Abstract
The extract of the plant species Satureja hortensis L. (often used as traditional ethno-therapy and in food processing) was prepared using the ultrasonic extraction technique, and contains a large quantity of secondary metabolites, with scientific evidence for antioxidant, antimicrobial, sedative, antispastic and antidiarrheal activities. Process optimization was carried out using a mathematical–statistical method (response surface methodology—RSM), which models and examines the effects of three levels and three variables on the observed response. The investigated responses were the content of total phenolic components (TPC) and total flavonoids (TFC), as well as tests of antioxidant activity at the level of radicals. The independent variables were ethanol concentration (40–80%), temperature (40–80 °C) and the liquid–solid ratio (10–30 mL/g). Results from this study were entered into a second-degree polynomial with multiple non-linear regression. Analysis of variance (ANOVA) was applied to find the most favorable environment for assessing the model’s performance and effectiveness with an ethanol concentration of 20%, temperature of 80 °C and LSR of 21.4 mL/g. ANOVA assessed the model’s significance, and a second-order polynomial model described the relationships between variables and responses. Diagnostic plots confirmed the model’s adequacy and reliability. The estimated values were 4.11 mg chlorogenic acid equivalents per gram of dry weight (CEA/g), 2.18 mg of rutin equivalents per gram of dry weight (RE/g), and 0.030 mg/mL and 0.030 mg/mL for TPC, TFC, IC50 and EC50, respectively. High-Performance Liquid Chromatography with diode array detection (HPLC-DAD) examination revealed that the prominent substance in the tested extract is rosmarinic acid (46,172 µg/mL), followed by chlorogenic acid (1519 µg/mL).
1. Introduction
Satureja hortensis L., commonly known as summer savory, is an annual herb from the Lamiaceae botanical family. It is widely distributed, and has use in traditional ethno-therapy and in food processing. Satureja hortensis L. is an annual aromatic, spicy and medicinal plant from the Lamiaceae family. This plant species has been used in nutrition since ancient times. The stems are branched from the base, 30 to 40 cm high. The leaves are opposite, sessile, narrow, very slender and bronze green in color. Purple or purple–white flowers are found in the upper nodes of the branches. The calyx is bell-shaped with five almost equal teeth, often purple. The plant grows on rocky slopes. It flowers from July to August, and the seeds ripen from August to September and are cultivated [1]. Different studies have shown that the herb possesses various activities such as antioxidant, antimicrobial, sedative, antispastic and antidiarrheal activities [2,3,4,5,6,7,8]. Further examination revealed that carvacrol and γ-terpinene are the main constituents of the essential oil [3,4]. Rosmarinic and caffeic acids were quantified in herbal extracts (HE) [5].
ULTRASOUND-assisted extraction employs ultrasound with the usual frequencies from 20 KHz to 100 MHz, which penetrates through a medium creating compression and expansion, which in turn induce a phenomenon called cavitation [9]. Cavitation allows a more extensive entry of the solvent into the cell matrix [10,11]. Some studies show that this technique is economical and requires cost-effective equipment [11]. The method enables less use of the dispersed substance, has a simpler use, has exceptional closeness of the value of the determined quantity and its reference value, as well as the rationalization of energy costs [12]. Besides those advantages mentioned, ultrasound diminishes the possibility of the thermal degradation of desired compounds as well as a reduction in the processes’ duration [10]. Beside the mentioned advantages, there are also some disadvantages of this approach. Some effects can contribute to the formation of new chemical compounds in HE and to the disruption of individual molecules and the creation of free electron species within the gas bubbles [13].
Optimization of the technological process represents a highly desirable and important task. This process aims to obtain the maximum benefit from the process with minimal losses [14]. The most common approach is the application of RSM. RSM is the collection of mathematical–statistical methods based on the influence of several different variables on the desired responses [15,16].
Given the optimal extraction conditions and the phytochemical and antioxidant profiles of the Satureja hortensis L. extract, it holds promise for various applications, including pharmaceuticals for supplements or therapeutic agents, functional foods to boost health benefits, and cosmetics for antioxidant and skin-protective properties. Future research could focus on exploring its bioactivity, developing effective formulations, and conducting safety and efficacy trials to fully realize and expand its potential uses. By using HPLC-DAD we detected two main compounds: rosmarinic acid and chlorogenic acid.
Rosmarinic acid is known for its strong antioxidant, anti-inflammatory and antimicrobial properties, contributing to cellular protection and immune modulation. Chlorogenic acid also exhibits potent antioxidant activity and helps regulate glucose and lipid metabolism, offering cardiovascular benefits and potential anti-carcinogenic effects.
This work was designed with the aim of determining the impact of three significant parameters, ethanol concentration, temperature and the liquid–sample ratio, on the ultrasound extraction of Satureja hortensis L. applying RSM. Such an investigation was further led to process optimization as well as experimental conformation of the obtained results. The extract prepared under optimal conditions was also assessed regarding the polyphenolic compounds, using chromatography analysis.
