Sated Pharmacopoeia. Official Monographs/Trypsin 5969 (35). Available online: https://www.drugfuture.com/Pharmacopoeia/usp35/PDF/4969-4970%20Crystallized%20Trypsin.pdf (accessed on 1 May 2012).
- Liu, K. Trypsin Inhibitor Assay: Expressing, Calculating, and Standardizing Inhibitor Activity in Absolute Amounts of Trypsin Inhibited or Trypsin Inhibitors. J. Am. Oil Chem. Soc. 2021, 98, 355–373. [Google Scholar] [CrossRef]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Nienow, A.W.; Hewitt, C.J.; Heathman, T.R.; Glyn, V.A.; Fonte, G.N.; Hanga, M.P.; Coopman, K.; Rafiq, Q.A. Agitation conditions for the culture and detachment of hMSCs from microcarriers in multiple bioreactor platforms. Biochem. Eng. J. 2016, 108, 24–29. [Google Scholar] [CrossRef]
- Heathman, T.R.; Nienow, A.W.; Rafiq, Q.A.; Coopman, K.; Kara, B.; Hewitt, C.J. Agitation and aeration of stirred-bioreactors for the microcarrier culture of human mesenchymal stem cells and potential implications for large-scale bioprocess development. Biochem. Eng. J. 2018, 136, 9–17. [Google Scholar] [CrossRef]
- Petry, F.; Salzig, D. Impact of Bioreactor Geometry on Mesenchymal Stem Cell Production in Stirred-Tank Bioreactors. Chem. Ing. Tech. 2021, 93, 1537–1554. [Google Scholar] [CrossRef]
- Nienow, A.W. Reactor engineering in large scale animal cell culture. Cytotechnology 2006, 50, 9–33. [Google Scholar] [CrossRef]
- Glaser, R.; Greenlea, Z.; Sha, M. Stimulating Growth. Cultivating Solutions: Power Number for Cell Culture Glass Vessels. Available online: https://www.eppendorf.com/product-media/doc/en/70270/Fermentors-Bioreactors_Brochure_Bioprocess-Family_Stimulating-Growth-Cultivating-Solutions.pdf (accessed on 28 August 2024).
- Yang, J.; Guertin, P.; Jia, G.; Lv, Z.; Yang, H.; Ju, D. Large-scale microcarrier culture of HEK293T cells and Vero cells in single-use bioreactors. AMB Express 2019, 9, 70. [Google Scholar] [CrossRef]
- Hewitt, C.J.; Lee, K.; Nienow, A.W.; Thomas, R.J.; Smith, M.; Thomas, C.R. Expansion of human mesenchymal stem cells on microcarriers. Biotechnol. Lett. 2011, 33, 2325–2335. [Google Scholar] [CrossRef]
- Goh, T.K.-P.; Zhang, Z.-Y.; Chen, A.K.-L.; Reuveny, S.; Choolani, M.; Chan, J.K.Y.; Oh, S.K.-W. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores. Open Access 2013, 2, 84–97. [Google Scholar] [CrossRef]
- Huang, H.-L.; Hsing, H.-W.; Lai, T.-C.; Chen, Y.-W.; Lee, T.-R.; Chan, H.-T.; Lyu, P.-C.; Wu, C.-L.; Lu, Y.-C.; Lin, S.-T.; et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 2010, 17, 36. [Google Scholar] [CrossRef]
- Fonte, G. The Effect of Key Process Parameters on Human Mesenchymal Stem Cell Expansion and Harvest. Available online: https://tinyurl.com/9w968chj (accessed on 1 November 2014).
- Cytiva. Cytodex 1, Cytodex 3 Cell Culture. Available online: https://cytiva-delivery.sitecorecontenthub.cloud/api/public/content/digi-11574-pdf (accessed on 1 June 2020).
