Lead Ion Adsorption on Glutathione-Modified Carbon
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Reagents and Equipment
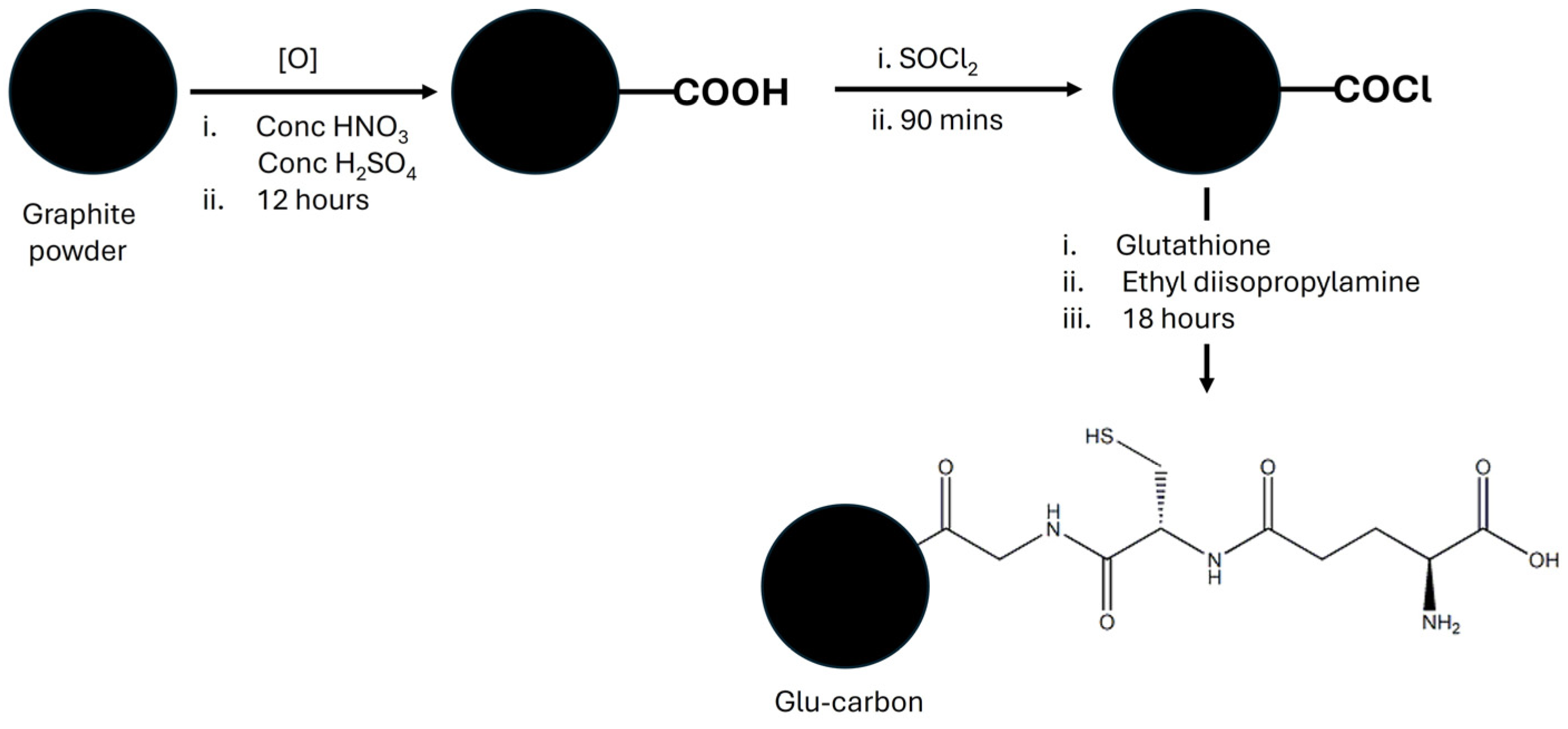
2.2. Preparation of L-Glutathione-Modified Carbon
2.2.1. Oxidation of Graphite Powder
2.2.2. Modification of Oxidized Graphite Powder Using L-Glutathione
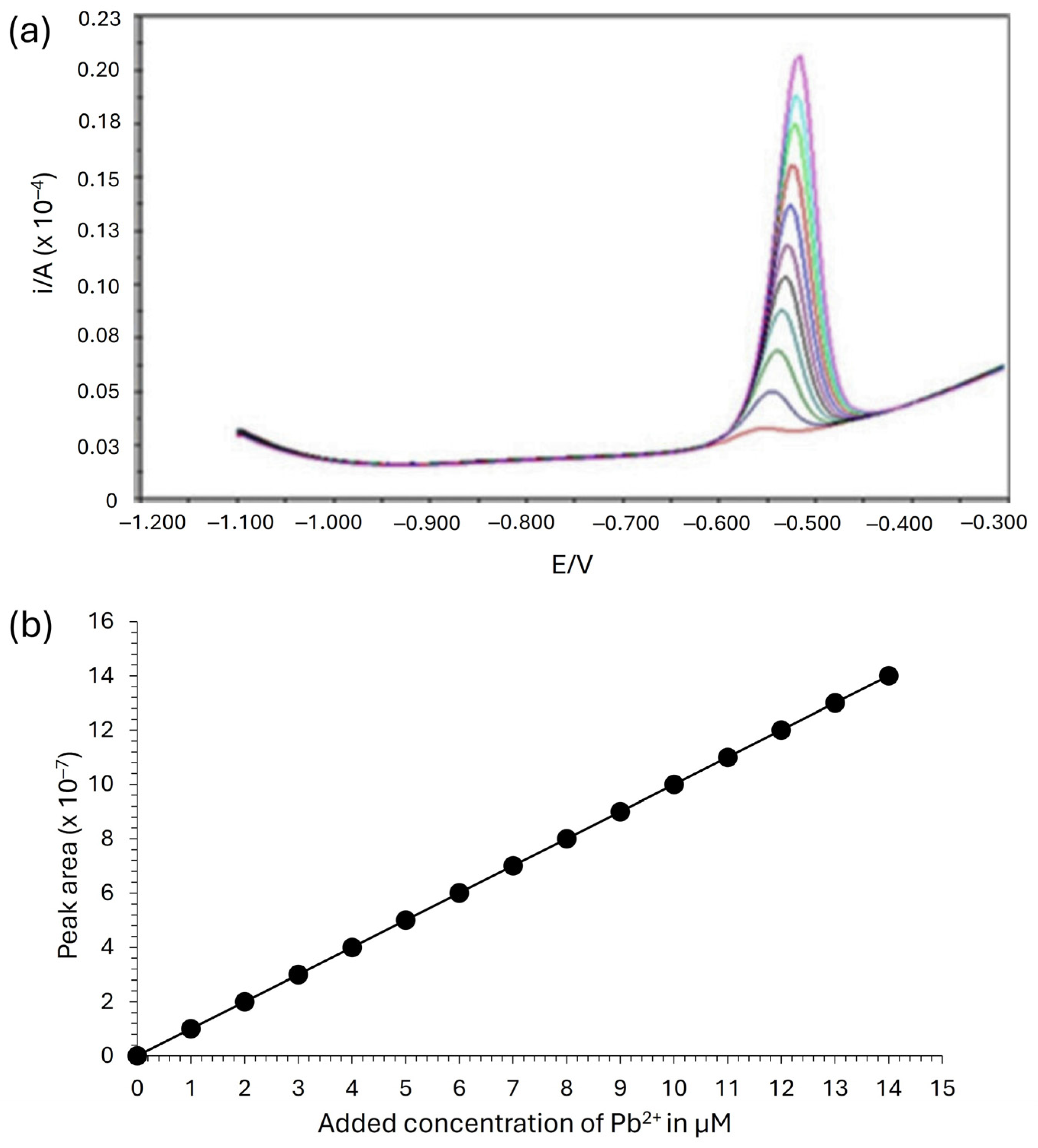
2.3. Determination of Pb2+ Ions Using SWASV
2.4. Optimal Concentration for the Adsorption of Pb2⁺ Ions
2.5. Optimal Dosage of Oxidized Graphite for the Adsorption of Pb2⁺ Ions
2.6. Calculations of the Adsorption Percentage of Pb2⁺ Ions
3. Results and Discussion
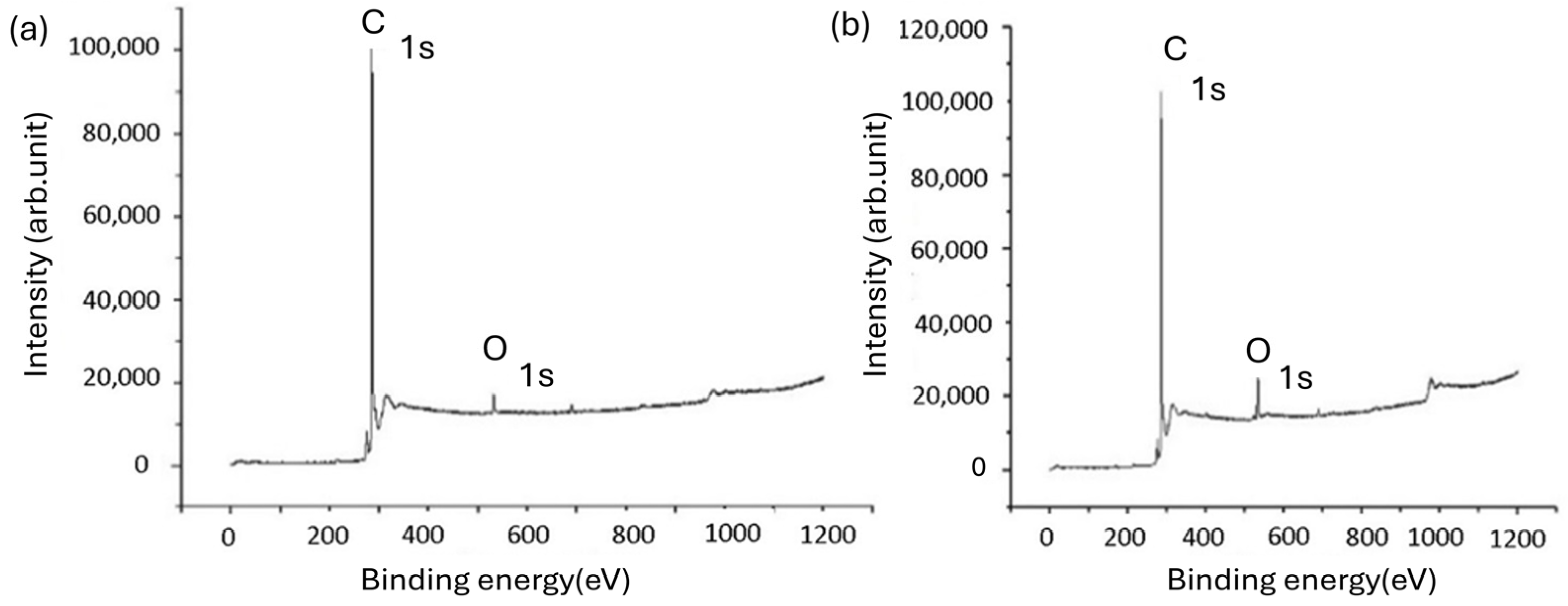
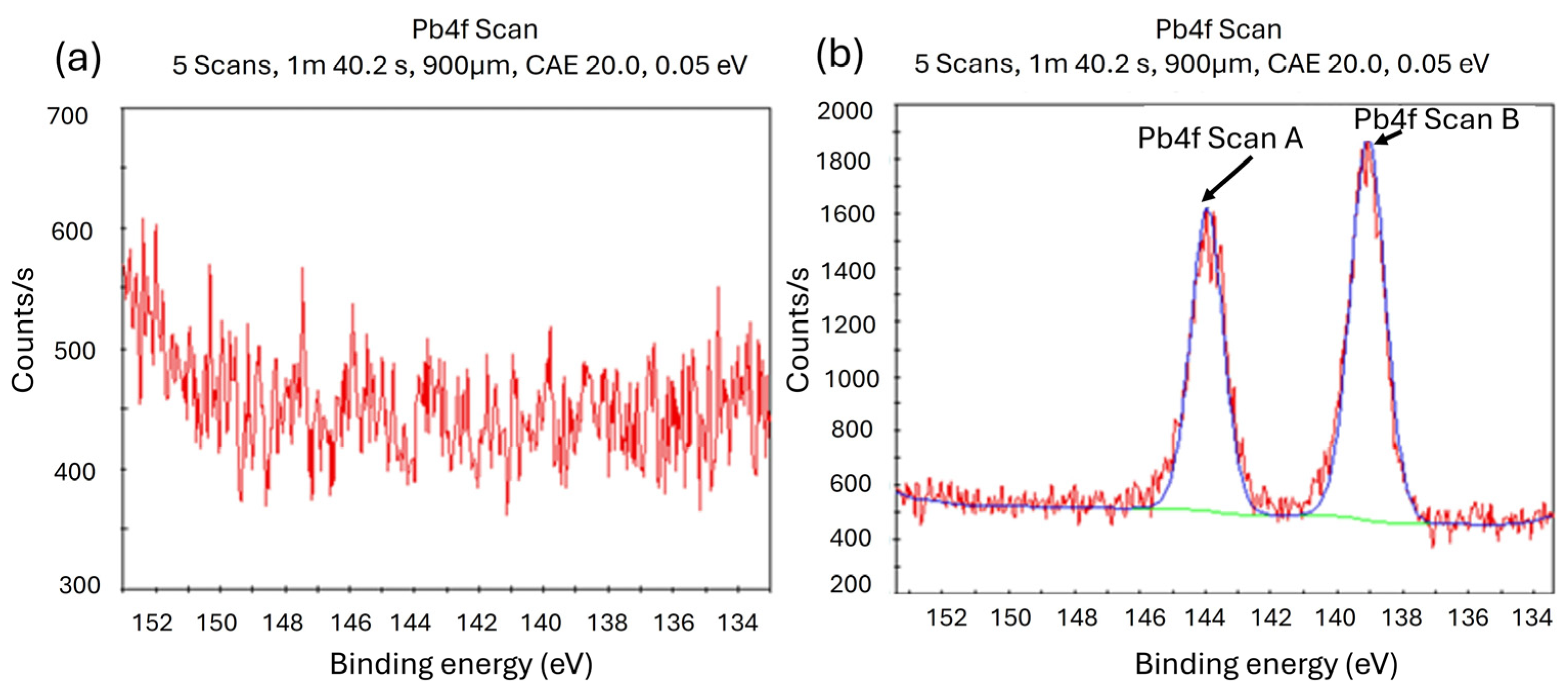
3.1. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.2. Impact of Pb2⁺ Ion Concentration on Adsorption
3.3. Impact of the Amount of Glutathione-Modified Carbon Powder on Adsorption
3.4. Thermodynamic Assessment
3.5. Comparison of Pb2+ Ion Adsorption by Various Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy Metal Pollution in the Environment and Its Impact on Health: Exploring Green Technology for Remediation. Environ. Health Insights 2023, 17, 11786302231201259. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Poater, A. Review on the Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef]
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic heavy metal ions contamination in water and their sustainable reduction by eco-friendly methods: Isotherms, thermodynamics and kinetics study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef]
