Phase Equilibria Study of the MgO–CaO–SiO2 Slag System with Ferronickel Alloy, Solid Carbon, and Al2O3 Additions
Abstract
:1. Introduction
2. Materials and Methods
3. Thermodynamic Calculations
4. Results and Discussion
4.1. Determination of Equilibrium Time
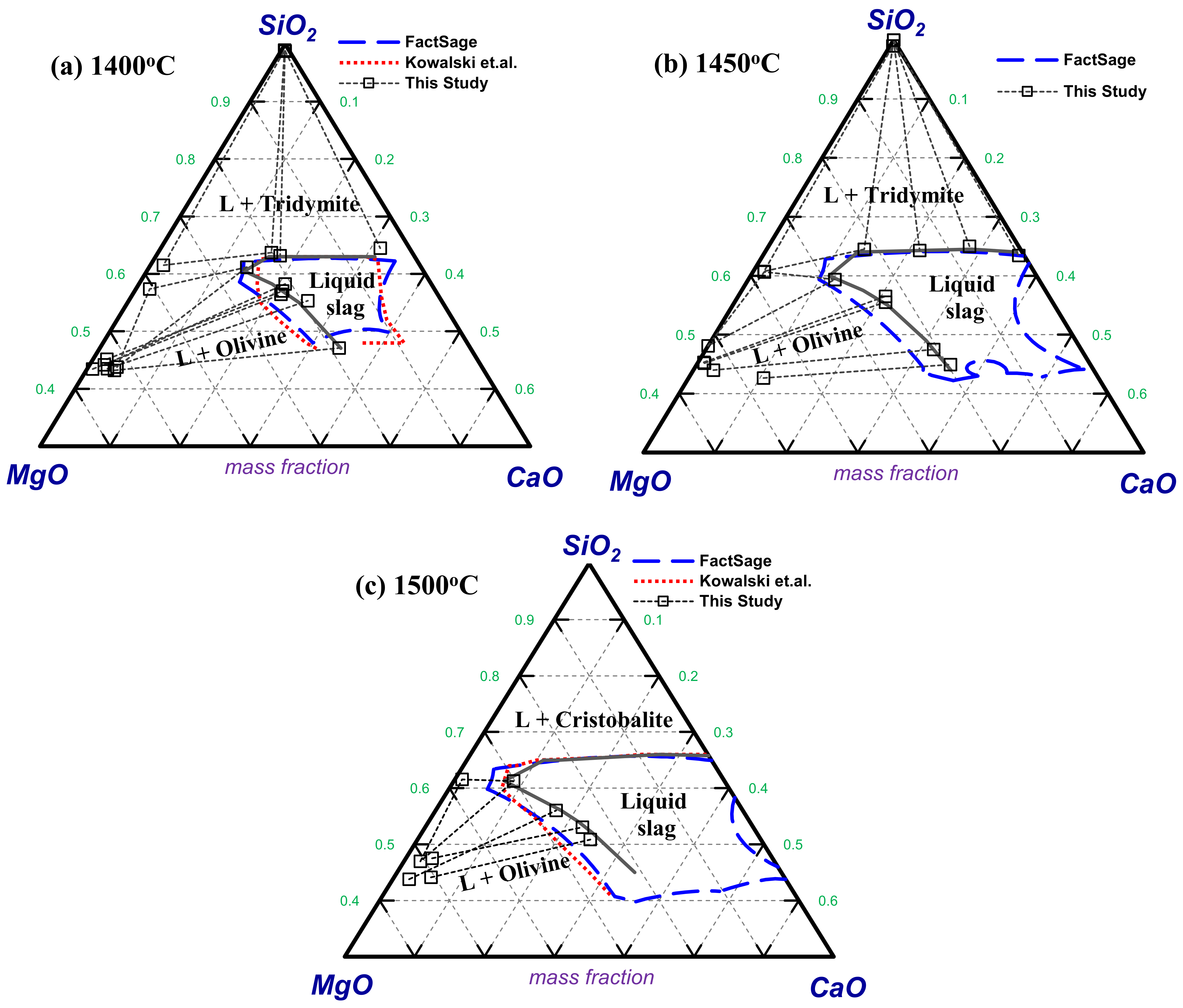
4.2. Experimentally Determined Phase Equilibria
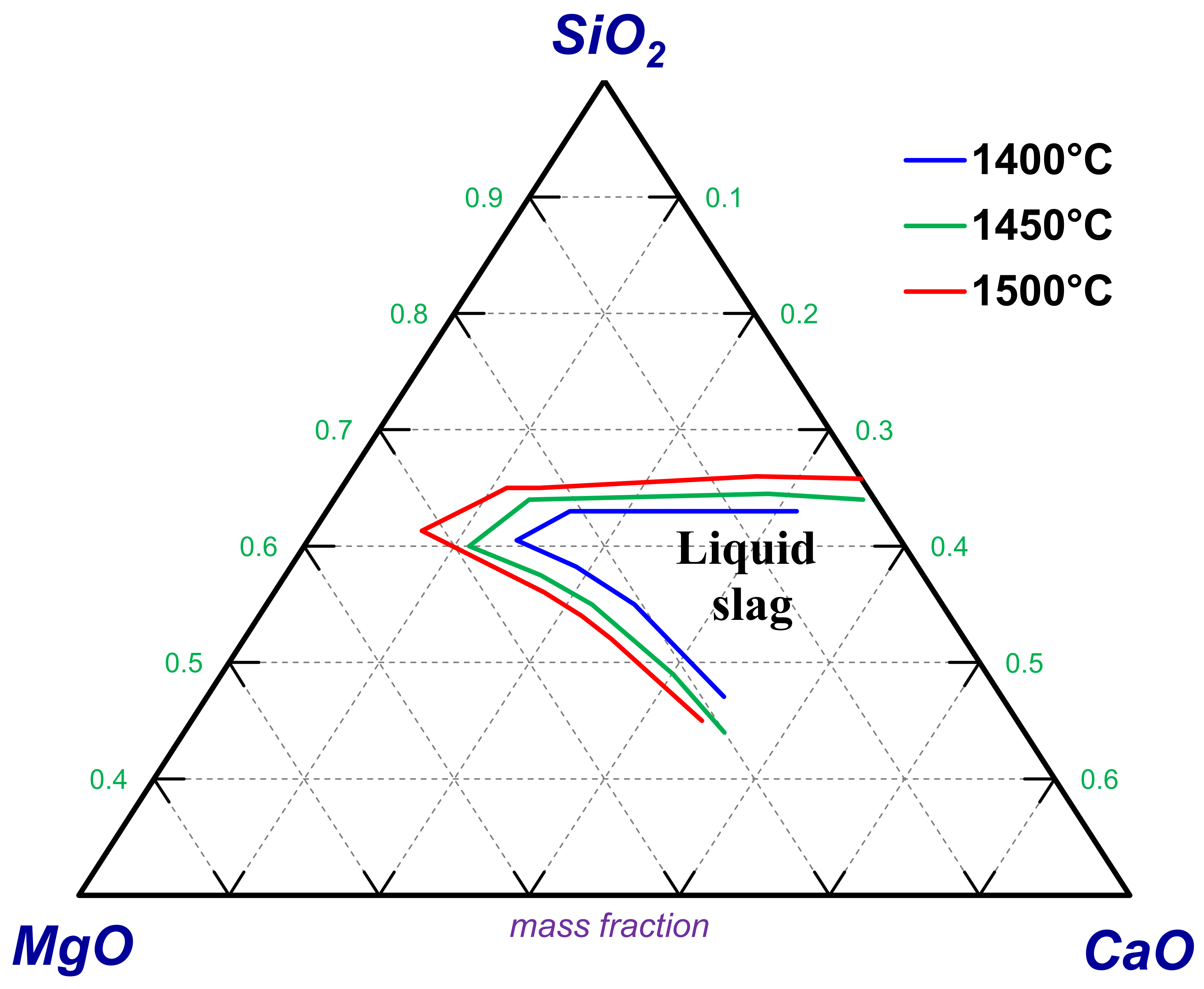
4.2.1. MgO–CaO–SiO2 Slag System
4.2.2. MgO–CaO–SiO2 Slag System in Equilibrium with Ferronickel and Solid Carbon