- Motamedvaziri, S.; Armenante, P.M. Flow regimes and surface air entrainment in partially filled stirred vessels for different fill ratios. Chem. Eng. Sci. 2012, 81, 231–250. [Google Scholar] [CrossRef]
- Martín, M.; Montes, F.J.; Galán, M.A. Influence of Impeller Type on the Bubble Breakup Process in Stirred Tanks. Ind. Eng. Chem. Res. 2008, 47, 6251–6263. [Google Scholar] [CrossRef]
- Souza, M.C.O.; Freire, M.S.; Schulze, E.A.; Gaspar, L.P.; Castilho, L.R. Production of yellow fever virus in microcarrier-based Vero cell cultures. Vaccine 2009, 27, 6420–6423. [Google Scholar] [CrossRef]
- Nienow, A.W.; Rafiq, Q.A.; Coopman, K.; Hewitt, C.J. A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochem. Eng. J. 2014, 85, 79–88. [Google Scholar] [CrossRef]
- Petiot, E.; El-Wajgali, A.; Esteban, G.; Gény, C.; Pinton, H.; Marc, A. Real-time monitoring of adherent Vero cell density and apoptosis in bioreactor processes. Cytotechnology 2012, 64, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Metze, S.; Ruhl, S.; Greller, G.; Grimm, C.; Scholz, J. Monitoring