Abstract
Mandarin peel (MP) has gained attention as a feedstock for flavonoid recovery via the extraction process based on the biorefinery concept, but residues remain after the extraction. Toward an integrated biorefinery concept, this study aimed to valorize extracted MP (eMP) by using it in bioethanol production. For efficient fermentable sugar production, the effect of enzymatic hydrolysis conditions on sugar conversion from eMP was investigated, and the results showed that combining cellulase and cellobiase resulted in a higher enzymatic glucose conversion (78.2%) than the use of the individual enzymes (37.5% and 45.6%). Pectinase played an essential role in enhancing enzymatic arabinose conversion, and the optimal conditions were determined to be pH 4 and 90 units of the three enzymes. Under optimal conditions, the sugar yield was 199 g glucose and 47 g arabinose/kg eMP, and the hydrolysate was used in bioethanol fermentation. The results showed that the bioethanol production was 3.78 g/L (73.9% yield), similar to the control medium (3.79 g/L; 74.2% yield), although the cell growth of the yeast was slightly delayed in the eMP hydrolysate medium. This study highlights the potential of eMP as a low-cost feedstock for sugar and bioethanol production.
1. Introduction
Mandarin is cultivated at about 160 million tons worldwide and is used as a raw material in the production of beverages, marmalades, essential oils, flavors, etc. [1]. By-products are generated during mandarin processing, of which mandarin peel (MP) accounts for 60–65% and is estimated to be approximately 48–62 million tons [2,3]. MP has high potential as a feedstock for biorefinery processes due to its valuable components, such as flavonoids, cellulose, and hemicellulose. In particular, flavonoids such as hesperidin, narirutin, and didymin contained in MP exhibit bioactivity, and thus, previous research has focused on the extraction of flavonoids. However, after the extraction process, a solid residue from MP remains [4,5]. For valorizing extracted MP (eMP), the biorefinery of eMP can be designed based on its residual fractions. It was reported that the eMP contains about 15–25% cellulose and about 20–30% hemicellulose fractions [6,7]; thus, sugar-platform-based biorefining is expected to be a promising route for eMP valorization. The eMP contains glucan, arabinan, galactan, and mannan, and these polysaccharides can be targeted in the saccharification process for the production of renewable energies and platform chemicals [8,9,10].
To produce fermentable sugars (i.e., monosaccharides) from the polysaccharides in biomass, a saccharification process is commonly performed, and the resulting sugars can then be utilized as a fermentation medium [11]. Among saccharification methods, the acid hydrolysis process generally produces fermentation inhibitors, including 5-(hydroxymethyl) furfural, acetic acid, and furfural [12]. For these reasons, the saccharification process of mandarin waste, citrus peel waste, and orange peel has been conducted by enzymatic hydrolysis, and the produced hydrolysates have been successfully utilized as fermentation media for the production of ethanol [13], methane [14], and lactic acid [15]. Until now, efficient conditions for the enzymatic hydrolysis of eMP have not been established; therefore, major variables, such as the enzyme combination, pH, and enzyme loading, should be optimized for the sugar-platform-based biorefinery of eMP. The improved sugar production from eMP is expected to contribute to the improvement of the overall production yield after the fermentation process.
Bioethanol is a renewable fuel that can replace fossil fuels, responsible for 35% of global greenhouse gas emissions [16]. The bioethanol market is expected to expand from USD 83.4 billion in 2023 to USD 114.7 billion in 2028 [17]. However, there are several challenges in fermentative bioethanol production, including high process costs and food security. Food resources such as corn, wheat, and barley have been used as feedstocks for bioethanol fermentation, and the feedstock costs accounted for about 45–75% of the overall process cost [18]. In addition, it is emphasized that non-edible feedstocks (e.g., food waste) should be used in biorefinery processes instead of food resources due to concerns raised about food security [19].
The aim of this study is to provide a concept for an integrated biorefinery of MP. In our previous study [19], high-value flavonoids such as hesperidin, narirutin, and didymin were recovered from MP with extraction yields of 39.57, 18.20, and 2.24 mg/g-biomass, respectively. However, we found that about 52% remained as solid waste containing polysaccharides after the extraction process. This motivated us to design a sugar-platform-based biorefinery of eMP for bioethanol production. First, the carbohydrate content was analyzed to select the target fermentable sugars of eMP. Then, the effects of the enzyme combination, pH, and enzyme loading on sugar conversion from eMP were investigated to determine the optimal enzymatic hydrolysis conditions. Finally, we evaluated the utilization potential of the eMP hydrolysate through fermentation by Saccharomyces cerevisiae K35.
2. Materials and Methods
2.1. Materials
Calcium carbonate (CaCO3), sodium hydroxide (NaOH), sulfuric acid (H2SO4), yeast extract, peptone, magnesium sulfate (MgSO4), and dipotassium phosphate (K2HPO4) were obtained from Duksan Chemical (Ansan-si, Gyeonggi-do, Republic of Korea). Glucose and arabinose were purchased from Sigma-Aldrich (St. Louis, MO, USA). Celluclast® 1.5 L (cellulase), Cellic® CTec2 (cellobiase), and Pectinex® USP were purchased from Novozymes (Krogshoejvej, Bagsværd, Denmark).