4.2.3. MgO–CaO–SiO2 Slag System in Equilibrium with Ferronickel and Solid Carbon with 5 and 10 wt% Al2O3
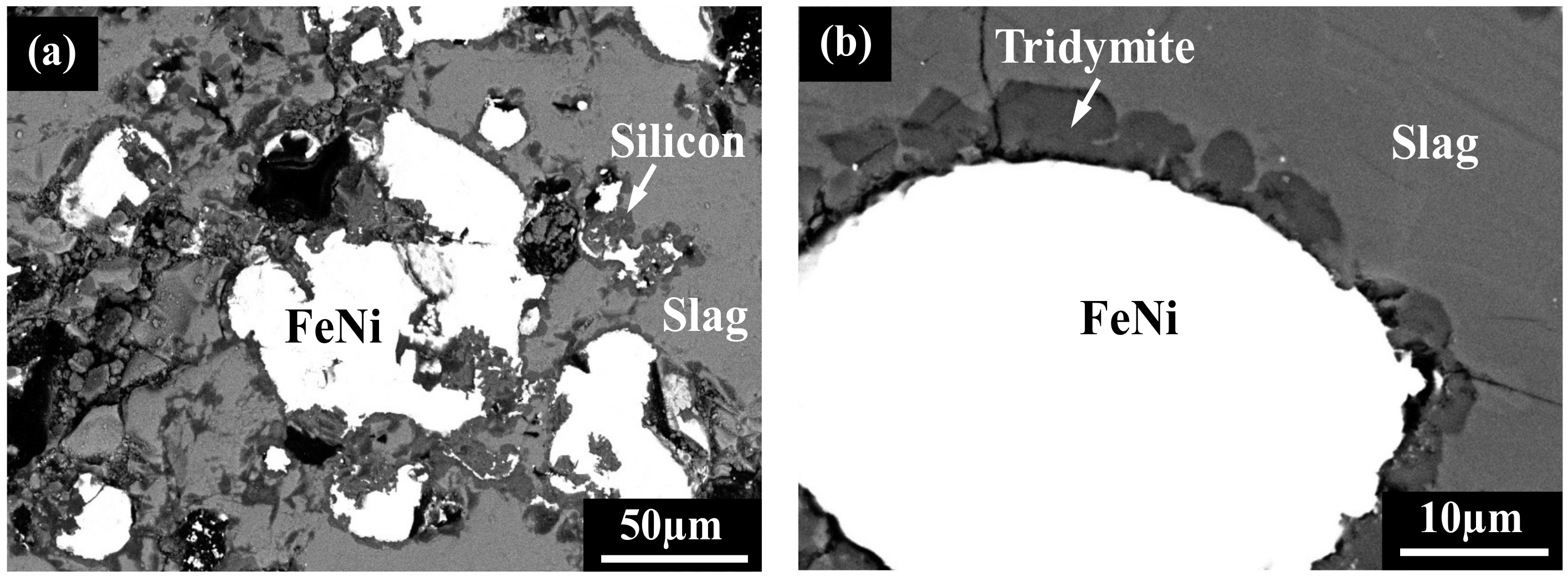
4.3. Analysis of Alloy Phase
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista Research Departement Chemicals & Resources: Mining, Metals & Minerals. Global Nickel Mining Industry Statistics & Facts. Available online: https://www.statista.com/topics/1572/nickel/#topicOverview (accessed on 23 May 2024).
