Hydrolytic Decomposition of Corncobs to Sugars and Derivatives Using Subcritical Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Proximate and Ultimate Analysis of Corncobs
2.2.2. Hydrolysis of Corncobs in Subcritical Water
2.2.3. Determination of Total Carbohydrates (TCH)
2.2.4. Determination of Sugars and Sugar Derivatives
2.2.5. Determination of the Total Phenol Content
2.2.6. Antioxidant Activity
2.2.7. Statistical Analysis
3. Results
3.1. Characterization of Corncobs
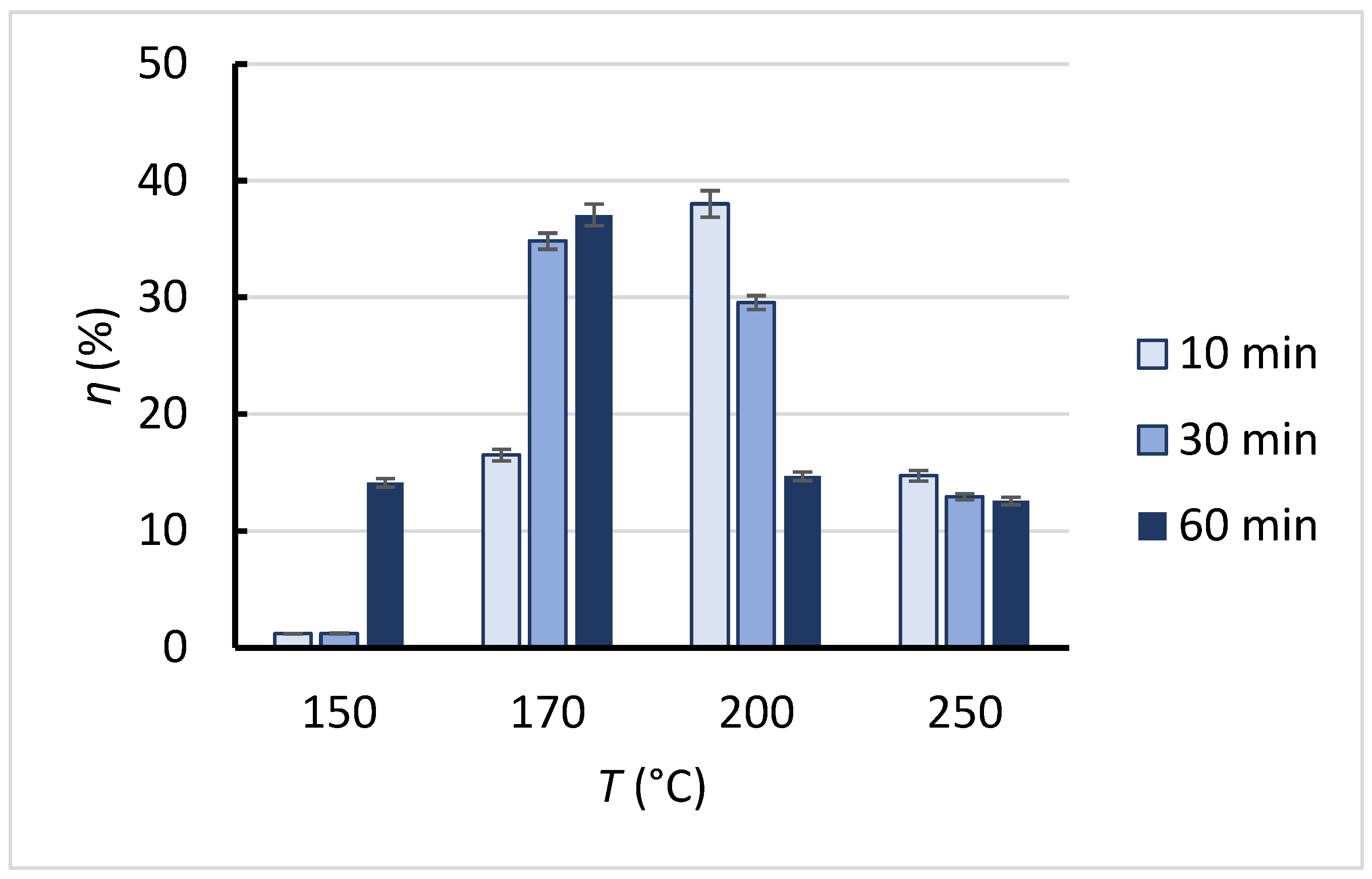
3.2. The Yield of Water-Soluble Product
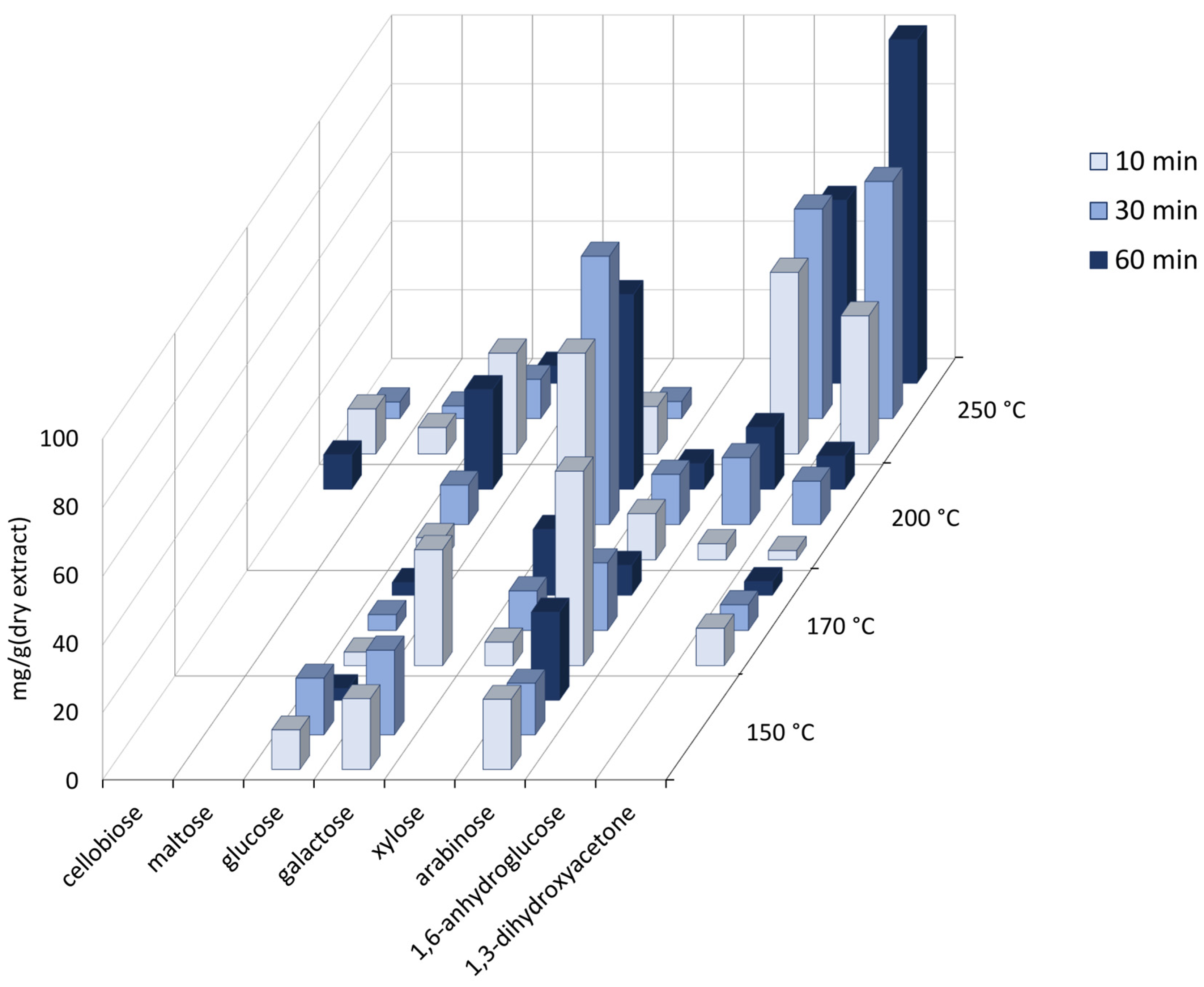
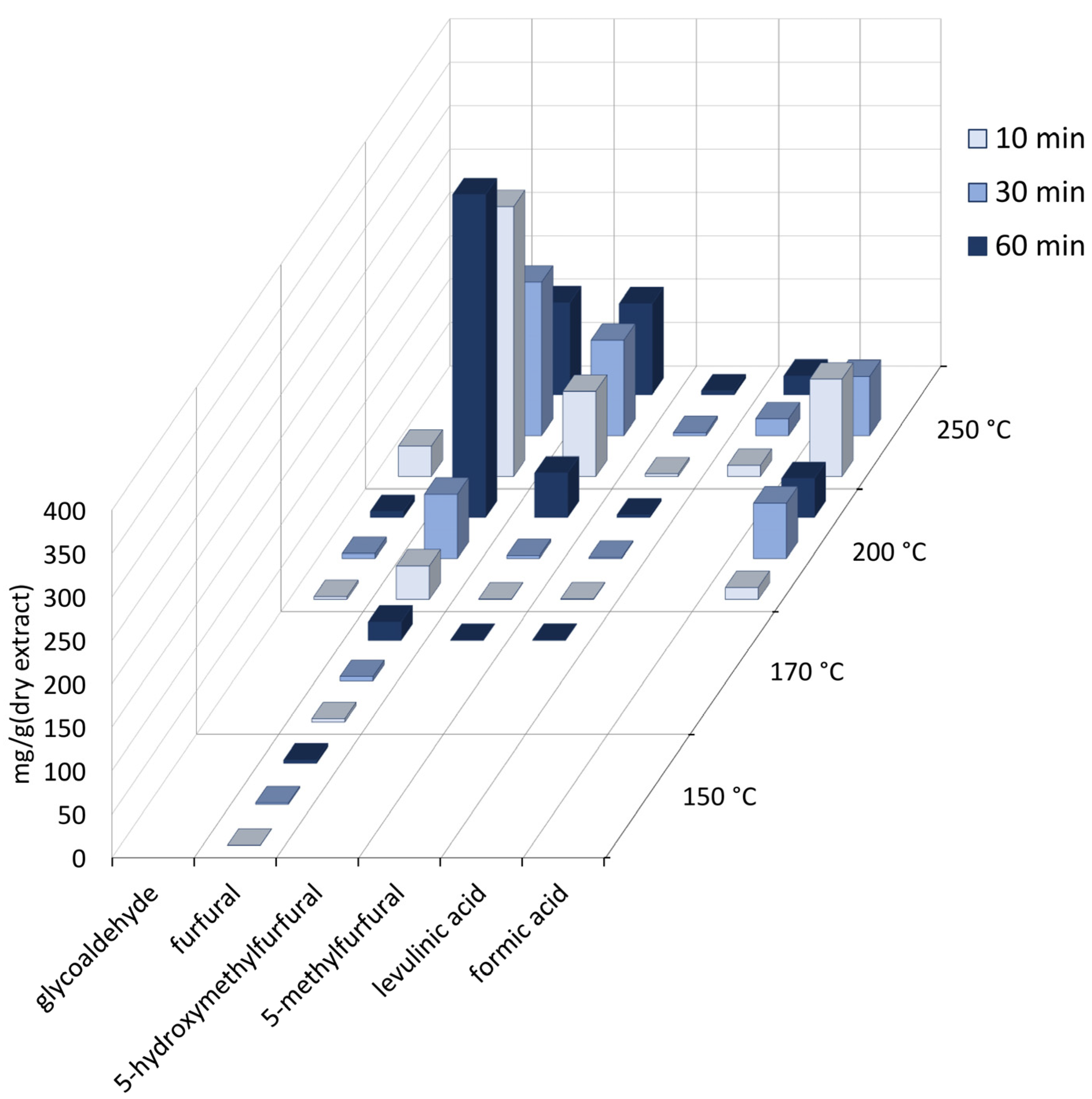
3.3. Content of Sugars and Sugar Derivatives
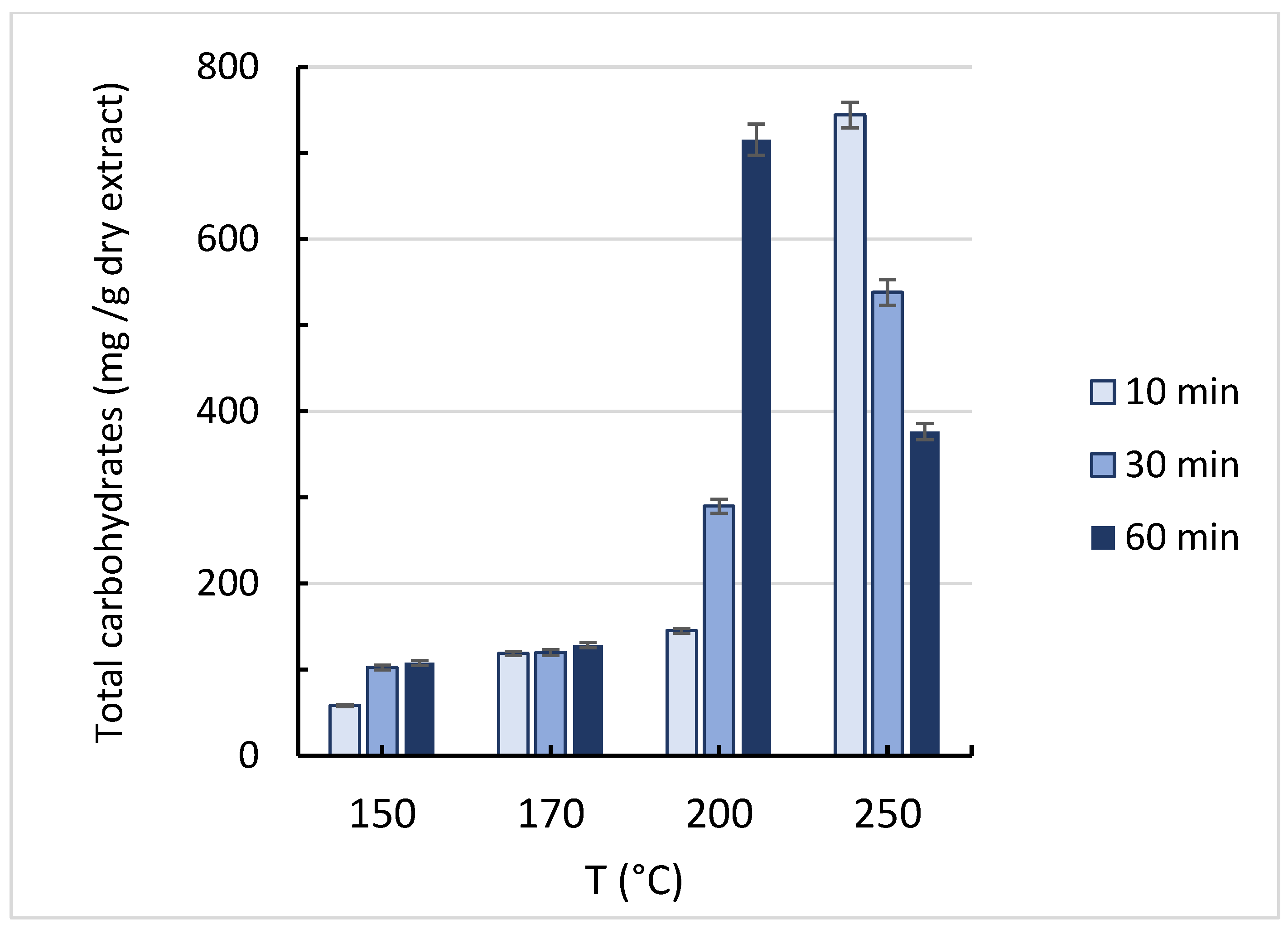
3.4. Total Carbohydrates
3.5. Antioxidant Activity and Content of Total Phenols
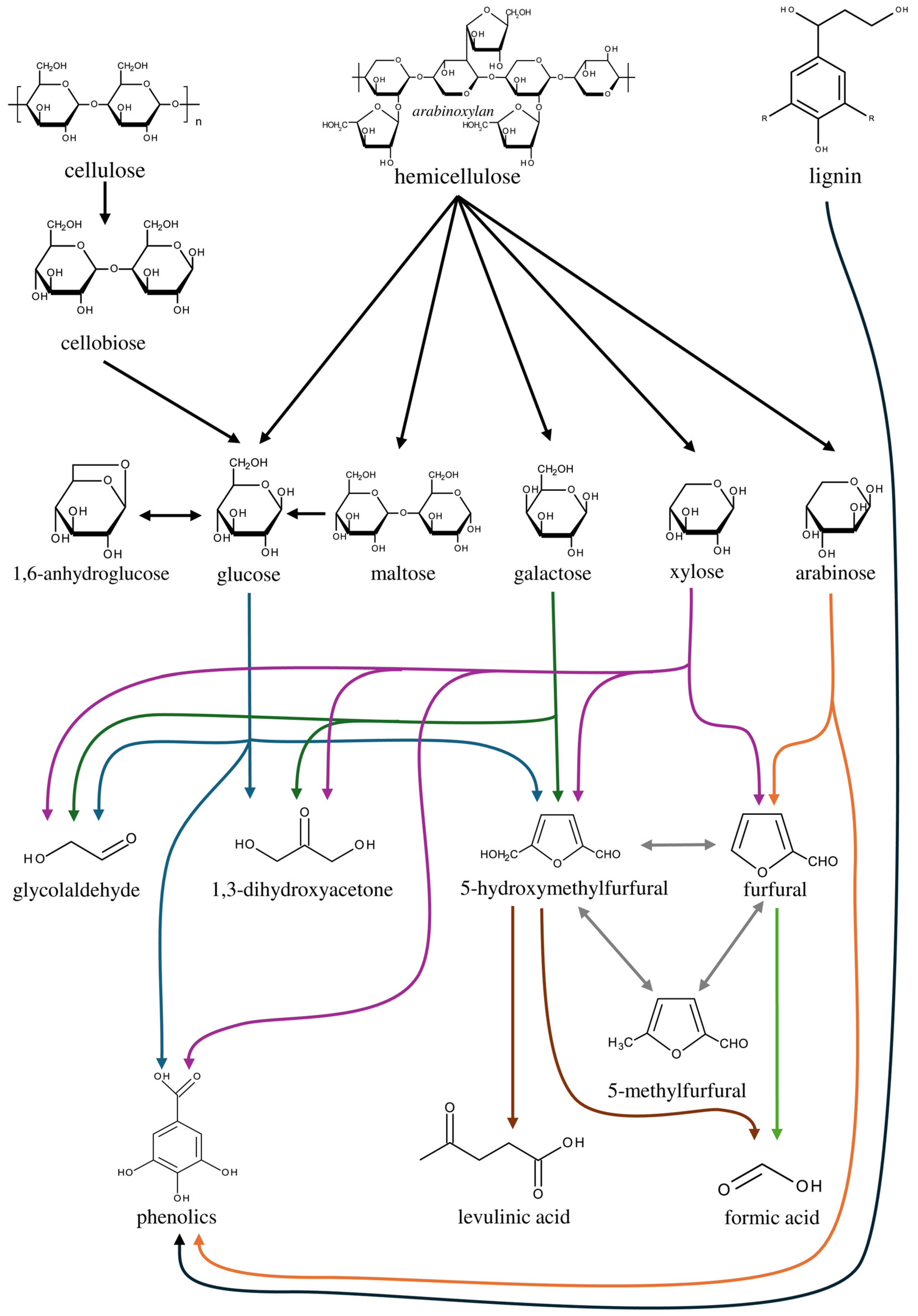
4. Degradation Mechanisms of Corncobs (Lignocellulosic Biomass) in Subcritical Water
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Knez, Ž.; Hrnčič, M.K.; Čolnik, M.; Škerget, M. Chemicals and Value Added Compounds from Biomass Using Sub- and Supercritical Water. J. Supercrit. Fluids 2018, 133, 591–602. [Google Scholar] [CrossRef]
- Bergamasco, S.; Zikeli, F.; Vinciguerra, V.; Sobolev, A.P.; Scarnati, L.; Tofani, G.; Scarascia Mugnozza, G.; Romagnoli, M. Extraction and Characterization of Acidolysis Lignin from Turkey Oak (Quercus cerris L.) and Eucalypt (Eucalyptus camaldulensis Dehnh.) Wood from Population Stands in Italy. Polymers 2023, 15, 3591. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.M.H.D.; Wickramasinghe, C.; Samarasiri, B.K.T.; Narayana, M. Modeling of Thermochemical Conversion of Waste Biomass—A Comprehensive Review. Biofuel Res. J. 2021, 8, 1481–1528. [Google Scholar] [CrossRef]