2.2. Biomass
MP was purchased from Theyundoo (Uijeongbu-si, Gyeonggi-do, Republic of Korea). MP was extracted under the optimal ultrasound-assisted extraction conditions (78.22% ethanol, 68 °C, solid loading 64.31 g/L, and amplitude 28.16%) for flavonoid recovery according to our previous study [19]. After removing the supernatant of the extract, the solid residue (i.e., eMP) was dried at 105 °C for 24 h and used as a feedstock for fermentable sugar recovery.
2.3. Analysis of Carbohydrate Composition
The chemical composition of the eMP was analyzed according to Lee et al. [20]. First, 0.3 g of the eMP was immersed in 3 mL of 72% (w/w) H2SO4 and reacted at 30 °C for 2 h. Distilled water was added to dilute the acid concentration to 4%, and the mixture was reacted at 121 °C for 1 h. Subsequently, the mixture was neutralized using CaCO3 and analyzed by high-performance liquid chromatography (HPLC). All experiments were performed in triplicate and averaged.
2.4. Enzymatic Hydrolysis
To determine the optimal combinations for the efficient hydrolysis of eMP, the effect of different combinations of three enzymes (cellulase, cellobiase, and pectinase) was investigated. The enzymatic hydrolysis of eMP (0.6 g) was performed at 50 °C for 24 h in 20 mL of sodium acetate buffer (pH 4.8) [21,22]. The enzymes used for hydrolysis were 30 U/g-biomass of cellulase, cellobiase, and pectinase. One enzyme unit (1 U) is the activity that catalyzes a substrate reaction to produce 1 μmol of each sugar per min.
In order to select the optimal pH for efficient enzymatic hydrolysis, the effect of pH (pH 3 to 8) on sugar conversion from eMP was investigated using citrate buffer (pH 3), sodium acetate buffer (pH 4, 5), phosphate buffer (pH 6, 7), and Tris-HCl buffer (pH 8). The enzymatic hydrolysis of eMP (0.6 g) was conducted in a reaction volume of 20 mL at 50 °C for 24 h using 30 FPU cellulase, 60 CBU cellobiase, and 30 U pectinase/g-biomass.
The effect of enzyme loading on sugar conversion from eMP was investigated. Enzymatic hydrolysis was performed for 3 h at 50 °C using 5, 15, 30, 60, 90, and 120 U/g-biomass of cellulase, cellobiase, and pectinase in a 20 mL reaction volume. The monosaccharide concentration in the eMP hydrolysate was quantified by HPLC analysis. Enzymatic sugar conversion from the polysaccharide fractions of eMP was calculated using Equations (1) and (2). All experiments were performed in triplicate and averaged.
Glucose conversion (%) = (weight of glucose released (g) × 0.9/weight of glucan (g)) × 100
Arabinose conversion (%) = (weight of arabinose released (g) × 0.88/weight of arabinan (g)) × 100
2.5. Bioethanol Fermentation
Saccharomyces cerevisiae K35 was precultured in 50 mL of YPD medium (10 g/L yeast extract, 10 g/L peptone, 10 g/L glucose, 1 g/L MgSO4, and 1 g/L K2HPO4; pH 6.0) at 30 °C and 180 rpm for 24 h. In the bioethanol production medium, a mixture of glucose and arabinose (control group) and eMP hydrolysate (with 10 g/L of glucose and 2.3 g/L of arabinose; experimental group) were used as carbon sources. A 0.2 mL volume of precultured cell suspension (OD600 nm = 1.2) was inoculated into the main medium and incubated at 30 °C for 24 h at 180 rpm. The bioethanol yield was calculated based on Equation (3). All experiments were performed in triplicate and averaged.
Bioethanol yield (%) = (weight of bioethanol released (g) × 0.9/weight of glucose consumed (g)) × 100
2.6. HPLC Analysis
Sugars and bioethanol were quantitatively analyzed using an HPLC system equipped with a refractive index detector. The Shodex SUGAR SH1011 H+ ion exclusion column (300 mm × 8 mm, Shodex, Tokyo, Japan) was used. The analysis was conducted at a temperature of 50 °C, using a mobile phase of 0.005 N H2SO4, a flow rate of 0.8 mL/min, and an injected volume of 20 μL.
3. Results and Discussion
3.1. Carbohydrate Composition of Extracted Mandarin Peel
In previous studies, MP has been utilized as a feedstock for fermentable sugar production [7,23]. However, few studies have been performed to utilize the extracted biomass as a feedstock for fermentable sugar production. Therefore, the carbohydrate composition of eMP was analyzed to investigate which fermentable sugars could be recovered. The carbohydrate compositions of MP and eMP are presented in Table 1, and it was confirmed that both are mainly composed of glucan and arabinan, which can be hydrolyzed into glucose and arabinose, respectively. The MP is composed of 31.00% glucan, 5.80% arabinan, and others, while the eMP is composed of 25.39% glucan, 12.03% arabinan, and others (Table 1). The other fractions of MP and eMP are presumed to contain pectin, lignin, ash, etc. In general, the lignin content of MP is within about 10%, and the pectin content is about 15–25% [24,25]. The main components of pectin (i.e., galacturonic acid) are non-fermentable sugars [26,27]. The glucan content decreased by 5.61% after extraction, while the arabinan content increased by 2.07-fold. During the extraction process, cellulose can be partially destroyed and dissolved [7]. The eMP contained similar amounts of polysaccharides, including glucan and arabinan, implying the high potential of eMP as a feedstock for sugar production.

