Abstract
This article presents a study on the precipitation of copper from the cyanide leaching solutions used for gold–copper ores, both with and without the addition of a sulphidizer (Na2S). Mathematical models were developed to summarize the effects of the pH, initial copper concentration, and Na2S stoichiometric ratio on the precipitation process, using an experimental design based on a probabilistic–deterministic method. Varying the stoichiometric ratio of Na2S has a minimal impact on the precipitation process. However, the presence of a sulphidizer is significant, as the precipitation process occurs at pH levels of 5 and below. The initial concentration of copper in the solution was identified as the most significant factor. At copper concentrations of 0.34% and 1.55% (pH = 3), the precipitation rates were 51.48% and 47.6%, respectively. This study also determined that the most effective method across the entire range of copper concentrations in the solution was the precipitation of copper in the form of copper cyanide hydrate (CuCN∙nH2O) without the addition of Na2S. At copper concentrations of 0.34% and 1.55% (pH = 3), the precipitation rates were 86.47% and 85%, respectively. The pH level was the most significant factor influencing this process, as copper deposition without Na2S did not occur at a pH of 5. The obtained models allow us to accurately predict the influence of factors on the deposition process. Aided by the mathematical model of precipitation (without Na2S), we selected the conditions for an enlarged experiment using 20 L of solution (Cu = 0.34%, pH = 3.2), which showed the high efficiency of the method. The calculated recovery amounted to 86%, where practical recovery was 87.2%, and divergence was ≥1.2%.
1. Introduction
Over the past 27 years, the volume of gold mining has increased from 2200 to ~3500 tons (1997–2023) [1]. Currently, the majority of gold is extracted from ores using an alkaline cyanide leaching process [2]. However, copper, which can be found in gold- and copper-bearing ores, drastically increases gold recovery costs. The presence of copper causes an increase in the cyanide consumption rate (since cyanide is required for gold dissolution). It is widely known that the reaction capacity of copper minerals (such as Cu2S, Cu2(CO3)(OH)2, CuS, Cu2O, and Cu3(CO3)2(OH)2) is quite high (except for CuFeS2 and CuSiO3nH2O) [3]. Apart from the increased consumption rate of cyanide, copper may cause a number of other issues. These include the contamination of the solution by recirculating flows, decreased adsorption operation efficiency caused by copper cyanides, and an increased copper grade in Dore bars, which makes them unconditional [4]. Because gold ores that contain copper minerals are widespread in the Republic of Kazakhstan [5], finding a way to increase the efficiency of their treatment is one of the most crucial problems that the industry currently faces. There are several methods for the recovery of copper and cyanide that can reduce gold production costs:
- -
- The AVR (acidification–volatilization–regeneration) method designed for the regeneration of cyanide and its modifications, namely, the AuGMENT (resin with a quarter naryamino group) and Vitrokele (cross-linked polystyrene structure-based resin) processes;
- -
- The modified Newton–Raphson MNR method, which includes cyanide regeneration and copper sulphide production;
- -
- Methods based on absorbent carbon use;
- -
- Methods based on ion-exchange resin use;
- -
- Other less common methods [6].
In addition, the technology of copper precipitation from cyanide solutions is based on the use of sodium dimethyldithiocarbamate C3H6NNaS2 (SDDC), followed by copper recovery using HCl and HNO3 solutions [7]. These operations include phosphonate-ion liquids [8], polymer surface-active aggregates [9], the acidification of copper- and cyanide-bearing solutions [10], leaching with thiosulphate and electric precipitation [11], and chloride leaching with simultaneous carbon sorption (CICL—carbon in chloride leach process) [12], all of which are well-known.
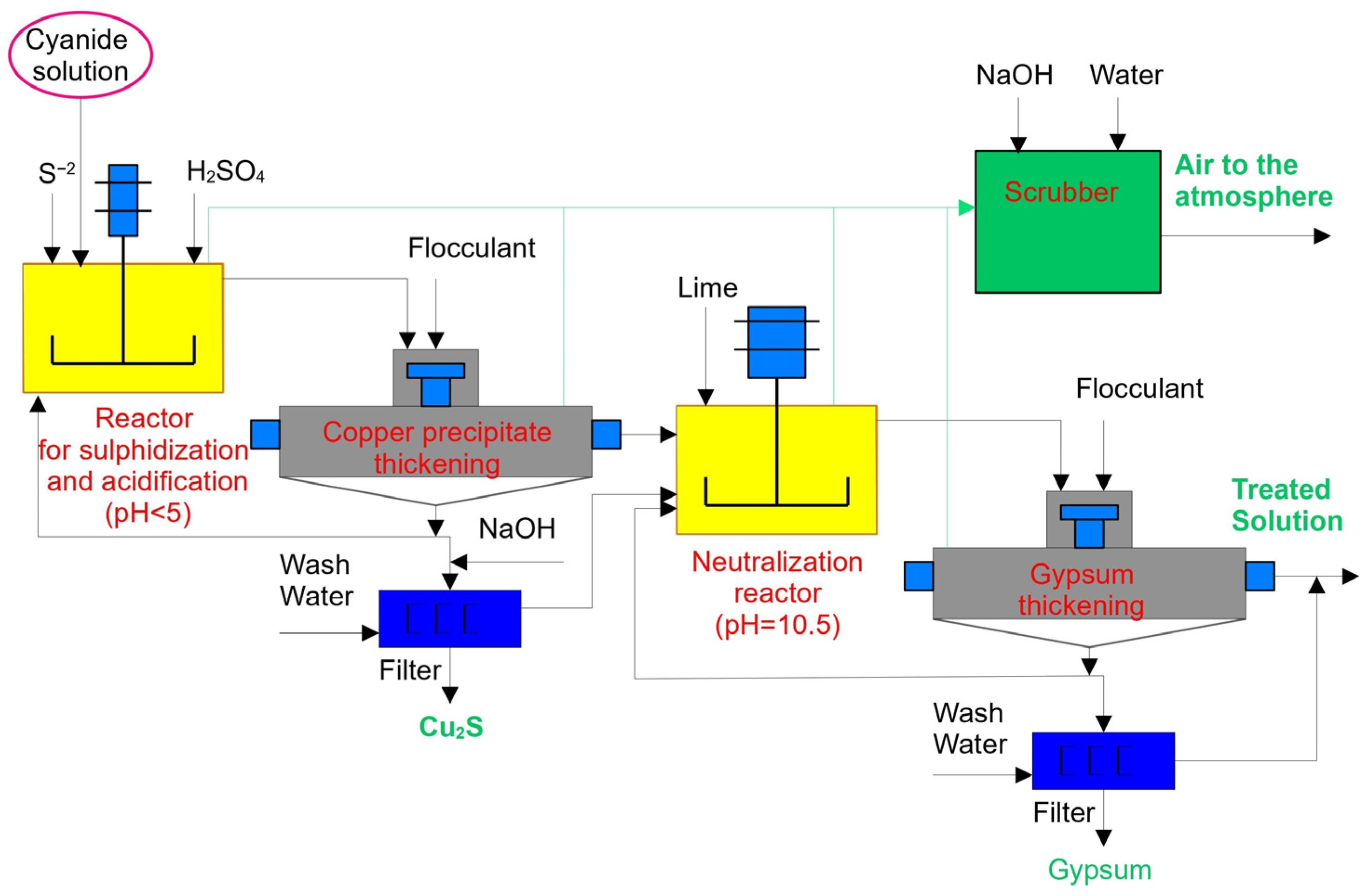
The SART process is considered one of the most popular technologies for processing gold- and copper-bearing ores [13,14,15]. This process includes sulphidization, acidification, recycling, and thickening. Figure 1 provides a general flowsheet of the SART process.