2. Materials and Methods
2.1. Herbal Material
Herbal species Satureja hortensis L. (summer gorse) was harvested in Central Serbia during 2015. The plant raw material was subjected to natural drying, after which it was ground using a mill, and stored and packed in paper bags at 22 °C until use. The aerial part of the plant was used for extraction.
2.2. Chemicals and Reagents
All chemicals and reagents were of high analytical grade and obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany) and Sigma (St. Louis, MO, USA).
2.3. Ultrasound Extractions
Ultrasound extractions were performed using a sonication water bath (Branson and SmithKline Company, B-220, USA) under constant ultrasonic power. Ultrasonic extraction is a green, innovative extraction process. During the experiments, ethanol concentration, the temperature of water and the liquid–solid ratio (LSR) were controlled. The Erlenmeyer flasks were equidistant from the transducer, and no mixing was used. Filtration of HEs was performed under a vacuum pump. In order to inhibit biochemical processes, HEs were stored at 4 °C. The yield of the extraction process was determined using 10 mL of extract, evaporation in a rotary vacuum evaporator (Devarot, Elektromedicina, Ljubljana, Slovenia) and drying to a constant mass at 110 °C.
2.4. TPC and TFC
The total phenolic components (TPC) was determined according to the previously described method [17]. The results are expressed as milligrams of chlorogenic acid equivalents per gram of dry weight (mg CEA/g). The total flavonoids (TFC) was determined using a previously described procedure [18]. The results are expressed as milligrams of rutin equivalents per gram of dry weight (mg RE/g).
2.5. DPPH and ABTS Assays
The DPPH assay was performed according to the previously described test [19] with suitable modifications [20]. The results (ABTS test and DPPH) were expressed in milligrams per milliliter (mg/mL). The ABTS test was performed using the previously described procedure [21]. The results were expressed in milligrams per milliliter (mg/mL).
2.6. Experimental Design
RSM was used in combination with the Box–Behnken method and [22], incorporating 3 numerical factors in three different degrees. Seventeen experiments were performed with five replicates, at central points. Variables independent of the trial outcome in this study were ethanol concentration (X1, 40–80%), temperature (X2, 40–80 °C) and liquid–solid ratio (X3, 10–30 mL/g). The parameters were normalized through the coding of the independent variables and their range from +1 to −1 to affect the response more evenly. Variables were calculated based on the equation created by Wang et al. (2008):
where X is the calculated value, Xi is the real value, X0 is the real value in the domain and ΔX is the yield Xi corresponding to a variation of 1 unit of X. The real and calculated values of the independent variables from the Box–Behnken methods are presented in Table 1.

Table 1.
Real and calculated levels of independent variables in RSM.
The types of response variables were fitted to a second-order polynomial describing the quotient of the response and independent variables [23]:
where Y is the measured response, β0 is a constant value, bj (linear coefficient), bjj (quadratic coefficient) and bij (interactive coefficient), while Xi and Xj are independent variables. The optimum conditions for the TPC, TFC, IC50 and EC50 values were determined. RSM and linear regression were tested using Design-Expert v.7 Trial (Stat-Ease, Minneapolis, MN, USA). ANOVA was applied, with significance levels set at 0.05. The adequacy of the model(s) was calculated using the coefficient of multiple determination (R2), coefficient of variance (CV) and p-value for the model, and lack of fit testing.
2.7. Quantitation Analysis of Optimized HE
Quantitation of active biomolecules was investigated by Agilent-1200 series high-performance liquid chromatography with a diode array detector (DAD) for multi-wavelength detection. The column was thermostated at 25 °C. Five μL of the sample was injected, after which separation was performed on a column (Agilent-Eclipse KSDB C-18, 4.6 150 mm). Two components served as eluent: eluent A is water with 2% formic acid and eluent B 80% acetonitrile + water with 2% acetic acid. Elution was performed as follows: 0–10 min 0% B; 10–28 min 25% B; 28–30 min 25% B; 30–35 min 50% B; 35–40 min 80% B, and during the last 5 min, it gradually decreases by 80–0% B. Phenolic substances were quantified by comparing retention times and spectra with retention times, as well as standard spectra for each individual component. Based on the calibration curve for each component, quantification was performed and the results were expressed in µg/mL HE [24,25].
2.8. Statistical Analysis with Experimental Design
The study used Response Surface Methodology (RSM) with a Box–Behnken design to evaluate the effects of ethanol concentration (40–80%), temperature (40–80 °C) and liquid–solid ratio (10–30 mL/g) across 17 experiments with five replicates at central points. Analysis of variance (ANOVA) assessed the model’s significance, and a second-order polynomial model described the relationships between variables and responses. Diagnostic plots confirmed the model’s adequacy and reliability.
3. Results and Discussion
3.1. Model System
Three parameters were determined (ethanol concentration, temperature and liquid–solid ratio, (TPC, TFC, DPPH and ABTS). The results obtained using the Box–Behnken experimental test are shown in Table 2.