online biomass with a capacitance sensor during scale-up of industrially relevant CHO cell culture fed-batch processes in single-use bioreactors. Bioprocess Biosyst. Eng. 2020, 43, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Negrete, A.; Esteban, G.; Kotin, R.M. Process optimization of large-scale production of recombinant adeno-associated vectors using dielectric spectroscopy. Appl. Microbiol. Biotechnol. 2007, 76, 761–772. [Google Scholar] [CrossRef]
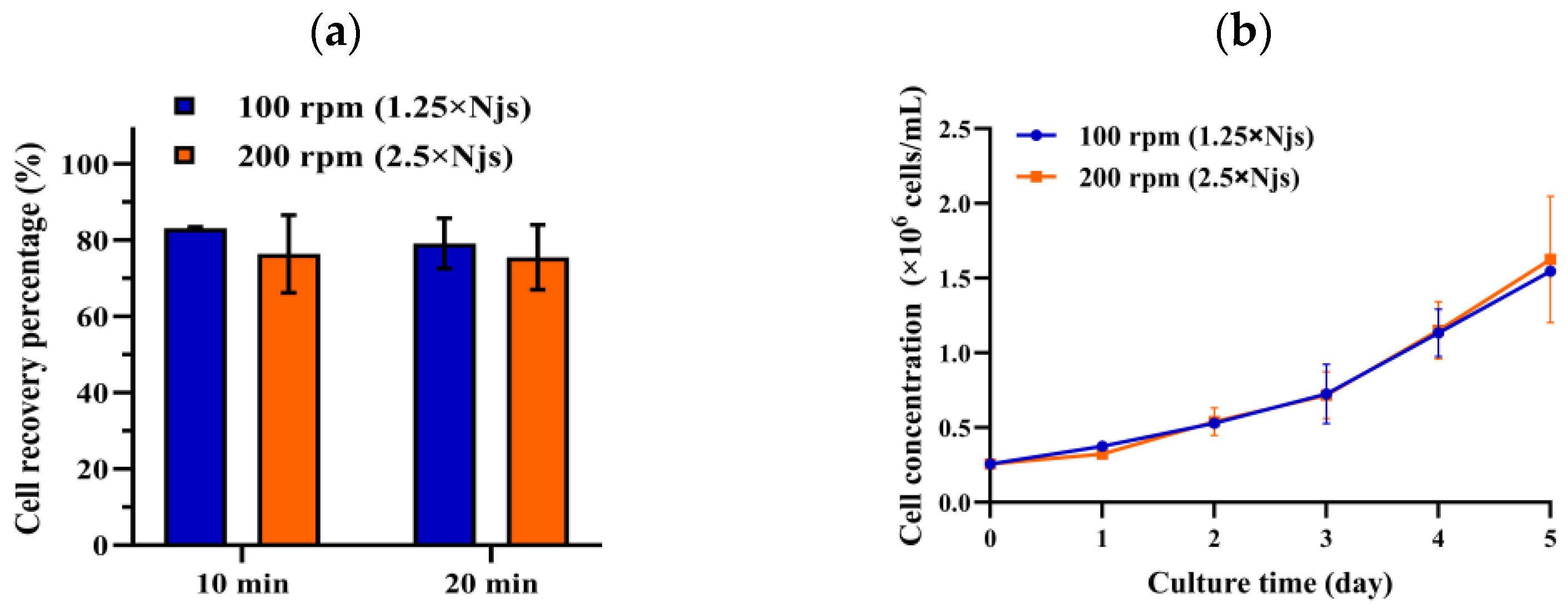
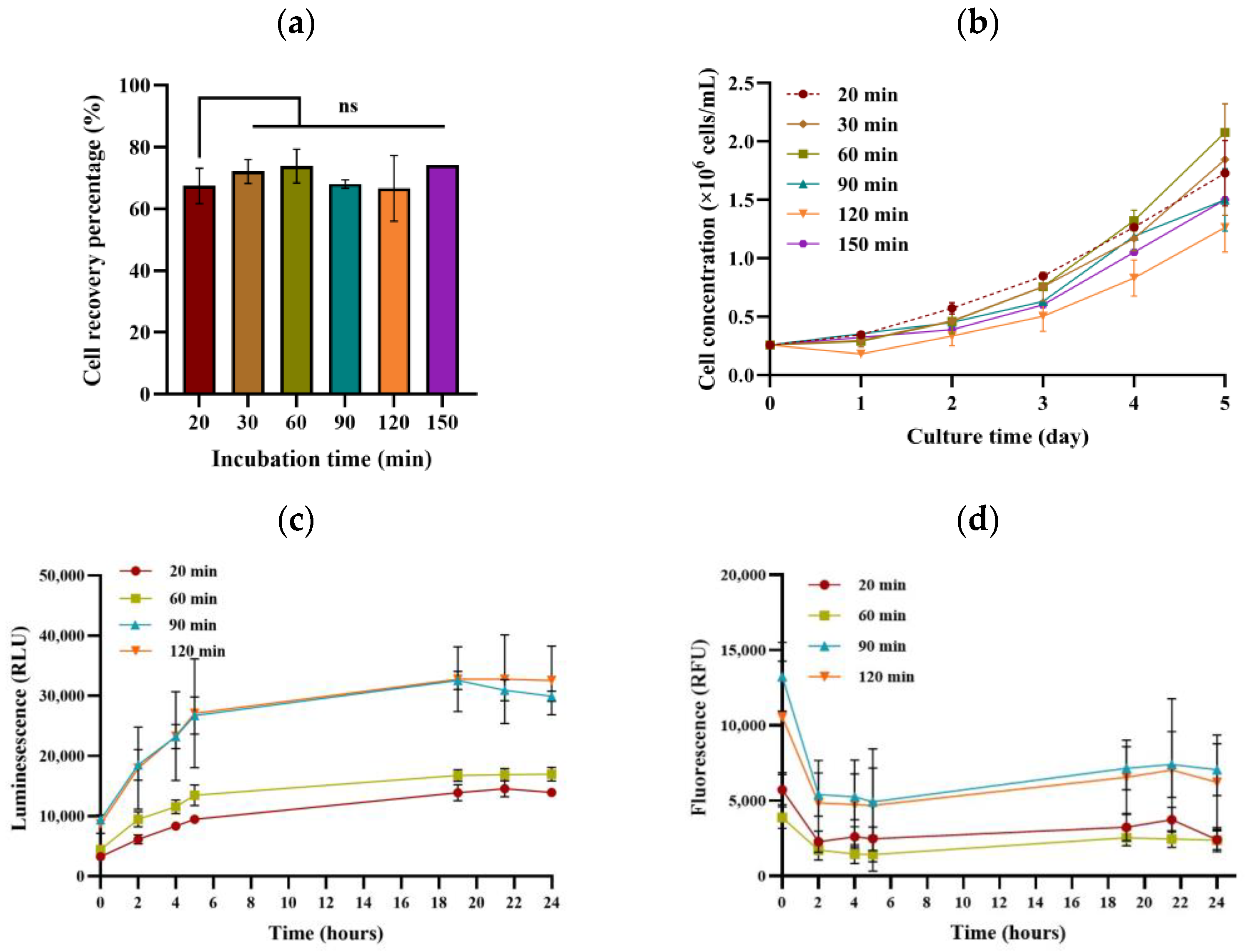
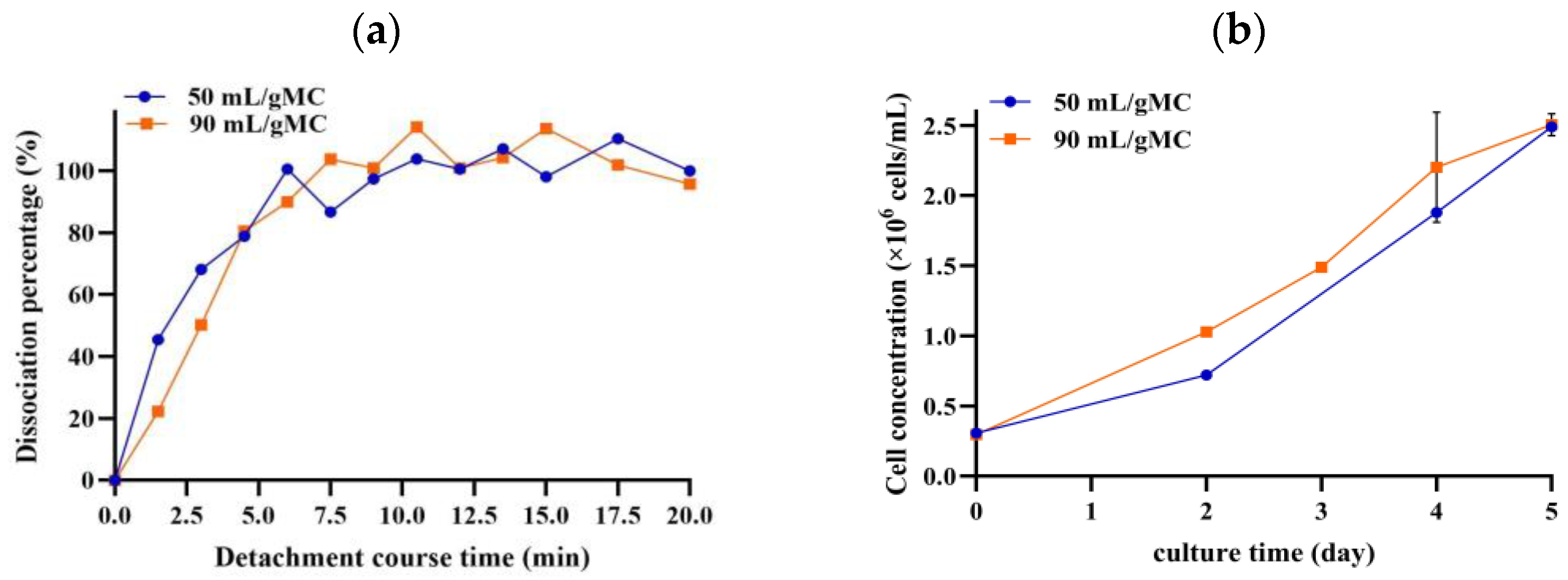
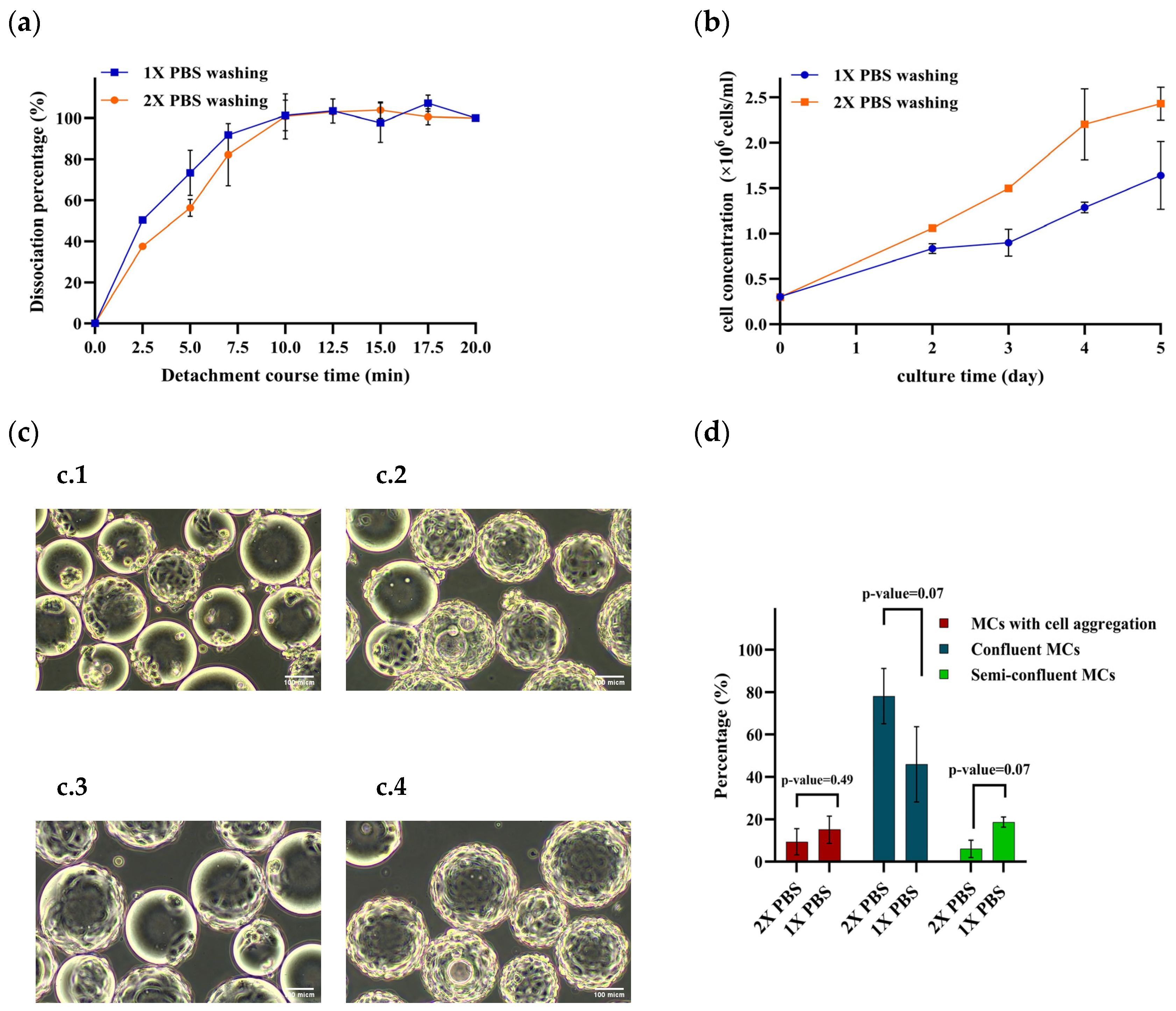

| Parameter | Lower Limit | Upper Limit | Other Parameters’ Setpoints |
|---|---|---|---|
| Agitation speed (rpm) | 100 * | 200 * | 90 mL/gMC, 30 min, 2X PBS wash |
| Incubation time with enzyme (min) | 20 | 150 | 90 mL/gMC; 100 rpm, 2X PBS wash |
| Trypsin–EDTA (0.25%) volume, mL/gMC | 50 | 90 | 20 min, 100 rpm, 2X PBS wash |
| PBS washing steps (40% of initial volume) | 1 | 2 | 20 min, 100 rpm, 90 mL/gMC |