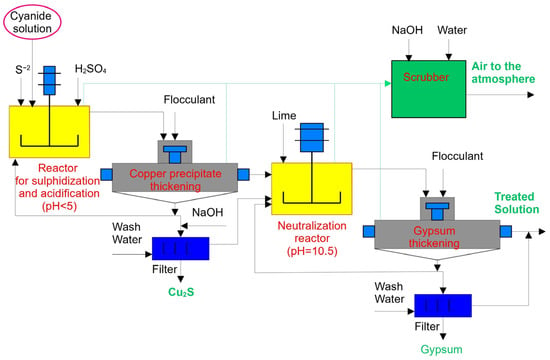
Figure 1.
General flowsheet of the SART process.
The SART process is a modification of the MNR process that includes a filtration stage only for separating solid residues from the solution. Furthermore, it includes thickening and recycling stages [16,17], followed by cyanide and copper recovery as marketable by-products. Equation (1) below displays the chemistry of the SART process [18].
2Cu (CN3)2− + 3H2SO4 + S2− ↔ Cu2S(S) + 6HCN(aq) + 3SO42−
Here, the pH level of the reaction is <5.5. Thickening and filtration operations are used to separate the precipitate from the solution. The solution becomes neutralized and returns to the leaching stage. This technology allows for the processing of gold-bearing ores with high copper grades. However, the SART process involves heavy CAPEX and requires strict operational control. These issues have not yet been resolved, so despite successfully implementing this process in plants worldwide, the gold mining industry as a whole is reluctant to spend money on new research aimed at optimizing both the process and principal design of the concentrating mills [19].
However, several modifications have been made to the SART process. For example, the so-called ‘SuCy process’ is composed of metal sulphide precipitation (the same as the SART process) coupled with a membrane filtration operation with the goal of clarifying copper residues [20]. It should also be mentioned that membrane processes represent separate scientific fields, with numerous variations of these membrane processes [21,22,23]. The microfiltration stage has been implemented to both decrease copper losses and simplify the equipment normally required for the precipitation of copper residues.
The Sceresini process is used to obtain copper-bearing by-products and carry out cyanide regeneration. In this process, eluted copper cyanide is free of contaminating agents, which can be generated by slimy precipitates accumulated during the direct treatment of process solutions within the MNR or SART processes. Two commercial Sceresini plants were commissioned. The first one, Mt. Gibson, precipitates CuCN at a pH level of 2.0–2.5. The second one, Red Done, precipitates Cu2S at the same pH level but also includes the addition of sodium sulphate. Both of the Sceresini plants used solid recirculation to initiate precipitation, resulting in very fine residues that accumulated porous filter precipitates [24].
A review of current copper-precipitating processes revealed that they all start by lowering the pH with sulphuric acid added to the cyanide leaching solution. Meanwhile, pH levels may vary from 5.4 to 2 depending on the process. Acidification operations can be conducted with or without a sulphidizer supplement, allowing the copper-bearing product to precipitate in the presence of either cyanide or sulphide. The next stage normally includes thickening, followed by the separation of copper-bearing residues using various techniques.
Thus, there is no consensus on the optimal parameters for precipitating copper-bearing products from cyanide leaching solutions. Industrial process optimization via experimental design has several positive outcomes [25,26]. Full factorial experiments and their variations are widely used to optimize metallurgical processes [27,28] and other research activities [29]. Despite full factorial experiments having clear advantages such as flexibility, impartiality, and ease of handling, there are disadvantages as well. For example, the mathematic model is applicable only within the range of factors that were initially set; therefore, there is a possibility that a target value may exceed either physical or logical limits. Should any particular value fall within a zero argument, this can lead to absurd results.
The combined probabilistic–deterministic method of experimental design compares favourably with other methods. The probabilistic approach is based on the Latin square [30]; it allows for dot dependences of specific functions to be obtained, which are further plotted on the chart and approximated using polynomials. The deterministic approach is based on Protodyakonov’s equation [31], where specific dependences are multiplied together. This allows us to avoid absurd values that may arise if any particular value belongs to a zero argument. Exponential functions can be used to provide upper limits of particular functions if necessary. The adequacy-checking procedure does not require the experiment to be repeated, but it assumes that the significance of the obtained results is determined via the confidence range.
This article is part of a comprehensive study on the development of technology for processing gold–copper ores from a Kazakhstan deposit. This article deals with selecting optimal parameters for the copper precipitation process from cyanide leaching solutions of gold–copper ores using the probabilistic–deterministic method of experimental planning.
2. Materials and Methods
All of the experiments were carried out at the NJSC ‘D. Serikbayev East-Kazakhstan Technical University’ (“https://www.ektu.kz/divisions/veritas.aspx (accessed on 27 September 2024)”) and the ‘Eastern Mining-and-metallurgical Research Institute for Non-ferrous Metals ‘VNIItsvetmet’’ (“http://vcm.ukg.kz (accessed on 27 September 2024)”), both located in the Republic of Kazakhstan, in the city of Ust-Kamenogorsk. Chemical compositions of the ores, solutions, and by-products were determined using a mass spectrometer ICP–MS 7500cx manufactured by the US company ‘Agilent Technologies’. The subject of this study was leaching solutions produced via copper ore leaching in a locked cycle with sodium cyanide. The initial ore sample was taken from one of the deposits located in the Republic of Kazakhstan.
The initial ore contained Au—1.2 g/t, Cu—0.15%, Fe—5.1%, Zn < 0.02%, and Pb < 0.02%. Grinding parameters included a solid-to-liquid ratio of 1:1, a residence time of 1 h, and a final ore material size of P80—0.057 mm. Optimal parameters for grinding the initial ore were determined via practical studies. After 60 min of grinding, the yield of particles with a size of P80 = 0.057 mm was 90.5%. After 80 min, the yield increased by no more than 0.2% (90.73%). For this reason, the grinding duration was chosen to be equal to 60 min.
As part of a comprehensive study, classes with particle sizes of P80 = 0.067 mm and P80 = 0.071 mm were also considered. However, when further examining the influence of particle size on the amount of gold transferred into the solution class P80 = 0.057 mm, the highest recovery of gold in solution was 87.5% compared to larger particle sizes, which yielded a recovery of 85.0% (Table 1). Therefore, all subsequent studies were conducted with the class P80 = 0.057 mm because the determining factor in the choice of class was the amount of gold transferred into the solution.

Table 1.
Main results of gold–copper ore sample leaching in bottle agitators.
A series of experiments were conducted to determine the dependence of gold recovery in solution on sodium cyanide consumption. These tests were conducted without the addition of activated carbon, using a pulp density of 40%, a sodium cyanide concentration in the initial solution set at 0.05%, and a leaching duration of 24 h. In the first experiment, sodium cyanide was administered only at the beginning of the process. In the second experiment, it was administered at the beginning of the process and also after 120 min. In the third experiment, it was administered at the beginning of the process, at 120 min, and at 240 min. In the fourth experiment, it was administered at the beginning of the process, at 120 min, 240 min, and 360 min (Table 2). The noted high consumption of sodium cyanide cannot be reduced without a significant decrease in gold recovery.

Table 2.
Ore leaching results at different NaCN flow rates.