Table 2.
A trial conducted including natural and calculated levels and trial-derived measured response results, including yield, TPC, TFC, IC50 and EC50 values.
ANOVA is used to determine the regression coefficients. The regression coefficients are presented in Table 3.

Table 3.
Regression coefficients of the second-order polynomial.
Each parameter was described as statistically significant (p > 0.05) and insignificant (p < 0.05), while coefficients of multiple determination (R2) were used as the first indicator of model adequacy. ANOVA results are shown in Table 4.

Table 4.
ANOVA of fitted second-order polynomial models.
The particularly high value of R2 for TPC (0.9548), as well as high values of the same parameter for TPC (0.8304), IC50 (0.8453) and EC50 (0.8336), indicated that the second-order polynomial model was a good approximation of the experimentally obtained results. The results provided by ANOVA indicated that regression for the model was significant (p < 0.05), while the lack of fit testing was insignificant (p > 0.05). Thus, regression equations may be successfully used as predictors of investigated responses in the investigated experimental design.
3.2. TPC
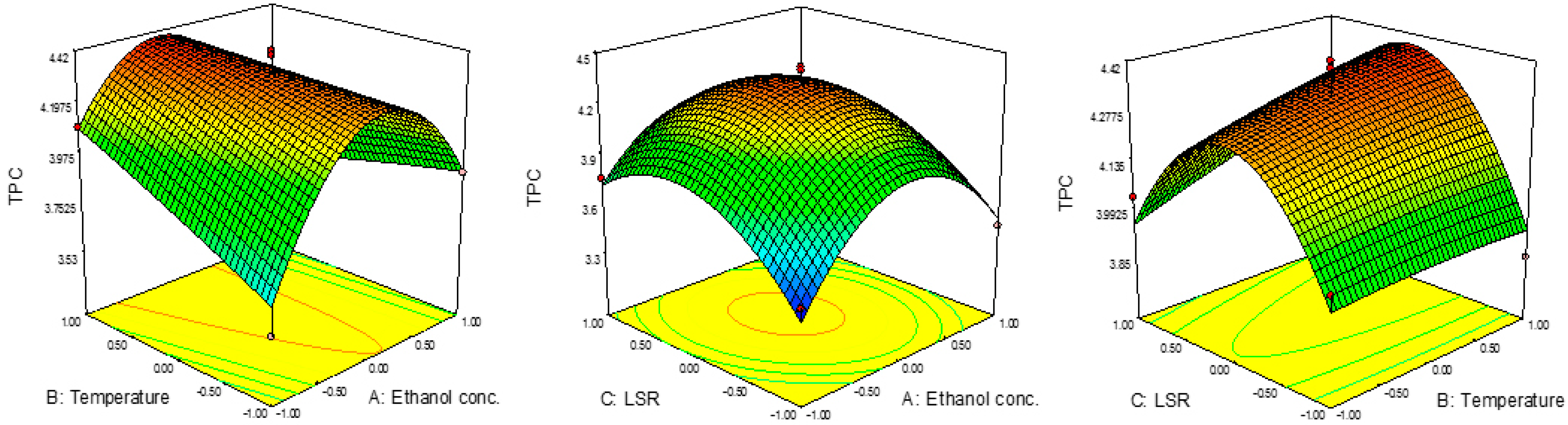
The experimentally obtained results for TPC are presented in Table 2. The results varied in the range of 3.36 to 4.40 mg CEA/g. The highest yield was noticed at 60%, 60 °C and 20 mL/g. On the other hand, the lowest yield was noticed at 80%, 60 °C and 30 mL/g. It might be noticed that the highest value for TPC was obtained at a lower ethanol concentration and LSR than in the case of the lowest value, while the temperature was the same.
The effects of the parameters on TPC are presented in Figure 1, while their significance was determined using RSM, are presented in Table 3. According to the ANOVA, quadratic terms of ethanol concentration and LSR, as well as the interaction term between ethanol concentration and temperature are significant (p < 0.05). The results presented in Table 3 show the negative influence of all three quadratic terms, while the linear term exhibited a rather positive effect with the exception of ethanol concentration. This suggests that TPC would increase with the increase in ethanol concentration and LSR until certain values, after which a decrease in TOC would occur. On the other hand, temperature would express a positive influence; thus, TPC would increase with temperature. Such an effect of temperature on TPC was expected due to the effect of this parameter on mass transfer throughout the increase in diffusion (Zekovic et al., 2016). The predicted second-order polynomial model for TPC is given by the following equation:
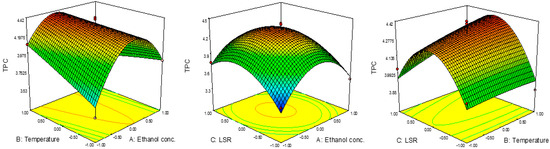
Figure 1.
Effect of ethanol concentration, temperature and TPC on RSM.
