Application of Optimized Dry Fractionation Process for Nutritional Enhancement of Different Sunflower Meals
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Processing and Dry Fractionation Process
2.3. Laboratory Analysis
2.3.1. Chemical Analysis
2.3.2. Amino Acid Analysis
2.3.3. Physical Analysis
2.3.4. Statistical Analysis
2.4. Process Efficiency Indicators
- -
- Protein content and relative protein enrichment Δpe (%);
- -
- Fraction yield ye (%);
- -
- Total specific grinding energy consumption Ete (Wh/kg).
2.4.1. Relative Protein Enrichment
2.4.2. Fraction Yield
2.4.3. Energy Consumption
3. Results and Discussion
3.1. Chemical Properties
3.2. Physical Properties
| Sunflower Meal | GMD (µm) | Bulk Density (kg/m3) | Flowability Properties | |
|---|---|---|---|---|
| Angle of Repose (°) | Flowability Rating | |||
| SFM1 | 1174.6 ± 16.6 a | 468.1 ± 2.0 b | 34.9 ± 0.2 a.b | Good |
| SFM1:HM | 462.2 ± 4.3 b | 499.5 ± 2.9 a | 33.0 ± 0.3 b | Good |
| SFM1:RM | 333.2 ± 1.2 c | 460.0 ± 4.4 b | 35.9 ± 0.1 a | Fair |
| SFM2 | 530.8 ± 7.8 a | 404.8 ± 1.5 b | 37.9 ± 0.1 a | Fair |
| SFM2:HM | 431.8 ± 1.7 b | 431.7 ± 4.4 a | 35.7 ± 0.1 b | Fair |
| SFM2:RM | 353.5 ± 2.1 c | 405.8 ± 8.4 b | 38.2 ± 0.3 a | Fair |
| SFM3 | 828.9 ± 17.8 a | 460.7 ± 2.6 a | 38.0 ± 0.1 b | Fair |
| SFM3:HM | 406.1 ± 11.1 b | 456.6 ± 3.5 a | 39.6 ± 0.1 a | Fair |
| SFM3:RM | 364.8 ± 2.7 c | 444.4 ± 2.6 b | 40.0 ± 0.1 a | Fair |
3.3. Dry Fractionation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aiking, H. Future protein supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Cuur. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Van der Poel, A.F.B.; Abdollahi, M.R.; Cheng, H.; Čolović, R.; Den Hartog, L.A.; Miladinović, D.; Page, G.; Sijssens, K.; Smililie, J.F.; Thomas, M.; et al. Future directions of animal feed technology research to meet the challenges of a changing world. Anim. Feed Sci. Technol. 2020, 270, 114692. [Google Scholar] [CrossRef]
- Pond, W.G.; Church, D.B.; Pond, K.R.; Schoknecht, P.A. Basic Animal Nutrition and Feeding; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zentek, J.; Goodarzi Boroojeni, F. (Bio) Technological processing of poultry and pig feed: Impact on the composition, digestibility, anti-nutritional factors and hygiene. Anim. Feed Sci. Technol. 2020, 268, 114576. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting Global Feed Protein Demand: Challenge, Opportunity, and Strategy. Annu. Rev. Anim. Biosci. 2019, 7, 221–243. [Google Scholar] [CrossRef]
- Parisi, G.; Tulli, F.; Fortina, R.; Marino, R.; Bani, P.; Dalle Zotte, A.; De Angelis, A.; Piccolo, G.; Pinnotti, L.; Schiavone, A.; et al. Protein hunger of the feed sector: The alternatives offered by the plant world. Ital. J. Anim. Sci. 2020, 19, 1205–1227. [Google Scholar] [CrossRef]
- Wu, G. Principles of Animal Nutrition; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Alagawany, M.; Farag, M.R.; Abd El-Hack, M.E.; Dhama, K. The practical application of sunflower meal in poultry nutrition. Adv. Anim. Vet. Sci. 2015, 3, 634–648. [Google Scholar] [CrossRef]
- Murru, M.; Calvo, C.L. Sunflower protein enrichment. Methods and potential applications. OCL—Oilseeds Fats Crops Lipids 2020, 27, 17. [Google Scholar] [CrossRef]