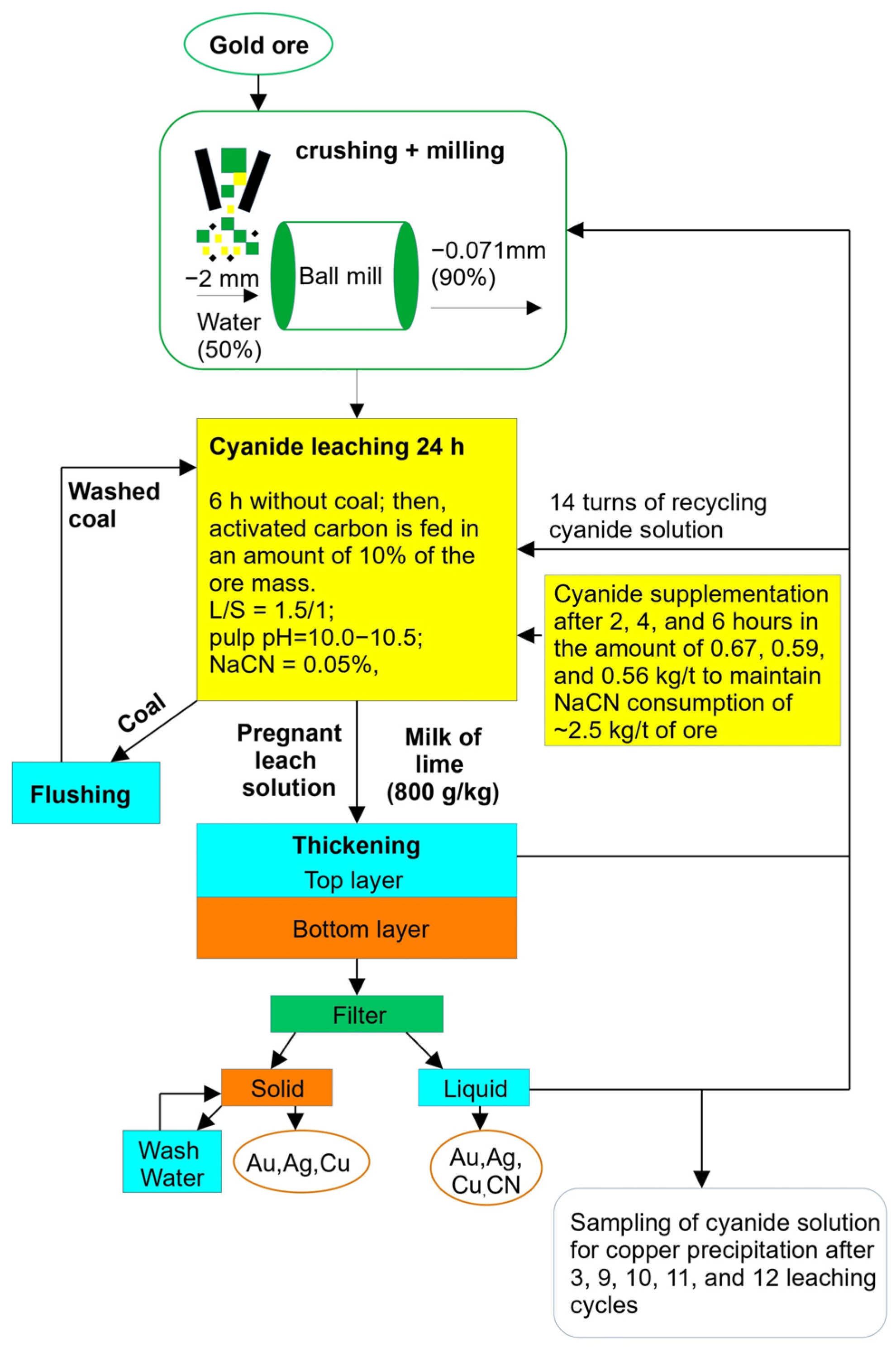
See Figure 2 for the flowsheet of gold ore leaching, followed by obtaining a cyanide solution for copper precipitation.
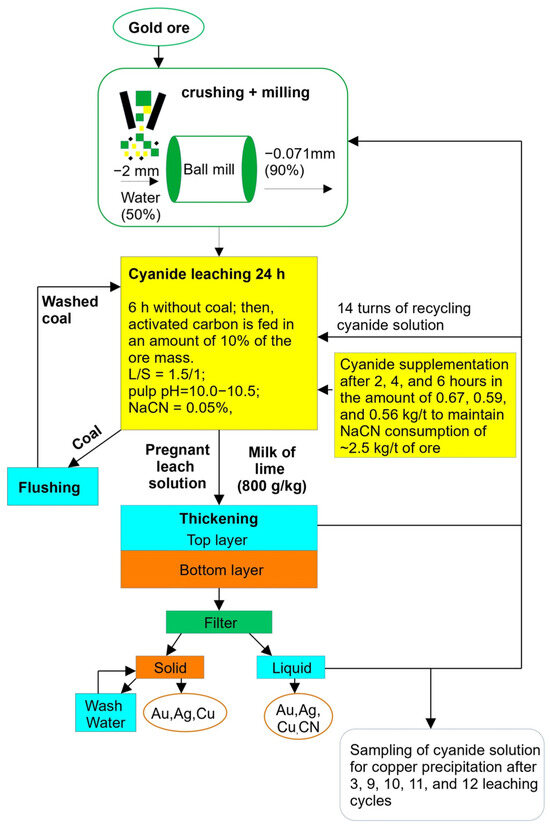
Figure 2.
Flowsheet of cyanide solution production for copper precipitation experiments.
The following chemical agents were used in the process:
- -
- ≥98% sodium cyanide (NaCN) tablets for ore leaching;
- -
- Sulphuric acid (H2SO4) to maintain the pH value;
- -
- Adsorbent carbon Norit RO 3515-B for gold extraction from the cyanide solutions;
- -
- Lime milk to thicken the pulps.
Gold- and copper-bearing ore leaching was carried out under the following conditions:
A temperature of 25 ± 2 °C;
A solid-to-liquid ratio of 1.5:1;
A residence time of 24 h (where during the first 6 h, there was no adsorbent carbon and then the adsorbent carbon was added in an amount of 10% of the ore mass);
An initial sodium cyanide (NaCN) concentration of 0.05%;
A pulp pH level of 10.0–10.5.
The leaching pulp was supplemented with sodium cyanide after 2, 4, and 6 h of residence time in amounts of 0.67, 0.59, and 0.56 kg/t. This allowed us to maintain a consistent sodium cyanide consumption level of 2.5 kg/t of ore in all cycles of the process. Each solution sample was analyzed for both the pH level and sodium cyanide concentration. PLS pH was maintained at a level of 10.5. After the tests were completed, the leaching pulp was subjected to filtration for the determination of gold, silver, and copper grades, as well as the concentration of sodium cyanide.
Leaching pulp thickening was carried out under the following conditions:
A residence time of 1 h;
A coagulating agent (lime milk) consumption of 800 g/kg;
A total of 14 thickening turnovers.
For the first 10 turnovers, all of the solutions (including the ones after the underflow was filtered) were returned to the beginning of the process. For the last 4 turnovers, only the overflow and a major part of the solution generated after underflow filtration were returned to the beginning of the process. Thus, the recycling water utilization rate equalled ~80% for the first 10 tests and ~70% for the last 4 tests.
In order to run the experiments, 7.5 L of cyanide solutions were collected after the 3rd, 9th, 10th, 11th, and 12th turnover in the locked cycle (see the details below). Moreover, 20 L of a cyanide solution after the 3rd turnover was collected for an extended pilot experiment.
2.1. Collection of the Solutions to Run Copper Precipitation Experiments
A total of 7.5 L of cyanide solutions were collected after the 3rd (Cu = 0.34 g/L), 9th (Cu = 1.35 g/L), 10th (Cu = 1.55 g/L), 11th (Cu = 1.15 g/L), and 12th (Cu = 1.1 g/L) turnovers (Table 3). Moreover, 20 L of a cyanide solution after the 3rd turnover was collected for an extended pilot experiment. Table 3 shows the quantity of gold, silver, and copper in the cake (Composition of Cake column) in the solution (Composition of the Solution column) and what percentage of the total gold, silver, and copper was recovered from the solid to the solution (Leaching Rate, % column) after each cycle. It can be seen that, after the first turn, the concentration of cyanide in the solution increases to ensure a high gold recovery rate. The highest copper concentration occurred after the 10th cycle.