Table 1.
The carbohydrate compositions of raw and extracted mandarin peel.
3.2. Determination of Efficient Enzymatic Hydrolysis Conditions
3.2.1. Effect of Enzyme Combination on Enzymatic Hydrolysis of eMP
Cellulase breaks the cellulose structure to induce hydrolysis, and cellobiase severs the β-D-glucosidic bonds of disaccharides to assist in glucose recovery [28,29]. Pectinase breaks down the pectin fractions, assisting the release of arabinose from biomass [30]. In order to efficiently convert the polysaccharides in the eMP to monosaccharides, an appropriate enzyme should be selected to improve the sugar conversion from eMP. Herein, three enzymes (cellulase, cellobiase, and pectinase) were tested for hydrolyzing eMP to recover fermentable sugars such as glucose and arabinose. As a result, the sole use of cellulase, cellobiase, and pectinase showed glucose conversion of 37.47, 45.58, and 20.82% and arabinose conversion of 3.18, 7.75, and 27.93%, respectively (Figure 1). Cellulase and cellobiase efficiently hydrolyze glucan to glucose, and pectinase is essential in the conversion of arabinan into arabinose. The synergistic effects of the combination of three enzymes were investigated (Figure 1). In the case of enzymatic hydrolysis using two enzymes (cellulase + cellobiase, cellulase + pectinase, and cellobiase + pectinase), the glucose conversion was 78.21, 40.22, and 57.92%, respectively, and the arabinose conversion was 8.53, 27.00, and 27.70%, respectively. For glucose conversion, combining two enzymes was more efficient than using a single enzyme. This result is similar to Pocan et al. [31], who reported that the synergistic effect of pectinase and cellulase is required for the efficient enzymatic hydrolysis of biomass containing pectin. In particular, the conversion was significantly improved by using a combination of cellulase and cellobiase. This is presumably due to the different functions that cellulase and cellobiase perform in the hydrolysis of cellulose, causing a significant synergistic effect on glucose conversion [32]. In contrast, arabinose conversion was not affected by combining other enzymes (cellulase and cellobiase), showing that arabinose conversion was only dependent on pectinase. Citrus peel with pectin has been reported to contain 3.84–11.52% arabinose [27], and the hydrolysis of pectin contributes to the release of arabinose. Nahar et al. [33] and Wilkins et al. [34] reported that pectinase had a greater positive effect on arabinose conversion than cellulose because pectinase contains a variety of activities that can degrade polysaccharides such as pectin. Based on (1) the synergistic effects of cellulase and cellobiase and (2) the essential role of pectinase in hydrolyzing arabinan, all three enzymes were tested, resulting in the highest enzymatic sugar conversion: enzymatic glucose conversion, 81.09%; enzymatic arabinose conversion, 27.74%. Therefore, a combination of cellulase, cellobiase, and pectinase was finally selected as a catalyst mixture for efficient sugar production from eMP.
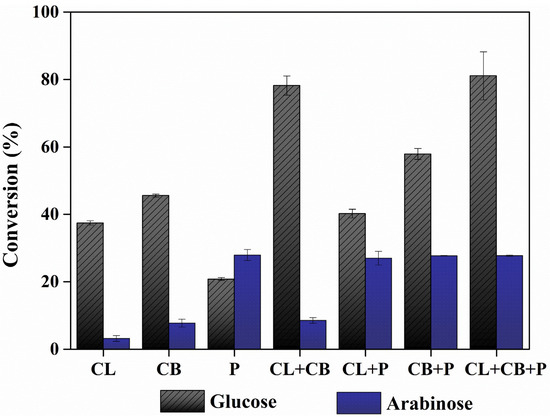
Figure 1.
The effects of enzyme types and their combination on enzymatic glucose and arabinose conversion from extracted mandarin peel (CL, cellulase; CB, cellobiase; P, pectinase). The data are presented as mean values. Error bars indicate standard deviations (n = 3).