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Overview of the Smelting of Nickel Laterite to Ferronickel. In Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Amsterdam, The Netherlands, 2011; pp. 49–53. [Google Scholar]
- Wei, W.; Samuelsson, P.B.; Tilliander, A.; Gyllenram, R.; Jönsson, P.G. Energy Consumption and Greenhouse Gas Emissions of Nickel Products. Energies 2020, 13, 5664. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Y.; Li, X.; Yang, S.; Zhao, J.; Tang, H.; Li, X. Molecular Dynamics Simulation on Microstructure and Physicochemical Properties of FexO-SiO2-CaO-MgO-“NiO” Slag in Nickel Matte Smelting under Modulating CaO Content. Minerals 2020, 10, 149. [Google Scholar] [CrossRef]
- Nelson, L.R.; Geldenhuis, J.M.A.; Miraza, T.; Badrujaman, T.; Hidyat, A.T.; Jauhari, I.; Stober, F.A.; Voermann, N.; Wasmund, B.O.; Jahnsen, E.J.M. Role of Operational Support in Ramp-Up of The FeNi Furnace at PT ANTAM in Pomala. In Proceedings of the International Conference on Innovations in the Ferro Alloy Industry (IFACON XI), New Delhi, India, 18–21 February 2007; pp. 798–813. [Google Scholar]
- Osborn, E.F.; Muan, A. Phase Equilibrium Diagrams of Oxide System; American Ceramic Society with the Edward Orton Jr. Ceramic Foundation: Columbus, OH, USA, 1960. [Google Scholar]
- Kushiro, I. Determination of Liquidus Relations in Synthetic Silicate Systems with Electron Probe Analysis: The System Forsterite-Diopside-Silica at 1 Atmosphere. Am. Mineral. J. Earth Planet. Mater. 1972, 57, 1260–1271. [Google Scholar]
- Longhi, J.; Boudreau, A.E. The Orthoenstatite Liquidus Field in The System Forsterite–Diopside–Silica at One Atmosphere. Am. Mineral. 1980, 65, 563–573. [Google Scholar]
- Liu, T.; Presnall, D.C. Diopside—Tridymite Liquidus Boundary Line in The System Mg2SiO4—CaMgSi2O6—SiO2 at Atmospheric Pressure. Mineral. Soc. Am. 1989, 7, 1032. [Google Scholar]
- Kowalski, M.; Spencer, P.J. Slag Atlas; Verlag Stahleisen GmbH: Duesseldorf, Germany, 1995; p. 134. [Google Scholar]
- Prince, A.T. Phase Equilibrium Relationships in a Portion of the System MgO-Al2O3-2CaO.SiO2. J. Am. Ceram. Soc. 1951, 34, 44–51. [Google Scholar] [CrossRef]
- DeVries, R.C.; Osborn, E.F. Phase Equilibria in High-Alumina Part of the System CaO–MgO–Al2O3–SiO2. J. Am. Ceram. Soc. 1957, 40, 6–15. [Google Scholar] [CrossRef]
- Osborn, E.F.; Devries, R.C.; Gee, K.H.; Kraner, H.M. Optimum Composition of Blast Furnace Slag as Deduced from Liquidus Data for The Quaternary System CaO-MgO-AI2O3-SiO2. J. Met. 1954, 200, 33–45. [Google Scholar]
- Prince, A.T. Liquidus Relationships on 10% MgO Plane of The System Lime—Magnesia—AIumina—SiIica. Am. Ceram. Soc. 1953, 37, 402–408. [Google Scholar] [CrossRef]
- Gutt, W.; Russell, A.D. Studies of the System CaO-SiO2-Al2O3-MgO in Relation to the Stability of Blast Furnace Slag. J. Mater. Sci. 1977, 12, 1869–1878. [Google Scholar] [CrossRef]
- Dahl, F.; Brandberg, J.; Sichen, D. Characterization of Melting of Some Slags in the Al2O3–CaO–MgO–SiO2 Quaternary System. ISIJ Int. 2006, 46, 614–616. [Google Scholar] [CrossRef]