- Govumoni, S.P.; Koti, S.; Kothagouni, S.Y.; Venkateshwar, S.; Linga, V.R. Evaluation of Pretreatment Methods for Enzymatic Saccharification of Wheat Straw for Bioethanol Production. Carbohydr. Polym. 2013, 91, 646–650. [Google Scholar] [CrossRef]
- Tshikovhi, A.; Motaung, T.E. Technologies and Innovations for Biomass Energy Production. Sustainability 2023, 15, 12121. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol Production from Agricultural Wastes: An Overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K. Agricultural Wastes and Their By-Products for the Energy Market. Energies 2024, 17, 2099. [Google Scholar] [CrossRef]
- Corn Production Worldwide 2023/24. Available online: https://www.statista.com/statistics/1156213/global-corn-production/ (accessed on 2 December 2024).
- Samanta, A.K.; Senani, S.; Kolte, A.P.; Sridhar, M.; Sampath, K.T.; Jayapal, N.; Devi, A. Production and in Vitro Evaluation of Xylooligosaccharides Generated from Corn Cobs. Food Bioprod. Process. 2012, 90, 466–474. [Google Scholar] [CrossRef]
- Ayeni, A.O.; Daramola, M.O. Lignocellulosic Biomass Waste Beneficiation: Evaluation of Oxidative and Non-Oxidative Pretreatment Methodologies of South African Corn Cob. J. Environ. Chem. Eng. 2017, 5, 1771–1779. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Ren, J.; Deng, H.; Peng, F.; Sun, R. Functional Relationship of Furfural Yields and the Hemicellulose-Derived Sugars in the Hydrolysates from Corncob by Microwave-Assisted Hydrothermal Pretreatment. Biotechnol. Biofuels 2015, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Oni, O.; Olajuyigbe, F.; Fatokun, C. Effective Substrate Loading for Saccharification of Corn Cob and Concurrent Production of Lignocellulolytic Enzymes by Fusarium Oxysporum and Sporothrix Carnis. Curr. Biotechnol. 2019, 8, 109–115. [Google Scholar] [CrossRef]