- Mushtaq, T.; Sarwar, M.; Ahmad, G.; Nisa, M.U.; Jamil, A. The influence of exogenous multienzyme preparation and graded levels of digestible lysine in sunflower meal-based diets on the performance of young broiler chicks two weeks posthatching. Poult. Sci. 2006, 85, 2180–2185. [Google Scholar] [PubMed]
- Raza, S.; Ashraf, M.; Pasha, T.N.; Latif, F. Effect of enzyme supplementation of broiler diets containing varying level of sunflower meal and crude fiber. Pak. J. Bot. 2009, 41, 2543–2550. [Google Scholar]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond oil: Press cakes and meals supplying global protein requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Van der Goot, A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Technol. 2011, 22, 154–164. [Google Scholar] [CrossRef]
- Banjac, V.; Čolović, R.; Vukmirović, Ð.; Sredanović, S.; Čolović, D.; Lević, J.; Teodosin, S. Protein enrichment of sunflower meal by air classification. Food Feed Res. 2013, 40, 77–83. [Google Scholar]
- Banjac, V.; Pezo, L.; Pezo, M.; Vukmirović, Ð.; Čolović, D.; Fišteš, A.; Čolović, R. Optimization of the classification process in the zigzag air classifier for obtaining a high protein sunflower meal–Chemometric and CFD approach. Adv. Powder Technol. 2017, 28, 1069–1078. [Google Scholar] [CrossRef]
- Laudadio, V.; Bastoni, E.; Introna, M.; Tufarelli, V. Production of low-fiber sunflower (Helianthus annuus L.) meal by micronization and air classification processes. CYTA J. Food 2013, 11, 398–403. [Google Scholar] [CrossRef]
- Laguna, O.; Barakat, A.; Alhamada, H.; Durand, E.; Baréa, B.; Fine, F.; Villeneuve, P.; Citeau, M.; Dauguet, S.; Lecomte, J. Production of proteins and phenolic compounds enriched fractions from rapeseed and sunflower meals by dry fractionation processes. Ind. Crops Prod. 2018, 118, 160–172. [Google Scholar] [CrossRef]
- Vidosavljević, S.; Bojanić, N.; Ilić, P.; Rakić, D.; Đuragić, O.; Banjac, V.; Fišteš, A. Optimization of Grinding Process of Sunflower Meal for Obtaining Protein-Enriched Fractions. Processes 2022, 10, 2704. [Google Scholar] [CrossRef]
- ISO 6496; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6492; Animal Feeding Stuffs—Determination of Fat Content. International Standards Organization: Genewa, Switzerland, 2001.
- ISO 5984; Animal Feeding Stuffs—Determination of Crude Ash. International Organization for Standardization: Genewa, Switzerland, 2013.
- ISO 5983-1; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content-Part 1: Kjeldahl Method (ISO 5983-1:2005). International Organization for Standardization: Genewa, Switzerland, 2010.
- AOCS. Official Methods and Recommended Practices of the AOCS; Official Method Ba 6-a05; The American Oil Chemists Society: Champaigns, IL, USA, 2005. [Google Scholar]
- Spackman, D.H.; Stein, W.H.; Moore, S. Automatic Recording Apparatus for Use in the Chromatography of Amino Acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- ISO 2591-1; Test Sieving-Part 1: Methods Using Test Sieves of Woven Wire Cloth and Perforated Metal Plate. International Organization for Standardization: Genewa, Switzerland, 1988.