3.2.2. Effect of pH on Enzymatic Hydrolysis of eMP
Depending on the type of enzyme, the pH at which it exhibits the highest activity is different [35]. Therefore, the optimal pH should be determined for enzymatic hydrolysis using an enzyme complex [36,37]. Herein, the effect of pH (pH 3–8) on sugar conversion from eMP was investigated. The glucose conversion was determined to be about 37.45, 74.74, 73.07, 73.10, 54.97, and 5.87% at pH 3 to 8, respectively (Figure 2). Glucose conversion increased between pH 3 and 4 and then gradually decreased after pH 6. The highest glucose conversion was observed at pH 4, 5, and 6, with comparable efficiency. The arabinose conversion was determined to be about 12.27, 39.70, 39.36, and 40.37% at pH 3 to 6, respectively. Similar to glucose conversion, arabinose conversion increased significantly in the reaction at pH 3 to pH 4, whereas arabinose was not detected at pH 7 and 8. Using filter paper or pectin as substrates rather than eMP, the three enzymes showed high activity around pH 4, 5, and 6 (Figure A1). Similarly, previous research reported that the activities of cellulase, cellobiase, and pectinase are highest in the range of pH 4 to 5, and the different enzyme activities at each pH are due to changes in the charge of the substrate and the ionic composition of the substrate with pH [38,39]. On the other hand, the initial pH of eMP in water is about pH 3.8; thus, pH 4 was determined to be the optimal pH for efficient sugar production from eMP.
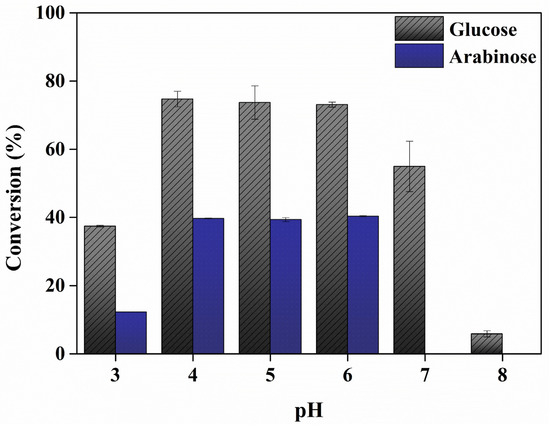
Figure 2.
Effect of pH on enzymatic glucose and arabinose conversion from extracted mandarin peel. The data are presented as mean values. Error bars indicate standard deviations (n = 3).
3.2.3. Effect of Enzyme Loading on Enzymatic Hydrolysis of eMP
Enzyme loading affects sugar conversion and should be optimized for efficient sugar production from biomass [40,41]. Figure 3 illustrates the sugar conversion from eMP with different enzyme loadings at pH 4. As the enzyme loading increased to 5, 15, 30, 60, 90, and 120 U, the glucose conversion improved to 43.46, 53.12, 57.87, 65.33, 78.29, and 81.77%, respectively. The results are consistent with previous studies [42,43,44]. The arabinose conversion was 35.24, 37.84, 37.23, 38.94, 38.14, and 38.06%, respectively, with increasing enzyme loading, and no significant changes were observed. To design an economical saccharification process, the enzyme loading needs to be minimized [45]. The sugar conversion was comparable at 90 U and 120 U; thus, the enzyme loading for efficient sugar recovery via saccharification was determined to be 90 U. The time course of enzymatic sugar conversion from eMP showed no further increase after 3 h. Therefore, the optimal saccharification conditions for eMP were determined to be pH 4, an enzyme loading of 90 U, and a 3 h reaction time.
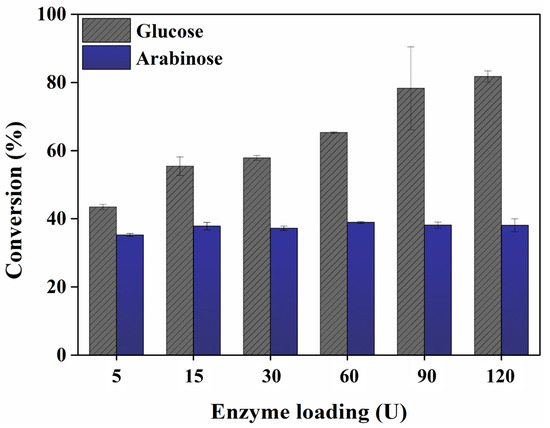
Figure 3.
The effect of enzyme loading on glucose and arabinose conversion from extracted mandarin peel. The data are presented as mean values. Error bars indicate standard deviations (n = 3).
In general, pretreatment processes to remove lignin are conducted to enhance the sugar conversion from lignocellulosic biomass [46]. For instance, chemical pretreatment improved sugar conversion from chestnut by-products [43] and corn by-products [47] by up to 75.7% and 85.0%, respectively. In contrast, MP contains a relatively low content of lignin; thus, a pretreatment process has not been required, as reported by Pocan et al. [31]. Similarly, the enzymatic sugar conversion from eMP was relatively high (glucose conversion: 78.29%) without additional pretreatment processes in the present study. The produced eMP hydrolysate contains an abundance of glucose, which is the preferred substrate for most fermentation strains, and thus, the eMP hydrolysate is expected to be utilized as a carbon source for the production of various fermentation products, such as bioethanol [48], lactic acid [49], and 2,3-butanediol [50].