| 3c | 10c | 50c | 250 L | 1000 L | |
|---|---|---|---|---|---|
| Bioreactor working volume (L) | 1.25–3.75 | 3.3–10 | 18–40 L | 125–250 | 500–1000 |
| Actual culture volume (L) | 2.5 | 10 | 40 | 190 | 900 |
| MC concentration (g/L) * | 3.0 | 3.8 | 3.9 | 3.8 | 3.8 |
| Vessel diameter (cm) | 14.7 | 20.4 | 33.7 | 59.7 | NA ** |
| Blade width + off-bottom clearance (cm) | 7.0 | 9.0 | 16.5 | 30.0 | NA |
| Minimum required volume to cover the impeller | 1.18 | 2.943 | 14.71 | 83.93 | NA |
| 20% remaining (L) *** | 0.7 | 2 | 8 | 50 | NA |
| Minimum volume of enzyme solution (L) | 0.44 | 0.94 | 6.71 | 33.93 | NA |
| Minimum volume of enzyme solution/gMC | 46.2 | 24.8 | 43.1 | 47.0 | NA |
| Run No. | MC Conc. (g/L) | Culture Volume (mL) | Trypsin–EDTA Conc. (mg/mL) * | Trypsin–EDTA Volume (mL/gMC) | Final Trypsin Conc. (mg/mL) | Final Trypsin Activity (USP/mL) ** |
|---|---|---|---|---|---|---|
| 1 *** | 3 | 700 | 2.5 | 90 | 1.65 | 1465.2 |
| 2 **** | 3 | 700 | 2.5 | 50 | 1.30 | 1150.9 |
| 3 | 3 | 700 | 1.25 | 90 | 0.86 | 767.2 |
