Analysis and Simulation of Adsorption Efficiency of Herbicides Diuron and Linuron on Activated Carbon from Spent Coffee Beans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Production of the ACs
2.3. Adsorption
2.4. Evaluation of the Phytotoxic, Cytotoxic, and Genotoxic Potential of Diuron and Linuron in Aqueous Medium before and after Adsorption with AC on Allium cepa L. (Onion) Roots
3. Results
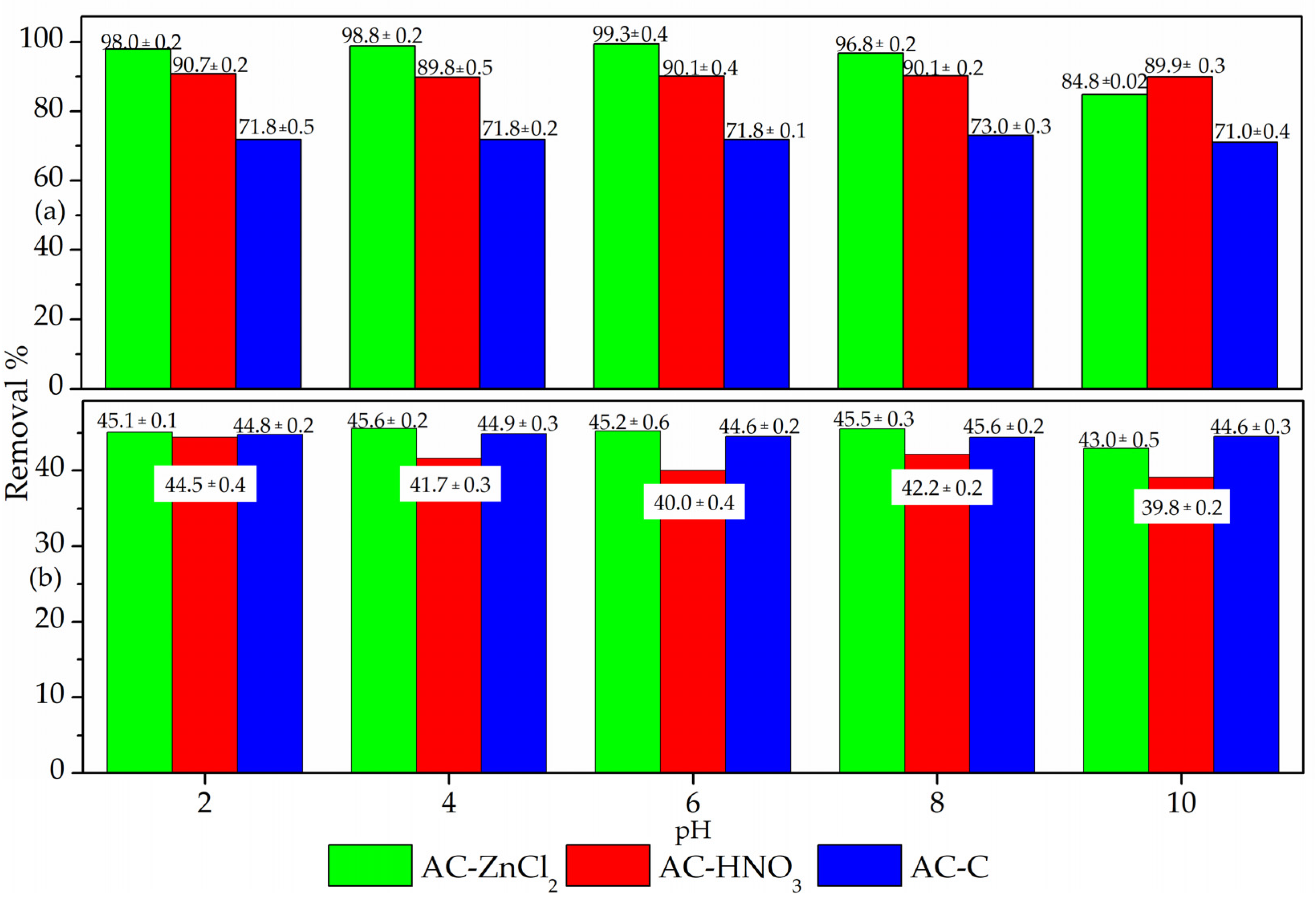
3.1. Adsorption
3.2. Toxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caioni, G.; Merola, C.; Perugini, M.; D’angelo, M.; Cimini, A.M.; Amorena, M.; Benedetti, E. An Experimental Approach to Study the Effects of Realistic Environmental Mixture of Linuron and Propamocarb on Zebrafish Synaptogenesis. Int. J. Environ. Res. Public Health 2021, 18, 4664. [Google Scholar] [CrossRef]
- Muyesaier, T.; Huada, D.R.; Li, W.; Jia, L.; Ross, S.; Des, C.; Cordia, C.; Tri, P.D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Lin, Z.; Huang, Y.; Bhatt, P.; Chen, S. Emerging Strategies for the Bioremediation of the Phenylurea Herbicide Diuron. Front. Microbiol. 2021, 12, 686509. [Google Scholar] [CrossRef] [PubMed]
- Agbaogun, B.K.; Fischer, K. Adsorption of Phenylurea Herbicides by Tropical Soils. Environ. Monit. Assess. 2020, 192, 212. [Google Scholar] [CrossRef]
- Ma, Y.; Pedersen, M.; Vinggaard, A.M. In Vitro Antiandrogenic Effects of the Herbicide Linuron and Its Metabolites. Chemosphere 2024, 349, 140773. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, S.; El Ahmadie, N.; Rheingold, S.; El Chehouri, J.; Yang, L.; Souders, C.L.; Martyniuk, C.J. Sub-Lethal Toxicity Assessment of the Phenylurea Herbicide Linuron in Developing Zebrafish (Danio Rerio) Embryo/Larvae. Neurotoxicol. Teratol. 2020, 81, 106917. [Google Scholar] [CrossRef] [PubMed]
- Barakan, S.; Aghazadeh, V. The Advantages of Clay Mineral Modification Methods for Enhancing Adsorption Efficiency in Wastewater Treatment: A Review. Environ. Sci. Pollut. Res. 2021, 28, 2572–2599. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Sun, R.; Lin, H.; Gong, L.; Fang, M.; Hu, W.H. HPV Infection and Anemia Status Stratify the Survival of Early T2 Laryngeal Squamous Cell Carcinoma. J. Voice 2015, 29, 356–362. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A Review on Experimental Chemically Modified Activated Carbon to Enhance Dye and Heavy Metals Adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface Oxides on Carbon and Their Analysis: A Critical Assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Blacks and Other Carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of Emerging Contaminants from Water and Wastewater by Modified Biochar: A Review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Saberian, M.; Li, J.; Donnoli, A.; Bonderenko, E.; Oliva, P.; Gill, B.; Lockrey, S.; Siddique, R. Recycling of Spent Coffee Grounds in Construction Materials: A Review. J. Clean. Prod. 2021, 289, 125837. [Google Scholar] [CrossRef]