- Vukmirović, Ð.; Lević, J.; Fišteš, A.; Čolović, R.; Brlek, T.; Čolović, D.; Đuragić, O. Influence of grinding method and grinding intensity of corn on mill energy consumption and pellet quality. Hem. Ind. 2016, 70, 67–72. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating the flow properties of solids. Chem. Eng. J. 1965, 72, 163–168. [Google Scholar]
- TIBCO Software Inc. Data Science Workbench; Version 14; Statistica 14; TIBCO Software Inc.: Palo Alto, CA, USA, 2020. [Google Scholar]
- Ditta, Y.A.; King, A.J. Recent advances in sunflower seed meal as an alternate source of protein in broilers. World’s Poult. Sci. J. 2017, 73, 527–542. [Google Scholar] [CrossRef]
- Official Gazette of Republic of Serbia. Regulation on Animal Feed Quality: 4/2010, 113/2012, 27/2014, 25/2015, 39/2016, 54/2017; Official Gazette of Republic of Serbia: Belgrade, Serbia, 2010. [Google Scholar]
- Syamsu, J.A.; Yusuf, M.; Abdullah, A. Evaluation of physical properties of feedstuffs in supporting the development of feed mill at farmers group scale. J. Adv. Agric. Technol. 2015, 2, 147–150. [Google Scholar] [CrossRef][Green Version]
- Dragojlović, D.; Đuragić, O.; Pezo, L.; Popović, L.; Rakita, S.; Tomičić, Z.; Spasevski, N. Comparison of nutritional profiles of super worm (Zophobas morio) and yellow mealworm (Tenebrio molitor) as alternative feeds used in animal husbandry: Is super worm superior? Animals 2022, 12, 1277. [Google Scholar] [CrossRef]
- Lević, J.; Delić, I.; Ivić, M.; Rac, M.; Stefanović, S. Removal of cellulose from sunflower meal by fractionation. J. Am. Oil Chem. Soc. 1992, 69, 890–893. [Google Scholar] [CrossRef]
- Sredanović, S.; Lević, J.; Đuragić, O.; Ivanov, D. Upgraded technology for sustainable sunflower meal production. PTEP 2009, 13, 265–267. [Google Scholar]
- Sredanović, S.A.; Lević, J.D.; Jovanović, R.D.; Đuragić, O.M. The nutritive value of poultry diets containing sunflower meal supplemented by enzymes. Acta Period. Technol. 2012, 43, 79–91. [Google Scholar] [CrossRef]
- Wang, W.W.; Feng, Q.Q.; Wang, J.; Wu, S.G.; Qi, G.H.; Zhang, H.J. Cyst (e) ine fortification in low crude protein diet improves growth performance of broilers by modulating serum metabolite profile. J. Proteom. 2021, 238, 104154. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, F.A.; Van Milgen, J.; Duperray, J.; Sergheraert, R.; Le Floc’h, N. Feeding intact proteins, peptides, or free amino acids to monogastric farm animals. Amino Acids 2022, 54, 157–168. [Google Scholar] [CrossRef]
