Synergistically Enhanced Enzymatic Hydrolysis of Sugarcane Bagasse Mediated by a Recombinant Endo-Xylanase from Streptomyces ipomoeae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Enzymes
2.2. Pretreatment of SCB
2.3. Selection of Pretreatment Methods
2.4. Effect of pH and Temperature on Synergistic Characteristics
2.5. Enzymatic Hydrolysis
2.6. Xyn10A Pre-Adsorption Experiments and Enzymatic Hydrolysis
2.7. Analytical Methods for SCB Chemical Composition
2.8. Calculation Method of the Hydrolysis Efficiency and the Degree of Synergy
2.9. Field Emission Scanning Electron Microscope (FE-SEM) Analysis
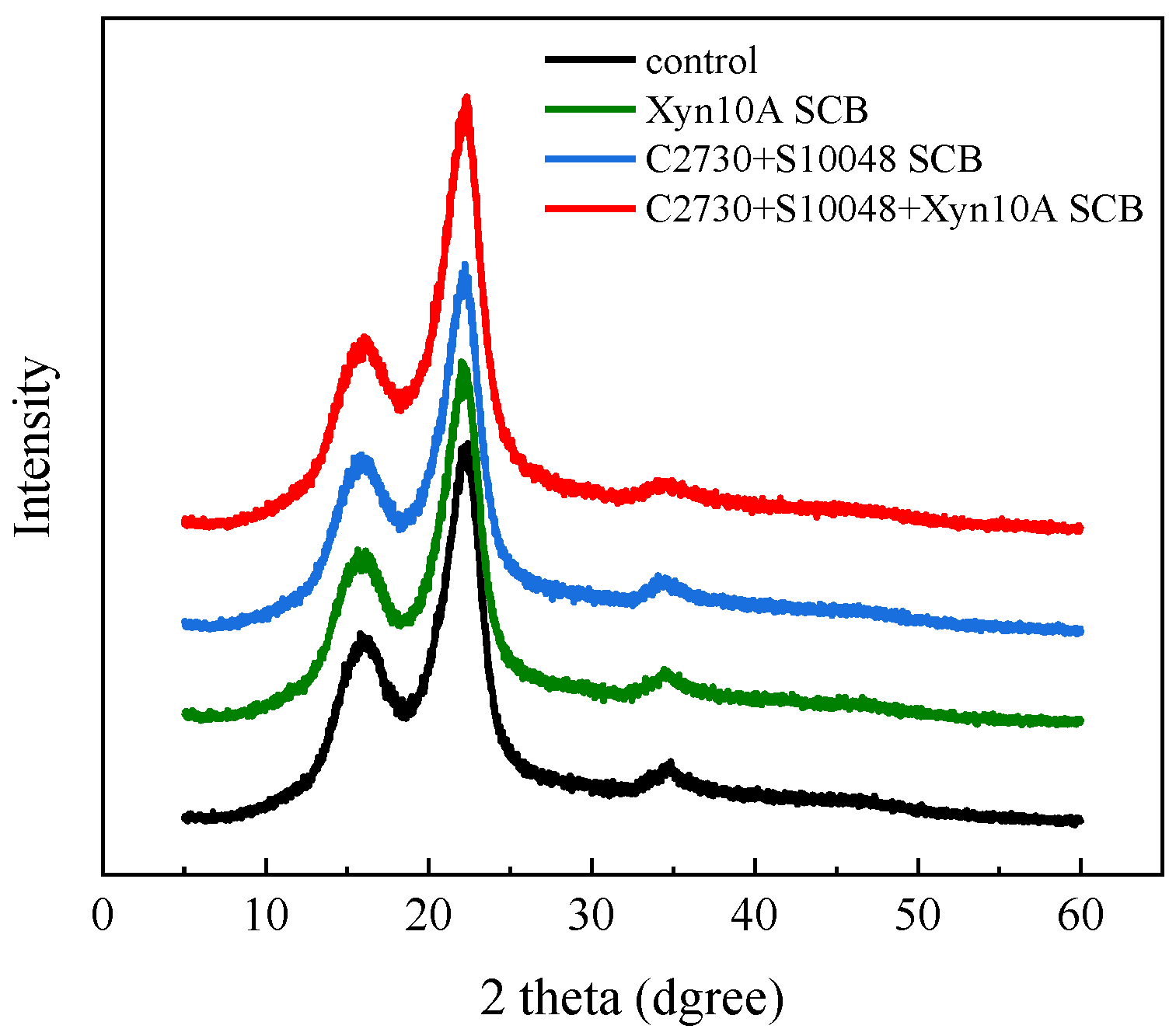
2.10. Crystallinity Analysis
2.11. Fourier Transform Infrared Spectroscopy (FT-IR)
3. Results and Discussion
3.1. Determination of Pretreatment Methods
3.2. Effect of pH and Temperature on Synergistic Characteristics
3.3. Effect of Enzyme Loading on Synergistic Behavior
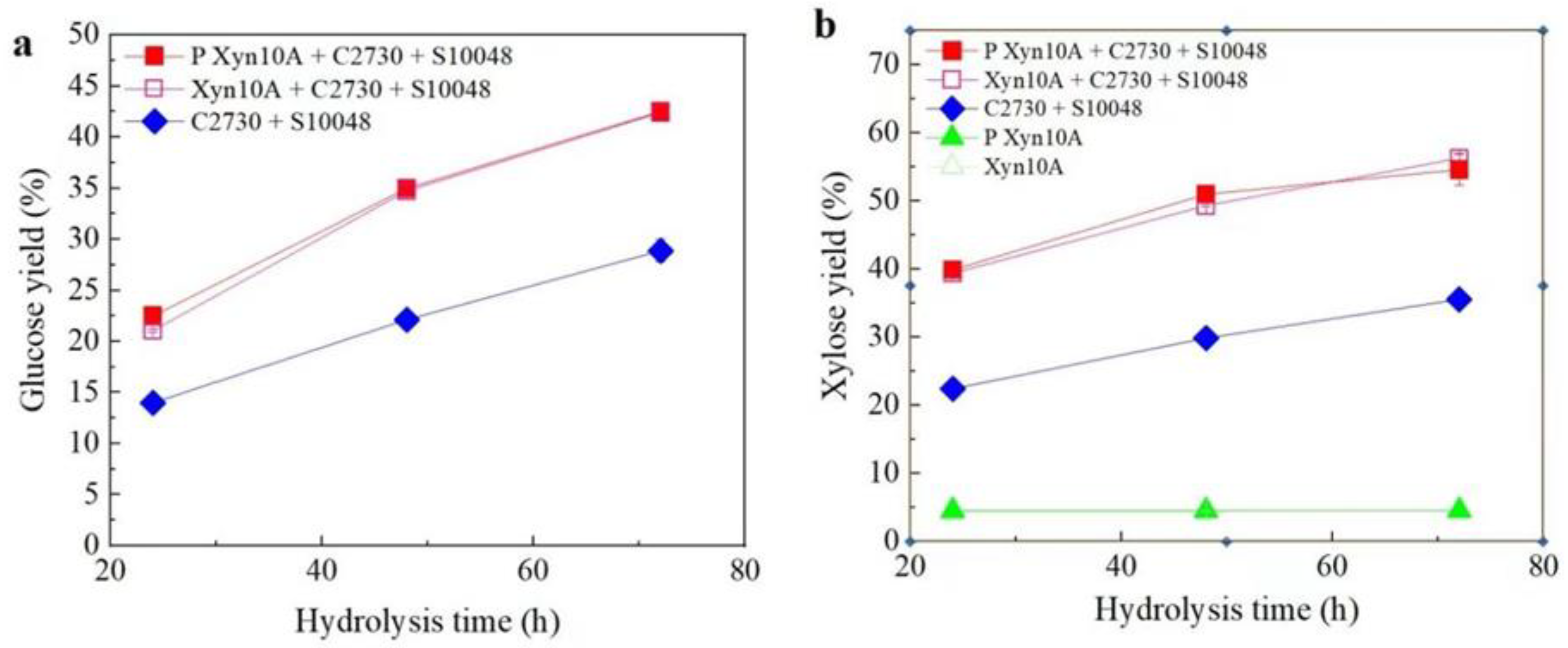
3.4. Synergistic Degradation Process
3.5. Synergy Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.W.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the-art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Janaswamy, S.; Yadav, M.P.; Hoque, M.; Bhattarai, S.; Ahmed, S. Cellulosic fraction from agricultural biomass as a viable alternative for plastics and plastic products. Ind. Crops Prod. 2022, 179, 114692. [Google Scholar] [CrossRef]
- Anselm, E. Sustainable Production of Second-Generation Biofuels: Potential and Perspectives in Major Economies and Developing Countries; IEA Energy Papers; OECD: Paris, France, 2010. [Google Scholar] [CrossRef]
- Huang, J.; Khan, M.T.; Perecin, D.; Coelho, S.T.; Zhang, M. Sugarcane for bioethanol production: Potential of bagasse in Chinese perspective. Renew. Sustain. Energy Rev. 2020, 133, 110296. [Google Scholar] [CrossRef]
- Wanderley Siqueira, J.G.; Rodrigues, C.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Escobar, E.L.N.; da Silva, T.A.; Pirich, C.L.; Corazza, M.L.; Pereira Ramos, L. Supercritical Fluids: A Promising Technique for Biomass Pretreatment and Fractionation. Front. Bioeng. Biotechnol. 2020, 8, 252. [Google Scholar] [CrossRef]
- Rubin, E.M. Genomics of cellulosic biofuels. Nature 2008, 454, 841–845. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, E.; Ma, F.; Zhou, X.; Jiang, K. Improved Release of Monosaccharides and Ferulic Acid Using Enzyme Blends From Aspergillus Niger and Eupenicillium Parvum. Front. Bioeng. Biotechnol. 2022, 9, 814246. [Google Scholar] [CrossRef]