- Siregar, C.A.; Siregar, A.M.; Lubis, R.W.; Marpaung, D. Rancang Bangun Mesin Giling Kopi Untuk Menunjang Dan Membuka Unit Usaha Baru Mitra Deli Coffe. ABDI SABHA (J. Pengabdi. Kpd. Masy.) 2022, 3, 174–180. [Google Scholar] [CrossRef]
- da S. Rocha, B.C.; de Moraes, L.E.Z.; Santo, D.E.; Peron, A.P.; de Souza, D.C.; Bona, E.; Valarini, O. Removal of Bentazone Using Activated Carbon from Spent Coffee Grounds. J. Chem. Technol. Biotechnol. 2024, 99, 1342–1355. [Google Scholar] [CrossRef]
- Javier Sánchez, A. Characterization of Activated Carbon Produced from Coffee Residues by Chemical and Physical Activation. KTH Chem. Sci. Eng. 2011, 66, 14–28. [Google Scholar]
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.M.; Tagliaferro, A. Waste Coffee Ground Biochar: A Material for Humidity Sensors. Sensors 2019, 19, 801. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honório, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of Discarded Coffee Waste on Human and Environmental Health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef]
- Ferraz, F.M.; Yuan, Q. Organic Matter Removal from Landfill Leachate by Adsorption Using Spent Coffee Grounds Activated Carbon. Sustain. Mater. Technol. 2020, 23, e00141. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Brković, S.; Potkonjak, N.; Unterweger, C.; Bajuk-Bogdanović, D.; Pašti, I.; Lazarević-Pašti, T. Spent Coffee Grounds-Derived Carbon Material as an Effective Adsorbent for Removing Multiple Contaminants from Wastewater: A Comprehensive Kinetic, Isotherm, and Thermodynamic Study. J. Water Process Eng. 2024, 63, 105507. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Pejčić, M.; Pašti, I.; Lazarević-Pašti, T. Spent Coffee Grounds as an Adsorbent for Malathion and Chlorpyrifos—Kinetics, Thermodynamics, and Eco-Neurotoxicity. Foods 2023, 12, 2397. [Google Scholar] [CrossRef] [PubMed]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R.; Lavagnolo, M.C.; Moretti, E.; Talon, A.; Mozzon, M.; Sgarbossa, P. Activated Carbon from Spent Coffee Grounds: A Good Competitor of Commercial Carbons for Water Decontamination. Appl. Sci. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Zbair, M.; El Hadrami, A.; Bellarbi, A.; Monkade, M.; Zradba, A.; Brahmi, R. Herbicide Diuron Removal from Aqueous Solution by Bottom Ash: Kinetics, Isotherm, and Thermodynamic Adsorption Studies. J. Environ. Chem. Eng. 2020, 8, 103667. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Gama, B.M.V.; Fernandes, D.P.; Sepúlveda, P.; Silva, L.F.O.; Meili, L. Effective Adsorption of Harmful Herbicide Diuron onto Novel Activated Carbon from Hovenia Dulcis. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 654, 129900. [Google Scholar] [CrossRef]
