Recent Advances in the Strategies for Developing and Modifying Photocatalytic Materials for Wastewater Treatment
Abstract
1. Introduction
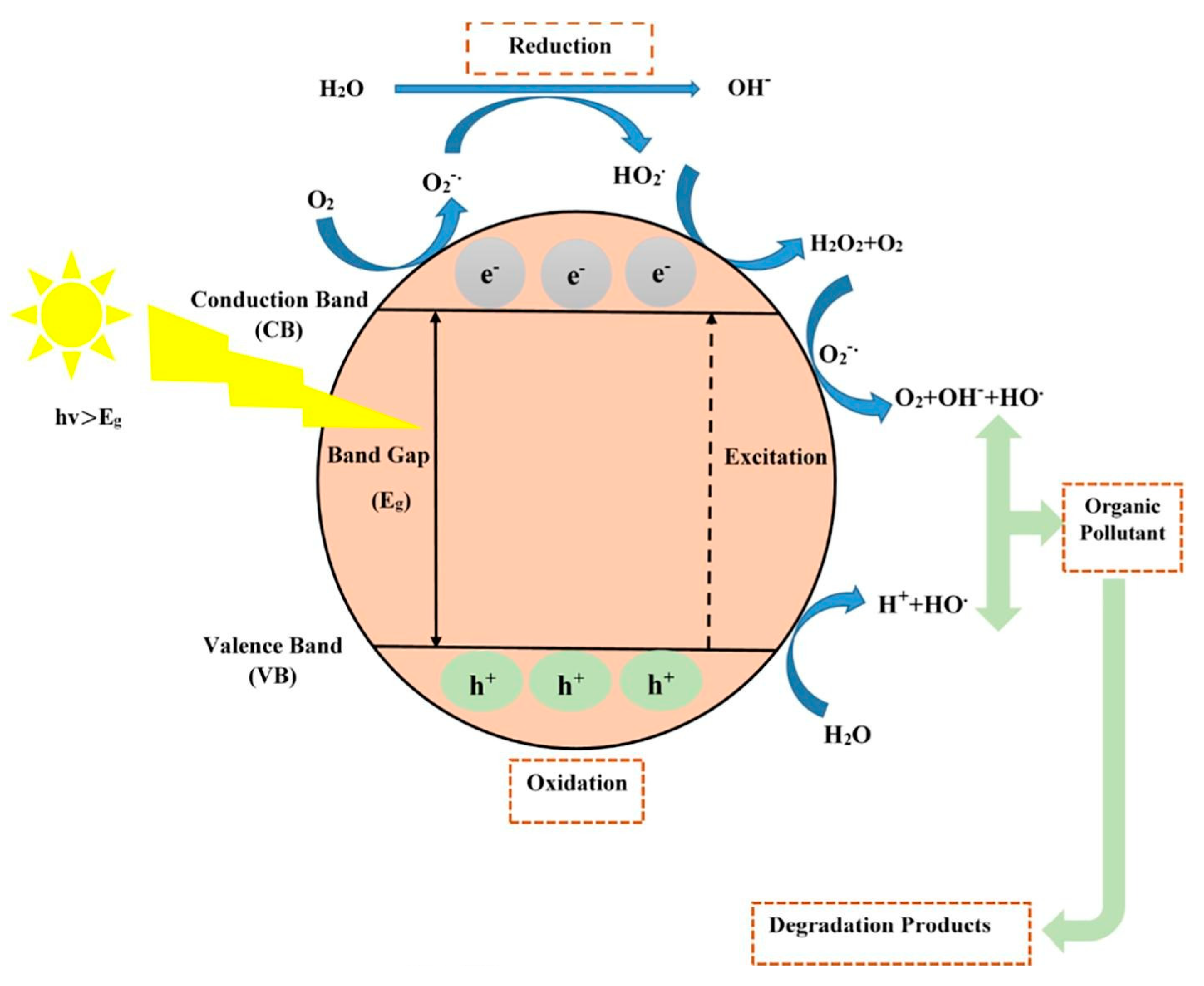
2. Principles of Photocatalytic Oxidation
- Adsorption of the pollutant from the surrounding environment onto the photocatalyst surface;
- Absorption of light with energy greater than the band gap of the photocatalyst, generating e−/h+ pairs in the bulk phase;
- Separation of e−/h+ pairs and their diffusion to the photocatalyst surface. Some photogenerated carriers simultaneously recombine, both on the surface and within the photocatalyst;
- Photo-oxidation and photoreduction reactions between the trapped electrons and holes with adsorbents on catalytic active sites. Specifically, holes in the valence band (VB) oxidize H2O molecules on the photocatalyst surface to •OH, while electrons in the conduction band (CB) reduce O2 adsorbed on the surface to superoxide radicals (•O2−). This process simultaneously degrades pollutants into smaller molecules such as H2O and CO2;
- Desorption of reaction products from the interface into the bulk solution, allowing for a new photoreaction cycle to begin.
Photocatalytic Mechanism
3. Photocatalytic Materials
3.1. Metal Oxides
3.1.1. TiO2
3.1.2. ZnO
3.1.3. WOx
3.1.4. Cu-Based Oxides
3.1.5. SnO2
3.1.6. Bi2O3
3.1.7. Bismuth-Based Multi-Component Oxides
3.2. Metal Sulfides
3.2.1. ZnS
3.2.2. CdS
3.2.3. PbS
3.2.4. Bi2S3
3.3. Non-Metal-Based Semiconductors: SiC
3.4. Organic Materials
3.4.1. Graphene
3.4.2. Graphitic Carbon Nitride
4. Strategies for Improving Photocatalytic Efficiency
4.1. Structural Engineering (Metal and Non-Metal Doping)
4.2. Morphology Design
- −
- High surface area, which leads to an increased number of active sites available for photocatalytic reactions, resulting in improved overall efficiency;
- −
- The planar structure, which improves light absorption, resulting in more effective excitation of electrons and holes, and enhanced charge separation and electron transfer, resulting in reduced charge recombination;
- −
- The unique electronic properties and high surface energy, as well as the tunability of their electronic, optical, and chemical properties;
- −
- The reduced structural defects compared to their bulk counterparts;
- −
- The easier development of 2D-based composite photocatalysts facilitated by their unique characteristics, which introduce new functionalities;
- −
- The easier development of large-scale photocatalytic systems for industrial applications.
4.3. Facets Engineering
4.4. Photosensitization
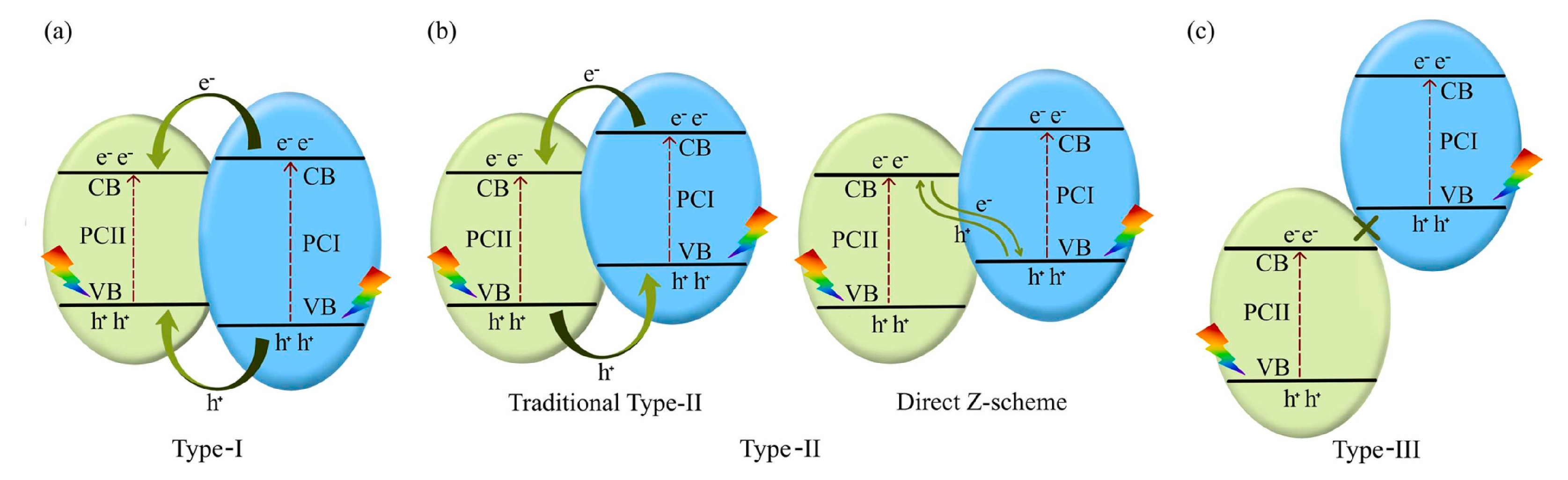
4.5. Design of Semiconductor Heterojunction
5. Summary of Photocatalysis Application
- −
- The intrinsic properties, including morphology, textural, and structural characteristics, vary significantly among different photocatalysts, making them challenging to standardize and compare;
- −
- Reaction conditions such as the type of reactor and the nature of light irradiation—including the light spectrum and intensity—vary among photocatalytic systems. Light absorption and scattering play an important and often underrated role in the overall photocatalytic process, significantly affecting the reaction rate and being difficult to standardize;
- −
- The type of pollutant, its concentration, the catalyst mass, and the pollutant-to-catalyst ratio differ between photocatalytic systems.