3.3. Application of Extracted Mandarin Peel Hydrolysate in Bioethanol Production
Herein, eMP hydrolysate was applied to bioethanol production to evaluate its potential as an alternative carbon source. Figure 4 illustrates the fermentation profiles of S. cerevisiae for bioethanol production. The initial glucose concentration in each medium was 10 g/L. In the control group, 10 g/L of glucose was completely consumed after 12 h and produced 3.79 g/L of bioethanol. On the other hand, the experimental group required 24 h for the consumption of all glucose due to lower growth (OD600 nm at 12 h = 0.41) compared to the control group (OD600 nm at 12 h = 1.26). It has been reported that several compounds in Citrus plants, including limonene, can inhibit bacterial growth. Limonene inhibits yeast growth due to alterations in H+ and K+ transport and the disruption of cell walls [25,51]. In particular, low concentrations of limonene (0.05–0.15%, v/w) and those higher than 0.16% (v/w) completely inhibit bioethanol fermentation [52,53]. Siddiqui et al. [54] reported that organic solvents can efficiently extract limonene from mandarin orange. The eMP was prepared after extraction using an organic solvent (ethanol); thus, the partial removal of limonene is assumed. However, the eMP hydrolysate still causes a slight inhibition of growth, requiring additional removal strategies. It is important to note that the final bioethanol production in the control and experimental groups was 3.79 g/L (bioethanol yield 74.2%) and 3.78 g/L (bioethanol yield 73.9%), respectively. Overall, the eMP hydrolysate has been demonstrated to have high potential as a low-cost carbon source for sustainable bioethanol production.
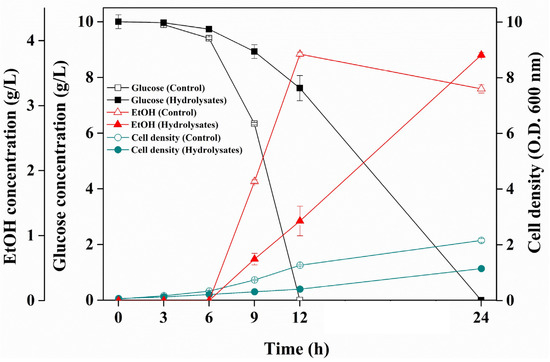
Figure 4.
Bioethanol fermentation by Saccharomyces cerevisiae using extracted mandarin peel hydrolysates. The data are presented as mean values. Error bars indicate standard deviations (n = 3).
3.4. Evaluation of the Overall Process for Sugar and Bioethanol Production from Extracted Mandarin Peel
Figure 5 presents the mass balance of the overall process based on 1000 g of eMP, which contains 254 g of glucan and 120 g of arabinan. Under the optimal saccharification conditions (pH 4, 90 U of enzyme loading, 3 h), glucose conversion and arabinose conversion were 78.3% and 38.1%, respectively, which resulted in 199 g of glucose and 47 g of arabinose recovered. The obtained eMP hydrolysate was applied to a fermentation medium for S. cerevisiae, and it was estimated that about 38.9 g of bioethanol could be produced (bioethanol yield: 73.9%). The control medium produced 39.0 g of bioethanol with a yield of 74.2%, and it was 99.7% consistent with the yield of the eMP hydrolysate medium. Therefore, eMP has high potential as a renewable feedstock for bioethanol production.
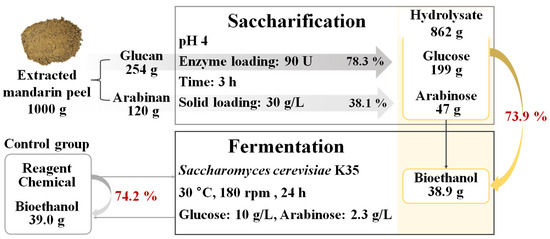
Figure 5.
Mass balance of extracted mandarin peel to produce sugars and bioethanol through saccharification and fermentation processes.
Previous studies related to bioethanol production from fruit peels reported that 60 and 158 g of bioethanol were produced from 1 kg of lemon peel [55] and durian peel [56], respectively. The overall yield of bioethanol production from eMP was estimated to be lower (about 39 g/kg eMP), but we designed integrated bioconversion processes; thus, various biochemicals and bioethanol were produced from MP. From 1 kg of raw MP, 39.57 of hesperidin, 18.20 g of narirutin, and 2.24 g of didymin were recovered [19], and about 52% of solids remained. The eMP was successfully converted to bioethanol with a yield of 39 g/kg eMP. To improve the potential of the industrial utilization of eMP, it is important to design a saccharification process to produce sugars more efficiently for economic feasibility. Therefore, future research is required on the optimization of the saccharification process by investigating the effects of variables such as the enzyme ratio, solid loading, and temperature, which will be carried out in our follow-up studies. This study highlights the possibility of the sustainable production of valuable chemicals and fuel from MP for valorizing food processing waste.