- Alicia Vázquez, B.; Caballero, Á.; Pena, P. Quaternary System Al2O3-CaO-MgO-SiO2: Study of the Crystallization Volume of MgAl2O4. J. Am. Ceram. Soc. 2005, 88, 1949–1957. [Google Scholar] [CrossRef]
- Gran, J.; Wang, Y.; Sichen, D. Experimental Determination of The Liquidus in The High Basicity Region in the Al2O3 (30 Mass%)—CaO—MgO—SiO2 System. Calphad Comput. Coupling Phase Diagr. Thermochem. 2011, 35, 249–254. [Google Scholar] [CrossRef]
- Gran, J.; Yan, B.; Sichen, D. Experimental Determination of the Liquidus in The High-Basicity Region in the Al2O3(25 Mass Pct)-CaO-MgO-SiO2 and Al2O3(35 Mass Pct)-CaO-MgO-SiO2 Systems. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2011, 42, 1008–1016. [Google Scholar] [CrossRef]
- Ma, X.; Wang, G.; Wu, S.; Zhu, J.; Zhao, B. Phase Equilibria in the CaO-SiO2-Al2O3-MgO System Related to Iron Blast Furnace Slag. In Proceedings of the High Temperature Processing Symposium 2014 Swinburne University of Technology, Melbourne, Australia, 7–8 February 2014; pp. 90–92. [Google Scholar]
- Ma, X.; Wang, G.; Wu, S.; Zhu, J.; Zhao, B. Phase Equilibria in the CaO-SiO2-Al2O3-MgO System with CaO/SiO2 Ratio of 1.3 Relevant to Iron Blast Furnace Slags. ISIJ Int. 2015, 55, 2310–2317. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, D.; Zhao, Z.; Evans, T.; Zhao, B. Phase Equilibria Studies in The CaO-SiO2- Al2O3-MgO System with CaO/SiO2 Ratio of 1.10. ISIJ Int. 2016, 56, 513–519. [Google Scholar] [CrossRef]
- Kou, M.; Wu, S.; Ma, X.; Wang, L.; Chen, M.; Cai, Q.; Zhao, B. Phase Equilibrium Studies of CaO-SiO2-MgO-Al2O3 System with Binary Basicity of 1.5 Related to Blast Furnace Slag. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2016, 47, 1093–1102. [Google Scholar] [CrossRef]
- Lyu, S.; Ma, X.; Chen, M.; Huang, Z.; Yao, Z.; Wang, G.; Zhao, B. Application of Phase Equilibrium Studies of CaO–SiO2–Al2O3–MgO System for Oxide Inclusions in Si-Deoxidized Steels. Calphad Comput. Coupling Phase Diagr. Thermochem. 2020, 68, 101721. [Google Scholar] [CrossRef]
- Wang, D.; Chen, M.; Jiang, Y.; Wang, S.; Zhao, Z.; Evans, T.; Zhao, B. Phase Equilibria Studies in The CaO–SiO2–Al2O3–MgO System with CaO/SiO2 Ratio of 0.9. J. Am. Ceram. Soc. 2020, 103, 7299–7309. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X.; Lyu, S. Phase Equilibria of the Al2O3–CaO–SiO2-(0%, 5%, 10%) MgO Slag System for Non-Metallic Inclusions Control. Calphad Comput. Coupling Phase Diagr. Thermochem. 2021, 72, 102227. [Google Scholar] [CrossRef]
- Liao, J.; Qing, G.; Zhao, B. Phase Equilibrium Studies in the CaO–SiO2–Al2O3–MgO System with MgO/CaO Ratio of 0.2. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2023, 54, 793–806. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. Reprint of: FactSage Thermochemical Software and Databases. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. Critical Thermodynamic Evaluation and Optimization of The CaO-MgO-SiO2 System. J. Eur. Ceram. Soc. 2004, 25, 313–333. [Google Scholar] [CrossRef]