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tang, X.; Tang, R.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Gong, D.; Deng, Y.; Su, L.; Liao, C. Application of natural minerals in photocatalytic degradation of organic pollutants: A review. Sci. Total Environ. 2022, 812, 152434. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, N. Recent Progress in photocatalytic removal of environmental pollution hazards in water using nanostructured materials. Separations 2022, 9, 264. [Google Scholar] [CrossRef]
- Cerrato, E.; Gaggero, E.; Calza, P.; Paganini, M.C. The role of cerium, europium and erbium doped TiO2 photocatalysts in water treatment: A mini-review. Chem. Eng. J. Adv. 2022, 10, 100268. [Google Scholar] [CrossRef]
- Sivaraman, C.; Vijayalakshmi, S.; Leonard, E.; Sagadevan, S.; Jambulingam, R. Current Developments in the Effective Removal of Environmental Pollutants through Photocatalytic Degradation Using Nanomaterials. Catalysts 2022, 12, 544. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar Senthil, P.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Djurišic, A.B.; He, Y.; Ng, A.M.C. Visible-light photocatalysts: Prospects and challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A review of graphene-based semiconductors for photocatalytic degradation of pollutants in wastewater. Chemosphere 2022, 300, 134391. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Kumari, H.; Suman, S.; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; Kumar, A.; et al. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil. Pollut. 2023, 234, 349. [Google Scholar] [CrossRef]
- Helmy, E.T.; El Nemr, A.; Mousa, M.; Arafa, E.; Eldafrawy, S. Photocatalytic degradation of organic dyes pollutants in the industrial textile wastewater by using synthesized TiO2, C-doped TiO2, S-doped TiO2 and C,S co-doped TiO2 nanoparticles. J. Water Environ. Nanotechnol. 2018, 3, 116. [Google Scholar]
- Ramalingam, G.; Pachaiappan, R.; Kumar, P.S.; Dharani, S.; Rajendran, S.; Vo, D.-V.N.; Hoang, T.K.A. Hybrid metal organic frameworks as an exotic material for the photocatalytic degradation of pollutants present in wastewater: A review. Chemosphere 2022, 288, 132448. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M.; Mishra, S.R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mater. Res. Bull. 2021, 143, 111417. [Google Scholar] [CrossRef]
- Hadnadjev-Kostic, M.; Karanovic, D.; Vulic, T.; Dostanić, J.; Lončarević, D. Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation. Green Process. Synth. 2023, 12, 20228153. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M. Potential Applications of Nanomaterials in Wastewater Treatment: Nanoadsorbents Performance in Advanced Treatment Techniques for Industrial Wastewater, 1st ed.; Hussain, A., Ahmed, S., Eds.; IGI Global: Hershey, PA, USA, 2019; pp. 51–61. [Google Scholar]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Noor, M.H.; Ngadi, N. Global research landscape on coagulation-flocculation for wastewater treatment: A 2000–2023 bibliometric analysis. J. Water Process Eng. 2024, 64, 105696. [Google Scholar] [CrossRef]
- Lasaki, B.A.; Maurer, P.; Schönberger, H. Uncovering the reasons behind high-performing primary sedimentation tanks for municipal wastewater treatment: An in-depth analysis of key factors. J. Environ. Chem. Eng. 2024, 12, 112460. [Google Scholar] [CrossRef]
- Behroozi, A.H.; Ataabad, M.R. Improvement in microfiltration process of oily wastewater: A comprehensive review over two decades. J. Environ. Chem. Eng. 2021, 9, 104981. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mahmoud, S.A.; Mohamed, A.A. Nanomaterials-modified reverse osmosis membranes: A comprehensive review. RSC Adv. 2024, 14, 18879–18906. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Rafiei, N.; Fatehizadeh, A.; Amin, M.M.; Pourzamani, H.R.; Ebrahimi, A.; Taheri, E.; Aminabhavi, T.M. Application of UV/chlorine processes for the DR83:1 degradation from wastewater: Effect of coexisting anions. J. Environ. Manag. 2021, 297, 113349. [Google Scholar] [CrossRef]
- Venkatesh, S.; Venkatesh, K.; Quaff, A.R. Dye decomposition by combined ozonation and anaerobic treatment: Cost effective technology. J. Appl. Res. Technol. 2017, 15, 340–345. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. Limitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: A critical review. Chemosphere 2022, 296, 134041. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem. Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Liu, K.; Chen, J.; Sun, F.; Liu, Y.; Tang, M.; Yang, Y. Historical development and prospect of intimately coupling photocatalysis and biological technology for pollutant treatment in sewage: A review. Sci. Total Environ. 2022, 835, 155482. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178. [Google Scholar] [CrossRef]
- Gaur, N.; Dutta, D.; Singh, A.; Dubey, R.; Kamboj, D.V. Recent advances in the elimination of persistent organic pollutants by photocatalysis. Front. Environ. Sci. 2022, 10, 872514. [Google Scholar] [CrossRef]
- Hossen, M.A.; Solayman, H.M.; Leong, K.H.; Sim, L.C.; Yaacof, N.; Aziz, A.A.; Wu, L.; Monir, M.U. Recent progress in TiO2-Based photocatalysts for conversion of CO2 to hydrocarbon fuels: A systematic review. Results Eng. 2022, 16, 100795. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloy Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Hadnađev-Kostić, M.; Vulić, T.; Dostanić, J.; Lončarević, D. Design and application of various visible light responsive metal oxide photocatalysts. In Handbook of Smart Photocatalytic Materials Fundamentals, Fabrications, and Water Resources Applications, 1st ed.; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–89. [Google Scholar]
- Karanovic, D.; Hadnadjev-Kostic, M.; Vulic, T.; Markov, S.; Tomic, A.; Miljevic, B.; Rajakovic-Ognjanovic, V. Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance. Green Process. Synth. 2024, 13, 20230269. [Google Scholar] [CrossRef]
- Osterloh, F.E. Kinetic and Thermodynamic Considerations for Photocatalyst Design in Heterogeneous Photocatalysis: From Fundamentals to Applications. In Energy Conversion and Depollution; Strunk, J., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2021; pp. 1–28. [Google Scholar]
- Chen, R.; Fan, F.; Li, C. Unraveling Charge-Separation Mechanisms in Photocatalyst Particles by Spatially Resolved Surface Photovoltage Techniques. Angew. Chem. Int. Ed. 2022, 61, e202117567. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B-Environ. 2010, 99, 398. [Google Scholar] [CrossRef]