4. Conclusions
The eMP remaining after the extraction process (a typical MP biorefinery process) was valorized by producing sugars and bioethanol. Glucose-rich hydrolysates from eMP were prepared by optimizing the enzyme combination, pH value, and enzyme loading. Under the optimized conditions, the sugar yield from eMP was estimated to be approximately 199 g of glucose and 47 g of arabinose, and these fermentable sugars can be applied as sustainable substrates for microbial conversion into high-value platform chemicals and fuel, including ethanol. The usability of the eMP hydrolysate as an alternative carbon source for bioethanol fermentation was investigated by profiling the fermentation results. Overall, S. cerevisiae consumed glucose more rapidly in the control medium than in the eMP hydrolysate medium, resulting in maximum bioethanol production at 12 h (titer = 3.79 g/L; conversion = 74.2%). In the eMP hydrolysate medium, the bioethanol titer and conversion were 3.78 g/L and 73.9% at 24 h, respectively. In conclusion, eMP hydrolysate can be considered a valuable feedstock for bioethanol production. In the near future, studies related to the identification and detoxification of fermentation inhibitors of the hydrolysate should be conducted to allow for more efficient glucose consumption, cell growth, and bioethanol production.
Author Contributions
Conceptualization, H.S. and H.Y.Y.; methodology, H.S. and J.L.; validation, H.Y.Y.; formal analysis, H.S.; investigation, H.S. and J.L.; writing—original draft preparation, H.S.; writing—review and editing, H.Y.Y.; visualization, H.S. and J.L.; supervision, H.Y.Y.; project administration, H.Y.Y.; funding acquisition, H.Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a 2022 Research Grant from Sangmyung University.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
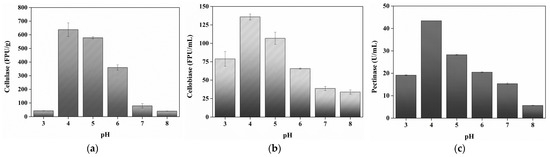
Figure A1.
Effect of pH on cellulase (a), cellobiase (b), and pectinase (c) activity.
References
- Gonzatto, M.P.; Santos, J.S. Introductory Chapter: World Citrus Production and Research. In Citrus Research-Horticultural and Human Health Aspects; IntechOpen: Rijeka, Croatia, 2023; Available online: https://www.intechopen.com/chapters/86388 (accessed on 6 May 2024).
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Benohoud, M.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. Green extraction of polyphenols from citrus peel by-products and their antifungal activity against Aspergillus flavus. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef] [PubMed]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green extraction techniques for obtaining bioactive compounds from mandarin peel (Citrus unshiu var. Kuno): Phytochemical analysis and process optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef]
- Jang, S.K.; Jung, C.D.; Seong, H.; Myung, S.; Kim, H. An integrated biorefinery process for mandarin peel waste elimination. J. Clean. Prod. 2022, 371, 133594. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, Y.G.; Chang, J.; Bae, H.J. A high-yield process for production of biosugars and hesperidin from mandarin peel wastes. Molecules 2022, 25, 4286. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.; Binns, M.; Kim, J.K. Process Design and Economic Assessment of Biomass-Based Hydrogen Production Processes. Korean J. Chem. Eng. 2024, 41, 2239–2249. [Google Scholar] [CrossRef]
- Çopur, M.; Pekdemir, T.; Kocakerim, M.M.; Korucu, H.; Guliyev, R. Industrial symbiosis: Boron waste valorization through CO2 utilization. Korean J. Chem. Eng. 2022, 39, 2600–2614. [Google Scholar] [CrossRef]
- Hiasa, S.; Iwamoto, S.; Endo, T.; Edashige, Y. Isolation of cellulose nanofibrils from mandarin (Citrus unshiu) peel waste. Ind. Crops Prod. 2014, 62, 280–285. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Kim, S.W. The Next-Generation Biomass for Biorefining. BioResources 2021, 16, 2188–2219. [Google Scholar] [CrossRef]
- Wang, T.; Lu, X. Overcome saccharification barrier: Advances in hydrolysis technology. In Advances in 2nd Generation of Bioethanol Production; Woodhead: Cambridge, UK, 2021; pp. 137–159. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Nanjundaswamy, A.; Bansal, S.; Singh, S.; Kaur, S.; Babbar, N. Enhanced ethanol production from Kinnow mandarin (Citrus reticulata) waste via a statistically optimized simultaneous saccharification and fermentation process. Bioresour. Technol. 2011, 102, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Patsalou, M.; Samanides, C.G.; Protopapa, E.; Stavrinou, S.; Vyrides, I.; Koutinas, M. A citrus peel waste biorefinery for ethanol and methane production. Molecules 2019, 24, 2451. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, D.; Tortajada, M.; Ramón, D.; Rojas, A. Production of D-lactic acid by the fermentation of orange peel waste hydrolysate by lactic acid bacteria. Fermentation 2019, 6, 1. [Google Scholar] [CrossRef]