- Du, L.; Ma, L.; Ma, Q.; Guo, G.; Han, X.; Xiao, D. Hydrolytic boosting of lignocellulosic biomass by a fungal lytic polysaccharide monooxygenase, AnLPMO15g from Aspergillus niger. Ind. Crops Prod. 2018, 126, 309–315. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Qu, M.; Pan, K.; OuYang, K.; Song, X.; Zhao, X. Construction and characterization of a chimeric enzyme of swollenin and xylanase to improve soybean straw hydrolysis. Int. J. Biol. Macromol. 2020, 156, 558–564. [Google Scholar] [CrossRef]
- Orlowski, A.; Artzi, L.; Cazade, P.-A.; Gunnoo, M.; Bayer, E.A.; Thompson, D. On the distinct binding modes of expansin and carbohydrate-binding module proteins on crystalline and nanofibrous cellulose: Implications for cellulose degradation by designer cellulosomes. Phys. Chem. Chem. Phys. 2018, 20, 8278–8293. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.; Antonopoulou, I.; Zerva, A.; Dimarogona, M.; Topakas, E.; Rova, U.; Christakopoulos, P. Thermophilic enzyme systems for efficient conversion of lignocellulose to valuable products: Structural insights and future perspectives for esterases and oxidative catalysts. Bioresour. Technol. 2019, 279, 362–372. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.; Jang, J.M.; Liu, J.; Zhang, Y.; Liu, L.; Yuan, H. Efficient ferulic acid and xylo-oligosaccharides production by a novel multi-modular bifunctional xylanase/feruloyl esterase using agricultural residues as substrates. Bioresour. Technol. 2020, 297, 122487. [Google Scholar] [CrossRef]
- Forsan, C.F.; de Freitas, C.; Masarin, F.; Brienzo, M. Xylooligosaccharide production from sugarcane bagasse and leaf using Aspergillus versicolor endoxylanase and diluted acid. Biomass Convers. Biorefinery 2023, 13, 3375–3390. [Google Scholar] [CrossRef]
- Li, H.; Xue, Y.; Wu, J.; Wu, H.; Qin, G.; Li, C.; Ding, J.; Liu, J.; Gan, L.; Long, M. Enzymatic hydrolysis of hemicelluloses from Miscanthus to monosaccharides or xylo-oligosaccharides by recombinant hemicellulases. Ind. Crops Prod. 2016, 79, 170–179. [Google Scholar] [CrossRef]
- Schropp, N.; Stanislas, V.; Michels, K.B.; Thriene, K. How Do Prebiotics Affect Human Intestinal Bacteria? Assessment of Bacterial Growth with Inulin and XOS In Vitro. Int. J. Mol. Sci. 2023, 24, 12796. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.T.F.; Ruschoni, U.C.M.; Ferraz, A.; Milagres, A.M.F. Xylan, Xylooligosaccharides, and Aromatic Structures With Antioxidant Activity Released by Xylanase Treatment of Alkaline-Sulfite-Pretreated Sugarcane Bagasse. Front. Bioeng. Biotechnol. 2022, 10, 940712. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. Technol. 2018, 82, 60–70. [Google Scholar] [CrossRef]
- Amorim, C.; Silverio, S.C.; Prather, K.L.J.; Rodrigues, L.R. From lignocellulosic residues to market: Production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 2019, 37, 107397. [Google Scholar] [CrossRef]