- Uscanga Olea, M.A.; Perez Bueno, J.d.J.; Maldonado Perez, A.X. Nanometric and surface properties of semiconductors correlated to photocatalysis and photoelectrocatalysis applied to organic pollutants—A review. J. Environ. Chem. Eng. 2021, 9, 106480. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Esmail Ebrahim, S. Recent advances in nano-semiconductors photocatalysis for degrading organic contaminants and microbial disinfection in wastewater: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100666. [Google Scholar] [CrossRef]
- Rauf, M.A.; Salman Ashraf, S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Ye, L.; Mao, J.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Synthesis of anatase TiO2 nanocrystals with {101}, {001} or {010} single facets of 90% level exposure and liquid-phase photocatalytic reduction and oxidation activity orders. J. Mater. Chem. A 2013, 35, 10532. [Google Scholar] [CrossRef]
- Karanović, Đ.; Hadnađev-Kostić, M.; Vulić, T.J.; Milanović, M.M.; Rajaković-Ognjanović, V.N.; Marinković-Nedučin, R.P. The influence of the coprecipitation synthesis methods on photodegradation efficiency of ZnFe based photocatalysts. J. Serb. Chem. Soc. 2024, 89, 667–678. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Kumar, R.; Kumar Thakur, V.; Nguyen, V.H.; Asiri, A.M.; Parwaz Khan, A.A.; Singh, P. Recent progress on bismuth-based Z-scheme semiconductor photocatalysts for energy and environmental applications. J. Environ. Chem. Eng. 2020, 8, 104505. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Huang, W. Bismuth-based photocatalysts for solar energy conversion. J. Mater. Chem. A 2020, 8, 24307. [Google Scholar] [CrossRef]
- Chen, L.; He, J.; Liu, Y.; Chen, P.; Au, C.T.; Yin, S.F. Recent advances in bismuth-containing photocatalysts with heterojunctions. Chin. J. Catal. 2016, 37, 780. [Google Scholar] [CrossRef]
- Kumar, R.; Raizada, P.; Verma, N.; Hosseini-Bandegharaei, A.; Kumar Thakur, V.; Van Le, Q.; Nguyen, V.H.; Selvasembian, R.; Singh, P. Recent advances on water disinfection using bismuth based modified photocatalysts: Strategies and challenges. J. Clean. Prod. 2021, 297, 126617. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Wang, S.; Huang, H. Controllable synthesis, characterization and photocatalytic performance of four kinds of bismuth-based materials. Colloids Surf. A 2019, 568, 419. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Yang, H.J.; Liu, Y.; Gurusamy, L.; Karuppasamy, L.; Wu, J.J. Photocatalytic Hydrogen Evolution from Water Splitting Using Core-Shell Structured Cu/ZnS/COF Composites. Nanomaterials 2021, 11, 3380. [Google Scholar] [CrossRef]
- Cervantes-Diaz, K.B.; Drobek, M.; Julbe, A.; Cambedouzou, J. SiC Foams for the Photocatalytic Degradation of Methylene Blue under Visible Light Irradiation. Materials 2023, 16, 1328. [Google Scholar] [CrossRef]
- Hao, J.Y.; Wang, Y.Y.; Tong, X.L.; Jin, G.Q.; Guo, X.Y. Photocatalytic hydrogen production over modified SiC nanowires under visible light irradiation. Int. J. Hydrogen Energy 2012, 37, 15038. [Google Scholar] [CrossRef]
- Das, S.; Daud, W.M.A.W. A review on advances in photocatalysts towards CO2 conversion. RSC Adv. 2014, 4, 20856–20893. [Google Scholar] [CrossRef]
- Žerjav, G.; Say, Z.; Zavašnik, J.; Finšgar, M.; Langhammer, C.; Pintar, A. Photo, thermal and photothermal activity of TiO2 supported Pt catalysts for plasmon-driven environmental applications. J. Environ. Chem. Eng. 2023, 11, 110209. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, M.; Wang, X.; Liu, S.; Zhang, L.; Liu, X.; Geng, Y.; Sun, X.; Liu, B. Pd nanoparticle-modified TiO2-x composites as efficient visible-light active photocatalysts for tetracycline degradation and microbial disinfection. Opt. Mater. 2023, 136, 113396. [Google Scholar] [CrossRef]
- Slapničar, Š.; Žerjav, G.; Zavašnik, J.; Finšgar, M.; Pintar, A. Synthesis and characterization of plasmonic Au/TiO2 nanorod solids for heterogeneous photocatalysis. J. Environ. Chem. Eng. 2023, 11, 109835. [Google Scholar] [CrossRef]
- Michalska, M.; Pavlovský, J.; Lemański, K.; Małecka, M.; Ptak, M.; Novák, V.; Kormunda, M.; Matějka, V. The effect of surface modification with Ag nanoparticles on 21 nm TiO2: Anatase/rutile material for application in photocatalysis. Mater. Chem. Tod. 2022, 26, 101123. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Liyanaarachchi, C.; Samarakoon, U. Efficient photocatalysis of Cu doped TiO2/g-C3N4 for the photodegradation of methylene blue. Arab. J. Chem. 2023, 16, 104749. [Google Scholar] [CrossRef]
- Dostanić, J.; Lončarević, D.; Pavlović, V.B.; Papan, J.; Nedeljković, J.M. Efficient photocatalytic hydrogen production over titanate/titania nanostructures modified with nickel. Ceram. Int. 2019, 45, 19447. [Google Scholar] [CrossRef]
- Aldao, C.M. Surface and interface electronic properties of tin oxide. In Tin Oxide Materials Synthesis, Properties, and Applications Metal Oxides, 1st ed.; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherland, 2020; pp. 101–132. [Google Scholar]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 3, 3001. [Google Scholar] [CrossRef]
- Anwer, S.; Bharath, G.; Iqbal, S.; Qian, H.; Masood, T.; Liao, K.; Cantwell, W.J.; Zhang, J.; Zheng, L. Synthesis of edge-site selectively deposited Au nanocrystals on TiO2 nanosheets: An efficient heterogeneous catalyst with enhanced visible-light photoactivity. Electrochim. Acta 2018, 283, 1095. [Google Scholar] [CrossRef]
- Whitney, A.V.; Elam, J.W.; Zou, S.; Zinovev, A.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Localized surface plasmon resonance Nanosensor: A high-resolution distance-dependence study using atomic layer deposition. J. Phys. Chem. B 2005, 109, 20522. [Google Scholar] [CrossRef]
- Li, Y.; Liao, D.; Li, T.; Zhong, W.; Wang, X.; Hong, X.; Yu, H. Plasmonic Z-scheme Pt-Au/BiVO4 photocatalyst: Synergistic effect of crystal-facet engineering and selective loading of Pt-Au cocatalyst for improved photocatalytic performance. J. Colloid. Interf. Sci. 2020, 570, 232. [Google Scholar] [CrossRef]
- Kamble, R.J.; Gaikwad, P.V.; Garadkar, K.M.; Sabale, S.R.; Puri, V.R.; Mahajan, S.S. Photocatalytic degradation of malachite green using hydrothermally synthesized cobalt-doped TiO2 nanoparticles. J. Iran. Chem. Soc. 2022, 19, 303. [Google Scholar] [CrossRef]
- Wong, R.S.; Feng, J.; Hu, X.; Yue, P.L. Discoloration and mineralization of non-biodegradable azo dye orange II by copper-doped TiO2 nanocatalysts. J. Environ. Sci. Health A 2004, 39, 2583. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, G.G.; Nikam, V.S.; Kanade, K.G.; Arbuj, S.; Kale, B.B.; Baeg, J.O. Hydrothermally derived nanosized Ni-doped TiO2: A visible light driven photocatalyst for methylene blue degradation. Mater. Chem. Phys. 2010, 124, 976. [Google Scholar] [CrossRef]