- Jung, I.-H.; Decterov, S.A.; Pelton, A.D. Critical Thermodynamic Evaluation and Optimization of The FeO-Fe2O3-MgO-SiO2 System. Met. Mater. Trans. B 2004, 35, 877–889. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. Critical Thermodynamic Evaluation and Optimization of the MgO-Al2O3, CaO-MgO-Al2O3, and MgO-Al2O3-SiO2 System. J. Phase Equilibria Diffus. 2004, 25, 329–345. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Stryzhyboroda, O.; Vinke, S.; Bobzin, K.; Hecht, U. Experimental Study and Thermodynamic Modelling of The Ternary System Fe–Ni–Si with Remodelling of The Constituent Binary Systems. J. Alloys Compd. 2023, 935, 168118. [Google Scholar] [CrossRef]
- Tangstad, M.; Olsen, J.E.; Ringdalen, E.; Ksiazek, M.; Reynolds, Q.; Steenkamp, J. Conceptual Tapping Model of Mn-Ferroalloy Furnaces. JOM 2022, 74, 3962–3970. [Google Scholar] [CrossRef]





| Phase | Composition (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| Mg * | Ca * | Si * | Fe * | Ni * | Al * | ||
| MgO | CaO | SiO2 | FeO | NiO | Al2O3 | ||
| 2 h from reductive initial condition | Alloy * | 0.02 | 0.13 | 20.5 | 72.9 | 5.8 | 0.04 |
| Si Alloy * | 0.00 | 0.18 | 96.2 | 1.96 | 0.35 | 0.05 | |
| Liquid Slag | 1.01 | 34.6 | 63.7 | 0.27 | 0.11 | 0.25 | |
| 2 h from oxidative initial condition | Alloy * | 0.09 | 0.15 | 2.6 | 59.5 | 36.4 | 0.07 |
| Tridymite | 0.22 | 8.3 | 89.1 | 1.28 | 0.37 | 0.47 | |
| Liquid Slag | 0.75 | 34.9 | 61.9 | 1.43 | 0.27 | 0.60 | |
| 24 h from reductive initial condition | Alloy * | 0.02 | 0.12 | 1.19 | 65.1 | 32.9 | 0.01 |
| Tridymite | 0.05 | 0.79 | 98.9 | 0.18 | 0.00 | 0.09 | |
| Liquid Slag | 0.58 | 32.2 | 60.2 | 5.7 | 0.85 | 0.49 | |
| No. | Temp (C) | Phase | Composition (wt%) | ||
|---|---|---|---|---|---|
| MgO | CaO | SiO2 | |||
| 1 | 1400 | Tridymite | 0.38 | 2.6 | 96.7 |
| CaSiO3 | 0.16 | 48.8 | 50.8 | ||
| Liquid Slag | 5.3 | 30.1 | 63.1 | ||
| 2 | 1400 | Tridymite | 0.97 | 3.2 | 95.5 |
| Liquid Slag | 9.7 | 28.6 | 61.1 | ||
| 3 | 1400 | Tridymite | 0.59 | 0.47 | 98.7 |
| Liquid Slag | 25.8 | 14.3 | 59.3 | ||
| 4 | 1400 | Olivine | 50.4 | 2.8 | 45.8 |
| Liquid Slag | 26.7 | 14.3 | 58.1 | ||
| 5 | 1400 | Olivine | 48.2 | 5.3 | 45.8 |
| Liquid Slag | 20.3 | 23.8 | 55.5 | ||
| 6 | 1500 | Cristobalite | 0.07 | 1.71 | 98.0 |
| Liquid Slag | 0.43 | 35.7 | 63.3 | ||
| 7 | 1500 | Cristobalite | 0.22 | 0.51 | 99.0 |
| Liquid Slag | 11.9 | 22.8 | 64.7 | ||
| 8 | 1500 | Cristobalite | 0.17 | 0.38 | 99.1 |
| Liquid Slag | 24.5 | 10.6 | 62.3 | ||
| 9 | 1500 | Olivine | 52.4 | 1.45 | 45.0 |
| Liquid Slag | 32.8 | 6.3 | 59.8 | ||
| 10 | 1500 | Olivine | 54.0 | 2.3 | 43.7 |
| Liquid Slag | 20.2 | 24.0 | 55.8 | ||
| No. | Temp (C) | Phase | Composition (wt%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mg * | Ca * | Si * | Fe * | Ni * | Al * | |||
| MgO | CaO | SiO2 | FeO | NiO | Al2O3 | |||
| 1. | 1400 | Alloy * | 0.43 | 3.0 | 22.8 | 71.2 | 2.4 | 0.11 |
| Tridymite | 0.58 | 0.61 | 98.0 | 0.17 | 0.22 | 0.41 | ||
| Liquid Slag | 4.1 | 30.8 | 63.4 | 0.63 | 0.41 | 0.43 | ||
| 2. | 1400 | Alloy * | 1.82 | 1.60 | 22.0 | 55.9 | 18.6 | 0.06 |
