The Valorization of Fruit and Vegetable Wastes Using an Anaerobic Fixed Biofilm Reactor: A Case of Discarded Tomatoes from a Traditional Market
Abstract
1. Introduction
2. Materials and Methods
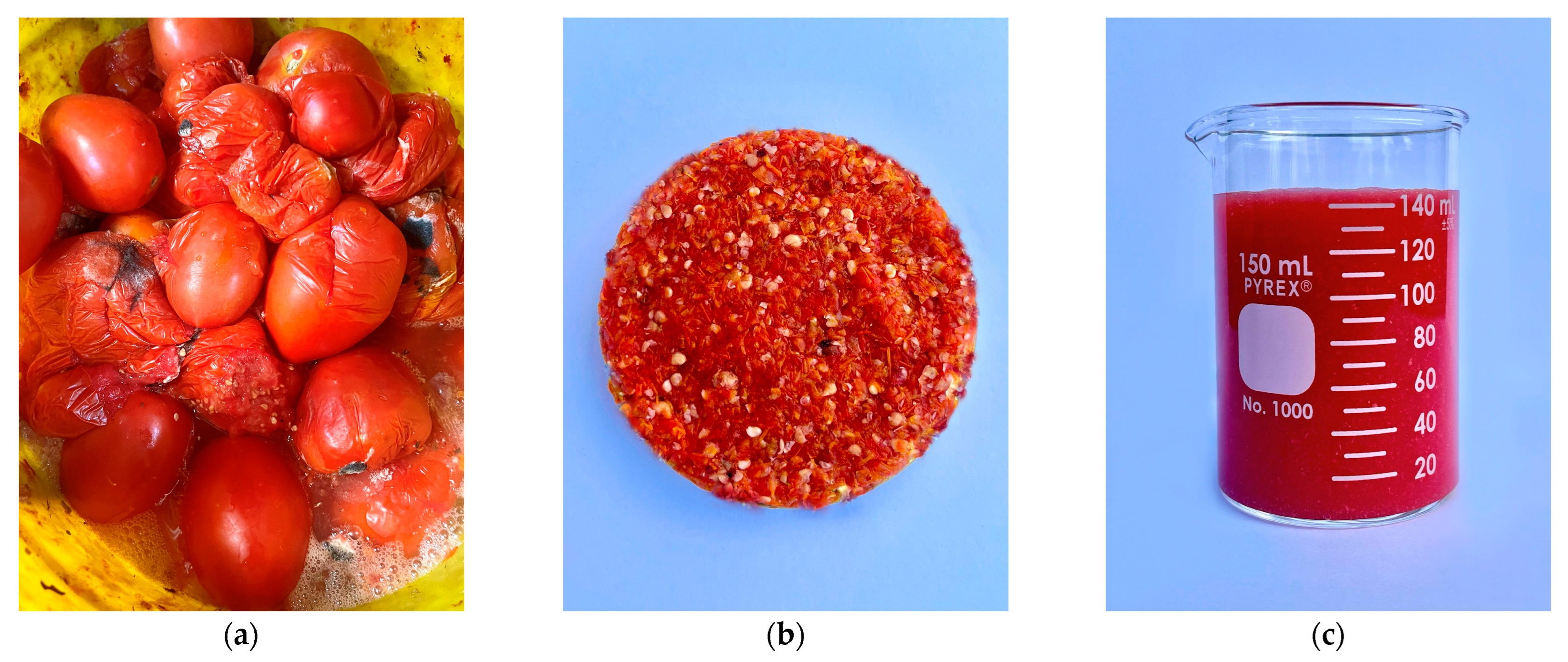
2.1. Tomato Waste and Inoculum Collection
2.2. Substrate Pretreatment
2.3. The Experimental Design of the Anaerobic Process
2.4. Operating Conditions
2.5. Monitoring and Physicochemical Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Substrate Characterization Results