- Chippada, M.L.V.P.; Sailaja, B.B.V.; Siva Rao, T.; Divya, G.; Nayak, S.R.; Satwika Manogna, B.; Jaishree, G. Structural modification of nano titania by doping with Barium and Copper—Impact on photocatalysis: Applications in degradation of dye and pathogens. Hybrid Adv. 2023, 3, 100033. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Iqbal Rokon, M.Z.; Rahim, M.A.; Hossain, M.I.; Islam, M.S.; Ali, M.R.; Bacchu, M.S.; Waizumi, H.; Komeda, T.; Hossain Khan, M.Z. Enhanced photocatalytic activity of Cu and Ni-doped ZnO nanostructures: A comparative study of methyl orange dye degradation in aqueous solution. Heliyon 2023, 9, e16506. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Moreira, A.J.; dos Santos, B.R.M.; Dias, J.A.; Rabello, P.T.; Coelho, D.; Mascaro, L.H.; Freschi, G.P.G.; Gobato, Y.G.; Galeti, H.V.A.; Mastelaro, V.R.; et al. Photoactivity of boron-or nitrogen-modified TiO2 for organic pollutants degradation: Unveiling the photocatalytic mechanisms and by-products. J. Environ. Chem. Eng. 2023, 11, 109207. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Lin, R.; Wang, Y. Exploring the mechanism of room temperature ferromagnetism in C-doped TiO2 nanoclusters by tuning the defects by different annealing temperature using citric acid as C source. Ceram. Int. 2022, 48, 26836–26845. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Z.; Li, Y.; Wang, W.; Li, J.; Li, J.; Ao, Y.; He, J.; Sharma, V.K.; Wang, J. Morphology and Phase Controlled Synthesis of Visible-Light-Activated S-doped TiO2 with Tunable S4+/S6+ Ratio. Chem. Eng. J. 2020, 402, 125549. [Google Scholar] [CrossRef]
- Divya, G.; Jaishree, G.; Rao, T.S.; Chippada, M.L.V.P.; Lakshmi, K.V.D.; Supriya, S.S. Improved catalytic efficiency by N-doped TiO2 via sol gel under microwave irradiation: Dual applications in degradation of dye and microbes. Hybrid Adv. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Filippatos, P.P.; Soultati, A.; Kelaidis, N.; Petaroudis, C.; Alivisatou, A.-A.; Drivas, C.; Kennou, S.; Agapaki, E.; Charalampidis, G.; bin Mohd Yusof, A.R.; et al. Preparation of hydrogen, fluorine and chlorine doped and co-doped titanium dioxide photocatalysts: A theoretical and experimental approach. Sci. Rep. 2021, 11, 5700. [Google Scholar] [CrossRef] [PubMed]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Kim, T.H.; Go, G.-M.; Cho, H.-B.; Song, Y.; Lee, C.-G.; Choa, Y.-H. A novel synthetic method for N doped TiO2 nanoparticles through plasma-assisted electrolysis and photocatalytic activity in the visible region. Front. Chem. 2018, 6, 458. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Photocatalytic and photoelectrochemical properties of nitrogen-doped titanium dioxide. Chemphyschem 2003, 4, 487–490. [Google Scholar] [CrossRef]
- Naik, B.; Moon, S.Y.; Kim, S.H.; Park, J.Y. Enhanced photocatalytic generation of hydrogen by Pt-deposited nitrogen-doped TiO2 hierarchical nanostructures. Appl. Surf. Sci. 2015, 354, 347–352. [Google Scholar] [CrossRef]
- Quesada-Cabrera, R.; Sotelo-Vázquez, C.; Quesada-González, M.; Pulido Melián, E.; Chadwick, N.; Parkin, I.P. On the apparent visible-light and enhanced UV-light photocatalytic activity of nitrogen-doped TiO2 thin films. J. Photoc. Photob. A 2017, 333, 49–55. [Google Scholar] [CrossRef][Green Version]
- Kuznetsov, V.N.; Serpone, N. On the origin of the spectral bands in the visible absorption spectra of visible-light-active TiO2 specimens analysis and assignments. J. Phys. Chem. C 2009, 113, 15110–15123. [Google Scholar] [CrossRef]
- Emeline, A.V.; Kuznetsov, V.N.; Rybchuk, V.K.; Serpone, N. Visible-light-active titania photocatalysts: The case of N-doped TiO2 s—Properties and some fundamental issues. Int. J. Photoenergy 2008, 2008, 258394. [Google Scholar] [CrossRef]
- Zeng, L.; Song, W.; Li, M.; Jie, X.; Zeng, D.; Xie, C. Comparative study on the visible light driven photocatalytic activity between substitutional nitrogen doped and interstitial nitrogen doped TiO2. Appl. Catal. A Gen. 2014, 488, 239–247. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.F.; Yu, H.; Wang, H.J.; Yang, J. Synthesis and characterization of substitutional and interstitial nitrogen-doped titanium dioxides with visible light photocatalytic activity. J. Solid State Chem. 2008, 181, 130–136. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B. 2005, 109, 11414–11419. [Google Scholar] [CrossRef]
- Fadlallah, M.M. Magnetic, electronic, optical, and photocatalytic properties of nonmetaland halogen-doped anatase TiO2 nanotubes. Physica E 2017, 89, 50–56. [Google Scholar] [CrossRef]
- Xing, H.; Wu, L.; Li, X. Zn/N co-doped TiO2 Nanotubes for Enhancement of Photocatalytic Degradation of Pentachlorophenol. Int. J. Electrochem. Sci. 2022, 17, 22066. [Google Scholar] [CrossRef]
- Liu, H. Improved visible light photocatalytic activity of Fe, N co-doped TiO2 for degradation of o-chlorophenol in water. Int. J. Electrochem. Sci. 2022, 17, 220322. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kalaivani, S.; Amala Infant Joice, J.; Sivakumar, T. Photocatalytic activity of multielement doped TiO2 in the degradation of congo red. Appl. Surf. Sci. 2012, 258, 2515–2521. [Google Scholar] [CrossRef]
- Jabbari, V.; Hamadanian, M.; Karimzadeh, S.; Villagra, D. Enhanced charge carrier efficiency and solar light-induced photocatalytic activity of TiO2 nanoparticles through doping of silver nanoclusters and C–N–S nonmetals. J. Ind. Eng. Chem. 2016, 35, 132–139. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Huang, B.; Shao, Q.; Xin, H.L.; Huang, X. Amorphization activated Ruthenium-Tellurium nanorods for efficient water splitting. Nat. Commun. 2019, 10, 5692. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Han, Z.-F.; Yan, X.; Lang, W.-Z.; Guo, Y.-J. Effective synthesis of vanadium-doped mesoporous silica nanospheres by sol-gel method for propane dehydrogenation reaction. Microporous Mesoporous Mater. 2022, 330, 111616. [Google Scholar] [CrossRef]
- Xia, Y.-J.; Song, J.-L.; Yuan, D.-N.; Guo, X.-N.; Guo, X. Synthesis and characterization of one-dimensional metal oxides: TiO2, CeO2, Y2O3-stabilized ZrO2 and SrTiO3. Ceram. Int. 2015, 41, 533–545. [Google Scholar] [CrossRef]
- Cao, Y.; Jia, D.; Wang, R.; Luo, J. Rapid one-step room-temperature solid-state synthesis and formation mechanism of ZnO nanorods as H2S-sensing materials. Solid State Electron. 2013, 82, 67. [Google Scholar] [CrossRef]
- Buscaglia, M.T.; Sennour, M.; Buscaglia, V.; Bottino, C.; Kalyani, V.; Nanni, P. Formation of Bi4Ti3O12 one-dimensional structures by solid-state reactive diffusion. From core−shell templates to nanorods and nanotubes. Cryst. Growth Des. 2011, 11, 1394. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, W.Z.; Du, G.H.; Peng, L.M. Trititanate nanotubes made via a single alkali treatment. Adv. Mater. 2002, 14, 1208–1211. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, L.M.; Chen, Q.; Du, G.H.; Dawson, G.; Zhou, W.Z. Formation mechanism of H2Ti3O7 nanotubes. Phys. Rev. Lett. 2003, 91, 256103. [Google Scholar] [CrossRef]