Table 3.
Results of the gold ore leaching process in the locked cycle.
The content of copper-related impurities, such as zinc and lead, is not given due to their insignificant content in the leach solution (less than 0.001 mg/L).
2.2. Flowsheet for the Copper Precipitation Experiment
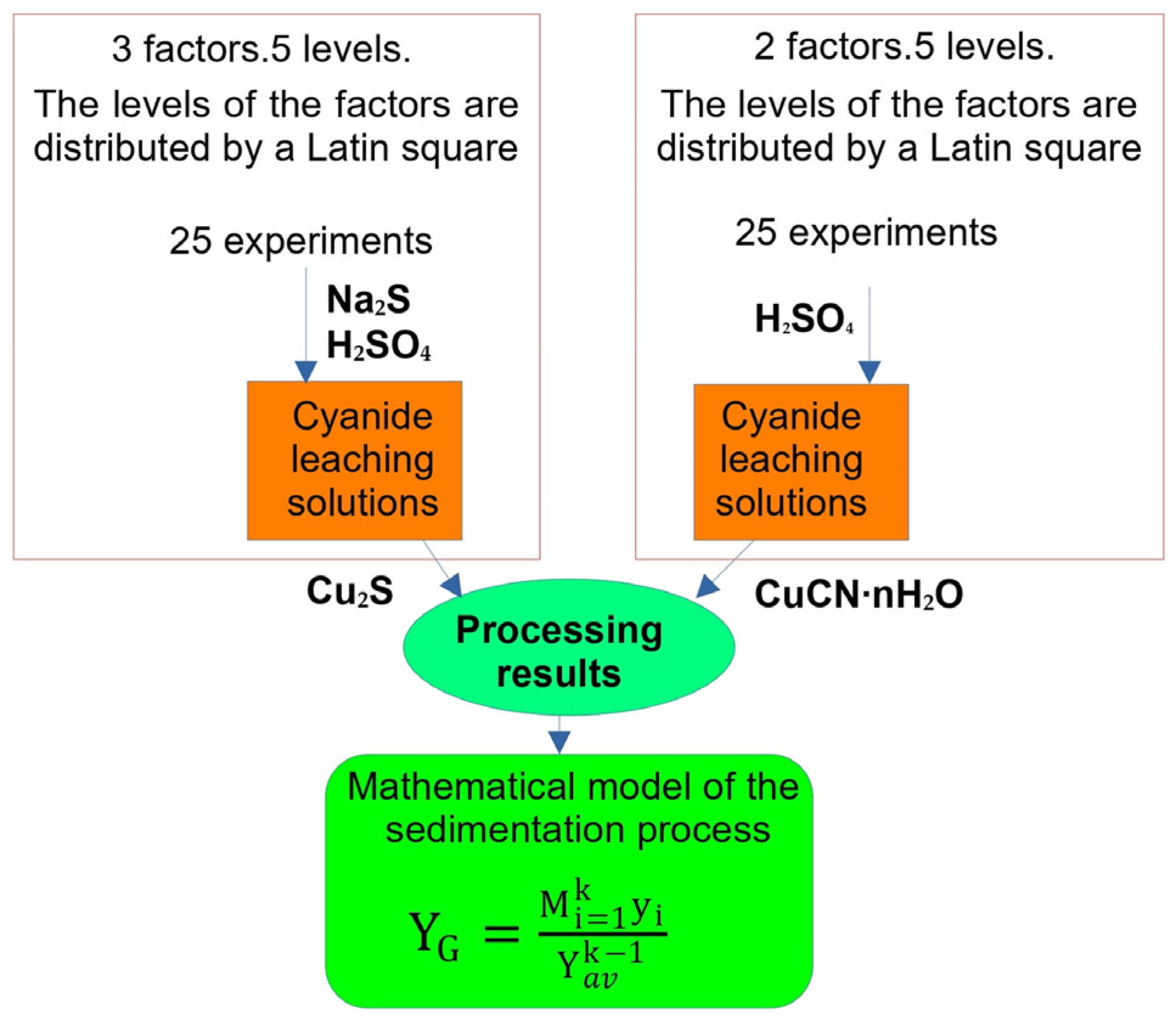
A flowsheet of the experiment design is given in Figure 3. Levels of the factors (copper concentration of cyanide solution, pH level, and sulphidizer consumption) were rotated via the Latin square method [30]. The experiments were repeated in triplicate. The mean (standard) deviation was not counted because the experiment was repeated if the values deviated by more than 1%.
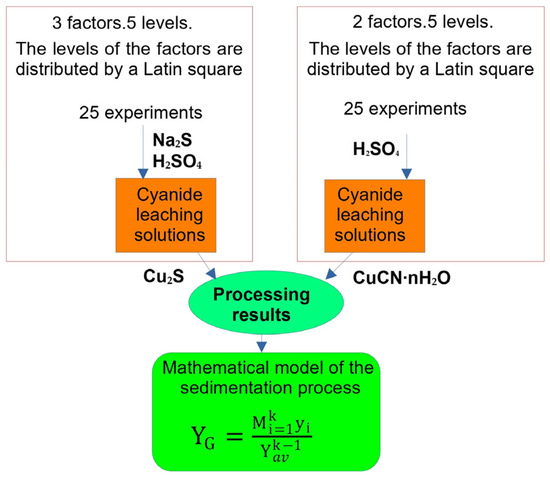
Figure 3.
Flowsheet of the experiment design.
2.3. Copper Precipitation from Cyanide Solutions
In order to model the process of copper precipitation from cyanide solutions, a table of factor levels (see Table 4) was compiled, where the X1, X2, and X3 factors (sulphidizer consumption, pH, and copper concentration in the cyanide solution) are the levels. To maintain the pH at a level of 3.0–5, a sulphuric acid solution (in a concentration of ~100 g/L) was used, while sodium sulphide solutions (in concentrations of ~100 g/L) were used for copper precipitation. An ambient air temperature of 25 ± 2 °C was maintained.

Table 4.
Levels of the three-factor experiment (X1–3) of copper precipitation and two-factor experiment both with and without Na2S(X4–5).
Specific dot dependences obtained experimentally were further approximated by the functions received using the least-squares method. Thus, charts plotting copper precipitation dependences on specific factors were compiled. In order to combine specific dependences, Protodyakonov’s equation, in which specific functions are combined as multiplicands, was applied:
The dependence adequacy was checked using a nonlinear multiple correlation coefficient, according to Formula (3) below.
where
- n—the number of the dots described;
- k—the number of operative factors (which equals 1 for specific dependences);
- yei—the experimental result value;
- yTi—the theoretical (calculated) result value;
- yav—the average experimental value.
The significance of both the correlation coefficient and the dependence can be assessed using Formula (4) as follows:
3. Results
The results of the experiments carried out according to the experimental matrix are summarized in Table 5. A total of 50 copper precipitation tests were conducted in total, including tests 1–25 with the inclusion of Na2S and tests 26–50 without the inclusion of Na2S.

Table 5.
Experimental conditions for copper deposition.
Each row in the table shows the experimental conditions. The point dependences of copper precipitation from the solution were selected by averaging the experimental results corresponding to each level of factors (1–5) given in Table 5. Table 6 shows the averaged data of dependences on five levels of the three-factor experiment with Na2S (Experiments 1–25). The actions of the factors are averaged via self-compensation between lower and upper values.

Table 6.
Factor levels of the five-factor experiment (x1–5) with Na2S (Experiments 1–25).