- Pointner, M.; Kuttner, P.; Obrlik, T.; Jäger, A.; Kahr, H. Composition of Corncobs as a Substrate for Fermentation of Biofuels. Agron. Res. 2014, 12, 391–396. [Google Scholar]
- Ranilla, L.G.; Rios-Gonzales, B.A.; Ramírez-Pinto, M.F.; Fuentealba, C.; Pedreschi, R.; Shetty, K. Primary and Phenolic Metabolites Analyses, In Vitro Health-Relevant Bioactivity and Physical Characteristics of Purple Corn (Zea mays L.) Grown at Two Andean Geographical Locations. Metabolites 2021, 11, 722. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple Corn (Zea mays L.) Phenolic Compounds Profile and Its Assessment as an Agent against Oxidative Stress in Isolated Mouse Organs. J. Med. Food. 2012, 15, 206–215. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanin, Phenolics and Antioxidant Activity Changes in Purple Waxy Corn as Affected by Traditional Cooking. Food Chem. 2014, 164, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.; Zhou, Q.; Zhang, L. Furfural Production from Biomass Residues: Current Technologies, Challenges and Future Prospects. Biomass Bioenergy 2022, 161, 106458. [Google Scholar] [CrossRef]
- Qu, W.-H.; Xu, Y.-Y.; Lu, A.-H.; Zhang, X.-Q.; Li, W.-C. Converting Biowaste Corncob Residue into High Value Added Porous Carbon for Supercapacitor Electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Anukam, A.I.; Goso, B.P.; Okoh, O.O.; Mamphweli, S.N. Studies on Characterization of Corn Cob for Application in a Gasification Process for Energy Production. J. Chem. 2017, 2017, 6478389. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, W.; Wang, W.; Zhang, Y.; Leksawasdi, N.; Zhuang, X.; Yu, Q.; Yuan, Z. Production of Furfural with High Yields from Corncob under Extremely Low Water/Solid Ratios. Renew. Energy 2019, 144, 139–146. [Google Scholar] [CrossRef]