- Vieira, Y.; Silveira, J.P.; Dotto, G.L.; Knani, S.; Vieillard, J.; Georgin, J.; Franco, D.S.P.; Lima, E.C. Mechanistic Insights and Steric Interpretations through Statistical Physics Modelling and Density Functional Theory Calculations for the Adsorption of the Pesticides Atrazine and Diuron by Hovenia Dulcis Biochar. J. Mol. Liq. 2022, 367, 120418. [Google Scholar] [CrossRef]
- Georgin, J.; Pinto, D.; Franco, D.S.P.; Schadeck Netto, M.; Lazarotto, J.S.; Allasia, D.G.; Tassi, R.; Silva, L.F.O.; Dotto, G.L. Improved Adsorption of the Toxic Herbicide Diuron Using Activated Carbon Obtained from Residual Cassava Biomass (Manihot esculenta). Molecules 2022, 27, 7574. [Google Scholar] [CrossRef]
- Asgari, G.; Abdipour, H.; Shadjou, A.M. A Review of Novel Methods for Diuron Removal from Aqueous Environments. Heliyon 2023, 9, e23134. [Google Scholar] [CrossRef]
- Elazabi, M.; Draskovic, B.; Novakovic, M.; Mihajlovic, I.; Hgeig, A. Adsorption of Linuron and Isoproturon Pesticides on Commercial Activated Carbon, Norit SA2. Fresenius Environ. Bull. 2021, 30, 1030–1043. [Google Scholar]
- Sirival, R.; Patdhanagul, N.; Preecharram, S.; Nantaphan, P.; Mahem, R. The Performance of Synthetic Zeolite Combined with Activated Carbon for Removal of Linuron Herbicide. Suranaree J. Sci. Technol. 2020, 27, 030027-1–030027-5. [Google Scholar]
- Yang, J.; Sun, H.; Liu, Y.; Wang, X.; Valizadeh, K. The Sorption of Tebuconazole and Linuron from an Aqueous Environment with a Modified Sludge-Based Biochar: Effect, Mechanisms, and Its Persistent Free Radicals Study. J. Chem. 2021, 2021, 2912054. [Google Scholar] [CrossRef]
- Belaroui, L.S.; Ouali, A.; Bengueddach, A.; Lopez Galindo, A.; Peña, A. Adsorption of Linuron by an Algerian Palygorskite Modified with Magnetic Iron. Appl. Clay Sci. 2018, 164, 26–33. [Google Scholar] [CrossRef]
- Rissouli, L.; Benicha, M.; Chabbi, M. Contribution to the Elimination of Linuron by the Adsorption Process Using Chitin and Chitosan Biopolymers. J. Mater. Environ. Sci. 2016, 7, 531–540. [Google Scholar]
- El-Nahhal, Y.; Abadsa, M.; Affifi, S. Adsorption of Diuron and Linuron in Gaza Soils. Am. J. Anal. Chem. 2013, 04, 94–99. [Google Scholar] [CrossRef]
- Debien, I.C.N.; Vardanega, R.; Santos, D.T.; Meireles, M.A.A. Pressurized Liquid Extraction as a Promising and Economically Feasible Technique for Obtaining Beta-Ecdysone-Rich Extracts from Brazilian Ginseng (Pfaffia glomerata) Roots. Sep. Sci. Technol. 2015, 50, 1647–1657. [Google Scholar] [CrossRef]
- Dean, J.R. Pressurized Liquid Extraction. In Extraction Techniques for Environmental Analysis; Wiley: Hoboken, NJ, USA, 2022; pp. 171–204. [Google Scholar] [CrossRef]
