Corrosion Failure Mechanism of 2507 Duplex Stainless Steel Circulation Pump Impeller
Abstract
1. Introduction
2. Physicochemical Experiments
2.1. Macroscopic Inspection
2.2. Chemical Composition Analysis
2.3. Metallographic Structure Analysis
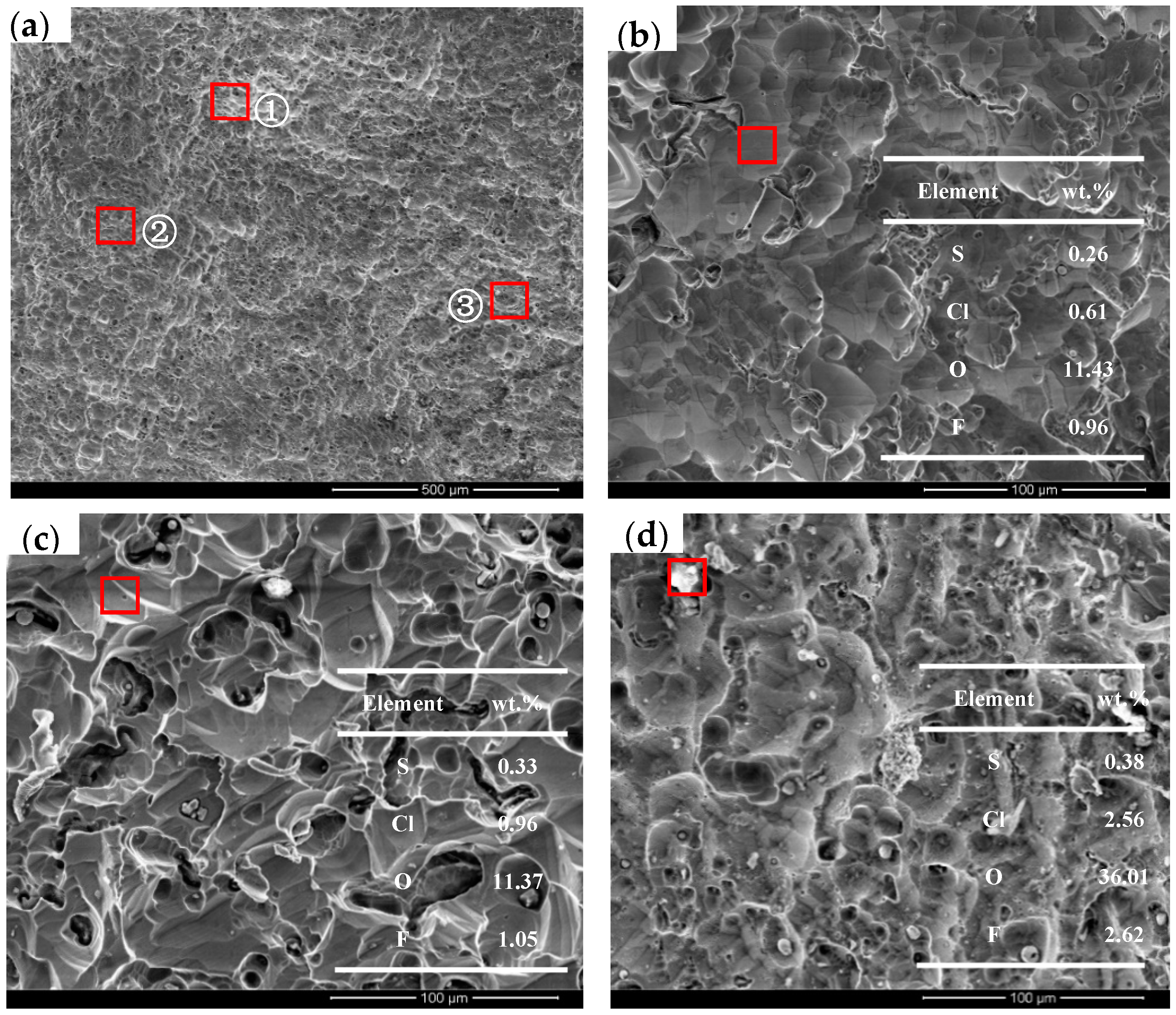
2.4. Scanning Electron Microscopy and Energy Spectrum Analysis
3. Impeller Failure Analysis
4. Conclusions
- (1)
- The chemical composition of the circulation pump impeller met standard requirements. The matrix microstructure consisted of ferrite and austenite, and no abnormalities were found, indicating that the impeller’s corrosion was unrelated to the material’s quality.