| Tridymite | 0.66 | 0.57 | 97.4 | 0.39 | 0.55 | 0.39 | ||
| Liquid Slag | 18.9 | 17.5 | 62.5 | 0.37 | 0.15 | 0.39 | ||
| 3. | 1400 | Alloy * | 3.2 | 1.29 | 5.03 | 30.9 | 59.2 | 0.17 |
| Pyroxene | 35.9 | 1.82 | 60.3 | 1.23 | 0.47 | 0.30 | ||
| Tridymite | 0.59 | 0.43 | 98.7 | 0.14 | 0.00 | 0.10 | ||
| Liquid Slag | 19.5 | 15.7 | 61.8 | 1.82 | 0.31 | 0.62 | ||
| 4. | 1400 | Alloy * | 5.23 | 1.70 | 23.4 | 43.3 | 25.9 | 0.21 |
| Pyroxene | 39.8 | 1.91 | 56.2 | 0.73 | 0.97 | 0.24 | ||
| Olivine | 51.9 | 4.06 | 43.6 | 0.23 | 0.00 | 0.16 | ||
| Liquid Slag | 24.3 | 13.6 | 59.7 | 1.04 | 0.40 | 1.00 | ||
| 5. | 1400 | Alloy * | 0.10 | 0.04 | 19.7 | 68.5 | 11.7 | 0.04 |
| Olivine | 53.4 | 2.09 | 43.9 | 0.13 | 0.08 | 0.34 | ||
| Liquid Slag | 20.6 | 20.6 | 57.5 | 0.19 | 0.19 | 0.98 | ||
| 6. | 1400 | Alloy * | 0.09 | 0.05 | 1.57 | 58.7 | 39.3 | 0.37 |
| Olivine | 52.7 | 1.97 | 45.0 | 0.20 | 0.00 | 0.13 | ||
| Liquid Slag | 21.6 | 20.9 | 56.4 | 0.35 | 0.06 | 0.59 | ||
| 7. | 1400 | Alloy * | 1.65 | 0.91 | 11.5 | 64.9 | 20.9 | 0.09 |
| Olivine | 55.3 | 0.80 | 43.1 | 0.54 | 0.00 | 0.19 | ||
| Liquid Slag | 22.1 | 20.9 | 55.7 | 0.40 | 0.21 | 0.57 | ||
| 8. | 1400 | Alloy * | 0.10 | 0.13 | 6.3 | 77.0 | 16.3 | 0.10 |
| Olivine | 53.4 | 2.90 | 43.3 | 0.14 | 0.17 | 0.17 | ||
| Liquid Slag | 18.9 | 25.3 | 54.8 | 0.10 | 0.05 | 0.77 | ||
| 9. | 1400 | Alloy * | 0.40 | 0.83 | 10.1 | 68.6 | 20.2 | 0.26 |
| Olivine | 52.6 | 4.03 | 43.1 | 0.15 | 0.00 | 0.14 | ||
| Liquid Slag | 18.6 | 33.9 | 46.7 | 0.04 | 0.00 | 0.84 | ||
| 10. | 1450 | Alloy * | 0.17 | 3.6 | 25.0 | 59.6 | 11.5 | 0.08 |
| Tridymite | 0.00 | 0.00 | 0.99 | 0.00 | 0.00 | 0.00 | ||
| Liquid Slag | 0.75 | 34.9 | 61.9 | 1.43 | 0.27 | 0.60 | ||
| 11. | 1450 | Alloy * | 0.83 | 2.78 | 24.1 | 49.3 | 22.8 | 0.14 |
| Tridymite | 0.00 | 0.00 | 0.99 | 0.00 | 0.00 | 0.00 | ||
| Liquid Slag | 6.7 | 27.5 | 63.6 | 1.30 | 0.22 | 0.46 | ||
| 12. | 1450 | Alloy * | 1.22 | 1.69 | 21.9 | 62.1 | 12.9 | 0.10 |
| Tridymite | 0.00 | 0.00 | 0.99 | 0.01 | 0.00 | 0.00 | ||
| Liquid Slag | 14.0 | 21.2 | 63.4 | 0.65 | 0.11 | 0.55 | ||
| 13. | 1450 | Alloy * | 1.58 | 0.27 | 2.3 | 45.4 | 47.3 | 0.08 |
| Pyroxene | 36.9 | 1.47 | 59.4 | 1.12 | 0.35 | 0.62 | ||
| Tridymite | 0.60 | 0.38 | 98.8 | 0.00 | 0.07 | 0.14 | ||
| Liquid Slag | 21.3 | 13.3 | 62.9 | 1.61 | 0.00 | 0.92 | ||
| 14. | 1450 | Alloy * | 1.73 | 0.23 | 1.7 | 62.5 | 33.6 | 0.16 |
| Pyroxene | 36.9 | 1.56 | 59.3 | 1.14 | 0.72 | 0.29 | ||
| Olivine | 49.5 | 0.00 | 45.9 | 2.2 | 2.10 | 0.36 | ||
| Liquid Slag | 27.6 | 11.7 | 57.4 | 1.54 | 0.62 | 1.12 | ||
| 15. | 1450 | Alloy * | 0.06 | 0.14 | 2.6 | 59.7 | 37.4 | 0.15 |
| Olivine | 53.1 | 0.94 | 44.5 | 0.57 | 0.10 | 0.79 | ||
| Liquid Slag | 23.1 | 20.9 | 54.9 | 0.20 | 0.09 | 0.73 | ||
| 16. | 1450 | Alloy * | 0.09 | 0.01 | 3.6 | 58.8 | 37.5 | 0.04 |
| Olivine | 53.3 | 0.79 | 44.8 | 0.66 | 0.31 | 0.15 | ||
| Liquid Slag | 22.6 | 20.4 | 55.9 | 0.33 | 0.02 | 0.59 | ||
| 17. | 1450 | Alloy * | 0.08 | 0.15 | 0.11 | 73.8 | 25.9 | 0.03 |
| Olivine | 52.9 | 2.8 | 43.7 | 0.37 | 0.11 | 0.14 | ||