- Winarsih, S.; Siskawardani, D.D. Hydrolysis of Corncobs Using a Mixture of Crude Enzymes from Trichoderma Reesei and Aspergillus Niger for Bioethanol Production. Energy Rep. 2020, 6, 256–262. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green Technologies for the Extraction of Bioactive Compounds in Fruits and Vegetables. CyTA—J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Pavlovič, I.; Knez, Ž.; Škerget, M. Hydrothermal Reactions of Agricultural and Food Processing Wastes in Sub- and Supercritical Water: A Review of Fundamentals, Mechanisms, and State of Research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.S.; Haghighi Asl, A.; Khajenoori, M. Production of Pharmaceutical Micro and Nano Particles by Subcritical Water Based Technologies: A Review. J. Drug Deliv. Sci. Technol. 2023, 85, 104621. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Gagić, T.; Knez, Ž.; Banožić, M.; Škerget, M. Separation of Active Compounds from Tobacco Waste Using Subcritical Water Extraction. J. Supercrit. Fluids 2019, 153, 104593. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical Water Extraction of Phenolic and Antioxidant Constituents from Pistachio (Pistacia vera L.) Hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Čolnik, M.; Irgolič, M.; Perva, A.; Škerget, M. The Conversion of Pistachio and Walnut Shell Waste into Valuable Components with Subcritical Water. Processes 2024, 12, 195. [Google Scholar] [CrossRef]
- Ravber, M.; Knez, Ž.; Škerget, M. Isolation of Phenolic Compounds from Larch Wood Waste Using Pressurized Hot Water: Extraction, Analysis and Economic Evaluation. Cellulose 2015, 22, 3359–3375. [Google Scholar] [CrossRef]
- Gagić, T.; Knez, Ž.; Škerget, M. Subcritical Water Extraction of Chestnut Bark and Optimization of Process Parameters. Molecules 2020, 25, 2774. [Google Scholar] [CrossRef]
- Daneshvar, S.; Hidemi, N.; Salak, F.; Mahinpey, N. Degradation of Textile Dyes under Subcritical Water Conditions in the Presence of Hydrogen Peroxide. Can. J. Chem. Eng. 2014, 92, 615–622. [Google Scholar] [CrossRef]