- (2)
- External factors such as surface wear, cavitation, and halogen element corrosion were the primary causes of corrosion failure in the circulation pump impeller. As the blades rotated, low-pressure zones formed generating bubbles that collapsed in high-pressure areas. The impact of the bubble collapse combined with the scouring force of internal particles damaged the passive film on the stainless steel surface, creating a large cathode–small anode catalytic corrosion system. Under the accelerated corrosive action of reactive halogen ions, the corrosion rate at the damaged areas of the passive film increased, eventually leading to component failure.
- (3)
- It is recommended that the impeller inlet be rounded, shaping it to be more streamlined, in order to reduce flow separation, enhance fluid flow efficiency, and improve the impeller’s resistance to cavitation. Wear resistance and pitting corrosion protection of circulation pumps can be achieved through both the appropriate selection of materials and the application of surface engineering techniques.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, L.Q.; Bai, Y.; Xu, B.Z.; Pan, W.; Li, J.; Qiao, L. Effect of hydrogen on pitting susceptibility of 2507 duplex stainless steel. Corros. Sci. 2013, 70, 140–144. [Google Scholar] [CrossRef]
- Liu, Z.B.; Liang, J.X.; Su, J.; Wang, X.; Sun, Y.; Wang, C.; Yang, Z. Research and application progress in ultra-high strength stainless steel. Acta Metall. Sin. 2020, 56, 549–557. [Google Scholar]
- Song, Z.G.; Feng, H.; Wu, X.H.; Heng, J.G.; Ling, G.P.; Zhang, Y.R. Development and research progress of duplex stainless steel in China. China Metall. 2022, 32, 2–14. [Google Scholar]
- Wang, L.; Dou, Y.; Han, S.; Wu, J.; Cui, Z. Influence of Sulfide on the Passivation Behavior and Surface Chemistry of 2507 Super Duplex Stainless Steel in Acidified Artificial Seawater. Appl. Surf. Sci. 2020, 504, 144340. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.M.; Li, F.T.; Liu, C.; Yang, S.; Dong, M. Corrosion behavior of 2507 duplex stainless steel in acidic and high chlorine environments. China Surf. Eng. 2024, 37, 91–100. [Google Scholar]
- Feng, M.; Peng, J.B.; Zhang, Y.Z.; Yu, C.; Kou, X.; Tian, Y. Corrosion and passivation behavior of 2507 duplex stainless steel in electrolytic seawater antifouling environment. J. Mater. Heat Treat. 2023, 44, 146–156. [Google Scholar]
- Zhu, M.; Zhu, T.; Chen, M.; Guo, S.-Y.; Yuan, Y.-F.; Yu, G.-H.; Nie, L. Corrosion behavior of 2507 duplex stainless steel in simulated S02-Polluted seawater. Chin. J. Eng. 2018, 40, 587–593. [Google Scholar]
- Zhang, G.; Jin, J.; Wang, Z.; Ren, Y.; Zhang, L. Corrosion behavior of 2507 super duplex stainless steel in H2S-free and H2S-containing acidic environments. J. Mater. Eng. Perform. 2023, 32, 1185–1195. [Google Scholar] [CrossRef]
- Li, Y.; Guan, L.; Wang, G.; Zhang, B. Influence of mechanical stresses on pitting corrosion of stainless steel. J. Chin. Soc. Corros. Prot. 2019, 39, 215–226. [Google Scholar]