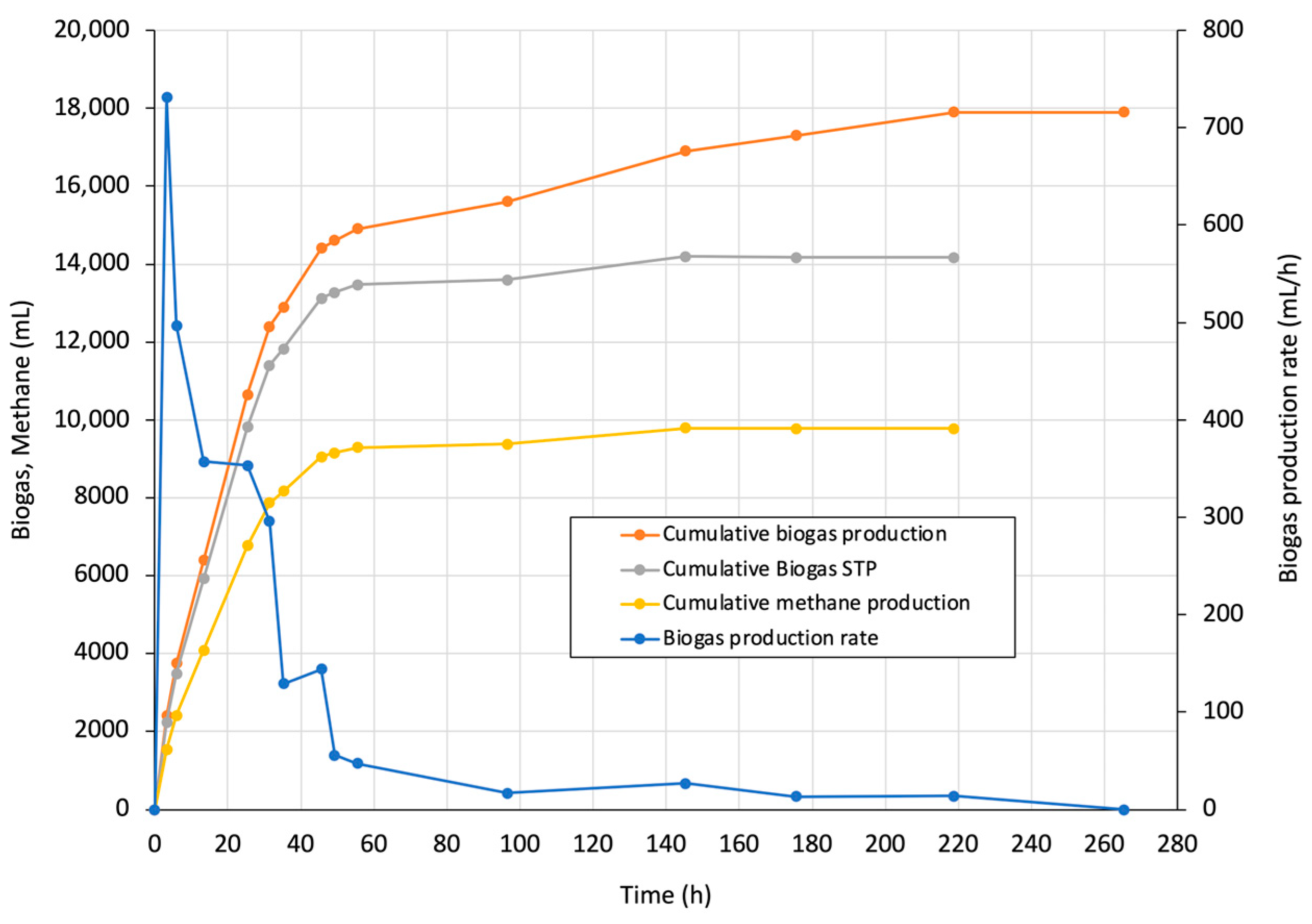
3.2. Startup Phase Evaluation
3.3. Operation Stages at Different OLRs
3.3.1. Stage 1: Semi-Continuous with 500 mL of TWLF1
3.3.2. Stage 2: Semi-Continuous with 700 mL of TWLF1
3.3.3. Stage 3: Semi-Continuous with 900 mL of TWLF1
3.3.4. Methane Content at Different Stages of Operation
3.4. Process Stability Indicators
3.4.1. Alkalinity
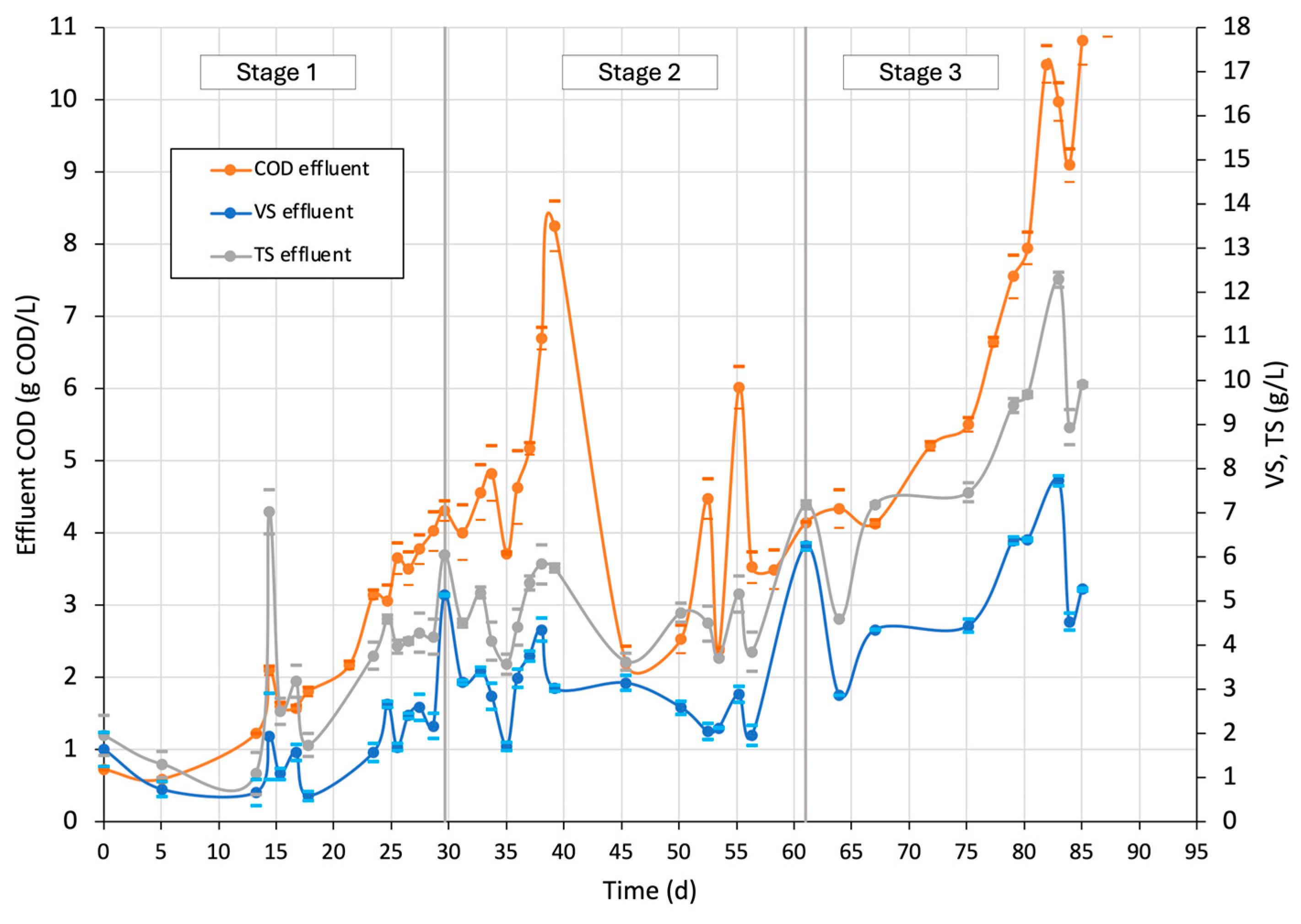
3.4.2. COD, VS, and TS Effluent
3.4.3. Cumulative Biogas and pH
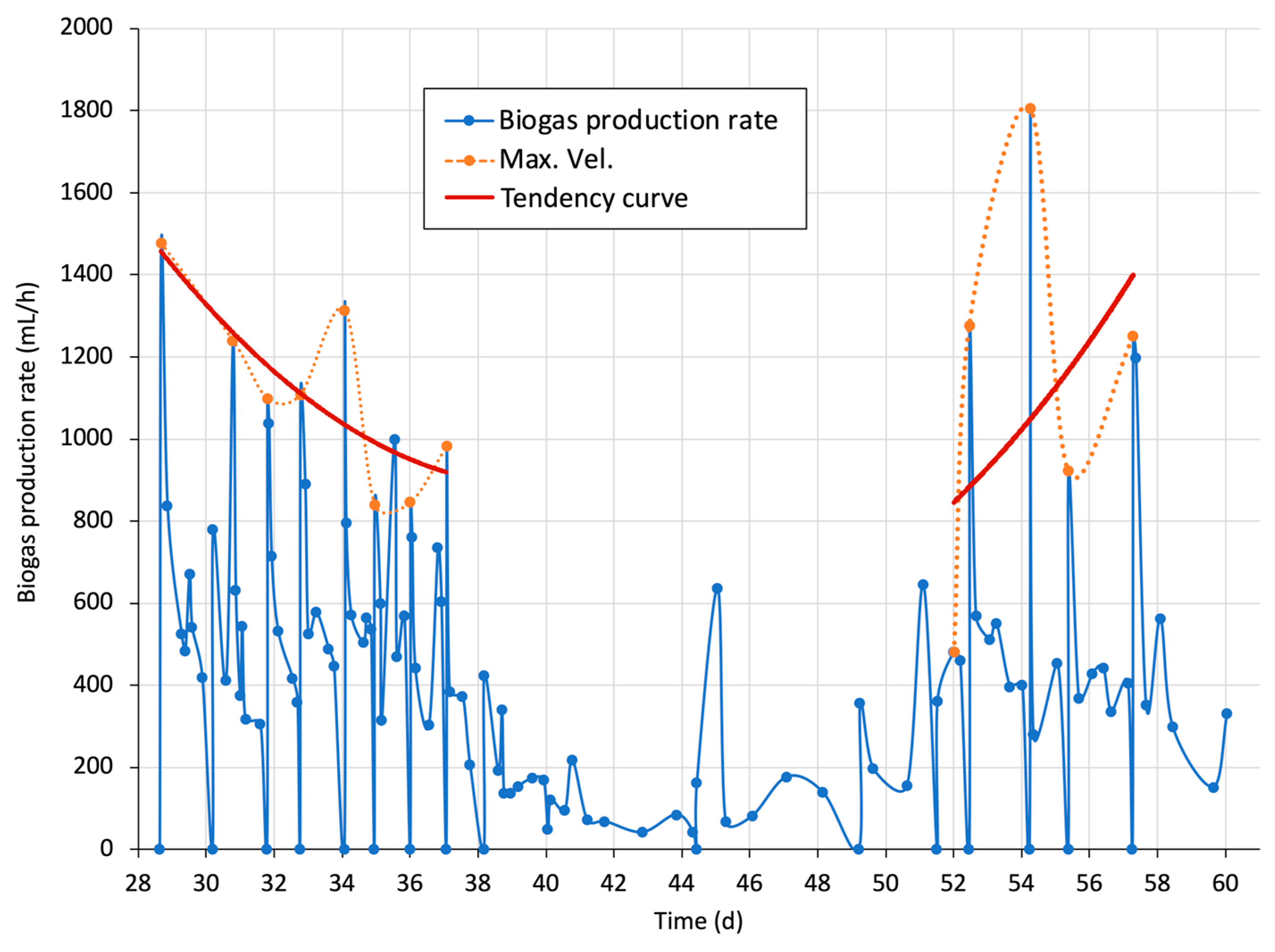