- An, H.-E.; Lee, K.H.; Jang, Y.W.; Kim, C.-B.; Yoo, H.Y. Improved Glucose Recovery from Sicyos angulatus by NaOH Pretreatment and Application to Bioethanol Production. Processes 2021, 9, 245. [Google Scholar] [CrossRef]
- Markets and Market. Bioethanol Market by Feedstock (Starch Based, Sugar Based, Cellulose-Based), Fuel Blend (E5, E10, E15 to E70, E75 & E85), End-Use (Transportation, Pharmaceutical, Cosmetic, Alcoholic Beverages), Generation and Region Global Forecast to 2028; Markets and Market: London, UK, 2023. [Google Scholar]
- Eisentraut, A. Sustainable Production of Second-Generation Biofuels: Potential and Perspectives in Major Economies and Developing Countries; IEA Energy Papers; OECD Publishing: Paris, France, 2010. [Google Scholar] [CrossRef]
- Son, H. Development of Bioconversion Process for Flavonoid and Sugar Recovery from Mandarin Peel. Master’s Thesis, Sangmyung University, Seoul, Republic of Korea, 2024. [Google Scholar]
- Lee, J.; Kim, S.; Lee, K.H.; Lee, S.K.; Chun, Y.; Kim, S.W.; Park, C.; Yoo, H.Y. Improvement of bioethanol production from waste chestnut shells via evaluation of mass balance-based pretreatment and glucose recovery process. Environ. Technol. Innov. 2022, 28, 102955. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Zhao, J.; Qin, Y. Pretreatment strategies to enhance enzymatic hydrolysis and cellulosic ethanol production for biorefinery of corn stover. Int. J. Mol. Sci. 2022, 23, 13163. [Google Scholar] [CrossRef]
- Kaur Sandhu, S.; Singh Oberoi, H.; Singh Dhaliwal, S.; Babbar, N.; Kaur, U.; Nanda, D.; Kumar, D. Ethanol production from Kinnow mandarin (Citrus reticulata) peels via simultaneous saccharification and fermentation using crude enzyme produced by Aspergillus oryzae and the thermotolerant Pichia kudriavzevii strain. Ann. Microbiol. 2012, 62, 655–666. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A. Sustainable approaches toward the production of bioethanol from biomass. In Sustainable Ethanol and Climate Change: Sustainability Assessment for Ethanol Distilleries; Springer: Cham, Switzerland, 2021; pp. 15–38. [Google Scholar] [CrossRef]
- Jeong, D.; Park, H.; Jang, B.K.; Ju, Y.; Shin, M.H.; Oh, E.J.; Kim, S.R. Recent advances in the biological valorization of citrus peel waste into fuels and chemicals. Bioresour. Technol. 2021, 323, 124603. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; García-Vidal, L.; del Pilar González-Castañeda, F.; López-Gómez, A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 2010, 101, 3506–3513. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sust. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Cho, H.S.; Lee, W.Y. Novel edible films fabricated with HG-type pectin extracted from different types of hybrid citrus peels: Effects of pectin composition on film properties. Int. J. Biol. Macromol. 2023, 253, 127238. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Alhazmi, A.; Kausar, T.; Haque, S.; Singh, R.; Gupta, V.K. Technological advances for improving fungal cellulase production from fruit wastes for bioenergy application: A review. Environ. Pollut. 2021, 287, 117370. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jang, H.; Park, S.; Kim, S.M.; Jeon, T.J. Colorimetric detection of milk spoilage at low temperatures: A novel PDA/ZnO@NC membrane for pH-based freshness monitoring. Biotechnol. Bioprocess Eng. 2024, 29, 177–183. [Google Scholar] [CrossRef]
- Coll-Almela, L.; Saura-Lopez, D.; Laencina-Sanchez, J.; Schols, H.A.; Voragen, A.G.; Ros-Garcia, J.M. Characterisation of cell-wall polysaccharides from mandarin segment membranes. Food Chem. 2015, 175, 36–42. [Google Scholar] [CrossRef]
- Pocan, P.; Bahcegul, E.; Oztop, M.H.; Hamamci, H. Enzymatic hydrolysis of fruit peels and other lignocellulosic biomass as a source of sugar. Waste Biomass Valorization 2018, 9, 929–937. [Google Scholar] [CrossRef]
- Park, S.H.; Ransom, C.; Mei, C.; Sabzikar, R.; Qi, C.; Chundawat, S.; Sticklen, M. The quest for alternatives to microbial cellulase mix production: Corn stover-produced heterologous multi-cellulases readily deconstruct lignocellulosic biomass into fermentable sugars. J. Chem. Technol. Biotechnol. 2011, 86, 633–641. [Google Scholar] [CrossRef]
- Nahar, N.; Pryor, S.W. Enzymatic hydrolysis and fermentation of crushed whole sugar beets. Biomass Bioenergy 2013, 59, 512–519. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K.; Cameron, R.G. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour. Technol. 2007, 98, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Espoui, A.H.; Larimi, S.G.; Darzi, G.N. Optimization of protease production process using bran waste using Bacillus licheniformis. Korean J. Chem. Eng. 2022, 39, 674–683. [Google Scholar] [CrossRef]
- Haile, S.; Ayele, A. Pectinase from microorganisms and its industrial applications. Sci. World J. 2022, 2022, 1881305. [Google Scholar] [CrossRef]