- Nugroho, F.A.; Herrnring, H.; Ehlers, S. Influence of corrosion pit geometry on stress distribution within a single artificial pit. Ship Technol. Res. 2023, 70, 73–89. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Geng, W.Y.; Liu, S.F.; Zhang, Z.Y.; Cong, L.L.; Wen, T.L. Cause of corrosion leakage off lash tank filter. Phys. Test. Chem. Anal. 2023, 59, 40–44. [Google Scholar]
- Bai, Q.Q.; Zhang, Z.H. Effect of solution treatment temperature on phase ration and mechanical properties of 2507 super duplex stainless steel. Heat Treat. Metals 2019, 44, 123–127. [Google Scholar]
- Liu, R.; Ge, H.H.; Gong, X.M.; Meng, X.J.; Zhao, Y.Z. Corrosion resistance of sodium hypochlorite and isothiazolone bactericides to stainless steel in cooling water. Mater. Prot. 2011, 44, 60–61. [Google Scholar]
- Xu, Z.; Xu, Q.; Fang, Y.; He, L. Effect of solution temperature on microstructure and properties of 2507 duplex stainless steel after aging. Hot Work. Technol. 2019, 48, 231–239. [Google Scholar]
- Kou, X.P.; Zhang, Y.Z.; Yu, C.L.; Feng, M.; Tuan, Y.-C. Corrosion and passivation behavior of 2507 duplex stainless steel at different stages of seawater desulfurization. J. Mater. Heat Treat. 2023, 44, 115–126. [Google Scholar]
- Ai, Y.K.; Wang, A.B. Analysis of Cavitation Factors of Centrifugal Pump and Study of Treatment Strategy. Mod. Chem. Res. 2020, 10, 21–22. [Google Scholar]
- Yu, R.Q.; He, J.J.; Li, W.; Ren, Y.; Yang, W. Erosive wear of Cr30A high chromium cast iron in a simulated circulating pump operation condition with slurry related to wet desulfuration process in thermal power plant. J. Chin. Soc. Corros. Prot. 2019, 39, 353–358. [Google Scholar]
- He, Z.H.; Jia, J.W.; Li, Y.; Zhang, W.; Xu, F.; Hou, L.; Wei, Y. Study on passivation behavior of super austenitic stainless steel in simulated flue gas desulfurization condensate. J. Chin. Soc. Corros. Prot. 2023, 43, 408–414. [Google Scholar]
- Xu, L.; Wu, P.; Zhu, X.; Zhao, G.; Ren, X.; Wei, Q.; Xie, L. Structural characteristics and chloride intrusion mechanism of the passive film. Corros. Sci. 2022, 207, 110563. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Su, Y.P.; Su, R. Influence of chlorine ion on corrosion behavior of metal materials used in power plants. Corros. Prot. 2021, 42, 14–18. [Google Scholar]
- Huang, J.D. Induction mechanism and evolution process of cavitation in expander impeller. Corros. Prot. 2023, 44, 24–29. [Google Scholar]
- Ge, F.; Wang, X.J.; Meng, X.J.; Huang, X.; Zhang, Y.; Song, Y.; Ge, H.; Zhao, Y. Comparison of corrosion behavior of 2205 and 2507 duplex stainless steel in simulated flue gas condensate of a waste incineration power plant. Corros. Rev. 2021, 39, 477–486. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, O.; Yuan, Y.; Guo, S.; Huang, Y. Sudy on the correlation between passive film and AC corrosion behavior of 2507 super duplexstainless steel in simulated marine environment. J. Electroanal. Chem. 2020, 864, 1–14. [Google Scholar] [CrossRef]