| Liquid Slag | 20.5 | 31.6 | 47.1 | 0.06 | 0.06 | 0.70 | ||
| 18. | 1450 | Alloy * | 1.76 | 2.5 | 2.6 | 62.4 | 29.5 | 1.35 |
| Olivine | 46.5 | 10.4 | 42.3 | 0.20 | 0.08 | 0.58 | ||
| Liquid Slag | 19.2 | 34.8 | 44.1 | 0.42 | 0.00 | 1.51 | ||
| 19. | 1500 | Alloy * | 5.9 | 1.12 | 27.6 | 43.4 | 21.6 | 0.24 |
| Pyroxene | 37.3 | 1.00 | 61.3 | 0.24 | 0.00 | 0.10 | ||
| Olivine | 49.8 | 2.3 | 46.1 | 1.00 | 0.28 | 0.53 | ||
| Liquid Slag | 29.4 | 8.3 | 59.6 | 1.16 | 0.47 | 1.09 | ||
| 20. | 1500 | Alloy * | 3.4 | 1.00 | 23.9 | 62.4 | 9.2 | 0.15 |
| Olivine | 53.3 | 2.3 | 43.3 | 0.44 | 0.28 | 0.44 | ||
| Liquid Slag | 25.9 | 16.7 | 54.3 | 0.68 | 0.60 | 1.69 | ||
| 21. | 1500 | Alloy * | 2.3 | 1.03 | 24.7 | 46.8 | 24.2 | 1.04 |
| Olivine | 46.7 | 3.6 | 45.5 | 2.4 | 1.39 | 0.46 | ||
| Liquid Slag | 23.6 | 21.7 | 51.2 | 1.27 | 0.86 | 1.25 | ||
| 22. | 1500 | Alloy * | 5.6 | 2.6 | 23.9 | 36.0 | 30.0 | 0.18 |
| Olivine | 49.8 | 5.2 | 43.4 | 0.55 | 0.53 | 0.42 | ||
| Liquid Slag | 23.5 | 23.8 | 48.9 | 0.90 | 0.57 | 2.13 | ||
| No. | Temp (C) | Phase | Composition (wt%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mg * | Ca * | Si * | Fe * | Ni * | Al * | |||
| MgO | CaO | SiO2 | FeO | NiO | Al2O3 | |||
| 1. | 1400 | Alloy * | 0.03 | 0.19 | 3.08 | 53.9 | 35.1 | 0.07 |
| Tridymite | 0.41 | 0.46 | 93.8 | 3.4 | 1.66 | 0.34 | ||
| Liquid Slag | 17.3 | 13.2 | 62.7 | 0.21 | 0.13 | 6.5 | ||
| 2. | 1400 | Alloy * | 1.04 | 1.86 | 23.6 | 29.1 | 43.8 | 0.67 |
| Tridymite | 2.2 | 5.6 | 88.6 | 1.03 | 0.57 | 1.93 | ||
| Liquid Slag | 7.9 | 18.4 | 66.1 | 0.89 | 0.33 | 6.4 | ||
| 3. | 1400 | Alloy * | 0.22 | 7.4 | 6.8 | 47.4 | 37.7 | 0.38 |
| Tridymite | 0.09 | 0.52 | 98.7 | 0.37 | 0.06 | 0.32 | ||
| Liquid Slag | 0.56 | 29.6 | 63.9 | 1.17 | 0.32 | 4.4 | ||
| 4. | 1400 | Alloy * | 5.7 | 1.49 | 19.0 | 34.7 | 38.5 | 0.30 |
| Olivine | 49.9 | 3.4 | 45.0 | 0.59 | 0.38 | 0.72 | ||
| Liquid Slag | 21.4 | 18.1 | 56.5 | 0.67 | 0.44 | 2.9 | ||
| 5. | 1400 | Alloy * | 0.06 | 0.08 | 16.0 | 48.9 | 34.8 | 0.04 |
| Olivine | 53.3 | 2.1 | 44.1 | 0.13 | 0.17 | 0.23 | ||
| Liquid Slag | 18.3 | 26.9 | 51.9 | 0.18 | 0.09 | 2.7 | ||
| 6. | 1400 | Alloy * | 0.05 | 0.13 | 19.8 | 53.9 | 25.7 | 0.07 |
| Tridymite | 5.1 | 4.2 | 87.4 | 0.34 | 0.08 | 2.9 | ||
| Liquid Slag | 15.5 | 11.7 | 63.2 | 0.59 | 0.16 | 8.8 | ||
| 7. | 1400 | Alloy * | 0.08 | 0.24 | 22.1 | 29.2 | 48.2 | 0.12 |
| Tridymite | 0.51 | 1.96 | 96.3 | 0.19 | 0.05 | 0.94 | ||
| Liquid Slag | 6.2 | 19.3 | 65.4 | 0.14 | 0.17 | 8.9 | ||
| 8. | 1400 | Alloy * | 0.01 | 0.19 | 21.9 | 40.6 | 37.1 | 0.06 |
| Tridymite | 0.13 | 4.8 | 92.8 | 0.23 | 0.04 | 1.98 | ||
| Liquid Slag | 0.44 | 24.4 | 65.9 | 0.15 | 0.02 | 9.2 | ||
| 9. | 1400 | Alloy * | 0.15 | 0.14 | 15.6 | 51.4 | 31.7 | 0.05 |
| Olivine | 55.4 | 0.61 | 43.6 | 0.15 | 0.08 | 0.18 | ||
| Liquid Slag | 18.2 | 17.4 | 57.4 | 0.05 | 0.05 | 6.9 | ||
| 10. | 1400 | Alloy * | 0.18 | 0.17 | 16.4 | 56.6 | 24.9 | 0.09 |
| Olivine | 54.7 | 1.14 | 43.8 | 0.00 | 0.02 | 0.31 | ||
| Liquid Slag | 17.9 | 23.5 | 51.3 | 0.12 | 0.12 | 6.8 | ||