- Duan, H.M.; McKinnon, J.T. Nanoclusters produced in flames. J. Phys. Chem. 1994, 98, 12815. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Papa, A.-L.; Millot, N.; Saviot, L.; Chassagnon, R.; Heintz, O. Titanate nanotubes: Towards a novel and safer nanovector for cardiomyocytes. J. Phys. Chem. C 2009, 113, 12682–12689. [Google Scholar] [CrossRef]
- Morgado, E., Jr.; De Abreu, M.A.S.; Moure, G.T.; Marinkovic, B.A.; Jardim, P.M.; Araujo, A.S. Effects of thermal treatment of nanostructured trititanates on their crystallographic and textural properties. Mater. Res. Bull. 2007, 42, 1748–1760. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Deng, Z.; Tong, H. Formation of titanate nanostructures under different NaOH concentration and their application in wastewater treatment. J. Solid State Chem. 2011, 184, 712–719. [Google Scholar] [CrossRef]
- Seo, H.-K.; Kim, G.-S.; Ansari, S.G.; Kim, Y.-S.; Shin, H.-S.; Shim, K.-H.; Suh, E.-K. A study on the structure/phase transformation of titanate nanotubes synthesized at various hydrothermal temperatures. Sol. Energy Mater. Sol. Cells 2008, 92, 1533–1539. [Google Scholar] [CrossRef]
- Ou, H.-H.; Lo, S.-L. Review of titania nanotubes synthesized via the hydrothermal treatment: Fabrication, modification, and application. Sep. Purif. Technol. 2007, 58, 179–191. [Google Scholar] [CrossRef]
- Lan, Y.; Gao, X.P.; Zhu, H.Y.; Zheng, Z.F.; Yan, T.Y.; Wu, F.; Ringer, S.P.; Song, D.Y. Titanate nanotubes and nanorods prepared from rutile powder. Adv. Funct. Mater. 2005, 15, 1310–1318. [Google Scholar] [CrossRef]
- Sikhwivhilu, L.M.; Ray, S.S.; Coville, N.J. Influence of bases on hydrothermal synthesis of titanate nanostructures. Appl. Phys. A Mater. Sci. Process. 2009, 94, 963–973. [Google Scholar] [CrossRef]
- Morgan, D.L.; Zhu, H.Y.; Frost, R.L.; Waclawik, E.R. Determination of a morphological phase diagram of titania/titanate nanostructures from alkaline hydrothermal treatment of Degussa P25. Chem. Mater. 2008, 20, 3800–3802. [Google Scholar] [CrossRef]
- Turki, A.; Kochkar, H.; Guillard, C.; Berhault, G.; Ghorbel, A. Effect of Na content and thermal treatment of titanate nanotubes on the photocatalytic degradation of formic acid. Appl. Catal. B Environ. 2013, 138/139, 401–415. [Google Scholar] [CrossRef]
- Lincho, J.; Domingues, E.; Mazierski, P.; Miodyńska, M.; Klimczuk, T.; Zaleska-Medynska, A.; Martins, R.C.; Gomes, J. The role of noble metals in TiO2 nanotubes for the abatement of parabens by photocatalysis, catalytic and photocatalytic ozonation. Sep. Purif. Technol. 2023, 326, 124747. [Google Scholar] [CrossRef]
- Zeng, L.; Lu, Z.; Li, M.; Yang, J.; Song, W.; Zeng, D.; Xie, C. A modular calcination method to prepare modified N-doped TiO2 nanoparticle with high photocatalytic activity. Appl. Catal. B-Environ. 2016, 183, 308–316. [Google Scholar] [CrossRef]
- Sheng, L.; Liao, T.; Kou, L.; Sun, Z. Single-crystalline ultrathin 2D TiO2 nanosheets: A bridge towards superior photovoltaic devices. Mater. Today Energy 2017, 3, 32–39. [Google Scholar] [CrossRef]
- He, Z.; Li, Y.; Zhang, Q.; Wang, H. Capillary microchannel-based microreactors with highly durable ZnO/TiO2 nanorod arrays for rapid, high efficiency and continuous-flow photocatalysis. Appl. Catal. B-Environ. 2010, 93, 376–382. [Google Scholar] [CrossRef]
- Weng, B.; Liu, S.; Tang, Z.; Xu, Y. One-dimensional nanostructure based materials for versatile photocatalytic applications. RSC Adv. 2014, 4, 12685. [Google Scholar] [CrossRef]
- Naito, K.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Single-molecule observation of photocatalytic reaction in TiO2 nanotube: Importance of molecular transport through porous structures. J. Am. Chem. Soc. 2008, 131, 934. [Google Scholar] [CrossRef] [PubMed]
- Samykano, M. Progress in one-dimensional nanostructures. Mater. Charact. 2021, 179, 111373. [Google Scholar] [CrossRef]
- He, Z.; Zhang, J.; Li, X.; Guan, S.; Dai, M.; Wang, S. 1D/2D heterostructured photocatalysts: From design and unique properties to their environmental applications. Small 2020, 16, 2005051. [Google Scholar] [CrossRef]
- Machín, A.; Fontánez, K.; Arango, J.C.; Ortiz, D.; De León, J.; Pinilla, S.; Nicolosi, V.; Petrescu, F.I.; Morant, C.; Márquez, F. One-dimensional (1D) nanostructured materials for energy applications. Materials 2021, 14, 2609. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, L. Synthesis strategies about 2D materials. In Two Dimensional Materials, 1st ed.; Kumar Nayak, P., Ed.; Intech Open: Vienna, Austria, 2016; pp. 1–20. [Google Scholar]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal Synthesis and Photoreactivity of Anatase TiO2 Nanosheets with Dominant {001} Facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Hong, Y.J.; Saroj, R.K.; Park, W.I.; Yi, G.-C. One-dimensional semiconductor nanostructures grown on two-dimensional nanomaterials for flexible device applications. Apl. Mater. 2021, 9, 060907. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.-M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 63, 155409. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kuang, Q.; Wang, Q.; Xie, Z. Engineering a high energy surface of anatase TiO2 crystals towards enhanced performance for energy conversion and environmental applications. RSC Adv. 2015, 5, 20396–20409. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.-O.; Uchida, S.; Shibayama, T.; Ohtani, B. Photocatalytic activity of octahedral single-crystalline mesoparticles of anatase titanium(iv) oxide. Chem. Commun. 2009, 17, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Wang, C.-H.; Zhai, Y.; Zhang, R.-Q.; Van Hove, M.A. Selective adsorption of l-serine functional groups on the anatase TiO2 (101) surface in benthic microbial fuel cells. Phys. Chem. Chem. Phys. 2014, 16, 20806–20817. [Google Scholar] [CrossRef]
- Dudziak, S.; Borzyszkowska, A.F.; Zielinska-Jurek, A. Photocatalytic degradation and pollutant-oriented structure-activity analysis of carbamazepine, ibuprofen and acetaminophen over faceted TiO2. J. Environ. Chem. Eng. 2023, 11, 109553. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, X. Reversing the photocatalytic activity orders of anatase TiO2 facets by surface treatment. Communication 2016, 1, 5838–5841. [Google Scholar] [CrossRef]