3.3. TFC
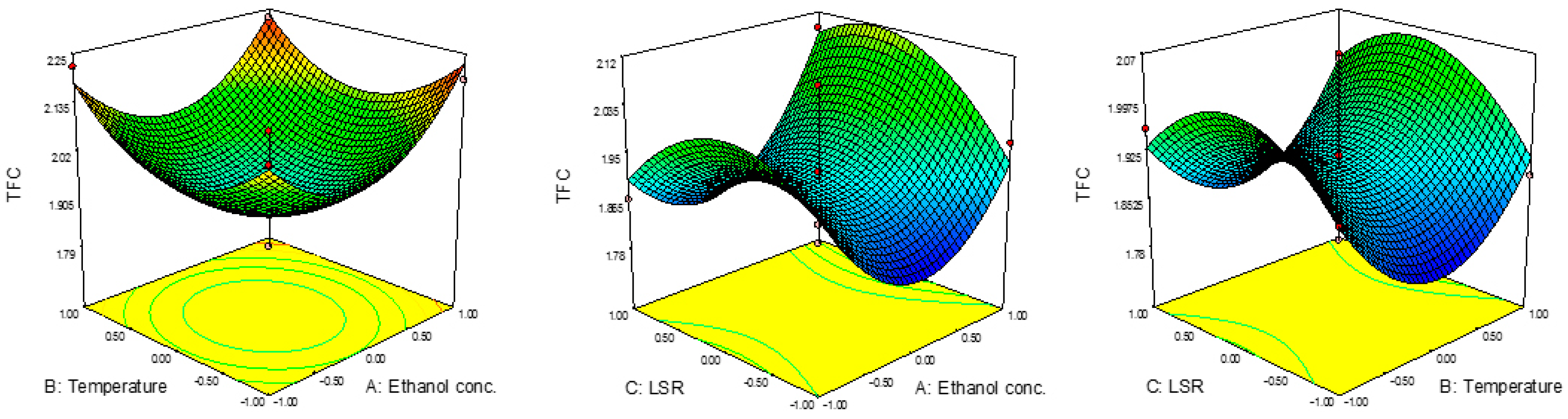
The yields for TFC are presented in Table 2, where TFC ranged from 1.79 mg ER/g to 2.23 mg ER/g. The lowest result for TFC was obtained at 60%, 60 °C and 20 mL/g, while the highest was noticed at 80%, 80 °C and 20 mL/g. It might be noticed that the conditions were quite different compared to those for TPC, i.e., the conditions for the highest TPC content revealed the lowest TFC content. The combined effects of the investigated parameters on TFC are presented in Figure 2. ANOVA results showed the significant influence of the quadratic terms of ethanol concentration and temperature on this investigated response. In this case, all linear terms exhibited a positive effect on TFC, while the quadratic term of LSR showed a negative effect. The combination of effects suggested that TFC would decrease up to certain value with the increase in ethanol concertation and temperature, then would start to increase. In the case of LSR, it would be vice versa, i.e., TFC would increase with LSR up to certain value and then start to decrease. The predicted second-order polynomial model for TFC is:
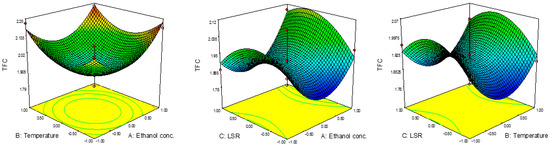
Figure 2.
Effect of ethanol concentration, temperature and TFC on RSM.