| 11. | 1450 | Alloy * | 0.09 | 0.10 | 8.3 | 57.3 | 32.7 | 0.03 |
| Tridymite | 6.00 | 1.64 | 90.9 | 0.34 | 0.06 | 0.98 | ||
| Liquid Slag | 20.7 | 8.3 | 63.9 | 1.37 | 0.15 | 5.6 | ||
| 12. | 1450 | Alloy * | 0.20 | 0.19 | 3.9 | 58.9 | 36.6 | 0.08 |
| Tridymite | 0.68 | 0.69 | 98.2 | 0.06 | 0.05 | 0.28 | ||
| Liquid Slag | 13.6 | 14.7 | 65.6 | 0.99 | 0.06 | 5.1 | ||
| 13. | 1450 | Alloy * | 0.09 | 0.51 | 9.7 | 54.7 | 34.9 | 0.01 |
| Tridymite | 0.35 | 0.73 | 98.1 | 0.17 | 0.12 | 0.49 | ||
| Liquid Slag | 7.2 | 18.21 | 66.8 | 0.29 | 0.16 | 7.3 | ||
| 14. | 1450 | Alloy * | 0.01 | 0.14 | 19.6 | 50.6 | 29.7 | 0.05 |
| Tridymite | 0.01 | 0.76 | 97.8 | 0.89 | 0.22 | 0.31 | ||
| Liquid Slag | 0.42 | 27.2 | 65.9 | 0.95 | 0.05 | 5.5 | ||
| 15. | 1450 | Alloy * | 0.25 | 0.56 | 16.5 | 42.1 | 37.7 | 0.17 |
| Olivine | 51.0 | 2.95 | 45.1 | 0.23 | 0.09 | 0.62 | ||
| Liquid Slag | 21.9 | 19.6 | 54.4 | 0.59 | 0.16 | 3.4 | ||
| 16. | 1450 | Alloy * | 0.04 | 0.27 | 15.1 | 44.1 | 40.3 | 0.10 |
| Olivine | 55.1 | 2.7 | 41.1 | 0.19 | 0.12 | 0.73 | ||
| Liquid Slag | 19.95 | 28.0 | 45.2 | 0.15 | 0.15 | 6.6 | ||
| 17. | 1450 | Alloy * | 3.89 | 0.96 | 26.6 | 35.0 | 32.2 | 1.28 |
| Tridymite | 6.37 | 2.4 | 87.1 | 0.71 | 0.37 | 3.1 | ||
| Liquid Slag | 17.70 | 6.1 | 66.4 | 0.70 | 0.58 | 8.5 | ||
| 18. | 1450 | Alloy * | 0.05 | 0.14 | 21.4 | 38.8 | 39.5 | 0.05 |
| Tridymite | 0.66 | 0.72 | 97.8 | 0.04 | 0.11 | 0.65 | ||
| Liquid Slag | 13.2 | 12.2 | 65.8 | 0.11 | 0.11 | 8.6 | ||
| 19. | 1450 | Alloy * | 0.26 | 0.72 | 22.1 | 36.9 | 39.6 | 0.30 |
| Tridymite | 0.78 | 2.9 | 94.7 | 0.15 | 0.10 | 1.39 | ||
| Liquid Slag | 5.5 | 19.2 | 66.6 | 0.14 | 0.00 | 8.5 | ||
| 20. | 1450 | Alloy * | 0.02 | 0.43 | 22.0 | 34.0 | 43.4 | 0.18 |
| Tridymite | 0.03 | 1.67 | 97.3 | 0.09 | 0.10 | 0.78 | ||
| Liquid Slag | 0.36 | 23.9 | 66.0 | 0.11 | 0.07 | 9.6 | ||
| 21. | 1450 | Alloy * | 0.12 | 0.15 | 16.4 | 48.9 | 33.7 | 0.03 |
| Olivine | 54.7 | 0.87 | 43.9 | 0.11 | 0.11 | 0.19 | ||
| Liquid Slag | 18.2 | 20.3 | 52.5 | 0.15 | 0.13 | 8.5 | ||
| 22. | 1450 | Alloy * | 0.06 | 0.11 | 13.0 | 46.0 | 39.8 | 0.03 |
| Olivine | 53.9 | 1.73 | 43.7 | 0.20 | 0.35 | 0.16 | ||
| Liquid Slag | 15.5 | 30.4 | 44.4 | 0.15 | 0.17 | 8.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prikusuma, N.K.P.; Algifari, M.G.; Harahap, R.A.; Zulhan, Z.; Hidayat, T. Phase Equilibria Study of the MgO–CaO–SiO2 Slag System with Ferronickel Alloy, Solid Carbon, and Al2O3 Additions. Processes 2024, 12, 1946. https://doi.org/10.3390/pr12091946
Prikusuma NKP, Algifari MG, Harahap RA, Zulhan Z, Hidayat T. Phase Equilibria Study of the MgO–CaO–SiO2 Slag System with Ferronickel Alloy, Solid Carbon, and Al2O3 Additions. Processes. 2024; 12(9):1946. https://doi.org/10.3390/pr12091946
Chicago/Turabian StylePrikusuma, Nandhya K. P., Muhammad G. Algifari, Rafiandy A. Harahap, Zulfiadi Zulhan, and Taufiq Hidayat. 2024. "Phase Equilibria Study of the MgO–CaO–SiO2 Slag System with Ferronickel Alloy, Solid Carbon, and Al2O3 Additions" Processes 12, no. 9: 1946. https://doi.org/10.3390/pr12091946