- Jones, A.D.; Morehead, A.T.; Yang, Y. Degradation and Extraction of Organochlorine Pollutants from Environmental Solids under Subcritical Water Conditions. Molecules 2023, 28, 5445. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-H.; Lee, J.-H.; Lee, C.-H. Corrosion Phenomena of Alloys by Subcritical and Supercritical Water Oxidation of 2-Chlorophenol. J. Supercrit. Fluids 2008, 44, 370–378. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass. Chem 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Brown, J.; Lindstrom, J.K.; Ghosh, A.; Rollag, S.A.; Brown, R.C. Production of Sugars from Lignocellulosic Biomass via Biochemical and Thermochemical Routes. Front. Energy Res. 2024, 12, 1347373. [Google Scholar] [CrossRef]
- Abaide, E.R.; Ugalde, G.; Di Luccio, M.; de FPM Moreira, R.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Obtaining Fermentable Sugars and Bioproducts from Rice Husks by Subcritical Water Hydrolysis in a Semi-Continuous Mode. Bioresour. Technol. 2019, 272, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.S.N.; Zabot, G.L.; Mazutti, M.A.; Ugalde, G.A.; Rezzadori, K.; Tres, M.V. Optimization of Subcritical Water Hydrolysis of Pecan Wastes Biomasses in a Semi-Continuous Mode. Bioresour. Technol. 2020, 306, 123129. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.M.; Vardanega, R.; Nogueira, G.C.; Forster-Carneiro, T.; Rostagno, M.A.; Maugeri Filho, F.; Meireles, M.A.A. Valorization of Residual Biomasses from the Agri-Food Industry by Subcritical Water Hydrolysis Assisted by CO2. Energy Fuels 2017, 31, 2838–2846. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Šubarić, D.; Škerget, M. Separation of Active Compounds from Food By-Product (Cocoa Shell) Using Subcritical Water Extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef]