- Haro, N.K.; Dávila, I.V.J.; Nunes, K.G.P.; de Franco, M.A.E.; Marcilio, N.R.; Féris, L.A. Kinetic, Equilibrium and Thermodynamic Studies of the Adsorption of Paracetamol in Activated Carbon in Batch Model and Fixed-Bed Column. Appl. Water Sci. 2021, 11, 38. [Google Scholar] [CrossRef]
- Milonjić, S.K.; Kopečni, M.M.; Ilić, Z.E. The Point of Zero Charge and Adsorption Properties of Natural Magnetite. J. Radioanal. Chem. 1983, 78, 15–24. [Google Scholar] [CrossRef]
- Gonçalves Júnior, D.R.; de Araújo, P.C.C.; Simões, A.L.G.; Voll, F.A.P.; Parizi, M.P.S.; de Oliveira, L.H.; Ferreira-Pinto, L.; Cardozo-Filho, L.; de Jesus Santos, E. Assessment of the Adsorption Capacity of Phenol on Magnetic Activated Carbon. Asia-Pacific J. Chem. Eng. 2022, 17, e2725. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J.N.; Jayakumar, N.S. Optimal Isotherm Parameters for Phenol Adsorption from Aqueous Solutions onto Coconut Shell Based Activated Carbon: Error Analysis of Linear and Non-Linear Methods. J. Taiwan Inst. Chem. Eng. 2017, 80, 472–487. [Google Scholar] [CrossRef]
- Fiskesjö, G. The Allium Test as a Standard in Environmental Monitoring. Hereditas 1985, 102, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Mrozik, W.; Minofar, B.; Thongsamer, T.; Wiriyaphong, N.; Khawkomol, S.; Plaimart, J.; Vakros, J.; Karapanagioti, H.; Vinitnantharat, S.; Werner, D. Valorisation of Agricultural Waste Derived Biochars in Aquaculture to Remove Organic Micropollutants from Water—Experimental Study and Molecular Dynamics Simulations. J. Environ. Manag. 2021, 300, 113717. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar Applications and Modern Techniques for Characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Fletcher, A.; Somorin, T.; Aladeokin, O. Production of High Surface Area Activated Carbon from Peanut Shell by Chemical Activation with Zinc Chloride: Optimisation and Characterization. Bioenergy Res. 2024, 17, 467–478. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Krishnaiah, D.; Bono, A.; Suali, E.; Abang, S.; Fai, L.M. Removal of Chlorinated Phenol from Aqueous Media by Guava Seed (Psidium guajava) Tailored Activated Carbon. Water Resour. Ind. 2016, 16, 29–36. [Google Scholar] [CrossRef]
- Gundogdu, A.; Duran, C.; Senturk, H.B.; Soylak, M.; Imamoglu, M.; Onal, Y. Physicochemical Characteristics of a Novel Activated Carbon Produced from Tea Industry Waste. J. Anal. Appl. Pyrolysis 2013, 104, 249–259. [Google Scholar] [CrossRef]
- Chen, W.S.; Chen, Y.C.; Lee, C.H. Modified Activated Carbon for Copper Ion Removal from Aqueous Solution. Processes 2022, 10, 150. [Google Scholar] [CrossRef]