Table 7 shows the averaged dependence data for the five levels of the two-factor experiment without Na2S (Experiments 26 through 50).

Table 7.
Factor levels of the five-factor experiment (x1–5) with Na2S (Experiments 26–50).
The obtained partial point dependencies are plotted on a graph and approximated. The equations describing the approximated graphs are partial dependencies of the copper deposition process. Further, the partial dependencies are combined using the Protodyakonov equation as a factor.
3.1. Results of the Experiments on Copper Precipitation with Na2S Supplement
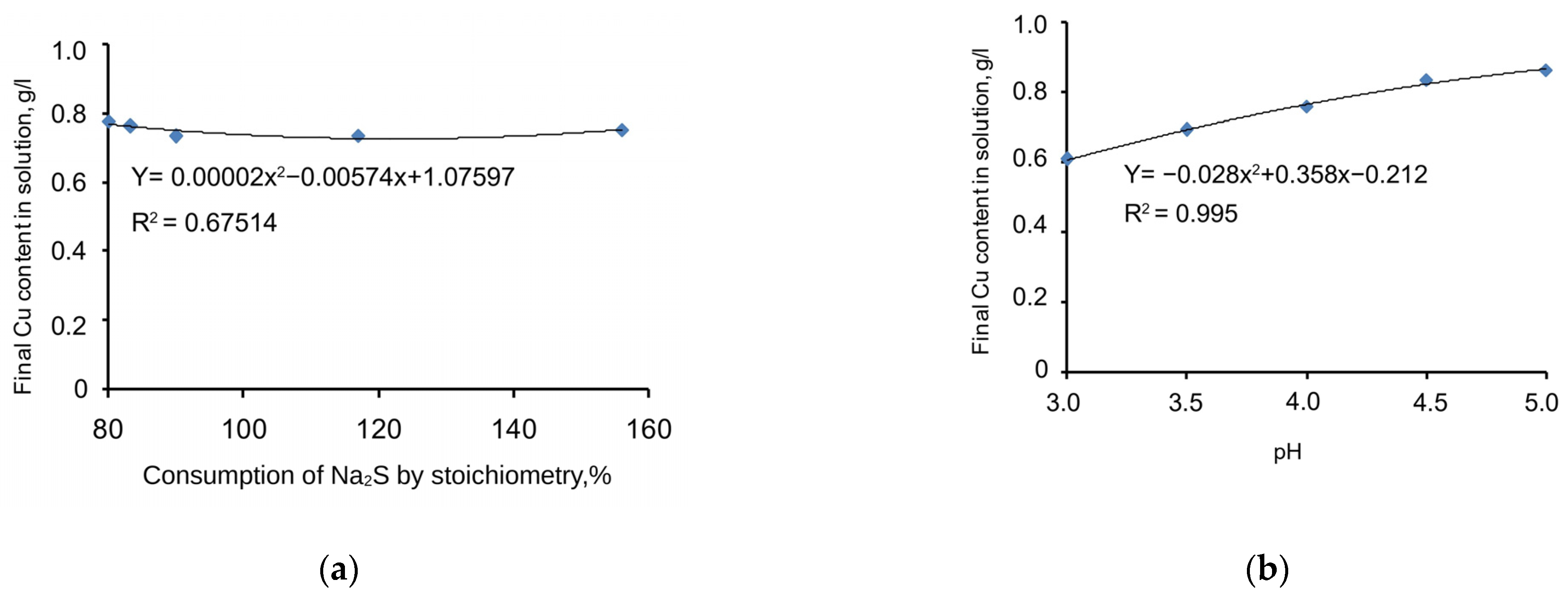
The chart in Figure 4a shows copper-precipitation-specific dependences on the Na2S stoichiometric ratio, expressed by the specific function given in Equation (5) below. Meanwhile, the correlation coefficient (R2) = 0.675, and the significance of the specific function correlation coefficient (tR) = 5.32 > 2.
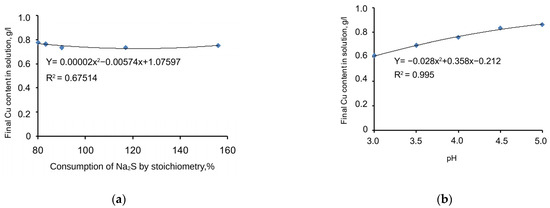
Figure 4.
Charts of copper precipitation dependence on the consumption of Na2S (a) and pH level (b).
Adding a sulphidizer to a cyanide solution significantly influences the precipitation process. However, if the sulphidizer stoichiometric ratio is changed from 80% to 156%, there is no considerable impact on the amount of copper sulphide precipitated because it is almost parallel to the X1 axis. Thus, the specific dependence of process behaviour on changes in the sulphidizer stoichiometric ratio is not considered in the mathematical modelling of the process.
The chart in Figure 4b shows copper-precipitation-specific dependence on the pH value with the supplementation of Na2S, expressed by the specific function given in Equation (6) below. Meanwhile, the correlation coefficient (R2) = 0.995, and the significance of the specific function correlation coefficient (tR) = ≥ 2.
This factor is considered among the most important. The copper sulphide precipitation process strongly depends on the pH value. If pH = 3, the grade of copper in the cyanide solution will be the lowest. If the pH increases to 5, the copper precipitation process will still be active, but its precipitation parameters will be the lowest. The approximated chart of the function is smooth and stable. Dots 4 and 5 achieve a plateau, which means that, if pH = 5, the process ceases. Thus, the specific dependence of process behaviour on pH is a considerable factor that should be considered for the mathematical modelling of the process.
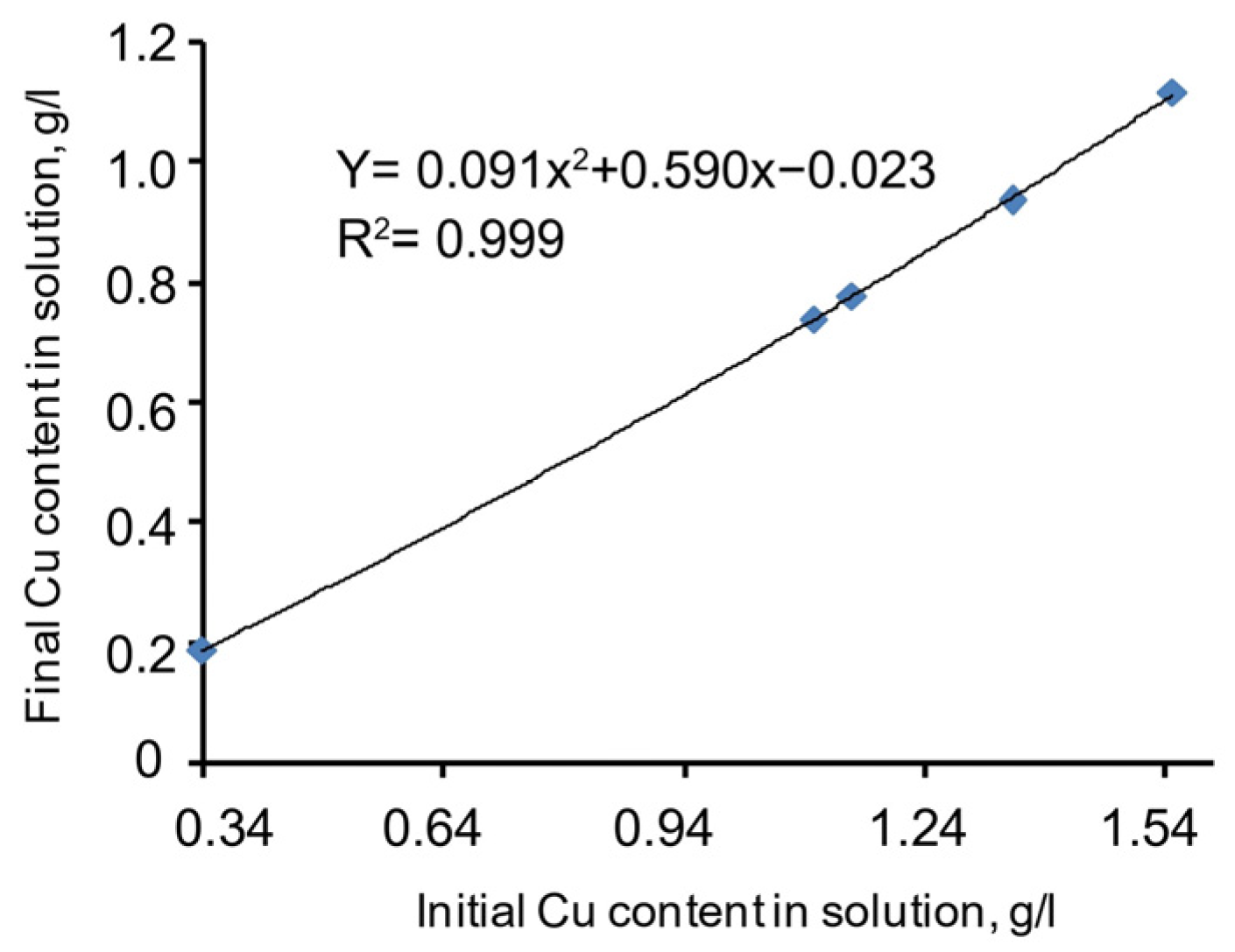
A chart of copper precipitation dependence on the initial copper grade in the cyanide solution (including the N2S supplement) is given in Figure 5. The specific function described in Equation (7) is presented below. Meanwhile, the correlation coefficient (R2) = 0.999, and the significance of the specific function correlation coefficient (tR) = ≥ 2.