- Dozier, W.A.; Hess, J.B. Soybean meal quality and analytical techniques. In Soybean and Nutrition; El-Shemy, H., Ed.; IntechOpen: London, UK, 2011; pp. 111–124. [Google Scholar]
- Liu, J.D.; Li, Q.Y.; Zeng, Z.K.; Li, P.; Xu, X.; Wang, H.L.; Zhang, S.; Piao, X.S. Determination and prediction of the amino acid digestibility of sunflower seed meals in growing pigs. Asian-Australas. J. Anim. Sci. 2015, 28, 86. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Jez, J.M. The promise and limits for enhancing sulfur-containing amino acid content of soybean seed. Plant Sci. 2018, 272, 14–21. [Google Scholar] [CrossRef] [PubMed]

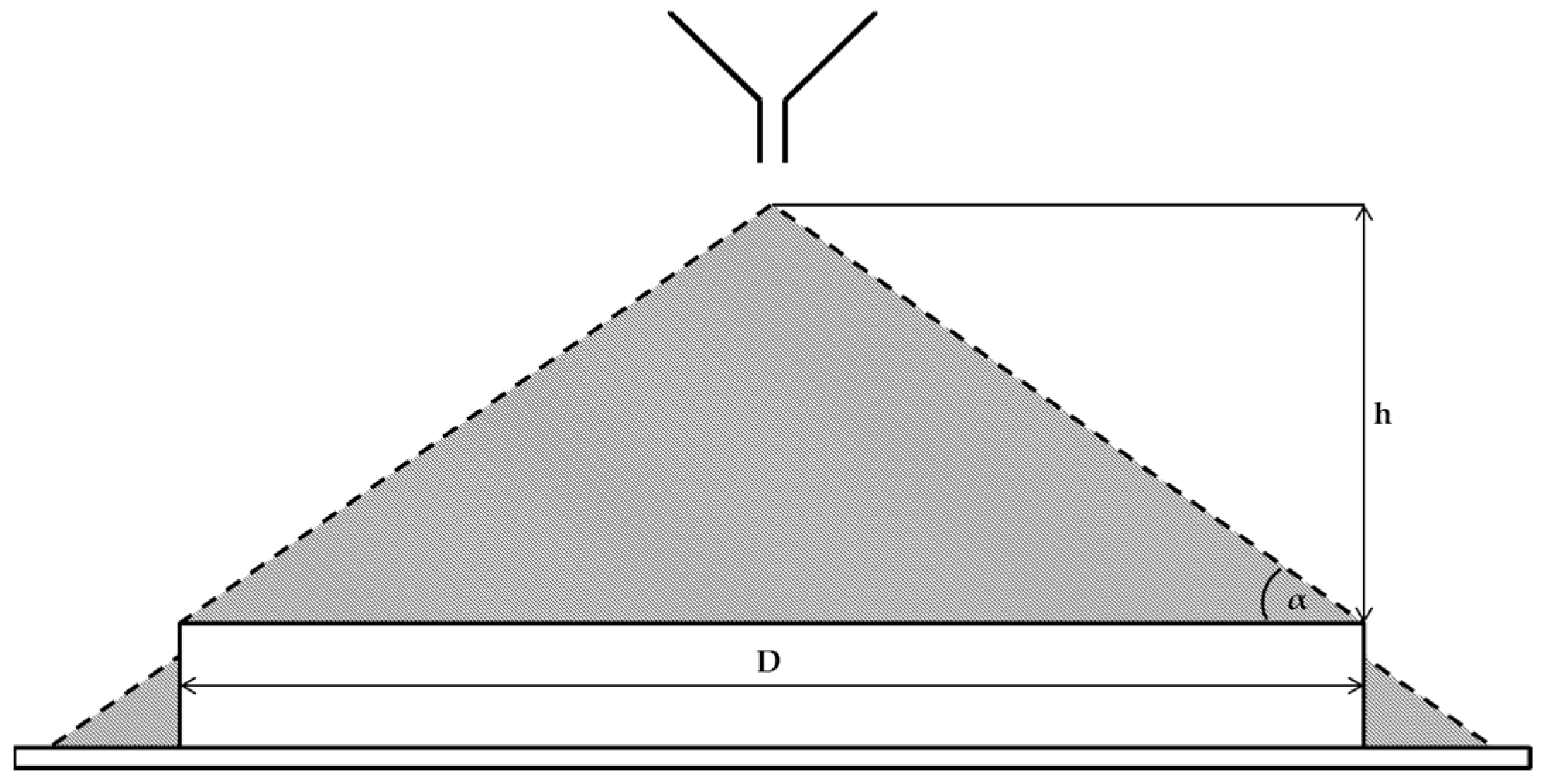
| Flowability Rating | Angle of Repose (°) |
|---|---|
| Excellent | 25–30 (or less) |
| Good | 31–35 |
| Fair | 36–40 |
| Passable | 41–45 |
| Poor | 46–55 |
| Very poor | 56–65 |
| Extremely poor | 66–90 |
| Chemical Composition | SFM1 | SFM2 | SFM3 | RSM | SBM |
|---|---|---|---|---|---|
| Moisture content (%) | 7.44 | 7.40 | 6.94 | 10.15 | 10.26 |
| Crude protein (%(dm)) | 35.64 | 32.89 | 35.82 | 35.51 | 48.04 |
| Crude fiber (%(dm)) | 21.70 | 23.53 | 22.44 | 7.51 | 7.25 |
| Crude fat (%(dm)) | 0.93 | 1.94 | 2.13 | 2.84 | 2.80 |
| Crude ash (%(dm)) | 7.44 | 7.05 | 6.01 | 6.51 | 6.52 |
| Observed Parameters | EFSFM1 * | EFSFM1 | EFSFM2 | EFSFM3 |
|---|---|---|---|---|
| Protein content after the process (%(dm)) | 45.50 | 44.20 | 43.16 | 42.09 |
| Relative protein enrichment (%) | / | 24.0 | 31.2 | 17.5 |
| Fraction yield (%) | 77.9 | 78.9 | 77.0 | 76.5 |
| Energy consumption (Wh/kg) | 8.31 | 8.44 | 9.30 | 9.93 |
| Aminoacid Content (%(dm)) | Starting SFMs and Enriched SFMs Fractions | RSM | SBM | |||||
|---|---|---|---|---|---|---|---|---|
| SFM1 | EFSFM1 | SFM2 | EFSFM2 | SFM3 | EFSFM3 | |||
| Total EAA | 10.77 d | 12.76 b | 9.71 e | 12.17 b,c | 10.62 d | 12.01 c | 12.30 b,c | 14.85 a |
| Leu | 1.47 d,e | 1.57 c | 1.40 e | 1.86 b | 1.53 c,d | 1.78 b | 1.79 b | 2.41 a |
| Val | 1.59 c | 1.93 a | 1.39 d | 1.77 b | 1.52 c | 1.70 b | 1.73 b | 1.90 a |
| Thr | 1.05 e | 1.37 b | 0.94 f | 1.21 d | 1.02 e | 1.20 d | 1.29 c | 1.49 a |
| Ile | 1.46 d | 1.84 b | 1.26 e | 1.65 c | 1.40 d | 1.60 c | 1.46 d | 2.02 a |
| Lys | 1.04 e | 1.33 c | 0.86 g | 1.17 d | 0.95 f | 1.13 d | 1.66 b | 2.19 a |