- Ye, L.; Mao, J.; Peng, T.; Zan, L.; Zhang, Y. Opposite photocatalytic activity orders of low-index facets of anatase TiO2 for liquid phase dye degradation and gaseous phase CO2 photoreduction. Phys. Chem. Chem. Phys. 2014, 16, 15675. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Hu, R.; Yuan, Q.; Zou, Z. Anatase TiO2 hierarchical microspheres with selectively etched high-energy {001} crystal facets for high-performance acetone sensing and methyl orange degradation. Mater. Res. Bull. 2017, 94, 272–278. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B-Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Wang, K.; He, S.; Lin, Y.; Chen, X.; Dai, W.; Fu, X. Photo-enhanced thermal catalytic CO2 methanation activity and stability over oxygen-deficient Ru/TiO2 with exposed TiO2 {001} facets: Adjusting photogenerated electron behaviors by metal-support interactions. Chin. J. Catal. 2022, 43, 391–402. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, Y.; Gao, T.; Zhang, J.; Sun, X.; Zhou, G. Fabrication of anatase TiO2 tapered tetragonal nanorods with designed {100}, {001} and {101} facets for enhanced photocatalytic H2 evolution. Int. J. Hydrogen Energy 2017, 42, 21775–21785. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Gratzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Tae, E.L.; Lee, S.H.; Lee, J.K.; Yoo, S.S.; Kang, E.J.; Yoon, K.B. A strategy to increase the efficiency of the dye-sensitized TiO2 solar cells operated by photoexcitation of dye-to-TiO2 charge-transfer bands. J. Phys. Chem. B 2005, 109, 22513. [Google Scholar] [CrossRef]
- Calzolari, A.; Ruini, A.; Catellani, A. Anchor group versus conjugation: Towards the gap-state engineering of functionalized ZnO(10-10) surface for optoelectronic applications. J. Am. Chem. Soc. 2011, 133, 5893. [Google Scholar] [CrossRef]
- Nawrocka, A.; Zdyb, A.; Krawczyk, S. Stark spectroscopy of charge-transfer transitions in catechol-sensitized TiO2 nanoparticles. Chem. Phys. Lett. 2009, 475, 272–276. [Google Scholar] [CrossRef]
- Tachan, Z.; Hod, I.; Zaban, A. The TiO2–catechol complex: Coupling type II sensitization with efficient catalysis of water oxidation. Adv. Energy Mater. 2014, 4, 1301249. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Shi, J.-L.; Hao, H.; Yuan, H.; Lang, X. Salicylic acid complexed with TiO2 for visible light-driven selective oxidation of amines into imines with air. Appl. Catal. B Environ. 2019, 244, 758–766. [Google Scholar] [CrossRef]
- Milićević, B.; Djordjević, V.; Lončarević, D.; Dostanić, J.M.; Ahrenkiel, S.P.; Dramićanin, M.D.; Sredojević, D.; Švrakić, N.M.; Nedeljković, J.M. Charge-transfer complex formation between TiO2 nanoparticles and thiosalicylic acid: A comprehensive experimental and DFT study. Opt. Mater. 2017, 73, 163–171. [Google Scholar] [CrossRef]
- Kay, A.; Graetzel, M. Artificial photosynthesis. 1. Photosensitization of titania solar cells with chlorophyll derivatives and related natural porphyrins. J. Phys. Chem. 1993, 97, 6272–6277. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of TiO2 by halogenated porphyrin derivatives for visible light biomedical and environmental photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.-H.; Wang, L.-N. Porphyrin-Based Organophotocatalysts. In Phthalocyanines and Some Current Applications, 1st ed.; Yilmaz, Y., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Zhang, P.; Song, T.; Wang, T.; Zeng, H. Enhancement of hydrogen production of a Cu–TiO2 nanocomposite photocatalyst combined with broad spectrum absorption sensitizer Erythrosin B. RSC Adv. 2017, 7, 17873–17881. [Google Scholar] [CrossRef]
- Le, T.T.; Akhtar, M.S.; Park, D.M.; Lee, J.C.; Yang, O.-B. Water splitting on Rhodamine-B dye ensitized Co-doped TiO2 catalyst under visible light. Appl. Catal. B Environ. 2012, 111–112, 397–401. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Peng, S.; Lu, G.; Li, S. Photosensitization of SiW11O398−-modified TiO2 by Eosin Y for stable visible-light H2 generation. Int. J. Hydrogen Energy 2013, 38, 1709–11719. [Google Scholar] [CrossRef]
- Flores, J.; Moya, P.; Bosca, F.; Marin, M.L. Photoreactivity of new rose bengal-SiO2 heterogeneous photocatalysts with and without a magnetite core for drug degradation and disinfection. Catal. Today 2023, 413–415, 113994. [Google Scholar] [CrossRef]
- Toor, R.A.; Sayyad, M.H.; Nasr, N.; Sajjad, S.; Shah, S.A.A.; Manzoor, T. Efficiency enhancement of dye sensitized solar cells with a low cost co-adsorbant in N719 dye. Int. J. Sustain. Energy Environ. Res. 2016, 5, 46–50. [Google Scholar] [CrossRef]
- Liu, S.-H.; Fu, H.; Cheng, Y.-M.; Wu, K.-L.; Ho, S.-T.; Chi, Y.; Chou, P.-T. Theoretical study of N749 dyes anchoring on the (TiO2)28 surface in DSSCs and their electronic absorption properties. J. Phys. Chem. C 2012, 116, 16338–16345. [Google Scholar] [CrossRef]
- Hao, S.; Wu, J.; Huang, Y.; Lin, J. Natural dyes as photosensitizers for dye-sensitized solar cell. Sol. Energy 2006, 80, 209–214. [Google Scholar] [CrossRef]
- Zdyb, A.; Krawczyk, S. Natural flavonoids as potential photosensitizers for dye-sensitized solar cells. Ecol. Chem. Eng. S 2019, 26, 29–36. [Google Scholar] [CrossRef]
- Tennakone, K.; Kumara, G.R.R.A.; Kumarasinghe, A.R.; Sirimanne, P.M.; Wijayantha, K.G.U. Efficient photosensitization of nanocrystalline TiO2 films by tannins and related phenolic substances. J. Photochem. Photobiol. A 1996, 94, 217–220. [Google Scholar] [CrossRef]
- Zhang, D.; Lanier, S.M.; Downing, J.A.; Avent, J.L.; Lum, J.; McHale, J.L. Betalain pigments for dye-sensitized solar cells. J. Photochem. Photobiol. A 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Zarubica, A.; Ljupković, R.; Papan, J.; Vukoje, I.; Porobić, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Visible-light-responsive Al2O3 powder: Photocatalytic study. Opt. Mater. 2020, 106, 110013. [Google Scholar] [CrossRef]
- Huang, J.-F.; Lei, Y.; Xiao, L.-M.; Chen, X.-L.; Zhong, Y.-H.; Qin, S.; Liu, J.-M. Photocatalysts for H2 generation from starburst triphenylamine/carbazole donor-based metal-free dyes and porous Anatase TiO2 Cube. ChemSusChem 2020, 13, 1037–1043. [Google Scholar] [CrossRef]
- Ortiz-Bustos, J.; del Hierro, I.; Sánchez-Ruiz, A.; García-Martínez, J.C.; Pérez, Y. Tuning of type-I and type-II mechanisms for visible light degradation in tris(styryl)benzene-sensitized TiO2 nanoparticles. Dye. Pigment. 2021, 184, 108802. [Google Scholar] [CrossRef]
- Hu, W.; Yang, J. Two-dimensional van der Waals heterojunctions for functional materials and devices. J. Mater. Chem. C 2017, 5, 12289. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide (TiO2)-based heterojunctions. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.G.; Jaroniec, M. All-solid-state Z-scheme hotocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Xu, F.Y.; Xiao, W.; Cheng, B.; Yu, J.G. Direct Z-scheme anatase/rutile bi-phase nanocomposite TiO2 nanofiber photocatalyst with enhanced photocatalytic H2-production activity. Int. J. Hydrogen Energy 2014, 39, 15394–15402. [Google Scholar] [CrossRef]
- Wei, Y.C.; Jiao, J.Q.; Zhao, Z.; Liu, J.; Li, J.M.; Jiang, G.Y.; Wang, Y.J.; Duan, A.J. Fabrication of inverse opal TiO2-supported Au@ CdS core–shell nanoparticles for efficient photocatalytic CO2 conversion. Appl. Catal. B Environ. 2015, 179, 422–432. [Google Scholar] [CrossRef]