- Ternero-Hidalgo, J.J.; Rosas, J.M.; Palomo, J.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Functionalization of Activated Carbons by HNO3 Treatment: Influence of Phosphorus Surface Groups. Carbon N. Y. 2016, 101, 409–419. [Google Scholar] [CrossRef]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of Persulfate by Biochar from Spent Malt Rootlets for the Degradation of Trimethoprim in the Presence of Inorganic Ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]
- Giannakopoulos, S.; Frontistis, Z.; Vakros, J.; Poulopoulos, S.G.; Manariotis, I.D.; Mantzavinos, D. Combined Activation of Persulfate by Biochars and Artificial Light for the Degradation of Sulfamethoxazole in Aqueous Matrices. J. Taiwan Inst. Chem. Eng. 2022, 136, 104440. [Google Scholar] [CrossRef]
- do Nascimento, R.F.; de Lima, A.C.A.; Vidal, C.B.; de Melo, D.Q.; Raulino, G.S.C. Adsorção: Aspectos Teóricos e Aplicações Ambientais; Imprensa Universitária: Fortaleza, Brazil, 2020; Volume 308. [Google Scholar]
- Benaouda, B.; Iman, G.; Michalkiewicz, B. Study on Anionic Dye Toxicity Reduction from Simulated Media by MnO2/Agro-Biomass Based-AC Composite Adsorbent. Ind. Crops Prod. 2024, 208, 117789. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L. From Langmuir Kinetics to First- and Second-Order Rate Equations for Adsorption. Langmuir 2008, 24, 11625–11630. [Google Scholar] [CrossRef]
- McCabe, W.C.; Smith, J.C.; Harriot, P. Unit Operations of Chemical Engineering. Choice Rev. Online 1993, 30, 30-6200. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A Review of the Hydrothermal Carbonization of Biomass Waste for Hydrochar Formation: Process Conditions, Fundamentals, and Physicochemical Properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- da Rocha, S.A.F.; da Rocha, B.C.S.; de Moraes, L.E.Z.; Villaça, J.M.P.; Scapin, D.; Santo, D.E.; da Gonzalez, R.; Junior, O.V.; Peron, A.P. Evaluation and Simulation of the Adsorption Capacity of Octocrylene Sunscreen on Commercial Carbon and Biochar from Spent Coffee Beans. Processes 2024, 12, 1249. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A.; et al. Use of Advanced Techniques for the Extraction of Phenolic Compounds from Tunisian Olive Leaves: Phenolic Composition and Cytotoxicity against Human Breast Cancer Cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef]
- Santo, D.E.; Dusman, E.; da Silva Gonzalez, R.; Romero, A.L.; dos Santos Gonçalves do Nascimento, G.C.; de Souza Moura, M.A.; Bressiani, P.A.; Filipi, Á.C.K.; Gomes, E.M.V.; Pokrywiecki, J.C.; et al. Prospecting Toxicity of Octocrylene in Allium cepa L. and Eisenia Fetida Sav. Environ. Sci. Pollut. Res. 2023, 30, 8257–8268. [Google Scholar] [CrossRef]
- Frâncica, L.S.; Gonçalves, E.V.; Santos, A.A.; Vicente, Y.S.; Silva, T.S.; Gonzalez, R.S.; Almeida, P.M.; Feitoza, L.L.; Bueno, P.A.A.; Souza, D.C.; et al. Antiproliferative, Genotoxic and Mutagenic Potential of Synthetic Chocolate Food Flavoring. Brazilian J. Biol. 2022, 82, e243628. [Google Scholar] [CrossRef]