- Raj, K.; Das, A.P. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Weldeslassie, T.; Naz, H.; Singh, B.; Oves, M. Chemical Contaminants for Soil, Air and Aquatic Ecosystem. In Modern Age Environmental Problems and their Remediation; Oves, M., Zain Khan, M., M.I. Ismail, I., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–22. [Google Scholar]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Dignam, T.; Kaufmann, R.B.; LeStourgeon, L.; Brown, M.J. Control of Lead Sources in the United States, 1970–2017: Public Health Progress and Current Challenges to Eliminating Lead Exposure. J. Public Health Manag. Pract. 2019, 25, S13–S22. [Google Scholar] [CrossRef]
- Olufemi, A.C.; Mji, A.; Mukhola, M.S. Potential Health Risks of Lead Exposure from Early Life through Later Life: Implications for Public Health Education. Int. J. Environ. Res. Public Health 2022, 19, 16006. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R. The Lead Industry and Lead Water Pipes “A MODEST CAMPAIGN”. Am. J. Public Health 2008, 98, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Green, D. Toward eliminating children’s lead exposure: A comparison of policies and their outcomes in three lead producing and using countries. Environ. Res. Lett. 2020, 15, 103008. [Google Scholar] [CrossRef]
- Angrand, R.C.; Collins, G.; Landrigan, P.J.; Thomas, V.M. Relation of blood lead levels and lead in gasoline: An updated systematic review. Environ. Health 2022, 21, 138. [Google Scholar] [CrossRef]