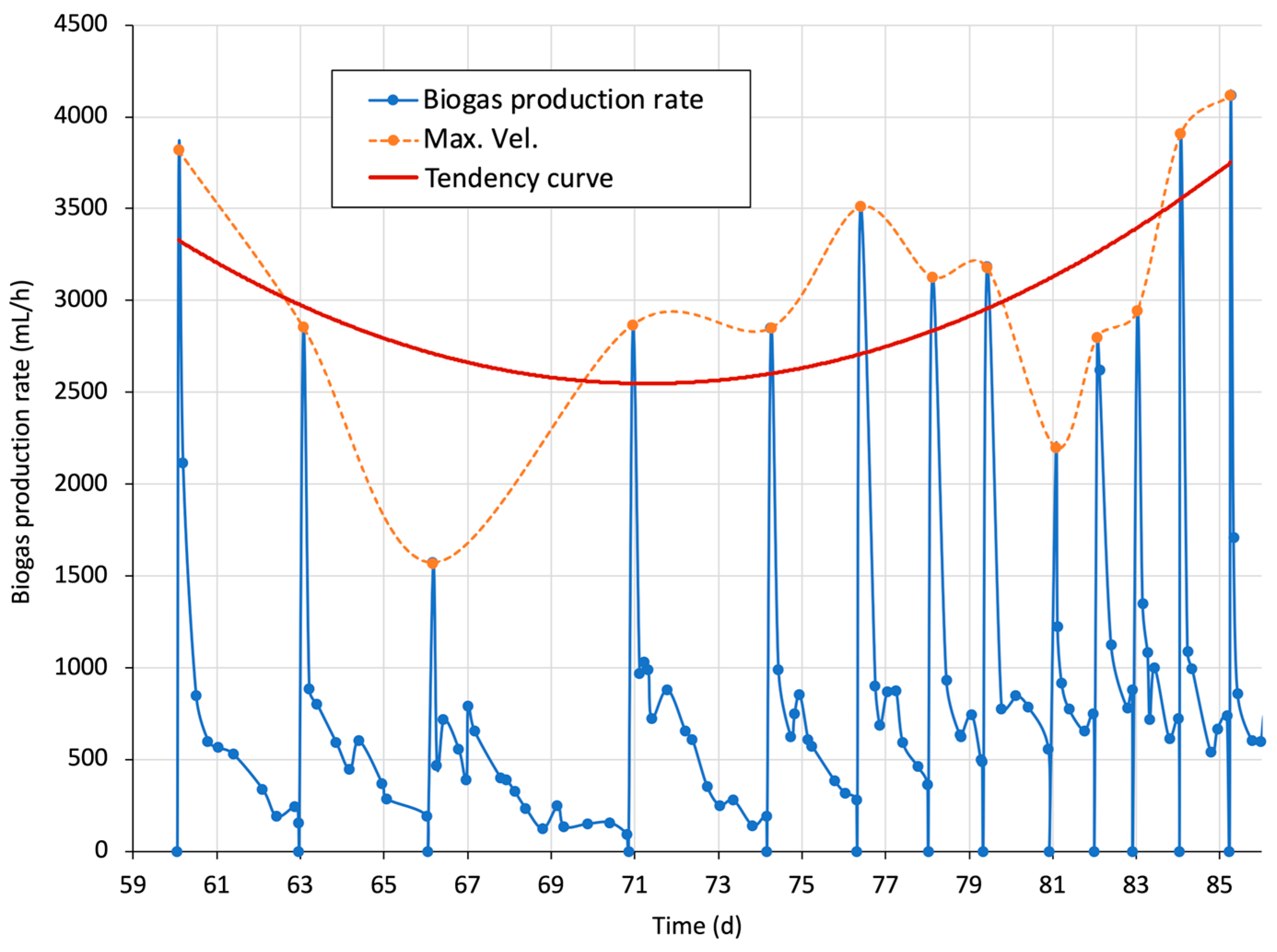
3.4.4. One-Day Cumulative Biogas and Biogas Production Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO-STAT. Datos Sobre Alimentación y Agricultura: Cultivos y Productos de Ganadería en México. 2023. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 23 April 2024).
- Stylianou, M.; Laifi, T.; Bennici, S.; Dutournie, P.; Limousy, L.; Agapiou, A.; Papamichael, I.; Khiari, B.; Jeguirim, M.; Zorpas, A.A. Tomato waste biochar in the framework of circular economy. Sci. Total Environ. 2023, 871, 161959. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Siles, J.A.; Gutiérrez, M.C.; Martín, M.Á. Evaluation of physicochemical pretreatment of tomato plant for aerobic and anaerobic biodegradation. Biomass Convers. Biorefinery 2019, 9, 489–497. [Google Scholar] [CrossRef]
- Tomatonews. The Global Tomato Processing Industry. The Tomato News Online Conference. 2021. Available online: https://www.tomatonews.com/en/background_47.html (accessed on 22 April 2024).