- Federico, C.; Palmieri, C.; Pappalardo, A.M.; Ferrito, V.; Pappalardo, M.; Librando, V.; Saccone, S. Mutagenic Properties of Linuron and Chlorbromuron Evaluated by Means of Cytogenetic Biomarkers in Mammalian Cell Lines. Environ. Sci. Pollut. Res. 2016, 23, 17018–17025. [Google Scholar] [CrossRef] [PubMed]
- Basal, W.T.; Ahmed, A.R.T.; Mahmoud, A.A.; Omar, A.R. Lufenuron Induces Reproductive Toxicity and Genotoxic Effects in Pregnant Albino Rats and Their Fetuses. Sci. Rep. 2020, 10, 19544. [Google Scholar] [CrossRef] [PubMed]
- Topal, A.; Gergit, A.; Özkaraca, M. Assessment of Oxidative DNA Damage, Oxidative Stress Responses and Histopathological Alterations in Gill and Liver Tissues of Oncorhynchus mykiss Treated with Linuron. Hum. Exp. Toxicol. 2021, 40, 1112–1121. [Google Scholar] [CrossRef]
- Ergin, T.; Inceer, H.; Ergin, B. Investigation of Antimutagenic Effect of Rosa canina L. Against Linuron Induced DNA Damage on Root Meristematic Cells of Allium cepa L. Cytologia 2020, 85, 233–237. [Google Scholar] [CrossRef]
- Akcha, F.; Spagnol, C.; Rouxel, J. Genotoxicity of Diuron and Glyphosate in Oyster Spermatozoa and Embryos. Aquat. Toxicol. 2012, 106–107, 104–113. [Google Scholar] [CrossRef]
- Behrens, D.; Rouxel, J.; Burgeot, T.; Akcha, F. Comparative Embryotoxicity and Genotoxicity of the Herbicide Diuron and Its Metabolites in Early Life Stages of Crassostrea Gigas: Implication of Reactive Oxygen Species Production. Aquat. Toxicol. 2016, 175, 249–259. [Google Scholar] [CrossRef]
- Sharma, S.; Vig, A. Genotoxicity of Atrazine, Avenoxan, Diuron and Quizalofop-P-Ethyl Herbicides Using the Allium cepa Root Chromosomal Aberration Assay. Terr. Aquat. Environ. Toxicol. 2012, 6, 90–95. [Google Scholar]
- Peraza-Vega, R.I.; Castañeda-Sortibrán, A.N.; Valverde, M.; Rojas, E.; Rodríguez-Arnaiz, R. Assessing Genotoxicity of Diuron on Drosophila Melanogaster by the Wing-Spot Test and the Wing Imaginal Disk Comet Assay. Toxicol. Ind. Health 2017, 33, 443–453. [Google Scholar] [CrossRef]





| Herbicide/Adsorbents | pH | Mass Carbon | C0 (mg·mL−1) | Removal (%) | Ref. |
|---|---|---|---|---|---|
| Diuron/bottom ash waste (BAW-200) | 2.0 | 10 mg | 20 | 80 | [24] |
| Diuron/carbon from Hovenia dulcis | 6.0 | 1 g·L−1 | 200 | 95 | [25] |
| Diuron/diochar Hovenia dulcis | 7.0 | 1 g·L−1 | 50 | 60 | [26] |
| Diuron/carbon activated cassava biomass (Manihot esculenta) | 7.0 | 0.5 g·L−1 | 50–200 | 68 | [27] |
| Diuron/carbon activated | 7.0 | 1 g·L−1 | 13–38 | 93 | [28] |
| Linuron/carbon activated NORIT A2 | 7.0 | 0.08 g·L−1 | 5 | 93 | [29] |
| Linuron/zeolite combined with activated carbon | 6.3 | 0.1 g·L−1 | 2 ppm | 77 | [30] |