- Delgado, C.F.; Ullery, M.A.; Jordan, M.; Duclos, C.; Rajagopalan, S.; Scott, K. Lead Exposure and Developmental Disabilities in Preschool-Aged Children. J. Public Health Manag. Pract. 2018, 24, e10–e17. [Google Scholar] [CrossRef]
- De Caroli, M.; Furini, A.; DalCorso, G.; Rojas, M.; Di Sansebastiano, G.-P. Endomembrane Reorganization Induced by Heavy Metals. Plants 2020, 9, 482. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Meez, E.; Rahdar, A.; Kyzas, G.Z. Sawdust for the Removal of Heavy Metals from Water: A Review. Molecules 2021, 26, 4318. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Obasi, P.N.; Akudinobi, B.B. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Suliman, M.A.; Sajid, M.; Nazal, M.K.; Islam, M.A. Carbon-based materials as promising sorbents for analytical sample preparation: Recent advances and trends in extraction of toxic metal pollutants from various media. TrAC Trends Anal. Chem. 2023, 167, 117265. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon Nanomaterials for the Treatment of Heavy Metal-Contaminated Water and Environmental Remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef]
- Krishna, R.H.; Chandraprabha, M.N.; Samrat, K.; Krishna Murthy, T.P.; Manjunatha, C.; Kumar, S.G. Carbon nanotubes and graphene-based materials for adsorptive removal of metal ions—A review on surface functionalization and related adsorption mechanism. Appl. Surf. Sci. Adv. 2023, 16, 100431. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Pham, V.V.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2022, 287, 131959. [Google Scholar] [CrossRef]