| 4 | 3 | 700 | 1.25 | 90 | 0.82 | 732.6 |
| 5 | 3 | 700 | 1.25 | 50 | 0.64 | 575.4 |
| 6 | 3 | 700 | 0.830 | 50 | 0.43 | 383.6 |
| Strain | Medium Condition | Washing Ratio (%iv *) | Washing Steps | Reference |
|---|---|---|---|---|
| Vero | SCM ** | 30 | 2–3 | [42] |
| hMSC | SCM | 41.5 | 3 | [44] |
| hMSC | SFM/SCM | NA | 2 | [37] |
| Vero | SFM *** | - | 0 | [17,50] |
| hMSC | SFM | 20 | 2 | [38] |
| hMSC | nm **** | 100 | 1 | [51] |
| Sensor | Final Enzyme Conc. (g/L) | Predicted Signal at 20 min | Predicted Status | Measured Signal at 20 min | Actual Status |
|---|---|---|---|---|---|
| Incyte Unit DN12 | 0.43 | 50.3 | No active enzyme, no detachment | 55.6 | No cell detachment |
| Incyte Arc | 57.1 | No active enzyme, no detachment | 59.8 | No cell detachment | |
| Incyte Unit DN12 | 0.86 | 0 | Fast and complete detachment | 6.54 | Complete cell detachment but slow |