| Linuron/modified sludge-based biochar | 7.0 | 0.075 g·L−1 | 10 | 90 | [31] |
| Linuron/hydrothermal treatment with FeOPal2 | 7.6 | 0.2 g·L−1 | 5 | 83 | [32] |
| Linuron/chitosan and chitin | 5.5 | 25 mg | 10 | 12.5 | [33] |
| TR | ARL/SD (%) | MI/SD (%) |
|---|---|---|
| Co | 100 ± 1.0 | 100 ± 0.9 |
| Diuron | ||
| DS before adsorption | 50.0 ± 1.3 * | 52.0 ± 1.4 * |
| DS after adsorption with AC-C | 90.4 ± 1.0 | 92.5 ± 1.5 |
| DS after adsorption with ACs | 97.9 ± 1.0 | 95.9 ± 1.1 * |
| Linuron | ||
| LS before adsorption | 52.8 ± 1.5 * | 50.8 ± 1.0 * |
| LS after adsorption with AC-C | 90.9 ± 1.5 | 90.5 ± 1.5 |
| LS after adsorption with ACs | 94.7 ± 0.9 | 97.9 ± 1.0 |
| Number and Types of Cellular Changes | ||||
|---|---|---|---|---|
| Micronucleus | Chromosomal Bridges | Chromosomal Disruptions | CAI ± SD (%) | |
| Co | 3 | 0 | 0 | 0.15 |
| DS before adsorption | 78 * | 44 * | 87 * | 10.45 * |
| DS after adsorption with AC-C | 1 | 0 | 2 | 0.15 |
| DS after adsorption with AC-ZnCl2 | 1 | 1 | 1 | 0.15 |
| DS after adsorption with AC-HNO3 | 2 | 1 | 1 | 0.25 |
| LS before adsorption | 92 * | 55 * | 76 * | 11.15 * |
| LS after adsorption with AC-C | 1 | 1 | 4 | 0.30 |
| LS after adsorption with AC-ZnCl2 | 1 | 1 | 0 | 0.10 |
| LS after adsorption with AC-HNO3 | 1 | 1 | 1 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, L.E.Z.d.; Marcoti, F.A.O.; Lucio, M.A.N.; Rocha, B.C.d.S.; Rocha, L.B.; Romero, A.L.; Bona, E.; Peron, A.P.; Junior, O.V. Analysis and Simulation of Adsorption Efficiency of Herbicides Diuron and Linuron on Activated Carbon from Spent Coffee Beans. Processes 2024, 12, 1952. https://doi.org/10.3390/pr12091952
Moraes LEZd, Marcoti FAO, Lucio MAN, Rocha BCdS, Rocha LB, Romero AL, Bona E, Peron AP, Junior OV. Analysis and Simulation of Adsorption Efficiency of Herbicides Diuron and Linuron on Activated Carbon from Spent Coffee Beans. Processes. 2024; 12(9):1952. https://doi.org/10.3390/pr12091952
Chicago/Turabian StyleMoraes, Luiz Eduardo Zani de, Felipe Augusto Olivo Marcoti, Marco Antônio Naves Lucio, Bianca Caroline da Silva Rocha, Lucas Bonfim Rocha, Adriano Lopes Romero, Evandro Bona, Ana Paula Peron, and Osvaldo Valarini Junior. 2024. "Analysis and Simulation of Adsorption Efficiency of Herbicides Diuron and Linuron on Activated Carbon from Spent Coffee Beans" Processes 12, no. 9: 1952. https://doi.org/10.3390/pr12091952
APA StyleMoraes, L. E. Z. d., Marcoti, F. A. O., Lucio, M. A. N., Rocha, B. C. d. S., Rocha, L. B., Romero, A. L., Bona, E., Peron, A. P., & Junior, O. V. (2024). Analysis and Simulation of Adsorption Efficiency of Herbicides Diuron and Linuron on Activated Carbon from Spent Coffee Beans. Processes, 12(9), 1952. https://doi.org/10.3390/pr12091952