- Servinis, L.; Beggs, K.M.; Scheffler, C.; Wölfel, E.; Randall, J.D.; Gengenbach, T.R.; Demir, B.; Walsh, T.R.; Doeven, E.H.; Francis, P.S.; et al. Electrochemical surface modification of carbon fibres by grafting of amine, carboxylic and lipophilic amide groups. Carbon 2017, 118, 393–403. [Google Scholar] [CrossRef]
- Hetemi, D.; Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Surface modification by electrochemical reduction of alkyldiazonium salts. Electrochem. Commun. 2016, 68, 5–9. [Google Scholar] [CrossRef]
- Adenier, A.; Bernard, M.-C.; Chehimi, M.M.; Cabet-Deliry, E.; Desbat, B.; Fagebaume, O.; Pinson, J.; Podvorica, F. Covalent Modification of Iron Surfaces by Electrochemical Reduction of Aryldiazonium Salts. J. Am. Chem. Soc. 2001, 123, 4541–4549. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, P.; Kots, P.A.; Cohen, M.; Chen, Y.; Quinn, C.M.; De Mello, M.D.; Anibal Boscoboinik, J.; Shaw, W.J.; Caratzoulas, S.; et al. Tuning the reactivity of carbon surfaces with oxygen-containing functional groups. Nat. Commun. 2023, 14, 2293. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jia, L.; Chen, X. Efficient Adsorption and Extraction of Glutathione S-Transferases with Glutathione-Functionalized Graphene Oxide-Polyhedral Oligomeric Silsesquioxane Composite. Molecules 2023, 28, 340. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Clarke, K.; Li, K. Covalent Functionalization of Graphene with Polysaccharides. Ind. Eng. Chem. Res. 2012, 51, 310–317. [Google Scholar] [CrossRef]