3.4. DPPH and ABTS Assays
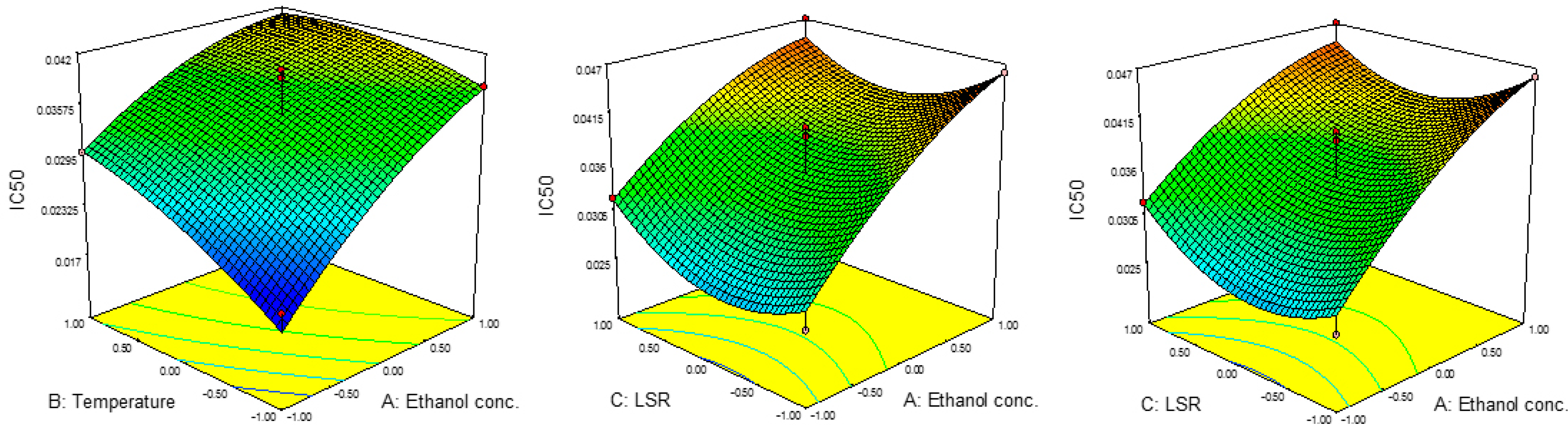
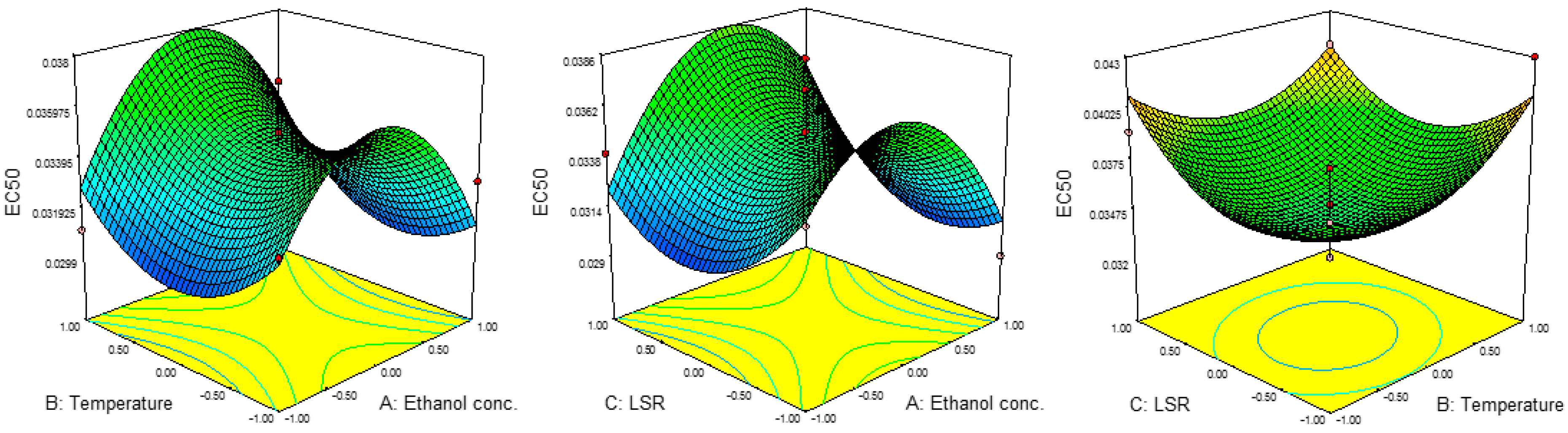
Results for the IC50 and EC50 values are presented in Table 2. The IC50 values were in the range of 0.020 to 0.047 mg/mL, while EC50 ranged from 0.029 mg/mL to 0.041 mg/mL. The highest value for IC50 was achieved at 80%, 60 °C and 30 mL/g, while those parameters for EC50 were 60%, 80 °C and 30 mL/g. The lowest IC50 was noticed at 40%, 40 °C and 20 mL/g, while EC50 reached its minimum at 80%, 60 °C and 10 mL/g.
Results for the ANOVA revealed the significant influence of the linear terms of ethanol concentration and temperature on IC50 values as well as the significant influence of all three quadratic terms on EC50 values. The combined effects of parameters on the IC50 and EC50 values are presented in Figure 3 and Figure 4. The results for regression coefficients (Table 3) showed the positive influence of linear terms in both cases, while the quadratic terms of ethanol concentration and temperature showed a negative influence on IC50 values, together with the negative influence of the quadratic term of ethanol concentration on EC50 values. The positive influence of the linear term of ethanol concentration on IC50 values suggested a linear increase in the IC50 value with the concentration. This would also be the case with the influence of the temperature, while with the increase in LSR, IC50 would decrease up to a certain value and then start to increase. In the case of EC50, this influence was different. In the case of ethanol concentration, EC50 would rise at first, but then would start to decrease. On the other hand, the influences of temperature and LSR were the same, i.e., the EC50 value decreased up to a certain value and then started to increase. The predicted second-order polynomial models for IC50 and EC50 are:
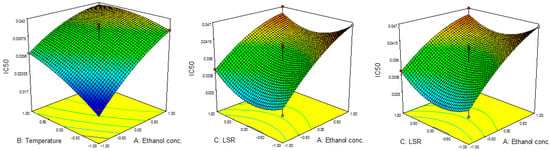
Figure 3.