- Shibata, N.; Suetsugu, M.; Kakeshita, H.; Igarashi, K.; Hagihara, H.; Takimura, Y. A novel GH10 xylanase from Penicillium sp accelerates saccharification of alkaline-pretreated bagasse by an enzyme from recombinant Trichoderma reesei expressing Aspergillus β-glucosidase. Biotechnol. Biofuels 2017, 10, 278. [Google Scholar] [CrossRef]
- Danso, B.; Ali, S.S.; Xie, R.; Sun, J. Valorisation of wheat straw and bioethanol production by a novel xylanase-and cellulase-producing Streptomyces strain isolated from the wood-feeding termite, Microcerotermes species. Fuel 2022, 310, 122333. [Google Scholar] [CrossRef]
- Alokika; Kumar, V.; Singh, B. Biochemical characteristics of a novel ethanol-tolerant xylanase from Bacillus subtilis subsp. subtilis JJBS250 and its applicability in saccharification of rice straw. Biomass Convers. Biorefinery 2023, 13, 1937–1949. [Google Scholar] [CrossRef]
- Xiao, W.; Li, H.; Xia, W.; Yang, Y.; Hu, P.; Zhou, S.; Hu, Y.; Liu, X.; Dai, Y.; Jiang, Z. Co-expression of cellulase and xylanase genes in Saccharomyces cerevisiae toward enhanced bioethanol production from corn stover. Bioengineered 2019, 10, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Fang, X.; Xu, Y.; Zhang, J. Combined acetic acid and enzymatic hydrolysis for xylooligosaccharides and monosaccharides production from poplar. Biomass Bioenergy 2022, 158, 106377. [Google Scholar] [CrossRef]
- Zhuo, R.; Yu, H.; Qin, X.; Ni, H.; Jiang, Z.; Ma, F.; Zhang, X. Heterologous expression and characterization of a xylanase and xylosidase from white rot fungi and their application in synergistic hydrolysis of lignocellulose. Chemosphere 2018, 212, 24–33. [Google Scholar] [CrossRef]
- Wang, K.; Cao, R.; Wang, M.; Lin, Q.; Zhan, R.; Xu, H.; Wang, S. A novel thermostable GH10 xylanase with activities on a wide variety of cellulosic substrates from a xylanolytic Bacillus strain exhibiting significant synergy with commercial Celluclast 1.5 L in pretreated corn stover hydrolysis. Biotechnol. Biofuels 2019, 12, 48. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, S.; Fan, Y.; Hu, C.; Lu, W.; Guo, L. Cloning and heterologous expression of a novel halo/alkali-stable multi-domain xylanase (XylM18) from a marine bacterium Marinimicrobium sp. strain LS-A18. Appl. Microbiol. Biotechnol. 2019, 103, 8899–8909. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mishra, C.; Behera, S.S.; Thatoi, H. Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass—A review. Renew. Sustain. Energy Rev. 2017, 78, 1007–1032. [Google Scholar] [CrossRef]
- Malgas, S.; Chandra, R.; Van Dyk, J.S.; Saddler, J.N.; Pletschke, B.I. Formulation of an optimized synergistic enzyme cocktail, HoloMix, for effective degradation of various pre-treated hardwoods. Bioresour. Technol. 2017, 245, 52–65. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, Y.; Yu, Q.; Tan, X.; Zhuang, X.; Yuan, Z. Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Ind. Crops Prod. 2020, 145, 112145. [Google Scholar] [CrossRef]