- Arana Juve, J.-M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.B.; Abd Rahim, S.Z.; Sadique, M.; Ming, L.Y.; Mohd Salleh, M.A.A.; Mohd Arif Zainol, M.R.R.; Ghazali, C.M.R. Diverse material based geopolymer towards heavy metals removal: A review. J. Mater. Res. Technol. 2023, 22, 126–156. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Abiman, P.; Wildgoose, G.G.; Crossley, A.; Compton, R.G. Quantitative Studies of Metal Ion Adsorption on a Chemically Modified Carbon Surface: Adsorption of Cd(II) and Hg(II) on Glutathione Modified Carbon. Electroanalysis 2009, 21, 897–903. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Dawodu, F.A.; Adebowale, K.O. Mechanism on the sorption of heavy metals from binary-solution by a low-cost montmorillonite and its desorption potential. Alex. Eng. J. 2015, 54, 757–767. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Impact of Surface Functional Groups and Their Introduction Methods on the Mechanisms of CO2 Adsorption on Porous Carbonaceous Adsorbents. Carbon Capture Sci. Technol. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Cherono, F.; Mburu, N.; Kakoi, B. Adsorption of lead, copper and zinc in a multi-metal aqueous solution by waste rubber tires for the design of single batch adsorber. Heliyon 2021, 7, e08254. [Google Scholar] [CrossRef]
- Neves, C.V.; Módenes, A.N.; Scheufele, F.B.; Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L.; Borba, C.E. Dibenzothiophene adsorption onto carbon-based adsorbent produced from the coconut shell: Effect of the functional groups density and textural properties on kinetics and equilibrium. Fuel 2021, 292, 120354. [Google Scholar] [CrossRef]