Effect of ethanol concentration, temperature and IC50 on RSM.
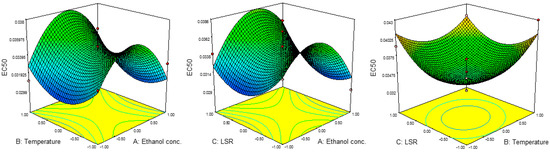
Figure 4.
Effect of ethanol concentration, temperature and EC50 on RSM.
3.5. Optimization Process
Optimization of the process represents the main goal of RSM application in this case. In order to accomplish this goal, the process was optimized searching for maximal TPC and TFC as well as the minimal IC50 and EC50 values simultaneously. The optimal conditions were 20%, 80 °C and 21.4 mL/g, for ethanol concentration, temperature and LSR, respectively. After optimization, the extract was prepared under the optimal conditions and predicted values were compared with those experimentally obtained (Table 5).

Table 5.
Predicted and experimentally obtained values for investigated responses.
From the presented results in Table 5, it might be concluded that the predicted and experimentally obtained values were quite similar; thus, optimization might be considered as successful. There are not a large amount of literature data that have dealt with the chemistry of this plant species. By comparing the results with the research of those authors who dealt with this plant species [25], we can state that the content of total phenols and flavonoids is high.
3.6. Polyphenolic Profile
Table 6 gives the relevant analytical parameters necessary for the identification of selected phenolic compounds, including a list of relevant analytical standards.

Table 6.