- Lan, T.Q.; Lou, H.; Zhu, J.Y. Enzymatic Saccharification of Lignocelluloses Should be Conducted at Elevated pH 5.2–6.2. Bioenergy Res. 2013, 6, 476–485. [Google Scholar] [CrossRef]
- Xian, L.; Li, Z.; Tan, A.-X.; Qin, Y.-M.; Li, Q.-Y.; Li, H.-B.; Liu, Y.-Y. A novel neutral and thermophilic endoxylanase from Streptomyces ipomoeae efficiently produced xylobiose from agricultural and forestry residues. Bioresour. Technol. 2019, 285, 121293. [Google Scholar] [CrossRef] [PubMed]
- Gongora, A.; Villafranco, D. Sugarcane bagasse cogeneration in Belize: A review. Renew. Sustain. Energy Rev. 2018, 96, 58–63. [Google Scholar] [CrossRef]
- Li, J.; Zhou, P.; Liu, H.; Xiong, C.; Lin, J.; Xiao, W.; Gong, Y.; Liu, Z. Synergism of cellulase, xylanase, and pectinase on hydrolyzing sugarcane bagasse resulting from different pretreatment technologies. Bioresour. Technol. 2014, 155, 258–265. [Google Scholar] [CrossRef]
- Cao, W.; Sun, C.; Liu, R.; Yin, R.; Wu, X. Comparison of the effects of five pretreatment methods on enhancing the enzymatic digestibility and ethanol production from sweet sorghum bagasse. Bioresour. Technol. 2012, 111, 215–221. [Google Scholar] [CrossRef]
- Monte, J.R.; Brienzo, M.; Milagres, A.M.F. Utilization of pineapple stem juice to enhance enzyme-hydrolytic efficiency for sugarcane bagasse after an optimized pre-treatment with alkaline peroxide. Appl. Energy 2011, 88, 403–408. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X.; Sun, Z.; Zhang, J. Effect of additives on adsorption and desorption behavior of xylanase on acid-insoluble lignin from corn stover and wheat straw. Bioresour. Technol. 2015, 186, 316–320. [Google Scholar] [CrossRef]
- Hu, J.; Mok, Y.K.; Saddler, J.N. Can We Reduce the Cellulase Enzyme Loading Required To Achieve Efficient Lignocellulose Deconstruction by Only Using the Initially Absorbed Enzymes? ACS Sustain. Chem. Eng. 2018, 6, 6233–6239. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP)/National Renewable Energy Laboratory: Golden, CO, USA, 2008; Volume 1617, pp. 1–16. [Google Scholar]
- Eriksson, T.; Karlsson, J.; Tjerneld, F. A model explaining declining rate in hydrolysis of lignocellulose substrates with cellobiohydrolase I (cel7A) and endoglucanase I (cel7B) of Trichoderma reesei. Appl. Biochem. Biotechnol. 2002, 101, 41–60. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover—a review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci. 2018, 67, 275–291. [Google Scholar] [CrossRef]
- Nicodeme, T.; Berchem, T.; Jacquet, N.; Richel, A. Thermochemical conversion of sugar industry by-products to biofuels. Renew. Sustain. Energy Rev. 2018, 88, 151–159. [Google Scholar] [CrossRef]