| Met | 0.62 c | 0.81 a | 0.49 d | 0.69 b | 0.59 c | 0.85 a | 0.61 c | 0.56 c |
| His | 0.73 c | 0.92 a | 0.60 d | 0.82 b | 0.67 c,d | 0.82 b | 0.81 b | 0.97 a |
| Phe | 2.81 c,d | 2.98 b | 2.75 d | 2.99 b | 2.96 b,c | 2.93 b,c | 2.95 b,c | 3.30 a |
| Total NEAA | 13.86 c | 17.24 a | 12.66 d | 16.16 b | 13.88 c | 15.76 b | 12.87 d | 17.95 a |
| Glu | 2.91 c | 3.50 a | 2.62 d | 3.34 b | 2.88 c | 3.26 b | 2.74 d | 3.51 a |
| Asp | 2.71 e | 3.39 b | 2.38 f | 3.09 c | 2.65 e | 2.95 d | 2.24 g | 4.17 a |
| Pro | 0.22 c | 0.35 b | 0.21 c | 0.32 b | 0.24 c | 0.38 b | 0.36 b | 0.60 a |
| Ala | 1.23 d,e | 1.53 a | 1.10 f | 1.40 b | 1.19 e,f | 1.34 b,c | 1.31 c,d | 1.56 a |
| Arg | 2.36 c | 3.07 a | 1.94 e | 2.80 b | 2.21 d | 2.73 b | 1.83 e | 2.75 b |
| Gly | 2.63 d | 3.06 a,b | 2.88 c | 3.12 a | 2.95 b,c | 3.07 a,b | 2.37 e | 2.54 d |
| Ser | 1.08 d,e | 1.40 b | 0.95 f | 1.27 c | 1.06 e | 1.23 c | 1.14 d | 1.63 a |
| Tyr | 0.72 d,e | 0.96 b | 0.58 f | 0.81 c | 0.69 e | 0.80 c,d | 0.89 b | 1.20 a |
| Cys | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total AA | 24.62 d | 30.00 b | 22.37 e | 28.33 c | 24.50 d | 27.77 c | 25.16 d | 32.80 a |
| The relative AA enrichment (%) | / | 21.85 | / | 26.64 | / | 13.35 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidosavljević, S.; Bojanić, N.; Dragojlović, D.; Stojkov, V.; Sedlar, T.; Banjac, V.; Fišteš, A. Application of Optimized Dry Fractionation Process for Nutritional Enhancement of Different Sunflower Meals. Processes 2025, 13, 255. https://doi.org/10.3390/pr13010255
Vidosavljević S, Bojanić N, Dragojlović D, Stojkov V, Sedlar T, Banjac V, Fišteš A. Application of Optimized Dry Fractionation Process for Nutritional Enhancement of Different Sunflower Meals. Processes. 2025; 13(1):255. https://doi.org/10.3390/pr13010255
Chicago/Turabian StyleVidosavljević, Strahinja, Nemanja Bojanić, Danka Dragojlović, Viktor Stojkov, Tea Sedlar, Vojislav Banjac, and Aleksandar Fišteš. 2025. "Application of Optimized Dry Fractionation Process for Nutritional Enhancement of Different Sunflower Meals" Processes 13, no. 1: 255. https://doi.org/10.3390/pr13010255
APA StyleVidosavljević, S., Bojanić, N., Dragojlović, D., Stojkov, V., Sedlar, T., Banjac, V., & Fišteš, A. (2025). Application of Optimized Dry Fractionation Process for Nutritional Enhancement of Different Sunflower Meals. Processes, 13(1), 255. https://doi.org/10.3390/pr13010255