- Milošević, K.; Lončarević, D.; Mudrinić, T.; Kalagasidis Krušić, M.; Dostanić, J. Mechanistic insights into the simultaneous visible-light induced photodegradation of organic pollutants by g-C3N4/titanate heterojunction. J. Nanopart. Res. 2023, 25, 26. [Google Scholar] [CrossRef]
- Yu, J.G.; Wang, S.H.; Low, J.X.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Suliman, Z.A.; Mecha, A.C.; Mwasiagi, J.I. Effect of TiO2/Fe2O3 nanopowder synthesis method on visible light photocatalytic degradation of reactive blue dye. Heliyon 2024, 10, e29648. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Luo, X.; Chen, Y.; Lei, K.; Mao, L.; Duan, Y.; Zeng, X.; Wan, G.; Zhao, Q.; et al. Facile construction of Bi2WO6/TiO2 microspheres heterojunction for boosting photocatalytic degradation of organic dyes. Inorg. Chem. Commun. 2024, 165, 112543. [Google Scholar] [CrossRef]
- Tao, P.; Wanga, Y. Enhanced photocatalytic performance of W-doped TiO2 nanoparticles for treatment of Procion Red MX-5B azo dye in textile wastewater. Int. J. Electrochem. Sci. 2023, 18, 100261. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci. 2018, 427B, 791–799. [Google Scholar] [CrossRef]
- Kumar Pal, V.; Kumar, D.; Gupta, A.; Neelratan Pashupati, P.; Purohit, L.P.; Singh, A.; Singh, V.; Lee, S.; Kumar Mishra, Y.; Kaushik, A.; et al. Nanocarbons decorated TiO2 as advanced nanocomposite fabric for photocatalytic degradation of methylene blue dye and ciprofloxacin. Diam. Relat. Mater. 2024, 148, 111435. [Google Scholar]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Rekha Buddiga, L.; Rao Gajula, G.; Sailaja, B.B.V.; Prasanna Ch, M.L.V. Visible light photocatalytic exploit of P/Zr doped TiO2 nano particles for dye degradation of rose Bengal. Appl. Surf. Sci. Adv. 2023, 18, 100492. [Google Scholar] [CrossRef]
- Saqib, M.; Rahman, N.; Safeen, K.; Mekkey, S.D.; Salem, M.A.; Safeen, A.; Husain, M.; u Zaman, S.; Abdullaev, S.; Kalsoom, A.; et al. Structure phase-induced photodegradation properties of cobalt-sulfur co-doped TiO2 nanoparticles synthesized by hydrothermal route. J. Mater. Res. Technol. 2023, 26, 8048–8060. [Google Scholar] [CrossRef]
- Khataeea, A.R.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A-Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Galindo, C.; Kalt, A. UV/H2O2 oxidation of azodyes in aqueous media: Evidence of a structure/degradability relationship. Dye. Pigment. 1999, 42, 199–207. [Google Scholar] [CrossRef]
- Hammett, L.P. The effect of structure upon the reactions of organic compounds. Benzene derivatives. J. Am. Chem. Soc. 1937, 59, 96103. [Google Scholar] [CrossRef]
- Parra, S.; Olivero, J.; Pacheco, L.; Pulgarin, C. Structural properties and photoreactivity relationships of substituted phenols in TiO2 suspensions. Appl. Catal. B-Environ. 2003, 43, 293–301. [Google Scholar] [CrossRef]
- Dostanic, J.; Loncarevic, D.; Zlatar, M.; Vlahovic, F.; Jovanovic, D.M. Quantitative structure-activity relationship analysis of substituted arylazo pyridone dyes in photocatalytic system: Experimental and theoretical study. J. Hazard. Mater. 2016, 316, 26–33. [Google Scholar] [CrossRef]
- Meetani, M.A.; Rauf, M.A.; Hisaindee, S.; Khaleel, A.A.; AlZamly, A.; Ahmed, A. Mechanistic studies of photoinduced degradation of Orange G using LC/MS. RSC Adv. 2011, 1, 490–497. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Sun, J.; Sun, R.; Sun, S.; Qiao, L. Photocatalytic Degradation and Kinetics of Orange G Using Nano-Sized Sn(IV)/TiO2/AC Photocatalyst. J. Mol. Catal. A Chem. 2006, 260, 241–246. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic degradation of Methylene Blue using a mixed catalyst and product analysis by LC/MS. J. Chem. Eng. 2010, 157, 373–378. [Google Scholar] [CrossRef]
- Benigni, R.; Passerini, L. Carcinogenicity of the aromatic amines: From structure-activity relationships to mechanisms of action and risk assessment. Mutat. Res. 2002, 511, 191–206. [Google Scholar] [CrossRef] [PubMed]

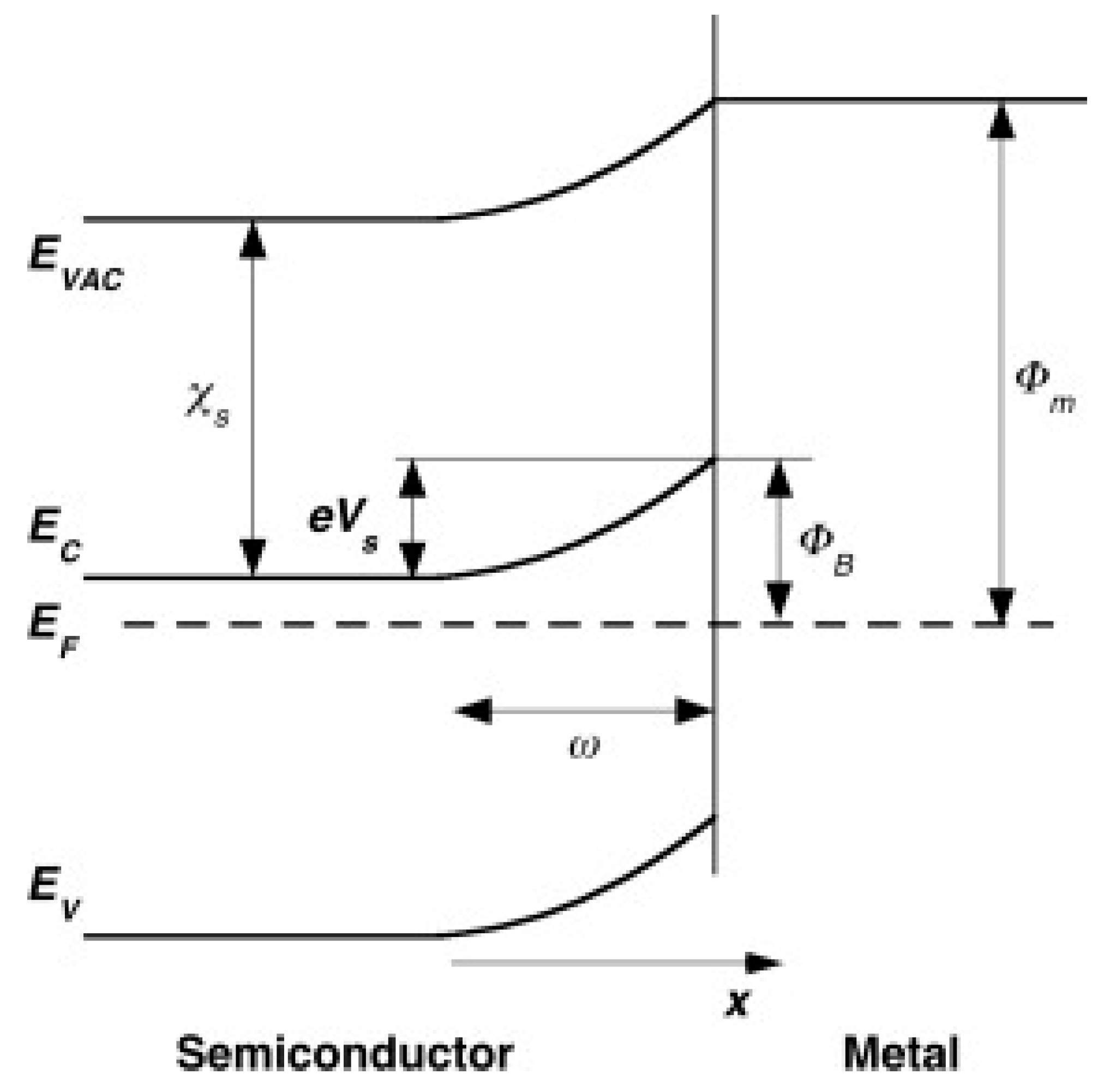
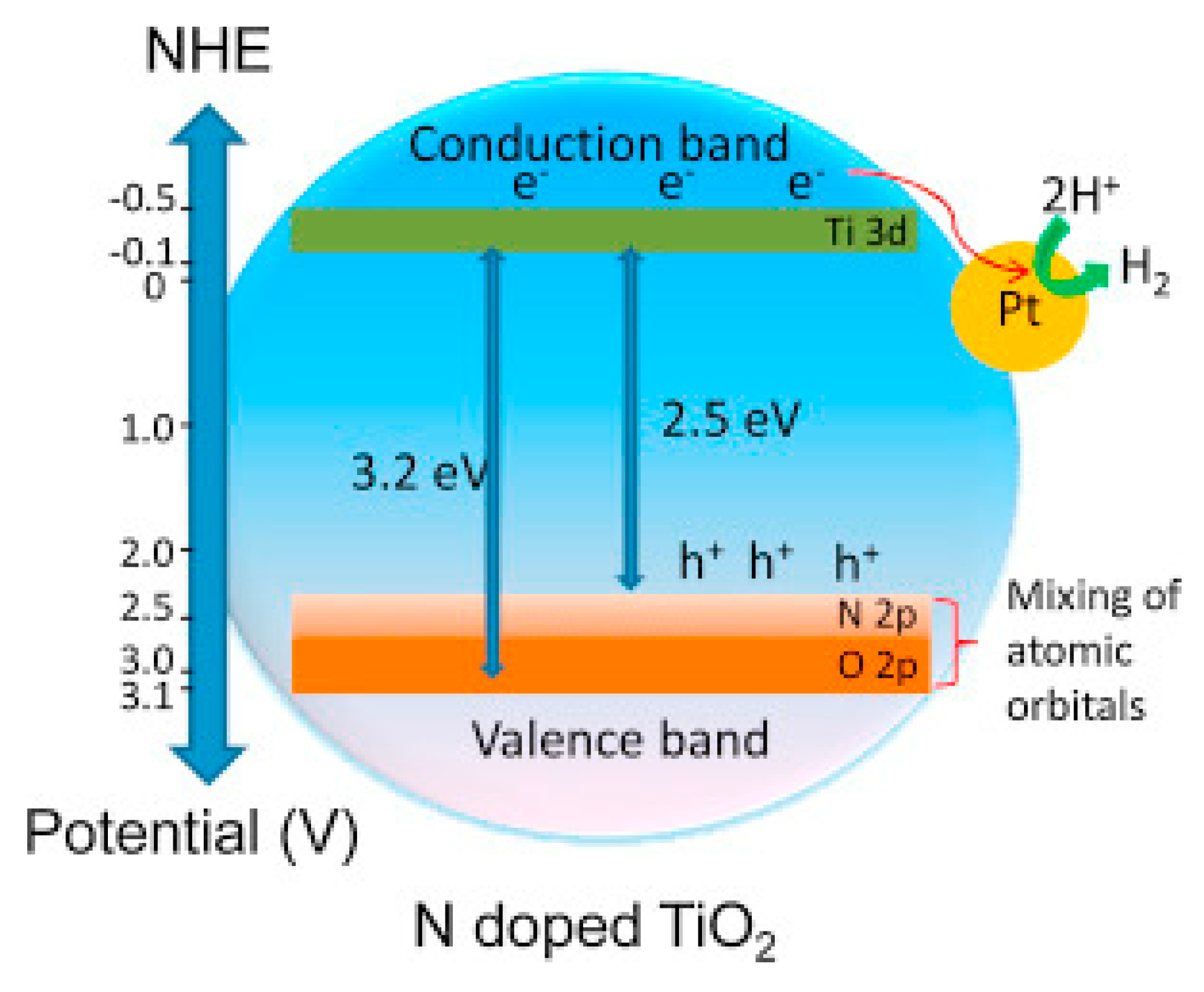



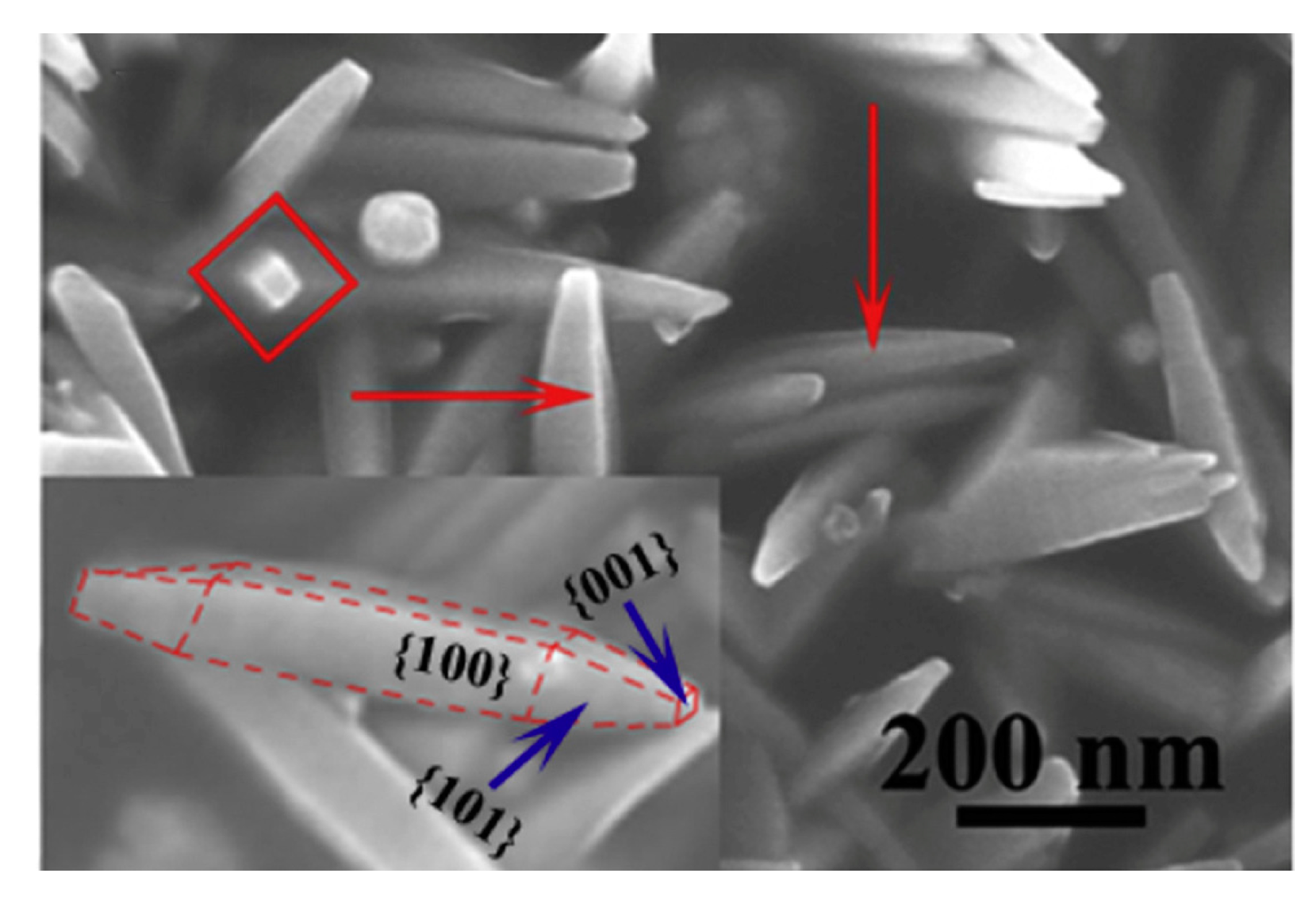

| Advantages | Disadvantages | References | |
|---|---|---|---|
| Physical-mechanical | |||
| Adsorption Activated carbon | Good efficiency | High cost, expensive regeneration, loss of adsorbents, non-destructive process, Disposal of adsorbents | [17] |
| Low-cost adsorbents | Low cost | Poor capacity, disposal of adsorbents | [18] |
| Coagulation/flocculation | Low cost, easy operation, high efficiency | High sludge production | [19] |
| Sedimentation | Relatively inexpensive, works by gravity and does not require energy | Lack of process control, poor environmental condition for treatment, not effective for removing colloidal solids or dissolved solids | [20] |
| Ultrafiltration-microfiltration | Low pressure | Unsatisfactory quality of the treated wastewater | [21] |
| Ion-exchange | Low efficiency for dispersive dyes, regeneration: no adsorbent loss | Concentrated sludge production | [22] |
| Reverse osmosis | High efficiency in removing hydrolized reactive dyes and salts | High pressure | [23] |
| Biological | Low cost, slow process | Low biodegradability of dyes | [24] |
| Chemical | |||
| Chlorine | Effectiveness | Dangerous gas | [25] |
| Ozone | Highest redox potential | Highly unstable, must be generated onsite | [26] |
| Fenton process | High efficiency, lack of high equipment requirements, availability of reagents, no harmful by-products | Excessive sludge generation, narrow range of operational pH, chemical consumption | [27] |
| Photocatalysis | Low cost, reusable, complete degradation, eco-friendly | Low photocatalytic efficiency and poor stability, long-scale applicability, light absorption and utilization | [28] |
| Photocatalytic Materials | Advantages | Drawbacks | Ref. |
|---|---|---|---|
| Metal oxides | |||
| TiO2 (anatase) | Significant activity in the degradation of organic pollutants (dyes, phenolic compounds, pesticides, herbicides, benzenes, furans, chlorinated alkanes, and dioxins), significant activity in the degradation of inorganic contaminants (including toxic metal ions like Pt2+, Rh3+, and Cr6+), excellent antibacterial and antiviral activity against various bacteria and viruses, durability, low cost, low toxicity, chemical and photochemical stability, superhydrophilicity. | Limited absorption, only in UV solar light region due to wide band gap (3.2 eV), rapid recombination of photogenerated e−/h+ pairs, reduced adsorption of hydrophobic organic pollutants on photocatalysts, aggregation of nanoparticles during photocatalytic degradation, difficult recovery of nanosized particles from the treated wastewater. | [9,11,33,34,40,42,43] |
| ZnO | Non-toxicity, insolubility in water, excellent piezoelectric effect, high electron mobility, high photosensitivity, environmentally friendly nature, relatively low cost. | Limited absorption, only in UV solar light region due to wide band gap (3.37 eV), susceptibility to photocorrosion, limited pH range of operation (ZnO dissolves in strong acids and alkalis). | [9,33,34,35,40,42,44] |