- Martinez, P.M.; Bakker, R.; Harmsen, P.; Gruppen, H.; Kabel, M. Importance of acid or alkali concentration on the removal of xylan and lignin for enzymatic cellulose hydrolysis. Ind. Crops Prod. 2015, 64, 88–96. [Google Scholar] [CrossRef]
- Ho, M.C.; Ong, V.Z.; Wu, T.Y. Potential use of alkaline hydrogen peroxide in lignocellulosic biomass pretreatment and valorization—A review. Renew. Sustain. Energy Rev. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, J.; Zhang, Y.; Guo, Y.; Xu, H.J.; Liang, C.Y.; Wang, Z.M.; Xu, J.L. Lignin prepared from different alkaline pretreated sugarcane bagasse and its effect on enzymatic hydrolysis. Int. J. Biol. Macromol. 2019, 141, 484–492. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Wei, W.; Zhang, J.; Xie, J. Investigation of alkaline hydrogen peroxide pretreatment and Tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol. Biofuels 2019, 12, 107. [Google Scholar] [CrossRef]
- dos Santos, A.C.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin-Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 2019, 37, 518–531. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, C.; Li, H.; Xiong, L.; Chen, X.; Luo, M.; Chen, X. Comparison of different pretreatments on the synergistic effect of cellulase and xylanase during the enzymatic hydrolysis of sugarcane bagasse. Rsc Adv. 2018, 8, 30725–30731. [Google Scholar] [CrossRef]
- Adney, W.S.; Rivard, C.J.; Ming, S.A.; Himmel, M.E. Anaerobic digestion of lignocellulosic biomass and wastes. Cellulases and related enzymes. Appl. Biochem. Biotechnol. 1991, 30, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.L.; Takasugi, Y.; Jia, L.; Mori, Y.; Noda, S.; Tanaka, T.; Ichinose, H.; Kamiya, N. Synergistic effect and application of xylanases as accessory enzymes to enhance the hydrolysis of pretreated bagasse. Enzym. Microb. Technol. 2015, 72, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Azelee, N.I.W.; Jahim, J.M.; Ismail, A.F.; Fuzid, S.F.Z.M.; Rahman, R.A.; Illias, R.M. High xylooligosaccharides (XOS) production from pretreated kenaf stem by enzyme mixture hydrolysis. Ind. Crops Prod. 2016, 81, 11–19. [Google Scholar] [CrossRef]
- Pavon-Orozco, P.; Santiago-Hernandez, A.; Rosengren, A.; Eugenia Hidalgo-Lara, M.; Stalbrand, H. The family II carbohydrate-binding module of xylanase CflXyn11A from Cellulomonas flavigenaincreases the synergy with cellulase TrCel7B from Trichoderma reesei during the hydrolysis of sugar cane bagasse. Bioresour. Technol. 2012, 104, 622–630. [Google Scholar] [CrossRef]
- Pengilly, C.; Garcia-Aparicio, M.P.; Diedericks, D.; Brienzo, M.; Goergens, J.F. Enzymatic hydrolysis of steam-pretreated sweet sorghum bagasse by combinations of cellulase and endo-xylanase. Fuel 2015, 154, 352–360. [Google Scholar] [CrossRef]