- Zhang, S.H.; Hou, L.F.; Wei, Y.H.; Du, H.Y.; Wei, H.; Liu, B.S.; Chen, X.B. Dual functions of chloride ions on corrosion behavior of mild steel in CO2 saturated aqueous solutions. Mater. Corros. 2019, 70, 888–896. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.X.; Jin, J.; Tang, D.-Z.; Zhang, L. Selective corrosion mechanism of CoCrFeMoNi high-entropy alloy in the transpassive region based on the passive film characterization by ToF-SIMS. Corros. Sci. 2023, 218, 111206. [Google Scholar] [CrossRef]
- Argandona, G.; Biezma, M.V.; Berrueta, J.M.; Berlanga, C.; Ruiz, A. Detection of secondary phases in UNS S32760 superduplex stainles steeldestnctive and non-destructive techniques. J. Mater. Eng. Perform. 2016, 25, 5269–5279. [Google Scholar] [CrossRef]
- Zhang, G.W.; Hu, Y.; Liu, F.; Wang, D.J.; Zhang, C.L.; Wang, F.X.; Liu, Y.J. Analysis on corrosion of low temperature reheater in a Russian made power plant. Therm. Power Gener. 2017, 462, 120–124. [Google Scholar]
- Wang, Y.B. Circulating water pump impeller corrosion cause analysis and treatment recommendations. Pet. Chem. Equip. 2023, 26, 184–185. [Google Scholar]
- Liu, K.; Zhou, F.; Luo, H.; Lin, X.Z.; Chen, X.D. Properties of pack boriding for 2205 duplex steel. Surf. Tech. 2016, 45, 183–188. [Google Scholar]
- Ji, D.S.; Peng, R.S. Experiential study on nitrogen containing duplex stainless steel coating of laser cladding. Mech. Res. Appl. 2020, 33, 88–90. [Google Scholar]
- Song, C.; Li, Y.; Wu, F.; Luo, J.; Li, L.; Li, G. Failure analysis of the crack and leakage of a crude oil pipeline under CO2-steam flooding. Processes 2023, 11, 1567. [Google Scholar] [CrossRef]






| Element | C | Si | Mn | P | S | Cr | Ni | Mo | N |
|---|---|---|---|---|---|---|---|---|---|
| Standard requirements | 0.030 | 0.80 | 1.20 | 0.035 | 0.020 | 24.0~26.0 | 6.0~8.0 | 3.0~5.0 | 0.24~0.32 |
| Sample composition | 0.015 | 0.46 | 0.52 | 0.023 | 0.016 | 24.89 | 7.26 | 3.83 | 0.28 |
| Phase Type | Fe | Cr | Mo | Ni | N | Si | Phase Category |
|---|---|---|---|---|---|---|---|
| Gray | 67.31 | 21.30 | 3.32 | 7.10 | 0.21 | 0.76 | α phase |
| Black | 64.56 | 24.32 | 5.67 | 4.53 | 0.27 | 0.65 | γ phase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hou, C.; Li, J.; Shi, M.; Chen, J.; Qian, G. Corrosion Failure Mechanism of 2507 Duplex Stainless Steel Circulation Pump Impeller. Processes 2024, 12, 1897. https://doi.org/10.3390/pr12091897
Wang W, Hou C, Li J, Shi M, Chen J, Qian G. Corrosion Failure Mechanism of 2507 Duplex Stainless Steel Circulation Pump Impeller. Processes. 2024; 12(9):1897. https://doi.org/10.3390/pr12091897
Chicago/Turabian StyleWang, Weihua, Chengbao Hou, Jiaxing Li, Mingxiao Shi, Jiugong Chen, and Gong Qian. 2024. "Corrosion Failure Mechanism of 2507 Duplex Stainless Steel Circulation Pump Impeller" Processes 12, no. 9: 1897. https://doi.org/10.3390/pr12091897
APA StyleWang, W., Hou, C., Li, J., Shi, M., Chen, J., & Qian, G. (2024). Corrosion Failure Mechanism of 2507 Duplex Stainless Steel Circulation Pump Impeller. Processes, 12(9), 1897. https://doi.org/10.3390/pr12091897