- Lopez, M.J.; Masaguer, A.; Paredes, C.; Roca, L.; Ros, M.; Salas, M.C.; Boluda, R. Residuos Orgánicos y Agricultura Intensiva III, 1st ed.; Mudi-Prensa: Madrid, Spain, 2015; p. 58. [Google Scholar]
- Calabrò, P.S.; Greco, R.; Evangelou, A.; Komilis, D. Anaerobic digestion of tomato processing waste: Effect of alkaline pretreatment. J. Environ. Manag. 2015, 163, 49–52. [Google Scholar] [CrossRef]
- Hirano, S.I.; Matsumoto, N. Analysis of a bio-electrochemical reactor containing carbon fiber textiles for the anaerobic digestion of tomato plant residues. Bioresour. Technol. 2018, 249, 809–817. [Google Scholar] [CrossRef]
- Ezieke, A.H.; Serrano, A.; Peces, M.; Clarke, W.; Villa-Gomez, D. Effect of feeding frequency on the anaerobic digestion of berry fruit waste. Waste Manag. 2024, 178, 66–75. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Serrano, A.; Alonso-Fariñas, B.; Fernández-Bolaños, J.; Borja, R.; Rodríguez-Gutiérrez, G. Valuable compound extraction, anaerobic digestion, and composting: A leading biorefinery approach for agricultural wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Chaudhari, P.K.; Ghosh, P. Anaerobic digestion of fruit and vegetable waste: A critical review of associated challenges. Environ. Sci. Pollut. Res. 2023, 30, 24987–25012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current technologies and uses for fruit and vegetable wastes in a sustainable system: A review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef]
- Ji, C.; Kong, C.X.; Mei, Z.L.; Li, J. A review of the anaerobic digestion of fruit and vegetable waste. Appl. Biochem. Biotechnol. 2017, 183, 906–922. [Google Scholar] [CrossRef]
- Azevedo, A.; Lapa, N.; Moldão, M.; Duarte, E. Opportunities and challenges in the anaerobic co-digestion of municipal sewage sludge and fruit and vegetable wastes: A review. Energy Nexus 2023, 10, 100202. [Google Scholar] [CrossRef]
- Assis, T.I.; Gonçalves, R.F. Valorization of food waste by anaerobic digestion: A bibliometric and systematic review focusing on optimization. J. Environ. Manag. 2022, 320, 115763. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.; Ahmed, S.; Kumar, A. Anaerobic digestion (AD) of fruit and vegetable market waste (FVMW): Potential of FVMW, bioreactor performance, co-substrates, and pre-treatment techniques. Biomass Convers. Biorefinery 2022, 12, 3573–3592. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, Z.; Lu, J.; Li, D.; Wang, G.; Li, Y.; Wu, Y.; Li, S.; Xu, F.; et al. Effect of inoculum and substrate/inoculum ratio on the performance and methanogenic archaeal community structure in solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover. Waste Manag. 2018, 81, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Salehiyoun, A.R.; Sharifi, M.; Di Maria, F.; Zilouei, H.; Aghbashlo, M. Effect of substituting organic fraction of municipal solid waste with fruit and vegetable wastes on anaerobic digestion. J. Mater. Cycles Waste Manag. 2019, 21, 1321–1331. [Google Scholar] [CrossRef]
- Chatterjee, B.; Mazumder, D. New approach of characterizing fruit and vegetable waste (FVW) to ascertain its biological stabilization via two-stage anaerobic digestion (AD). Biomass Bioenergy 2020, 139, 105594. [Google Scholar] [CrossRef]
- Mannina, G.; Ekama, G.A.; Capodici, M.; Cosenza, A.; Di Trapani, D.; Ødegaard, H. Integrated fixed-film activated sludge membrane bioreactors versus membrane bioreactors for nutrient removal: A comprehensive comparison. J. Environ. Manag. 2018, 226, 347–357. [Google Scholar] [CrossRef]
- di Biase, A.; Kowalski, M.S.; Devlin, T.R.; Oleszkiewicz, J.A. Moving bed biofilm reactor technology in municipal wastewater treatment: A review. J. Environ. Manag. 2019, 247, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Lassman, A.; Rustrián, E.; García-Alvarado, M.A.; Houbron, E. Simultaneous removal of carbon and nitrogen in an anaerobic inverse fluidized bed reactor. Water Sci. Technol. 2006, 54, 111–117. [Google Scholar] [CrossRef]
- Huang, C.; Shi, Y.; Xue, J.; Zhang, Y.; El-Din, M.G.; Liu, Y. Comparison of biomass from integrated fixed-film activated sludge (IFAS), moving bed biofilm reactor (MBBR) and membrane bioreactor (MBR) treating recalcitrant organics: Importance of attached biomass. J. Hazard. Mater. 2017, 326, 120–129. [Google Scholar] [CrossRef]
- Vallejo-Cantú, N.A.; Galván-Hernández, A.; Alvarado-Vallejo, A.; Méndez-Contreras, J.M.; Rosas-Mendoza, E.S.; Landeta-Escamilla, O. Enhancing biomethane production from citrus waste: An integrated approach of hydrothermal carbonization and anaerobic digestion for sustainable waste management. Renew. Energy Biomass Sustain. 2023, 5, 40–46. [Google Scholar] [CrossRef]
- ASTM E-11-70; Standard Practices for Simulating Vehicular Response to Longitudinal Profiles of Traveled Surfaces. ASTM International: West Conshohocken, PA, USA, 2017.
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved alkalimetric monitoring for anaerobic digestion of high-strength wastes. J. (Water Pollut. Control Fed.) 1986, 58, 406–411. [Google Scholar]
- ASTM D1293-18; Standard Test Methods for pH of Water. ASTM International: West Conshohocken, PA, USA, 2018.