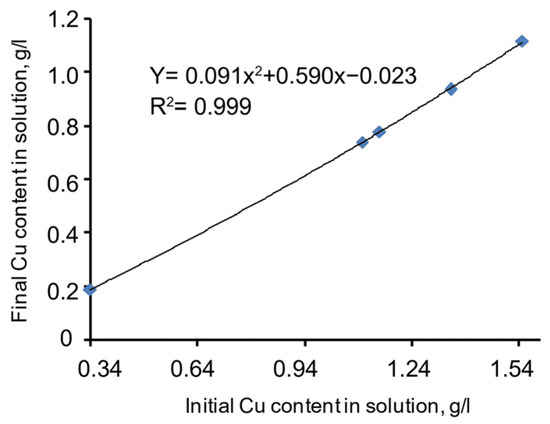
Figure 5.
Copper precipitation dependence on the initial copper grade in the cyanide solution.
The approximated chart of the function is smooth, stable, and almost linear. This proves the proportional dependence of the copper sulphide precipitation process on the initial copper grade in the cyanide solution. Thus, the specific dependence of the process behaviour on copper grade in the cyanide solution is a considerable factor and should be considered for the mathematical modelling of the process.
After data processing, by substituting the equations of partial dependences into Equation (2), Mathematic Model (8) demonstrates the influence of two factors (pH value and initial copper grade in cyanide solution) on the copper precipitation process from cyanide leaching solutions (including the Na2S supplement). The equation showing the specific dependence of the process behaviour on the amount of Na2S was not included, as this factor was inconsiderable.
In order to determine the adequacy of Model (8), experimental practical data were compared with theoretical data (experiments nos. 1, 7, 15, and 16). See Table 8 below for the comparison results.

Table 8.
Comparison of the theoretical calculated data and experimental practical data.
Based on Table 4, by considering pH ranges and the initial copper grade in the cyanide solution, the calculated values are deemed to be very close to the experimental results. Differences between the calculated and experimental values equal 5–8%, R = 0.85, and the significance of the specific function correlation coefficient (tR) = 4.33 > 2.
3.2. Results of the Experiments on Copper Precipitation with No Na2S Supplement
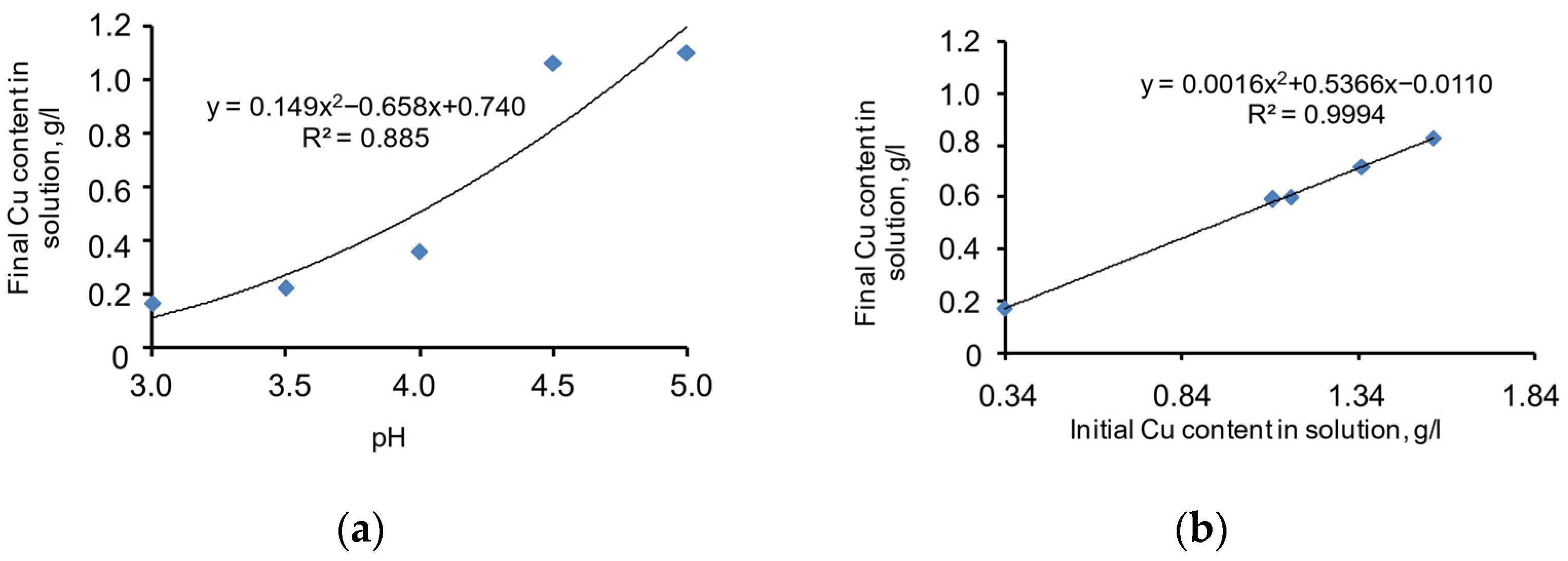
The chart in Figure 6a shows the copper-precipitation-specific dependence pH level without the Na2S supplement, expressed by the specific function Equation (9) given below. Meanwhile, the correlation coefficient (R2) = 0.885, and the significance of the specific function correlation coefficient (tR) = 15.06 > 2.
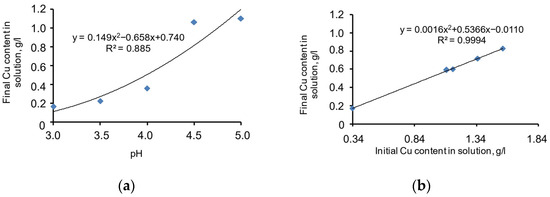
Figure 6.