Analytical parameters for 18 phenolic compounds used for HPLC-DAD analysis.
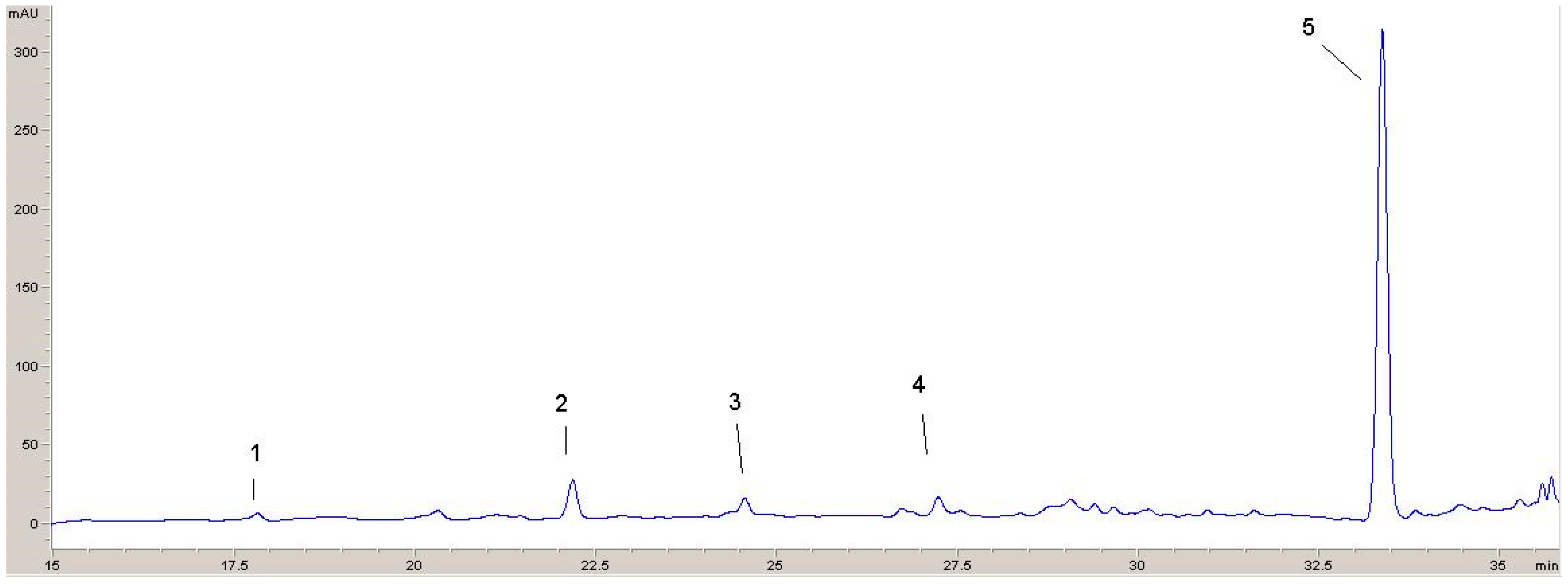
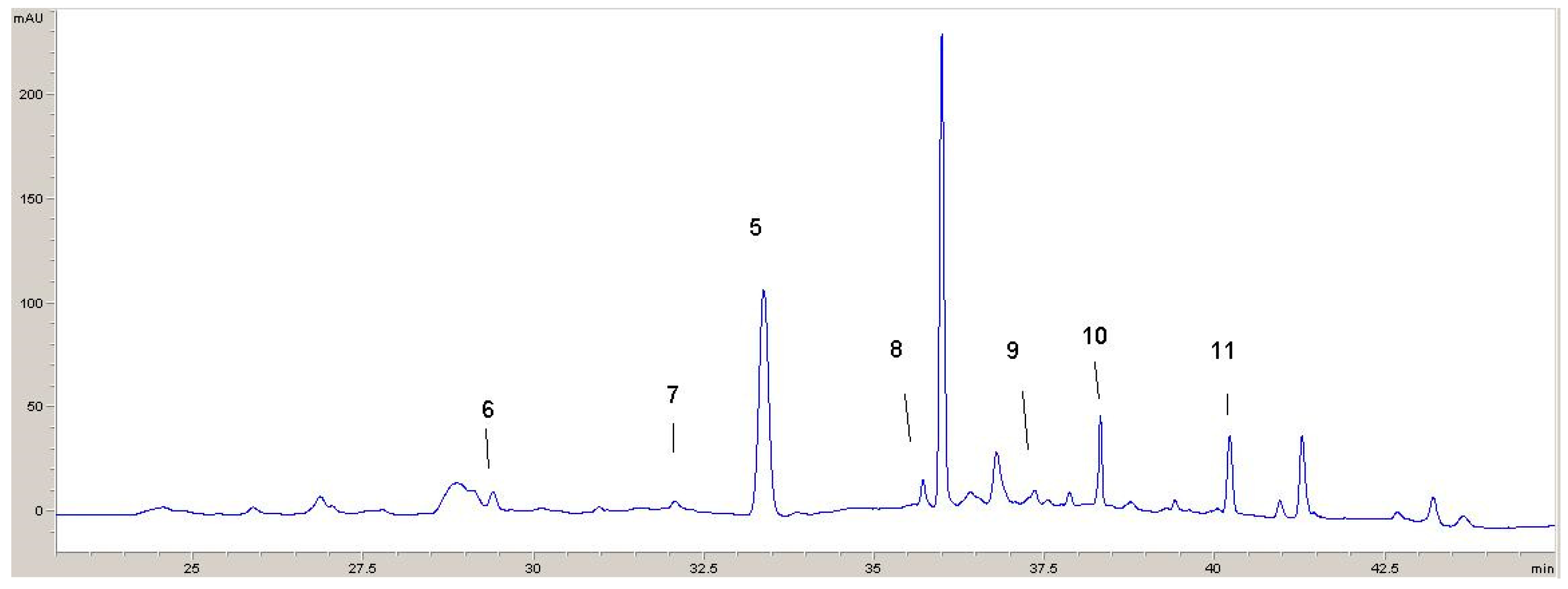
Two chromatograms recorded at 320 nm and 360 nm with identified phenolic compounds are shown (Figure 5 and Figure 6). From these chromatograms, the retention times for the various components can be seen.
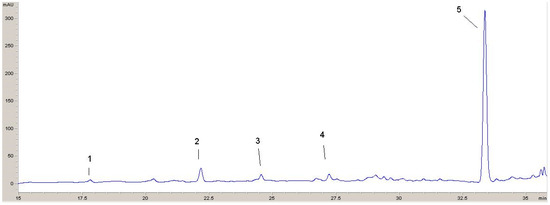
Figure 5.
HPLC chromatogram recorded at 320 nm: 1—p-hydroxybenzoic acid; 2—vanillic acid; 3—p-coumaric acid; 4—ferulic acid; 5—rosmarinic acid.
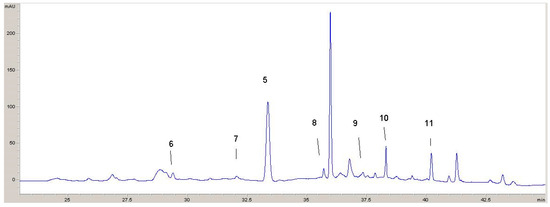
Figure 6.
HPLC chromatogram recorded at 360 nm: 5—rosmarinic acid; 6—rutin; 7—apigenin glucoside; 8—quercetin; 9—luteolin; 10—kaempferol; 11—apigenin.
The polyphenolic profile of the extract obtained under the optimal conditions was established by HPLC-DAD analysis, while the results are presented in Table 7.

Table 7.
Phenolic profile of S. hortensis L. extract.
The obtained results show that the dominant compound in the investigated extract was rosmarinic acid (46.172 µg/mL) followed by chlorogenic acid (1.519 µg/mL). Other compounds were detected and quantified in rather trace levels, especially compared to rosmarinic acid. The presence of rosmarinic acid, apigenin, luteolin and quercetin is inconsistent with the previously performed study (Exarchou et al., 2002). However, the authors also reported the presence of caffeic acid (Exarchou et al., 2002), which was not detected in this study.