| WOx | Narrow band gap (2.8 eV), effective in the blue region of the visible solar spectrum, chemical stability at various pH levels. | Synthesis complexity, cost, and scalability issues when compared to TiO2 and ZnO. | [33,40,42] |
| Cu-based oxides | CuO can be used as catalyst support, semiconductor, and catalyst; the band gap can be adjusted across a wide range within the sunlight spectrum, from approximately 1.2 eV to greater than 3.0 eV by varying transition metals and the Cu(I) coordination environment in ternary Cu-based metal oxides. | Cu2O is thermodynamically unstable in aqueous solutions and tends to reduce to Cu. A protective layer on a Cu2O electrode is necessary, | [33,34] |
| SnO2 | When coupled with other semiconductors, SnO as good electron acceptor, forms heterostructures with efficient charge separation and improved photocatalytic properties. | Limited absorption, only in UV solar light region due to wide band gap (3.6 eV), cannot be used independently as photocatalyst since it cannot reduce oxygen molecules due to its conduction band position. | [33,40,42] |
| Bi2O3 | Visible light responsive photocatalyst. | Recombination of photogenerated electrons and holes. | [45,46,47] |
| Bismuth-based multi-component oxides (Bi2WO6, BiVO4, Bi2MoO6, BiFeO3, Bismuth titanates) | Photocatalytic activity in visible light and NIR regions. | Charge recombination. | [45,46,47,48,49] |
| Bismuth oxyhalides, BiOX (X = F, Cl, Br, I) | Efficient separation of photoinduced charge carriers, the variation of the Bi and X contents can tailor the bandgap and band edge positions of the photocatalyst, non-hazardous and inert. | Fast recombination of photo-generated electrons and holes. | [46,48,50] |
| Metal sulfides | |||
| ZnS | Exceptional transport properties for reducing the scattering and recombination of carriers, thermal stability, high electron mobility, non-toxicity, insolubility in water, lower cost. | Limited absorption, only in UV solar light region due to wide band gap (3.6 eV). | [9,30,40] |
| CdS | Photocatalytic activity in visible light region | Poor e−/h+ separation, susceptibility to photocorrosion due to its self-oxidative nature, poor stability, high toxicity. | [6,9,33,40,42] |
| PbS | Narrow and small band gaps (0.41–0.78 eV), high carrier mobility, high dielectric constant. | Moderate photocatalytic activity. | [33] |
| Bi2S3 | Remarkable visible-light absorption properties. | Photocorrosion. | [45] |
| Non-metal-based semiconductors | |||
| SiC | Indirect and wide band gaps (2.3–3.3 eV) tunable to adsorb visible light, high mechanical strength, high melting point, excellent resistance to chemical oxidation, high thermal stability. | Very low surface area of SiC powder, moderate photocatalytic activity. | [33,52,53] |
| Organic materials | |||
| Graphene | Large surface area, high electrical conductivity, high thermal conductivity, high mechanical stiffness, high charge carrier mobility in room temperature, optical transparency, biocompatibility, efficient water dispersion, photocatalytic activity in UV-VIS region. | Pristine graphene is rarely used, graphene-based nanocomposites have enhanced photocatalytic performance. | [8,40,42] |
| g-C3N4 | tunable medium band gap (~2.7 eV), photocatalytic activity in UV-VIS region. Numerous surface groups that facilitate coupling with other photocatalysts, high stability, high chemical resistance, non-toxicity, high reduction ability, cost effectiveness. | Insufficient visible-light absorption, low oxidation ability, high recombination rate, low solvent-accessible surface area, susceptibility to degradation by hydroxyl radicals. | [6,10,15,40,42] |
| Composition | Morphology | Preparation Techniques | Ref. |
|---|---|---|---|
| RuTe2 | nanorods | Chemical precipitation and coprecipitation | [94] |
| vanadium-doped mesoporous silica | nanospheres | Sol–gel approach | [95] |
| TiO2, CeO2, Y2O3-ZrO2 and SrTiO3 | nanofibers | Electrospinning | [96] |
| ZnO | nanorods | Solid-state reaction | [97] |
| Bi4Ti3O12 | nanorods and nanotubes | [98] | |
| Titanates | nanotubes | Hydrothermal/solvothermal | [99] |
| Titanates | nanorods | Vapor-phase hydrothermal | [100] |
| Carbon | nanotubes | Aerosol flame synthesis | [101] |
| Type of Materials | Dye Pollutant | Source of Light | Removal Efficiency (%); Reaction Time | Stability vs. Number of Cycles | Ref. |
|---|---|---|---|---|---|
| Composites | |||||
| TiO2/Fe2O3 | Reactive blue dye | Sunlight irradiation | 100%; 120 min | 9.7% after 4th run | [174] |
| Bi2WO6/TiO2 | Rhodamine B (RhB), methylene blue (MB), and methyl orange (MO) | Simulated solar irradiation using a 500 W xenon lamp (AM1.5, 100 mW/cm2) | 95.2%; 30 min (RhB) 89.3% within 60 min (MB) 15.1% MO; 60 min (MO) | [175] | |
| Metal doped | |||||
| W-TiO2 | Procion Red MX-5B | Visible light irradiation (35 W Xe-arc) | 92.05%; 60 min | 90% after 5th run | [176] |
| Fe-TiO2 | Methyl orange | Visible light irradiation (300 W xenon lamp with a UV cut off filter (λ > 400 nm)) | 98%; 60 min | n.a. | [177] |
| Nonmetal doped | |||||
| C-TiO2 | Methylene blue | Sunlight irradiation | 98.86%; 140 min. | 85.94%, after 4th run | [178] |
| N-TiO2 | Methylene blue (MB) and Methyl orange (MO) | Visible light irradiation (300 W xenon lamp with a UV cut off filter (λ > 420 nm)) | 92%; 60 (MB); 95%; 180 min (MO) | 100% after 6th run | [179] |
| Metal/nonmetal co-doped | |||||
| P/Zr co-doped TiO2 | Rose Bengal | Visible light irradiation | 95%; 40 min | 78% in 4 th run | [180] |
| Co/S co-doped TiO2 | Methylene Blue | Visible light irradiation (200 W halogen lamp) | 93%; 120 min | n.a. | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dostanić, J.; Lončarević, D.; Hadnađev-Kostić, M.; Vulić, T. Recent Advances in the Strategies for Developing and Modifying Photocatalytic Materials for Wastewater Treatment. Processes 2024, 12, 1914. https://doi.org/10.3390/pr12091914
Dostanić J, Lončarević D, Hadnađev-Kostić M, Vulić T. Recent Advances in the Strategies for Developing and Modifying Photocatalytic Materials for Wastewater Treatment. Processes. 2024; 12(9):1914. https://doi.org/10.3390/pr12091914
Chicago/Turabian StyleDostanić, Jasmina, Davor Lončarević, Milica Hadnađev-Kostić, and Tatjana Vulić. 2024. "Recent Advances in the Strategies for Developing and Modifying Photocatalytic Materials for Wastewater Treatment" Processes 12, no. 9: 1914. https://doi.org/10.3390/pr12091914
APA StyleDostanić, J., Lončarević, D., Hadnađev-Kostić, M., & Vulić, T. (2024). Recent Advances in the Strategies for Developing and Modifying Photocatalytic Materials for Wastewater Treatment. Processes, 12(9), 1914. https://doi.org/10.3390/pr12091914