Chart of the dependence of copper precipitation function on the pH level (a) and the initial copper grade in the cyanide solution (b).
If pH = 3, the copper grade in the cyanide solution is the lowest. If the pH increases to 5, the copper precipitation process ceases. The approximated chart of the function is smooth and stable. Thus, the specific dependence of the process behaviour on pH value is a considerable factor and should be considered for the mathematical modelling of the process.
A chart of copper precipitation dependence on initial copper grade in the cyanide solution (without the N2S supplement) is given in Figure 6b. The specific function Equation (10) is presented below. Meanwhile, the correlation coefficient (R2) = 0.999, and the significance of the specific function correlation coefficient (tR) = ≥ 2.
The approximated chart of the function is almost linear. This proves the proportional dependence of the copper sulphide precipitation process on the initial copper grade in the cyanide solution. Thus, the specific dependence of the process behaviour on the copper grade in the cyanide solution is a considerable factor and should be considered for the mathematical modelling of the process.
After data processing, by substituting the equations of partial dependences into Equation (2), Mathematic Model (11) describes the influence of two factors (the pH value and the initial copper grade in the cyanide solution) on the process of copper precipitation from cyanide leaching solutions (without the Na2S supplement).
In order to determine the adequacy of Model (11), X4 and X5 were substituted for the experimental data (experiments Nos. 26, 43, 37, and 48); then, YG was compared with the calculated theoretical data (see Table 9). Based on Table 9, the calculated results are fairly similar to the experimental data at pH = 2 and 4. If the pH value = 3.5 and the initial copper grade in the solution = 1.55 g/L, the difference between the calculated and experimental data is <1%. Should the pH value increase to 4.5 and 5, the copper precipitation process ceases. Notably, if the sulphidizer is not supplemented, at pH ≤ 4 the copper precipitation rate equals ~70%. If the pH level decreases to ≤3.4, the copper precipitation rate is ~70%. If the pH level decreases to ≤3, the copper precipitation rate is ~87%, R = 0.75, and the significance of the specific function correlation coefficient (tR) = 2.42 > 2.

Table 9.
Comparison of the theoretical calculated data with experimental practical data.
In order to assess the model, output parameters of copper precipitation at a pH level of 3.2 and an initial copper grade in the cyanide solution of 0.34 g/L were calculated. Meanwhile, the calculated copper grade in the solution after precipitation equalled 0.0472 g/L (copper recovery rate = 86%). These conditions were the basis for the extended experiment using 20l of the solution. Other conditions of the copper precipitation process were as follows:
- -
- A sulphuric acid-specific consumption of 1.48 g/L;
- -
- A consumption of Magnafloc 351 flocculating agent at 0.5 g/L m3 of pulp;
A thickening rate of ~60 mm/min or 3.6 m3/m2 × h;
A solid grade in the initial pulp of 0.313 g/L;
A solid grade in the underflow pulp of 10.4 g/L;
An underflow filtration rate of 0.2 m3/m2 × h;
A precipitant moisture of 63–65%;
A copper grade in the precipitant of 52.1%.
The chemical composition of copper cyanide is summarized in Table 10. The copper grade in the solution after the precipitation process equalled 0.04352 g/L at a copper recovery rate of ~87.2%.

Table 10.
Chemical composition of copper cyanide.
4. Discussion
Using the probabilistic–deterministic method of experimental design, mathematic models were produced. These models describe the process of copper precipitation from gold- and copper-ore cyanide leaching solutions. The models included either three factors (models with a Na2S supplement) or two factors (models with no Na2S supplement). It was found that Na2S allows for the copper precipitation process to be run at pH values of 3 to 5. However, should Na2S (in any amount) be added to the process, the residual copper grade in the solutions remains high (0.15–0.20 g/L), even when considering a low initial copper grade in cyanide solutions of 0.34%. This was clearly caused by the reactions described in Equations (12) and (13) as follows:
2Na2Cu(CN)3 + Na2S → Cu2S + 6NaCN
Cu2S + 7NaCN + ½O2 + H2O → 2Na2Cu(CN)3 + 2NaOH + NaCNS
This can also be confirmed using the function chart of copper-precipitation-specific dependence on the Na2S stoichiometric ratio, which is parallel to the X1 axis. Thus, the specific dependence of the process behaviour on changes in the Na2S stoichiometric ratio was not considered for the mathematical modelling of the process. Meanwhile, the Na2S supplement influences the copper precipitation process considerably, which is why this factor must be considered as the one influencing the whole process. The mathematical model developed quite accurately forecasts changes in output parameters depending on changes in either the pH level or the initial copper grade in the cyanide solution. This is also confirmed by the correlation coefficient R = 0.85 and the significance of the specific function correlation coefficient (tR) = 4.33 > 2.
The most efficient way to recover copper from cyanide leaching solutions is the copper precipitation process in the presence of copper cyanide (CuCN∙nH2O). The residual copper grade in the solution (at a pH level of 3 to 4), considering an initial copper grade of 0.34%, equals 0.04–0.06 g/L. The mathematical model of the copper precipitation process (without Na2S) dependence on pH values and initial copper grade in the solution precisely describes the copper precipitation process at a pH level of 3.5. In this case, the difference between calculated and experimental data is <1%. Should the pH level increase to 5, the precipitation process ceases. Optimal values of the most significant factor of the mathematical model’s pH range from 2 to 3.5. The concentration of copper in the solution required to start deposition depends on economic feasibility because, by the second cycle, the concentration of NaCN in the solution—and consequently, its consumption—increases by six times. Both the model and practical tests have confirmed that deposition without a sulphidizer is effective at any copper concentration.
A model-extended pilot test was conducted on a 20 L cyanide solution containing 0.34 g/L of copper and a pH value of 3.2. In terms of copper recovery, the miscount between the calculated and experimental data equals 1.2%. The copper precipitant produced becomes thickened and can be filtered well. The copper grade in the precipitant equals 51–52%. The copper sulphide residue becomes thickened and filtered worse in comparison with copper cyanide; in this case, the copper grade on the precipitant equals ~69%. The residual grade of sodium cyanide in the solution after copper recovery in the presence of copper cyanide equals 0.015% and 0.028% after copper recovery in the presence of copper sulphide.
5. Conclusions
This study presented a practical comparison of two copper precipitation methods from a cyanide leaching solution of gold–copper ores, accompanied by the creation of mathematical models of the process based on the probabilistic–deterministic design of the experiment. It showed the advantage of the precipitation method without using a sulphidizer and the practical value of the probabilistic–deterministic design of experiment planning. The use of probabilistic–deterministic planning methods allows us to accurately predict the output parameters of the copper deposition process. This ultimately leads to optimized costs associated with the preparation and conduct of technological processes, which, as a result, contributes to reducing the carbon footprint.
Author Contributions
Conceptualization, D.K. and R.S.; methodology, R.S.; validation, L.K. and N.K.; formal analysis, L.K. and N.K.; investigation, D.K. and Z.S.; resources, D.K.; data curation, L.K. and M.A.; writing—original draft preparation, R.S.; writing—review and editing, R.S. and D.K.; visualization, R.S., Z.S. and M.A.; supervision, D.K.; project administration, D.K.; funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financially supported by the Eastern Mining and Metallurgical Research Institute for Non-ferrous Metals “VNIITSVETMET”, Ust-Kamenogorsk, Kazakhstan.
Data Availability Statement
The data are unavailable due to privacy, as they are a trade secret.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Gold Council Gold Demand Trends Full Year 2022. Available online: https://www.gold.org/goldhub/research/gold-demand-trends/gold-demand-trends-full-year-2022/supply (accessed on 21 August 2024).