| Incyte Arc | 0 | Fast and complete detachment | 6.91 | Complete cell detachment but slow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimian, A.; Schalk, M.; Dürkop, M.; Maurer, M.; Bliem, R.; Kühnel, H. Parameter Optimization and Capacitance-Based Monitoring of In Situ Cell Detachment in Microcarrier Cultures. Processes 2024, 12, 1887. https://doi.org/10.3390/pr12091887
Ebrahimian A, Schalk M, Dürkop M, Maurer M, Bliem R, Kühnel H. Parameter Optimization and Capacitance-Based Monitoring of In Situ Cell Detachment in Microcarrier Cultures. Processes. 2024; 12(9):1887. https://doi.org/10.3390/pr12091887
Chicago/Turabian StyleEbrahimian, Atefeh, Mona Schalk, Mark Dürkop, Michael Maurer, Rudolf Bliem, and Harald Kühnel. 2024. "Parameter Optimization and Capacitance-Based Monitoring of In Situ Cell Detachment in Microcarrier Cultures" Processes 12, no. 9: 1887. https://doi.org/10.3390/pr12091887
APA StyleEbrahimian, A., Schalk, M., Dürkop, M., Maurer, M., Bliem, R., & Kühnel, H. (2024). Parameter Optimization and Capacitance-Based Monitoring of In Situ Cell Detachment in Microcarrier Cultures. Processes, 12(9), 1887. https://doi.org/10.3390/pr12091887