- Inoue, H.; Kishishita, S.; Kumagai, A.; Kataoka, M.; Fujii, T.; Ishikawa, K. Contribution of a family 1 carbohydrate-binding module in thermostable glycoside hydrolase 10 xylanase from Talaromyces cellulolyticus toward synergistic enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Arantes, V.; Saddler, J.N. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: Is it an additive or synergistic effect? Biotechnol. Biofuels 2011, 4, 36. [Google Scholar] [CrossRef]
- Sanhueza, C.; Carvajal, G.; Soto-Aguilar, J.; Elena Lienqueo, M.; Salazar, O. The effect of a lytic polysaccharide monooxygenase and a xylanase from Gloeophyllum trabeum on the enzymatic hydrolysis of lignocellulosic residues using a commercial cellulase. Enzym. Microb. Technol. 2018, 113, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Wang, H.; Qi, X.; Gao, S.; Zhang, Y.; An, Y. Biochemical characterization of an engineered bifunctional xylanase/feruloyl esterase and its synergistic effects with cellulase on lignocellulose hydrolysis. Bioresour. Technol. 2022, 355, 127244. [Google Scholar] [CrossRef] [PubMed]
- Bura, R.; Chandra, R.; Saddler, J. Influence of Xylan on the Enzymatic Hydrolysis of Steam-Pretreated Corn Stover and Hybrid Poplar. Biotechnol. Prog. 2009, 25, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Viikari, L. Impact of Xylan on Synergistic Effects of Xylanases and Cellulases in Enzymatic Hydrolysis of Lignocelluloses. Appl. Biochem. Biotechnol. 2014, 174, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Malgas, S.; Thoresen, M.; van Dyk, J.S.; Pletschke, B.I. Time dependence of enzyme synergism during the degradation of model and natural lignocellulosic substrates. Enzym. Microb. Technol. 2017, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-T.; Gao, Y.; Yang, Y.-M.; Xiao, W.-J.; Liu, S.-H.; Xia, W.-C.; Liu, Z.-L.; Yi, L.; Jiang, Z.-B. Synergistic effect of cellulase and xylanase during hydrolysis of natural lignocellulosic substrates. Bioresour. Technol. 2016, 219, 710–715. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef]
- Pihlajaniemi, V.; Sipponen, M.H.; Kallioinen, A.; Nyyssola, A.; Laakso, S. Rate-constraining changes in surface properties, porosity and hydrolysis kinetics of lignocellulose in the course of enzymatic saccharification. Biotechnol. Biofuels 2016, 9, 18. [Google Scholar] [CrossRef]
- Li, M.; Yi, L.; Bin, L.; Zhang, Q.; Song, J.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Comparison of nonproductive adsorption of cellulase onto lignin isolated from pretreated lignocellulose. Cellulose 2020, 27, 7911–7927. [Google Scholar] [CrossRef]
- Ma, X.; Huang, H.; Huang, F.; Long, Y.; Cao, S.; Chen, L.; Huang, L.; Ni, Y. Synergistic effects of enzyme pretreatment for hemicellulose separation from paper-grade pulp in ionic liquid/water. Cellulose 2018, 25, 4193–4198. [Google Scholar] [CrossRef]