- Medina, D.; Anderson, C.G. A Review of the Cyanidation Treatment of Copper-Gold Ores and Concentrates. Metals 2020, 10, 897. [Google Scholar] [CrossRef]
- Sceresini, B. Gold-Copper Ores. In Developments in Mineral Processing; Mike, D., Adams, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 789–824. [Google Scholar] [CrossRef]
- Estay, H. Designing the SART process—A review. Hydrometallurgy 2018, 176, 147–165. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Xie, F.; Dreisinger, D.; Doyle, F. A Review on Recovery of Copper and Cyanide from Waste Cyanide Solutions. Miner. Process. Extr. Metall. Rev. 2013, 34, 387–411. [Google Scholar] [CrossRef]
- Yilmaz, E.; Yazici, E.Y.; Ahlatci, F.; Celep, O.; Deveci, H. Precipitation of copper from cyanide leach solutions using sodium dimethyldithiocarbamate (SDDC). Hydrometallurgy 2021, 202, 105610. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Liu, W.; Shen, Y.; Zhou, S.; Cui, B. A new strategy for extraction of copper cyanide complex ions from cyanide leach solutions by ionic liquids. J. Mol. Liq. 2023, 383, 122108. [Google Scholar] [CrossRef]
- Li, W.; Liu, W.; Liu, W.; Kou, W. Removal of copper and iron cyanide complex from cyanide solution by polymer-surfactant aggregates. Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134788. [Google Scholar] [CrossRef]
- Alonso-González, O.; Nava-Alonso, F.; Uribe-Salas, A. Copper removal from cyanide solutions by acidification. Miner. Eng. 2009, 22, 324–329. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Chen, J.; Shen, X.; Cui, S.; Liu, K.; Han, Q. A novel green method for copper recovery from cuprous thiocyanate-containing acidified sediments in the gold industry. J. Clean. Prod. 2021, 329, 129729. [Google Scholar] [CrossRef]
- Karppinen, A.; Seisko, S.; Nevatalo, L.; Wilson, B.P.; Yliniemi, K.; Lundström, M. Gold recovery from cyanidation residue by chloride leaching and carbon adsorption—Preliminary results from CICL process. Hydrometallurgy 2024, 226, 106304. [Google Scholar] [CrossRef]
- Botz, M.; Guzman, G.; Sevilla, L. Campaign testing the yanacocha sart plant with high-copper feed solution. In Proceedings of the Society for Mining, Metallurgy, and Exploration, Inc. (SME), Denver, CO, USA, 15–18 February 2015. [Google Scholar]
- Baker, B.; Rodriguez, F.; Littlejohn, P. SART implementation at Gold Mines in Latin America. In World Gold 2017; CIM/ICM: Vancouver, BC, Canada, 2017; Available online: https://onemine.org/documents/sart-implementation-at-gold-mines-in-latin-america (accessed on 21 August 2024).
- Kratochvil, D.; Salari, D.; Avilez, T. SART implementation at heap leach operations in Mexico, 2018. In Proceedings of the 50th Canadian Minerals Processors Conference in Ottawa, Ottawa, ON, Canada, 24 January 2018. [Google Scholar]
- MacPhail, P.K.; Fleming, C.; Sarbutt, K. Cyanide Recovery by the SART Process for the Lobo-Marte Project, Chile. In Randol Gold and Silver Forum, Society for Mining; Metallurgy & Exploration: Denver, CO, USA, 1998. [Google Scholar]
- Estay, H.; Gim-Krumm, M.; Quilaqueo, M. Two-Stage SART Process: A Feasible Alternative for Gold Cyanidation Plants with High Zinc and Copper Contents. Minerals 2018, 8, 392. [Google Scholar] [CrossRef]
- Fleming, C.A.; Melashvili, M. The SART process: Killing the sacred cows. In Proceedings of the XXVIII International Mineral Processing Congress (IMPC 2016), Quebec, QC, Canada, 11–15 September 2016; pp. 2107–2120. [Google Scholar]
- Estay, H.; Gim-Krumm, M.; Seriche, G.; Quilaqueo, M.; Barros, L.; Ruby-Figueroa, R.; Romero, J.; Troncoso, E. Optimizing the SART process: A critical assessment of its design criteria. Miner. Eng. 2020, 146, 106116. [Google Scholar] [CrossRef]
- Estay, H.; Ruby-Figueroa, R.; Quilaqueo, M.; Seriche, G.; Cortés, I.; Gim-Krumm, M.; Barros, L. Enhancing the effectiveness of copper and cyanide recovery in gold cyanidation: A new integrated membrane process. Hydrometallurgy 2021, 202, 105606. [Google Scholar] [CrossRef]
- Lu, D.; Chang, Y.; Wang, W.; Xie, F.; Asselin, E.; Dreisinger, D. Copper and Cyanide Extraction with Emulsion Liquid Membrane with LIX 7950 as the Mobile Carrier: Part 1, Emulsion Stability. Metals 2015, 5, 2034–2047. [Google Scholar] [CrossRef]
- Xie, F.; Wang, W. Recovery of copper and cyanide from waste cyanide solutions using emulsion liquid membrane with LIX 7950 as the carrier. Environ. Technol. 2017, 38, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Seriche, G.; Quilaqueo, M.; Barros, L.; Gim-Krumm, M.; Cortés, I.; Troncoso, E.; Ruby-Figueroa, R.; Estay, H. Integrated Membrane Process Coupled with Metal Sulfide Precipitation to Recover Zinc and Cyanide. Minerals 2022, 12, 229. [Google Scholar] [CrossRef]
- Sceresini, B.; Breuer, P. Chapter 43-Gold-Copper Ores. In Adams Gold Ore Processing, 2nd ed.; Mike, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 771–801. [Google Scholar] [CrossRef]
- Das, A.K.; Saikat, D. Chapter 3-Optimization of Extraction Using Mathematical Models and Computation. In Computational Phytochemistry; Satyajit, D., Sarker, L.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 75–106. ISBN 9780128123645. [Google Scholar] [CrossRef]
- Liu, P.-j.; Liu, Z.-g.; Chu, M.-s.; Yan, R.-j.; Li, F.; Tang, J. Multiobjective collaborative optimization of novel carbothermal reduction process of stainless steel dust and laterite nickel ore. Trans. Nonferrous Met. Soc. China 2023, 33, 1919–1931. [Google Scholar] [CrossRef]
- Gürkan, E.H.; Tibet, Y.; Çoruh, S. Application of Full Factorial Design Method for Optimization of Heavy Metal Release from Lead Smelting Slag. Sustainability 2021, 13, 4890. [Google Scholar] [CrossRef]
- Mihăilescu, M.; Negrea, A.; Ciopec, M.; Negrea, P.; Duțeanu, N.; Grozav, I.; Svera, P.; Vancea, C.; Bărbulescu, A.; Dumitriu, C.Ș. Full Factorial Design for Gold Recovery from Industrial Solutions. Toxics 2021, 9, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teo, P.T.; Zakaria, S.K.; Mohd Sharif, N.; Abu Seman, A.; Taib, M.A.A.; Mohamed, J.J.; Yusoff, M.; Yusoff, A.H.; Mohamad, M.; Ali, A.; et al. Application of General Full Factorial Statistical Experimental Design’s Approach for the Development of Sustainable Clay-Based Ceramics Incorporated with Malaysia’s Electric Arc Furnace Steel Slag Waste. Crystals 2021, 11, 442. [Google Scholar] [CrossRef]
- Hanrahan, G.; Zhu, J.; Gibani, S.; Patil, D.G. Chemometrics and Statistics|Experimental Design. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 8–13. [Google Scholar] [CrossRef]
- Malyshev, V.P. Probabilistic-deterministic planning of the experiment. Alma-Ata Sci. 1981, 116, 116. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).