- APHA 5220 D; Chemical Oxygen Demand: Standard Methods for the Examination of Water and Wastewater. American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Denver, CA, USA; Water Environment Federation (WEF): Alexandria, VA, USA, 1997.
- NMX-AA-034-SCFI-2015; Water Analysis-Measurement of Solids and Dissolved Salts in Natural, Waste and Treated Waste Waters-Test Method. Diario Oficial de la Federación: Ciudad de México, México, 2015.
- ASTM D1946-12; Standard Test Method for Analysis of Reformed Gas by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2016.
- Li, R.; Chen, S.; Li, X. Anaerobic co-digestion of kitchen waste and cattle manure for methane production. Energ. Source. Part A Recover. Util. Environ. Eff. 2009, 31, 1848–1856. [Google Scholar] [CrossRef]
- Zhang, W.; Xing, W.; Li, R. Real-time recovery strategies for volatile fatty acid-inhibited anaerobic digestion of food waste for methane production. Bioresour. Technol. 2018, 265, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Nkuna, R.; Roopnarain, A.; Rashama, C.; Adeleke, R. Insights into organic loading rates of anaerobic digestion for biogas production: A review. Crit. Rev. Biotechnol. 2022, 42, 487–507. [Google Scholar] [CrossRef]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef]
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Triolo, J.M.; Flotats, X.; de Mendonça Costa, M.S. Infuence of chemical composition on biochemical methane potential of fruit and vegetable waste. Waste Manag. 2018, 71, 618–625. [Google Scholar] [CrossRef]
- Edwiges, T.; Frare, L.M.; Alino, J.H.L.; Triolo, J.M.; Flotats, X.; de Mendonça Costa, M.S.S. Methane potential of fruit and vegetable waste: An evaluation of the semi-continuous anaerobic mono-digestion. Environ. Technol. 2020, 41, 921–930. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O.; Mbohwa, C. Experimental and feasibility assessment of biogas production by anaerobic digestion of fruit and vegetable waste from Joburg Market. Waste Manag. 2018, 75, 236–250. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour. Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef]
- Dinh, P.V.; Fujiwara, T.; Phu, S.T.P.; Hoang, M.G. Kinetic of biogas production in co-digestion of vegetable waste, horse dung, and sludge by batch reactors. IOP Conf. Ser. Earth Environ. Sci. 2018, 1, 012041. [Google Scholar] [CrossRef]
- Ferreira, T.B.; Passos, F.; Chernicharo, C.A.; de Souza, C.L. Anaerobic digestion of food waste: Effect of the organic load variation in a demo-scale system. Waste Biomass Valorization 2021, 12, 4407–4417. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Rajendran, K.; Taherzadeh, M.J. A comparative study between single-and two-stage anaerobic digestion processes: Effects of organic loading rate and hydraulic retention time. Int. Biodeterior. Biodegrad. 2014, 95, 181–188. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Cruz-Bournazou, M.N.; Torres-Lozada, P. Single-stage and two-stage anaerobic digestion of food waste: Effect of the organic loading rate on the methane production and volatile fatty acids. Water Air Soil Pollut. 2021, 232, 105. [Google Scholar] [CrossRef]
- Scano, E.A.; Asquer, C.; Pistis, A.; Ortu, L.; Demontis, V.; Cocco, D. Biogas from anaerobic digestion of fruit and vegetable wastes: Experimental results on pilot-scale and preliminary performance evaluation of a full-scale power plant. Energy Convers. Manag. 2014, 77, 22–30. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, J.; Chen, X.; Xing, W.; Xing, L.; Li, P.; Lu, X.; Li, C. Microbial community structures in an integrated two-phase anaerobic bioreactor fed by fruit vegetable wastes and wheat straw. J. Environ. Sci. 2014, 26, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Babaee, A.; Shayegan, J. Effect of organic loading rates (olr) on production of methane from anaerobic digestion of vegetables waste. In Proceedings of the World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011. [Google Scholar]
- Aramrueang, N.; Rapport, J.; Zhang, R. Effects of hydraulic retention time and organic loading rate on performance and stability of anaerobic digestion of Spirulina platensis. Biosyst. Eng. 2016, 147, 174–182. [Google Scholar] [CrossRef]
- Eslami, H.; Hashemi, H.; Fallahzadeh, R.A.; Khosravi, R.; Fard, R.F.; Ebrahimi, A.A. Effect of organic loading rates on biogas production and anaerobic biodegradation of composting leachate in the anaerobic series bioreactors. Ecol. Eng. 2018, 110, 165–171. [Google Scholar] [CrossRef]
- Dinh, P.V.; Hoang, M.G.; Pham Phu, S.T.; Fujiwara, T. Kinetics of carbon dioxide, methane and hydrolysis in co-digestion of food and vegetable wastes. Glob. J. Environ. Sci. Manag. 2018, 4, 401–412. [Google Scholar] [CrossRef]
- Pham Van, D.; Takeshi, F.; Hoang Minh, G.; Pham Phu, S.T. Comparison between single and two-stage anaerobic digestion of vegetable waste: Kinetics of methanogenesis and carbon flow. Waste Biomass Valorization 2020, 11, 6095–6103. [Google Scholar] [CrossRef]
- Niu, Q.; Kobayashi, T.; Takemura, Y.; Kubota, K.; Li, Y.Y. Evaluation of functional microbial community’s difference in full-scale and lab-scale anaerobic digesters feeding with different organic solid waste: Effects of substrate and operation factors. Bioresour. Technol. 2015, 193, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Michaud, S.; Bernet, N.; Buffière, P.; Delgenes, J.P. Use of the methane yield to indicate the metabolic behaviour of methanogenic biofilms. Process Biochem. 2005, 40, 2751–2755. [Google Scholar] [CrossRef]