- Amadi, O.C.; Awodiran, I.P.; Moneke, A.N.; Nwagu, T.N.; Egong, J.E.; Chukwu, G.C. Concurrent production of cellulase, xylanase, pectinase and immobilization by combined Cross-linked enzyme aggregate strategy-advancing tri-enzyme biocatalysis. Bioresour. Technol. Rep. 2022, 18, 101019. [Google Scholar] [CrossRef]
- Farinas, C.S.; Loyo, M.M.; Junior, A.B.; Tardioli, P.W.; Neto, V.B.; Couri, S. Finding stable cellulase and xylanase: Evaluation of the synergistic effect of pH and temperature. New Biotechnol. 2010, 27, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.G.; Knox, A.; Di Profio, F. Evaluation of macerating pectinase enzyme activity under various temperature, pH and ethanol regimes. Beverages 2018, 4, 10. [Google Scholar] [CrossRef]
- Gao, M.T.; Yano, S.; Inoue, H.; Sakanishi, K. Combination of wet disk milling and hydrogen peroxide treatments for enhancing saccharification of sugarcane bagasse. Biochem. Eng. J. 2014, 68, 152–158. [Google Scholar] [CrossRef]
- Kuglarz, M.; Alvarado-Morales, M.; Dąbkowska, K.; Angelidaki, I. Integrated production of cellulosic bioethanol and succinic acid from rapeseed straw after dilute-acid pretreatment. Bioresour. Technol. 2018, 265, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Pistone, L.; Ottolina, G.; Xu, P.; Riva, S. Hemp hurds biorefining: A path to green l-(+)-lactic acid production. Bioresour. Technol. 2015, 191, 59–65. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Son, H.; Lee, K.H.; Park, C.; Yoo, H.Y. Physicochemical Characterization of Potassium Hydroxide Pretreated Chestnut Shell and Its Bioconversion to Lactic Acid by Lacticaseibacillus rhamnosus. Processes 2023, 11, 3340. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Lu, J.; Zhu, B.; Pan, Q.; Cheng, Y.; Wang, H. Integrating surfactants with low enzyme loading to increase the glucan conversion and ethanol concentration of reed after combined pretreatment. Ind. Crops Prod. 2023, 204, 117360. [Google Scholar] [CrossRef]
- Salimi, A.; Khodaiyan, F.; Askari, G.; Hosseini, S.S. A zero-waste approach towards a sustainable waste management of apple: Extraction of value-added products and their application as edible coating. Food Hydrocoll. 2024, 147, 109304. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Menana, Z.; Chrusciel, L.; Chalot, M.; Bert, V.; Brosse, N. Steam explosion pretreatment of willow grown on phytomanaged soils for bioethanol production. Ind. Crops Prod. 2019, 140, 111722. [Google Scholar] [CrossRef]
- Zheng, J.; Choo, K.; Bradt, C.; Lehoux, R.; Rehmann, L. Enzymatic hydrolysis of steam exploded corncob residues after pretreatment in a twin-screw extruder. Biotechnol. Rep. 2014, 3, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, S.K.; Lee, J.; Kim, S.; Kim, S.W.; Park, C.; Yoo, H.Y. Energy-efficient glucose recovery from chestnut shell by optimization of NaOH pretreatment at room temperature and application to bioethanol production. Environ. Res. 2022, 208, 112710. [Google Scholar] [CrossRef]
- Lee, J.; Bae, J.; Shin, H.; Kim, M.; Yang, E.; Lee, K.H.; Yoo, H.Y.; Park, C. Improved recovery of mannitol from Saccharina japonica under optimal hot water extraction and application to lactic acid production by Lacticaseibacillus rhamnosus. GCB Bioenergy 2024, 16, e13166. [Google Scholar] [CrossRef]
- Fernández-Delgado, M.; Rodríguez-Sarmiento, M.; Medina, J.D.C.; Lucas, S.; García-Cubero, M.T.; Coca, M.; López-Linares, J.C. Bio-2, 3-butanediol production from banana waste: Preliminary techno-economic evaluation of processing strategies. Biomass Bioenergy 2024, 184, 107218. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, J.H.; Wi, S.G.; Kim, K.H.; Bae, H.J. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy 2013, 102, 204–210. [Google Scholar] [CrossRef]
- Tomita, H.; Okazaki, F.; Tamaru, Y. Direct IBE fermentation from mandarin orange wastes by combination of Clostridium cellulovorans and Clostridium beijerinckii. AMB Express 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- John, I.; Muthukumar, K.; Arunagiri, A. A review on the potential of citrus waste for D-Limonene, pectin, and bioethanol production. Int. J. Green Energy 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Pahmeyer, M.J.; Assadpour, E.; Jafari, S.M. Extraction and purification of d-limonene from orange peel wastes: Recent advances. Ind. Crops Prod. 2022, 177, 114484. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; López-Gómez, A. Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. Ind. Crops Prod. 2013, 41, 188–197. [Google Scholar] [CrossRef]
- Panakkal, E.J.; Cheenkachorn, K.; Gundupalli, M.P.; Kitiborwornkul, N.; Sriariyanun, M. Impact of sulfuric acid pretreatment of durian peel on the production of fermentable sugar and ethanol. J. Indian Chem. Soc. 2021, 98, 100264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).