- Ding, S.Y.; Himmel, M.E. The maize primary cell wall microfibril: A new model derived from direct visualization. J. Agric. Food Chem. 2006, 54, 597–606. [Google Scholar] [CrossRef]
- Baramee, S.; Siriatcharanon, A.-K.; Ketbot, P.; Teeravivattanakit, T.; Waeonukul, R.; Pason, P.; Tachaapaikoon, C.; Ratanakhanokchai, K.; Phitsuwan, P. Biological pretreatment of rice straw with cellulase-free xylanolytic enzyme-producing Bacillus firmus K-1: Structural modification and biomass digestibility. Renew. Energy 2020, 160, 555–563. [Google Scholar] [CrossRef]
- Velasco, J.; Oliva, B.; Mulinari, E.J.; Quintero, L.P.; da Silva Lima, A.; Goncalves, A.L.; Goncalves, T.A.; Damasio, A.; Squina, F.M.; Ferreira Milagres, A.M.; et al. Heterologous expression and functional characterization of a GH10 endoxylanase from Aspergillus fumigatus var. niveus with potential biotechnological application. Biotechnol. Rep. 2019, 24, e00382. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, Z.; Wu, W.; Chen, J.; Xia, Q. Physicochemical properties and structural changes of bamboo shoots dietary fiber with enzymatic modification. Food Ferment. Ind. 2019, 45, 36–41. [Google Scholar]
- Zhao, C.; Cao, Y.; Ma, Z.; Shao, Q. Optimization of liquid ammonia pretreatment conditions for maximizing sugar release from giant reed (Arundo donax L.). Biomass Bioenergy 2017, 98, 61–69. [Google Scholar] [CrossRef]
- Long, L.; Tian, D.; Zhai, R.; Li, X.; Zhang, Y.; Hu, J.; Wang, F.; Saddler, J. Thermostable xylanase-aided two-stage hydrolysis approach enhances sugar release of pretreated lignocellulosic biomass. Bioresour. Technol. 2018, 257, 334–338. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Zhai, S.; Liang, C.; Huang, C.; Lai, C.; Yong, Q. Improving enzymatic hydrolysis efficiency of wheat straw through sequential autohydrolysis and alkaline post-extraction. Bioresour. Technol. 2018, 251, 374–380. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Zambon, M.D.; Area, M.C.; Curvelo, A.A.S. Influence of the Chemical Composition on the Enzymatic Hydrolysis of Hot Water and Organosolv Pretreated Sugarcane Bagasse. Waste Biomass Valorization 2020, 11, 3337–3344. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Sharma, S.; Bajaj, B.K. Efficacy and functional mechanisms of a novel combinatorial pretreatment approach based on deep eutectic solvent and ultrasonic waves for bioconversion of sugarcane bagasse. Renew. Energy 2021, 163, 1910–1922. [Google Scholar] [CrossRef]
- Lan, T.; Jiang, Y.; Zheng, W.; Wang, S.; Sang, S.; Li, H. Comprehensively Understanding Enzymatic Hydrolysis of Lignocellulose and Cellulase–Lignocellulose Adsorption by Analyzing Substrates’ Physicochemical Properties. BioEnergy Res. 2020, 13, 1108–1120. [Google Scholar] [CrossRef]