- Namburath, M.; Alappat, B.J.; Ramaswamy, S.T. A critical review of inverse fluidized bed reactors—Start-up optimization strategies and wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 108370–108392. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, A.A.; Mohammadi, P.; Mirghorayshi, M.; Ibrahim, S.; Younesi, H.; Mohamed, A.R. An anaerobic hybrid bioreactor of granular and immobilized biomass for anaerobic digestion (AD) and dark fermentation (DF) of palm oil mill effluent: Mass transfer evaluation in granular sludge and role of internal packing. Bioenergy 2017, 103, 1–10. [Google Scholar] [CrossRef]
- Carneiro, R.B.; Gomes, G.M.; Zaiat, M.; Santos-Neto, Á.J. Two-phase (acidogenic-methanogenic) anaerobic fixed bed biofilm reactor enhances the biological domestic sewage treatment: Perspectives for recovering bioenergy and value-added by-products. J. Environ. Manag. 2022, 317, 115388. [Google Scholar] [CrossRef]
- Lv, P.L.; Jia, C.; Guo, X.; Zhao, H.P.; Chen, R. Microbial stratification protects denitrifying anaerobic methane oxidation archaea and bacteria from external oxygen shock in membrane biofilm reactor. Bioresour. Technol. 2024, 391, 129966. [Google Scholar] [CrossRef]
- Hussain, A.; Dubey, S.K. Specific methanogenic activity test for anaerobic degradation of influents. Appl. Water Sci. 2017, 7, 535–542. [Google Scholar] [CrossRef]
- Puñal, A.; Trevisan, M.; Rozzi, A.; Lema, J.M. Influence of C:N ratio on the start-up of up-flow anaerobic filter reactors. Water Res. 2000, 34, 2614–2619. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, B.; Zhang, L.; Zhang, H.; Liu, Y. Microbial community dynamics in granular activated carbon enhanced up-flow anaerobic sludge blanket (UASB) treating municipal sewage under sulfate reducing and psychrophilic conditions. Chem. Eng. J. 2021, 405, 126957. [Google Scholar] [CrossRef]
- de Lemos Chernicharo, C.A. Anaerobic Reactors; IWA Publishing: London, UK, 2007. [Google Scholar] [CrossRef]
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresour. Technol. 2017, 227, 286–296. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.; Yang, P.; Peng, X. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Wang, X.; Wu, D. Anaerobic digestion of food waste: A review focusing on process stability. Bioresour. Technol. 2018, 248, 20–28. [Google Scholar] [CrossRef]
- Dhar, H.; Kumar, P.; Kumar, S.; Mukherjee, S.; Vaidya, A.N. Effect of organic loading rate during anaerobic digestion of municipal solid waste. Bioresour. Technol. 2016, 217, 56–61. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Liu, X.; Mei, Z.; Ren, H.; Cao, Q.; Yan, Z. Instability mechanisms and early warning indicators for mesophilic anaerobic digestion of vegetable waste. Bioresour. Technol. 2017, 245, 90–97. [Google Scholar] [CrossRef]
- Li, D.; Ran, Y.; Chen, L.; Cao, Q.; Li, Z.; Liu, X. Instability diagnosis and syntrophic acetate oxidation during thermophilic digestion of vegetable waste. Water Res. 2018, 139, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, L.; Peng, Y.; Yang, P.; Peng, X.; Sun, Y.; Wang, X. State indicators of anaerobic digestion: A critical review on process monitoring and diagnosis. Renew. Sustain. Energy Rev. 2021, 148, 111260. [Google Scholar] [CrossRef]
- Espinoza-Escalante, F.M.; Pelayo-Ortíz, C.; Navarro-Corona, J.; González-García, Y.; Bories, A.; Gutiérrez-Pulido, H. Anaerobic digestion of the vinasses from the fermentation of Agave tequilana Weber to tequila: The effect of pH, temperature and hydraulic retention time on the production of hydrogen and methane. Biomass Bioenergy 2009, 33, 14–20. [Google Scholar] [CrossRef]
- Sun, H.; Ni, P.; Angelidaki, I.; Dong, R.; Wu, S. Exploring stability indicators for efficient monitoring of anaerobic digestion of pig manure under perturbations. Waste Manag. 2019, 91, 139–146. [Google Scholar] [CrossRef]
- Casallas-Ojeda, M.R.; Marmolejo-Rebellón, L.F.; Torres-Lozada, P. Identification of factors and variables that influence the anaerobic digestion of municipal biowaste and food waste. Waste Biomass Valorization 2021, 12, 2889–2904. [Google Scholar] [CrossRef]
- Belhadj, S.; Joute, Y.; El Bari, H.; Serrano, A.; Gil, A.; Siles, J.Á.; Chica, A.F.; Martín, M.Á. Evaluation of the anaerobic co-digestion of sewage sludge and tomato waste at mesophilic temperature. Appl. Biochem. Biotechnol. 2014, 172, 3862–3874. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Q.; Wei, Y.; He, Q.; Peng, X. Early warning indicators for monitoring the process failure of anaerobic digestion system of food waste. Bioresour Technol. 2014, 171, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Boe, K.; Angelidaki, I. Pilot-scale application of an online VFA sensor for monitoring and control of a manure digester. Water Sci. Technol. 2012, 66, 2496–2503. [Google Scholar] [CrossRef]
- Boe, K.; Batstone, D.J.; Steyer, J.; Angelidaki, I. State indicators for monitoring the anaerobic digestion process. Water Res. 2010, 44, 5973–5980. [Google Scholar] [CrossRef] [PubMed]
- Labatut, R.A.; Gooch, C.A. Monitoring of Anaerobic Digestion Process to Optimize Performance and Prevent System Failure. 2014. Available online: https://ecommons.cornell.edu/server/api/core/bitstreams/9074a3d3-057f-497d-a2f5-ffd7d43fe2f3/content (accessed on 23 August 2024).