The results show that the predicted values of the investigated responses (TPC, TFC, antioxidant activity) were very close to the experimentally obtained values, indicating the model’s predictive capability and reliability. This validates the use of the RSM approach for optimizing the extraction process. The study suggests that additional information or analyses could be conducted to further characterize the bioactive phytochemical composition and potential applications of the S. hortensis L. extract, such as compound identification, bioactivity testing or formulation development.
4. Conclusions
The preparation of extracts with a high content of biologically active species such as phenolic and flavonoid compounds and high antioxidant activity at the same time requires deep investigation and careful selection of extraction parameters. In this study, RSM was successfully applied for optimization of the ultrasound extraction of S. hortensis L. The obtained results demonstrated that the second-order polynomial model was able to successfully describe the process of the extraction of phenolics and flavonoids from the investigated plant. The available data obtained from ANOVA showed different influences of the tested parameters on the investigated responses. In the end, RSD in combination with analysis of variance successfully predicted the values of the investigated parameters, which in turn proved to be very close to the experimentally obtained ones. The study contributes to the understanding of optimization strategies for extracting valuable secondary metabolites from plant sources and can be extended to other plant species or natural matrices.
Author Contributions
Conceptualization, J.M.; Methodology, L.V.K.; Formal analysis, M.M.; Investigation, P.Z.M.; Writing—original draft, J.M.M. and P.Z.M.; Supervision, V.J. and V.Ž. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plánder, S.; Gontaru, L.; Blazics, B.; Veres, K.; Kéry, Á.; Kareth, S.; Simándi, B. Major antioxidant constituents from Satureja hortensis L. extracts obtained with different solvents. Eur. J. Lipid Sci. Technol. 2012, 114, 772–779. [Google Scholar] [CrossRef]
- Deans, S.G.; Svoboda, K.P. Antibacterial activity of summer savory (Satureja hortensis L) essential oil and its constituents. J. Hortic. Sci. 1989, 64, 205–210. [Google Scholar] [CrossRef]
- Dorman, H.J.; Hiltunen, R. Fe(III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L.) extract and subfractions. Food Chem. 2004, 88, 193–199. [Google Scholar] [CrossRef]
- Esquível, M.M.; Ribeiro, M.A.; Bernardo-Gil, M.G. Supercritical extraction of savory oil: Study of antioxidant activity and extract characterization. J. Supercrit. Fluids 1999, 14, 129–138. [Google Scholar] [CrossRef]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant Activities and Phenolic Composition of Extracts from Greek Oregano, Greek Sage, and Summer Savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Güllüce, M.; Sökmen, M.; Daferera, D.; Aǧar, G.; Özkan, H.; Kartal, N.; Polissiou, M.; Sökmen, A.; Şahïn, F. In Vitro Antibacterial, Antifungal, and Antioxidant Activities of the Essential Oil and Methanol Extracts of Herbal Parts and Callus Cultures of Satureja hortensis L. J. Agric. Food Chem. 2003, 51, 3958–3965. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Pezeshkian, S.K. Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J. Ethnopharmacol. 2002, 82, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F.; Karaman, İ.; Güllüce, M.; Öğütçü, H.; Şengül, M.; Adıgüzel, A.; Öztürk, S.; Kotan, R. Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol. 2003, 87, 61–65. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A.; Pappas, C.S.; Polissiou, M.G. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 2012, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Tomao, V.; Virot, M. Ultrasound-Assisted Extraction in Food Analysis. In Handbook of Food Analysis Instruments; Ötles, S., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 85–94. [Google Scholar]
- Paniwnyk, L.; Beaufoy, E.; Lorimer, J.; Mason, T. The extraction of rutin from flower buds of Sophora japonica. Ultrason. Sonochem. 2001, 8, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Zekovic, Z.; Djurovic, S.; Pavlic, B. Optimization of ultrasound-assisted extraction of polyphenolic compounds from coriander seeds using response surface methodology. Acta Period. Technol. 2016, 249–263. [Google Scholar] [CrossRef]
- Adamczyk, J.; Horny, N.; Tricoteaux, A.; Jouan, P.-Y.; Zadam, M. On the use of response surface methodology to predict and interpret the preferred c-axis orientation of sputtered AlN thin films. Appl. Surf. Sci. 2008, 254, 1744–1750. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorometry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Takao, T.; Kitatani, F.; Watanabe, N.; Yagi, A.; Sakata, K. A Simple Screening Method for Antioxidants and Isolation of Several Antioxidants Produced by Marine Bacteria from Fish and Shellfish. Biosci. Biotechnol. Biochem. 1994, 58, 1780–1783. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of some Scottish plants for free radical scavenging activity. Phyther. Res. 2007, 21, 615–621. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Jabbari, N.; Goli, M.; Shahi, S. Optimization of Bioactive Compound Extraction from Saffron Petals Using Ultrasound-Assisted Acidified Ethanol Solvent: Adding Value to Food Waste. Foods 2024, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Mašković, P.; Veličković, V.; Mitić, M.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted in enhanced bilogical activity. Ind. Crop. Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).