- Hashem, M. Adsorption of lead ions from aqueous solution by okra wastes. Int. J. Phys. Sci. 2007, 2, 178–184. [Google Scholar]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019, 179, 105151. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. Z. Für Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Selvanantharajah, N.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Removal of Lead by Oxidized Graphite. C 2021, 7, 23. [Google Scholar] [CrossRef]




| Equilibrium Concentration of Lead(II) Ion in µM [Cbulk]Na Local Hop | Mass of Glutathione-Modified Carbon in mg | Adsorbed Moles of Pb2+ in mol mg−1(×10−8) [Nads] | Log[Cbulk] | Log[Nads] |
|---|---|---|---|---|
| 33.4 | 12.5 | 5.33 | −4.48 | −7.27 |
| 20.5 | 25 | 3.18 | −4.69 | −7.49 |
| 8.1 | 50 | 1.84 | −5.09 | −7.74 |
| 3.7 | 75 | 1.28 | −5.43 | −7.89 |
| 33.4 | 12.5 | 5.33 | −4.48 | −7.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvanantharajah, N.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Lead Ion Adsorption on Glutathione-Modified Carbon. Processes 2024, 12, 1972. https://doi.org/10.3390/pr12091972
Selvanantharajah N, Iyngaran P, Abiman P, Kuganathan N. Lead Ion Adsorption on Glutathione-Modified Carbon. Processes. 2024; 12(9):1972. https://doi.org/10.3390/pr12091972
Chicago/Turabian StyleSelvanantharajah, Namasivayam, Poobalasuntharam Iyngaran, Poobalasingam Abiman, and Navaratnarajah Kuganathan. 2024. "Lead Ion Adsorption on Glutathione-Modified Carbon" Processes 12, no. 9: 1972. https://doi.org/10.3390/pr12091972