- Wu, K.; Ying, W.; Shi, Z.; Yang, H.; Zheng, Z.; Zhang, J.; Yang, J. Fenton Reaction-Oxidized Bamboo Lignin Surface and Structural Modification to Reduce Nonproductive Cellulase Binding and Improve Enzyme Digestion of Cellulose. ACS Sustain. Chem. Eng. 2018, 6, 3853–3861. [Google Scholar] [CrossRef]
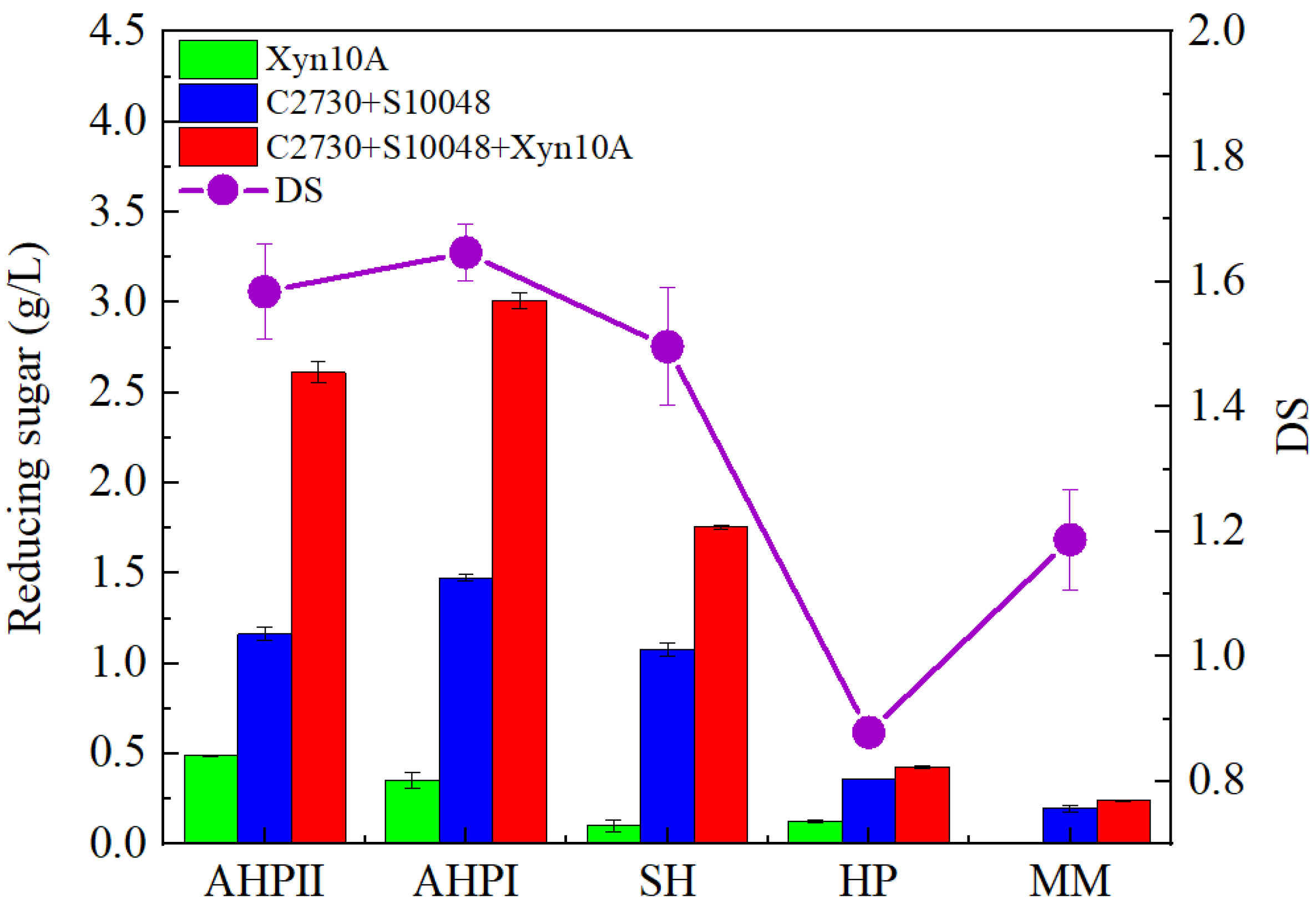
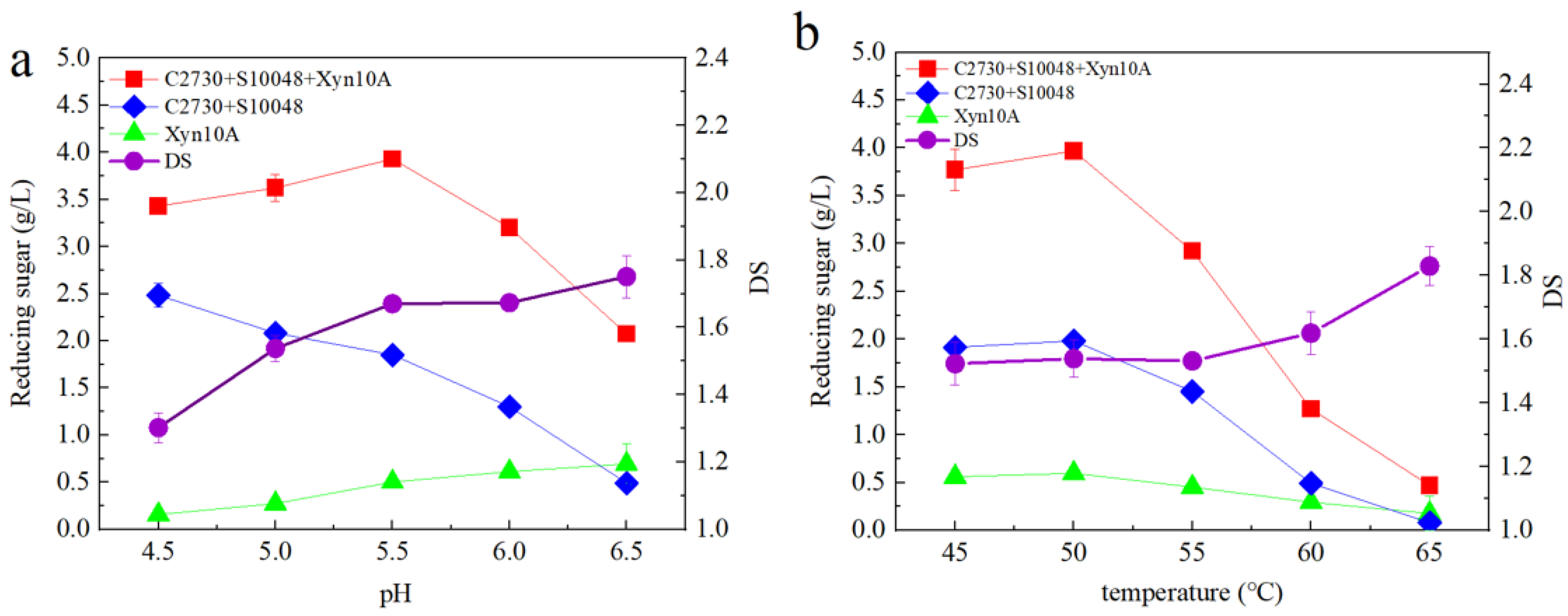
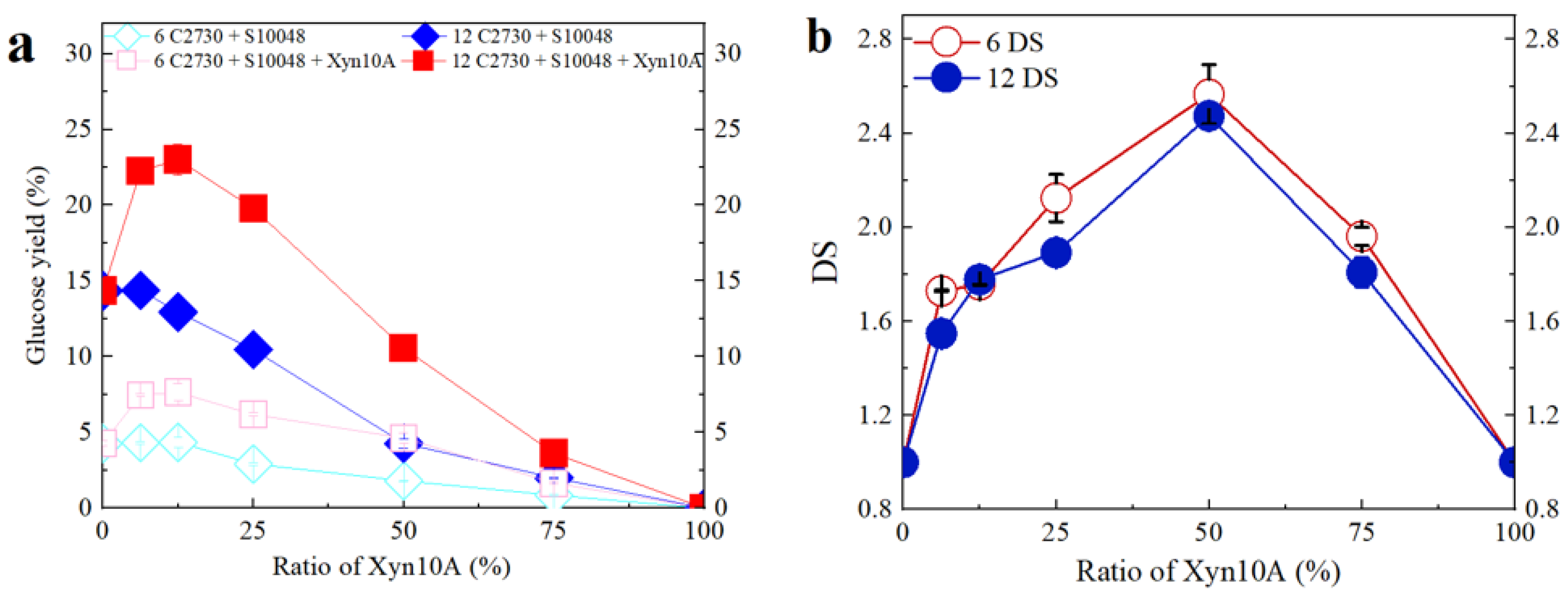

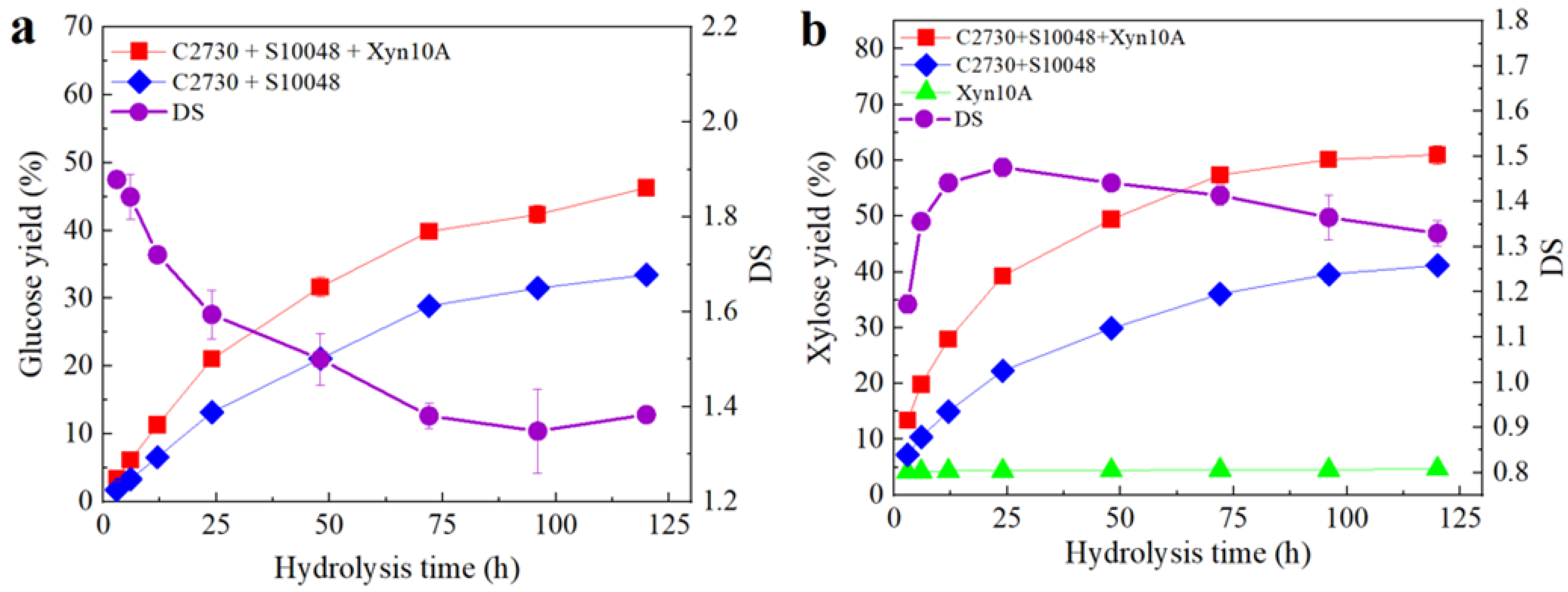

| Pretreatment Methods of SCB a | Cellulose (%) b | Xylan (%) | AIL (%) c |
|---|---|---|---|
| Mechanical Milling (MM) | 43.00 ± 1.42 | 20.35 ± 0.83 | 22.47 ± 1.46 |
| Hydrogen Peroxide (HP) | 47.14 ± 0.15 | 17.02 ± 0.35 | 22.24 ± 0.33 |
| Sodium Hydroxide (SH) | 61.37 ± 0.54 | 23.83 ± 1.29 | 7.02 ± 0.26 |
| Alkaline Hydrogen Peroxide I (AHP I) | 63.66 ± 0.09 | 22.39 ± 0.03 | 5.17 ± 0.19 |
| Alkaline Hydrogen Peroxide II (AHP II) | 60.02 ± 1.70 | 25.05 ± 0.31 | 5.02 ± 0.16 |
| Enzymatic Hydrolysis Method | Crystallinity Index (CrI) |
|---|---|
| No enzyme | 42.8% |
| Single Xyn10A | 42.6% |
| single cellulase group | 44.5% |
| Mixed enzyme | 48.3% |
| Enzymatic Hydrolysis Method | Scale Bar | ||
|---|---|---|---|
| 10 μm | 1 μm | 500 nm | |
| Control (No enzyme) | 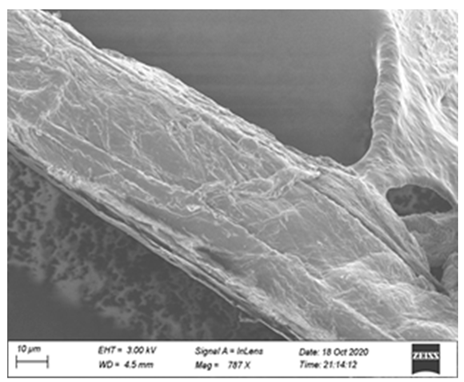 | 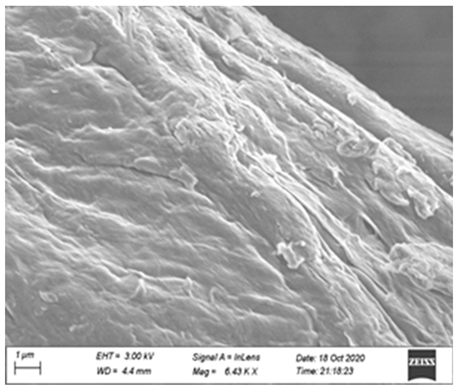 |  |
| Single Xyn10A | 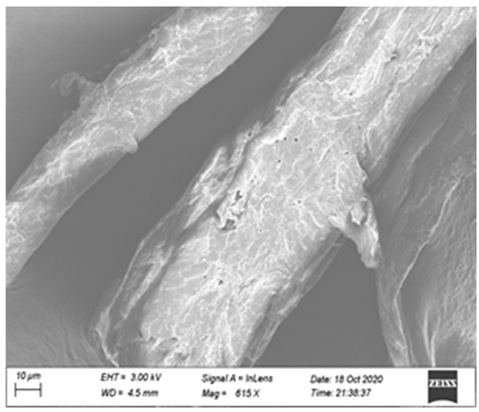 |  |  |
| single cellulase group |  | 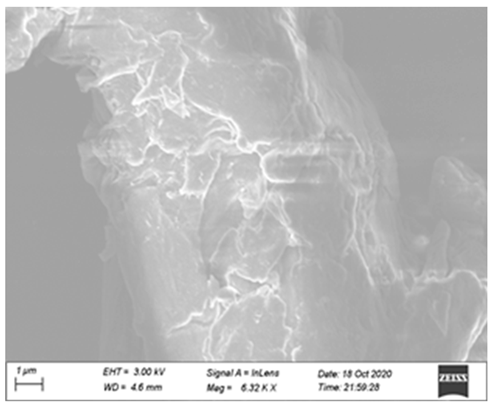 | 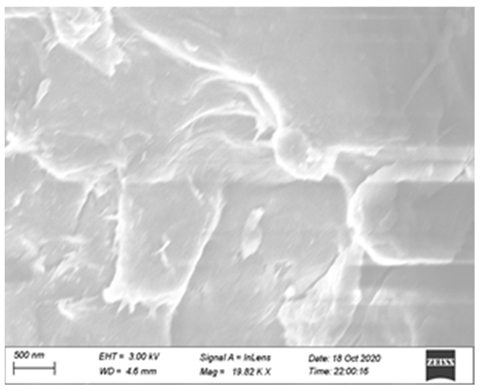 |
| Mixed enzyme |  |  | 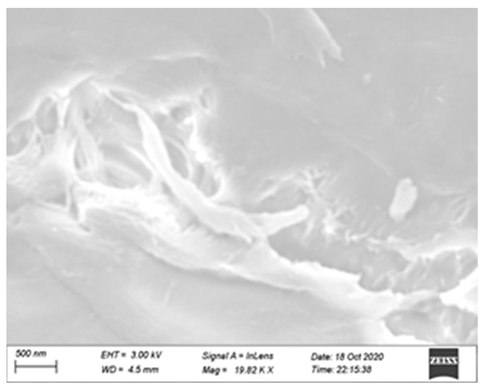 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Dong, Y.; Liu, J.; Xian, L.; Tang, A.; Li, Q.; Li, Q.; Liu, Y. Synergistically Enhanced Enzymatic Hydrolysis of Sugarcane Bagasse Mediated by a Recombinant Endo-Xylanase from Streptomyces ipomoeae. Processes 2024, 12, 1997. https://doi.org/10.3390/pr12091997
Li Z, Dong Y, Liu J, Xian L, Tang A, Li Q, Li Q, Liu Y. Synergistically Enhanced Enzymatic Hydrolysis of Sugarcane Bagasse Mediated by a Recombinant Endo-Xylanase from Streptomyces ipomoeae. Processes. 2024; 12(9):1997. https://doi.org/10.3390/pr12091997
Chicago/Turabian StyleLi, Zhong, Youqing Dong, Junli Liu, Liang Xian, Aixing Tang, Qingyun Li, Qunliang Li, and Youyan Liu. 2024. "Synergistically Enhanced Enzymatic Hydrolysis of Sugarcane Bagasse Mediated by a Recombinant Endo-Xylanase from Streptomyces ipomoeae" Processes 12, no. 9: 1997. https://doi.org/10.3390/pr12091997
APA StyleLi, Z., Dong, Y., Liu, J., Xian, L., Tang, A., Li, Q., Li, Q., & Liu, Y. (2024). Synergistically Enhanced Enzymatic Hydrolysis of Sugarcane Bagasse Mediated by a Recombinant Endo-Xylanase from Streptomyces ipomoeae. Processes, 12(9), 1997. https://doi.org/10.3390/pr12091997