- Ciftci, D.; Saldaña, M.D.A. Hydrolysis of Sweet Blue Lupin Hull Using Subcritical Water Technology. Bioresour. Technol. 2015, 194, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Treatment of Sugars to Obtain High-Value Products. J. Serbian Chem. Soc. 2020, 85, 97–109. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Illera, A.E.; Benito-Román, O.; Melgosa, R.; Bermejo-López, A.; Beltrán, S.; Sanz, M.T. Degradation Kinetics of Sugars (Glucose and Xylose), Amino Acids (Proline and Aspartic Acid) and Their Binary Mixtures in Subcritical Water: Effect of Maillard Reaction. Food Chem. 2024, 442, 138421. [Google Scholar] [CrossRef]
- Lu, Y.J.; Jin, H.; Guo, L.J.; Zhang, X.M.; Cao, C.Q.; Guo, X. Hydrogen Production by Biomass Gasification in Supercritical Water with a Fluidized Bed Reactor. Int. J. Hydrog. Energy 2008, 33, 6066–6075. [Google Scholar] [CrossRef]
- Li, H.; Deng, A.; Ren, J.; Liu, C.; Lu, Q.; Zhong, L.; Peng, F.; Sun, R. Catalytic Hydrothermal Pretreatment of Corncob into Xylose and Furfural via Solid Acid Catalyst. Bioresour. Technol. 2014, 158, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Donahue, C.J.; Rais, E.A. Proximate Analysis of Coal. J. Chem. Educ. 2009, 86, 222. [Google Scholar] [CrossRef]
- Gani, A.; Adisalamun; Arkan, D.M.R.; Suhendrayatna; Reza, M.; Erdiwansyah; Saiful; Desvita, H. Proximate and Ultimate Analysis of Corncob Biomass Waste as Raw Material for Biocoke Fuel Production. Case Stud. Chem. Environ. Eng. 2023, 8, 100525. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant Potential of Corncob Extracts for Stabilization of Corn Oil Subjected to Microwave Heating. Food Chem. 2007, 104, 997–1005. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of Lignocellulosic Biomass with Ionic Liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Knierim, L.; Uhl, A.; Schmidt, A.; Flemming, M.; Höß, T.; Treutwein, J.; Strube, J. Pressurized Hot Water Extraction as Green Technology for Natural Products as Key Technology with Regard to Hydrodistillation and Solid–Liquid Extraction. ACS Omega 2024, 9, 31998–32010. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Saka, S. New Insights on Monosaccharides’ Isomerization, Dehydration and Fragmentation in Hot-Compressed Water. J. Supercrit. Fluids 2012, 61, 146–156. [Google Scholar] [CrossRef]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Cellulose at Temperature from 200 to 300 °C. Ind. Eng. Chem. Res. 2018, 57, 6576–6584. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef]
- Yu, F.; Ruan, R.; Chen, P.; Deng, S.; Liu, Y.; Lin, X.; Deng, S.; Paul, S.; Liu, Y. Liquefaction of Corn Cobs with Supercritical Water Treatment. Trans. ASABE 2007, 50, 175–180. [Google Scholar] [CrossRef]
- Mok, W.S.L.; Antal, M.J., Jr. Uncatalyzed Solvolysis of Whole Biomass Hemicellulose by Hot Compressed Liquid Water. Ind. Eng. Chem. Res. 1992, 31, 1157–1161. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Dong, J.; Cai, L.; Zhu, X.; Huang, X.; Yin, T.; Fang, H.; Ding, Z. Antioxidant Activities and Phenolic Compounds of Cornhusk, Corncob and Stigma Maydis. J. Braz. Chem. Soc. 2014, 25, 1956–1964. [Google Scholar] [CrossRef]
- Wolf, M.; Berger, F.; Hanstein, S.; Weidenkaff, A.; Endreß, H.-U.; Oestreich, A.M.; Ebrahimi, M.; Czermak, P. Hot-Water Hemicellulose Extraction from Fruit Processing Residues. ACS Omega 2022, 7, 13436–13447. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of Degradation Compounds from Lignocellulosic Biomass in the Biorefinery: Sugar Reaction Mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Tongtummachat, T.; Akkarawatkhoosith, N.; Kaewchada, A.; Jaree, A. Conversion of Glucose to 5-Hydroxymethylfurfural in a Microreactor. Front. Chem. 2020, 7, 951. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Amit, T.A.; Jadhav, B. Recent Advances in Lignin Depolymerization Techniques: A Comparative Overview of Traditional and Greener Approaches. Biomass 2022, 2, 130–154. [Google Scholar] [CrossRef]
- Yong, T.L.-K.; Matsumura, Y. Kinetic Analysis of Lignin Hydrothermal Conversion in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 2013, 52, 5626–5639. [Google Scholar] [CrossRef]
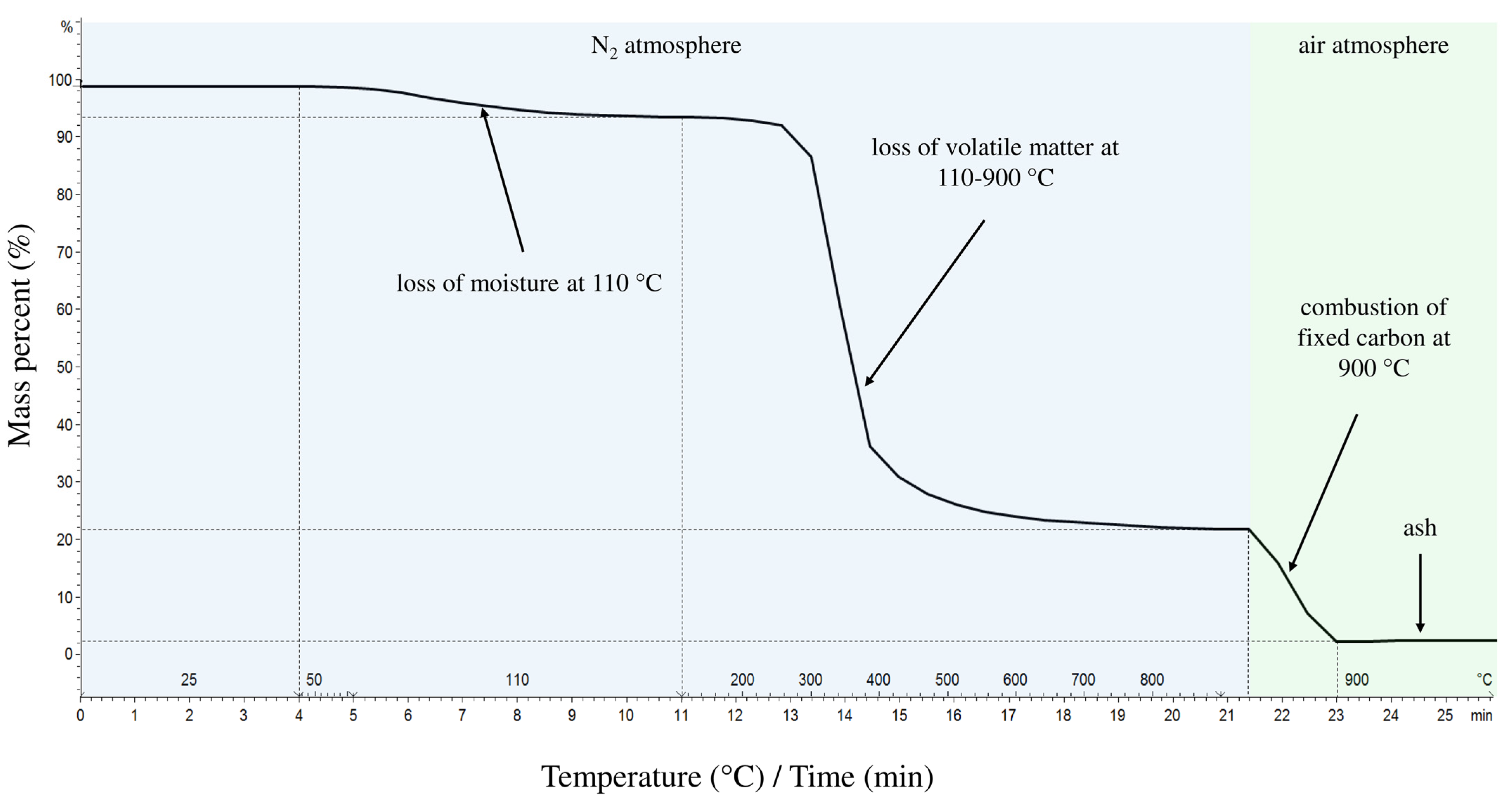

| Characteristics | Proximate Analysis |
|---|---|
| Moisture (wt.%) | 5.39 ± 0.12 |
| Volatile matter (wt.%) | 71.46 ± 1.47 |
| Fixed carbon (wt.%) | 19.36 ± 0.54 |
| Ash content (wt.%) | 3.79 ± 0.10 |
| Ultimate analysis | |
| Carbon (wt.%) | 45.51 ± 1.16 |
| Hydrogen (wt.%) | 7.49 ± 0.13 |
| Nitrogen (wt.%) | 0.67 ± 0.02 |
| Sulfur (wt.%) | 0.26 ± 0.01 |
| Oxygen (wt.%) | 42.28 ± 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čolnik, M.; Irgolič, M.; Perva, A.; Škerget, M. Hydrolytic Decomposition of Corncobs to Sugars and Derivatives Using Subcritical Water. Processes 2025, 13, 267. https://doi.org/10.3390/pr13010267
Čolnik M, Irgolič M, Perva A, Škerget M. Hydrolytic Decomposition of Corncobs to Sugars and Derivatives Using Subcritical Water. Processes. 2025; 13(1):267. https://doi.org/10.3390/pr13010267
Chicago/Turabian StyleČolnik, Maja, Mihael Irgolič, Amra Perva, and Mojca Škerget. 2025. "Hydrolytic Decomposition of Corncobs to Sugars and Derivatives Using Subcritical Water" Processes 13, no. 1: 267. https://doi.org/10.3390/pr13010267
APA StyleČolnik, M., Irgolič, M., Perva, A., & Škerget, M. (2025). Hydrolytic Decomposition of Corncobs to Sugars and Derivatives Using Subcritical Water. Processes, 13(1), 267. https://doi.org/10.3390/pr13010267