| Stage | Specific Stage | Period (Days) | Conditions |
|---|---|---|---|
| Preliminary stages | Startup | 68 | Recirculation: every 12 h, flow rate: 300 mL/min. |
| Initial semi-continuous | 7 | Feed: TWFL2, 125 mL for 7 days, then 250 mL for 5 days. | |
| Stabilization | 114 | Feed: TWLF1, 500 mL for 114 days, HRT: 1–8 days. | |
| Operational stages | Initial kinetic | 48 | Feed: TWLF1, 500 mL. All substrate was consumed. |
| Stage 1 | 28 | Feed: TWLF1, 500 mL, 3.2 gCOD/L·d. | |
| Stage 2 | 32 | Feed: TWLF1, 700 mL, 4.35 gCOD/L·d. | |
| Stage 3 | 26 | Feed: TWLF1, 900 mL, 6.26 gCOD/L·d. |
| Parameter | Method of Analysis | Reference Method |
|---|---|---|
| pH | Digital pH meter with buffer | ASTM D1293-18 [27] |
| COD and sCOD | HACH spectrophotometer at 620 nm | APHA Standard Methods 5220 D [28] |
| TSs and VSs | Gravimetric method | NMX-AA-034-SCFI-2015 [29] |
| Biogas production | Liquid displacement method | Not applicable |
| CH4 content | Gas chromatography (Buck Mod. 310) | ASTM D1946-12 [30] |
| Alkalinity | Acid–base titration | Method according to Ripley et al. [26] |
| Parameter | Unit | Value |
|---|---|---|
| pH | pH unit | 4.36 ± 0.11 |
| COD | g/L | 58.44 ± 4.75 |
| sCOD | g/L | 46.70 ± 5.04 |
| TSs | g/L | 3.95 ± 0.336 |
| VSs (% of TSs) | % | 87.75 ± 1.87 |
| Ash | % | 12.25 ± 1.87 |
| Moisture | % | 96.05 ± 0.36 |
| Stage | Sampling Period (Day) | CH4 Content (%) |
|---|---|---|
| 1 (500 mL of TWLF1) | 15 | 69 |
| 21 | 69 | |
| 29 | 69 | |
| 2 (700 mL of TWLF1) | 30 | 65 |
| 36 | 69 | |
| 40 | 69 | |
| 3 (900 mL of TWLF1) | 68 | 72 |
| 75 | 75 | |
| 80 | 71 | |
| 84 | 78 | |
| Outside the study | 87 | 62 |
| 91 | 73 | |
| 94 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Vallejo, A.; Marín-Peña, O.; Rosas-Mendoza, E.S.; Méndez-Contreras, J.M.; Alvarado-Lassman, A. The Valorization of Fruit and Vegetable Wastes Using an Anaerobic Fixed Biofilm Reactor: A Case of Discarded Tomatoes from a Traditional Market. Processes 2024, 12, 1923. https://doi.org/10.3390/pr12091923
Alvarado-Vallejo A, Marín-Peña O, Rosas-Mendoza ES, Méndez-Contreras JM, Alvarado-Lassman A. The Valorization of Fruit and Vegetable Wastes Using an Anaerobic Fixed Biofilm Reactor: A Case of Discarded Tomatoes from a Traditional Market. Processes. 2024; 12(9):1923. https://doi.org/10.3390/pr12091923
Chicago/Turabian StyleAlvarado-Vallejo, Andrea, Oscar Marín-Peña, Erik Samuel Rosas-Mendoza, Juan Manuel Méndez-Contreras, and Alejandro Alvarado-Lassman. 2024. "The Valorization of Fruit and Vegetable Wastes Using an Anaerobic Fixed Biofilm Reactor: A Case of Discarded Tomatoes from a Traditional Market" Processes 12, no. 9: 1923. https://doi.org/10.3390/pr12091923
APA StyleAlvarado-Vallejo, A., Marín-Peña, O., Rosas-Mendoza, E. S., Méndez-Contreras, J. M., & Alvarado-Lassman, A. (2024). The Valorization of Fruit and Vegetable Wastes Using an Anaerobic Fixed Biofilm Reactor: A Case of Discarded Tomatoes from a Traditional Market. Processes, 12(9), 1923. https://doi.org/10.3390/pr12091923









