Comparative Study on the Effect of Carbon Existence Form and Sulfur on the Hydrophilicity of Coal Pyrite Surface Based on the Density Functional Theory
Abstract
1. Introduction
2. Calculation Methods and Model
2.1. Calculation Methods
2.2. Surface Model
3. Results and Discussion
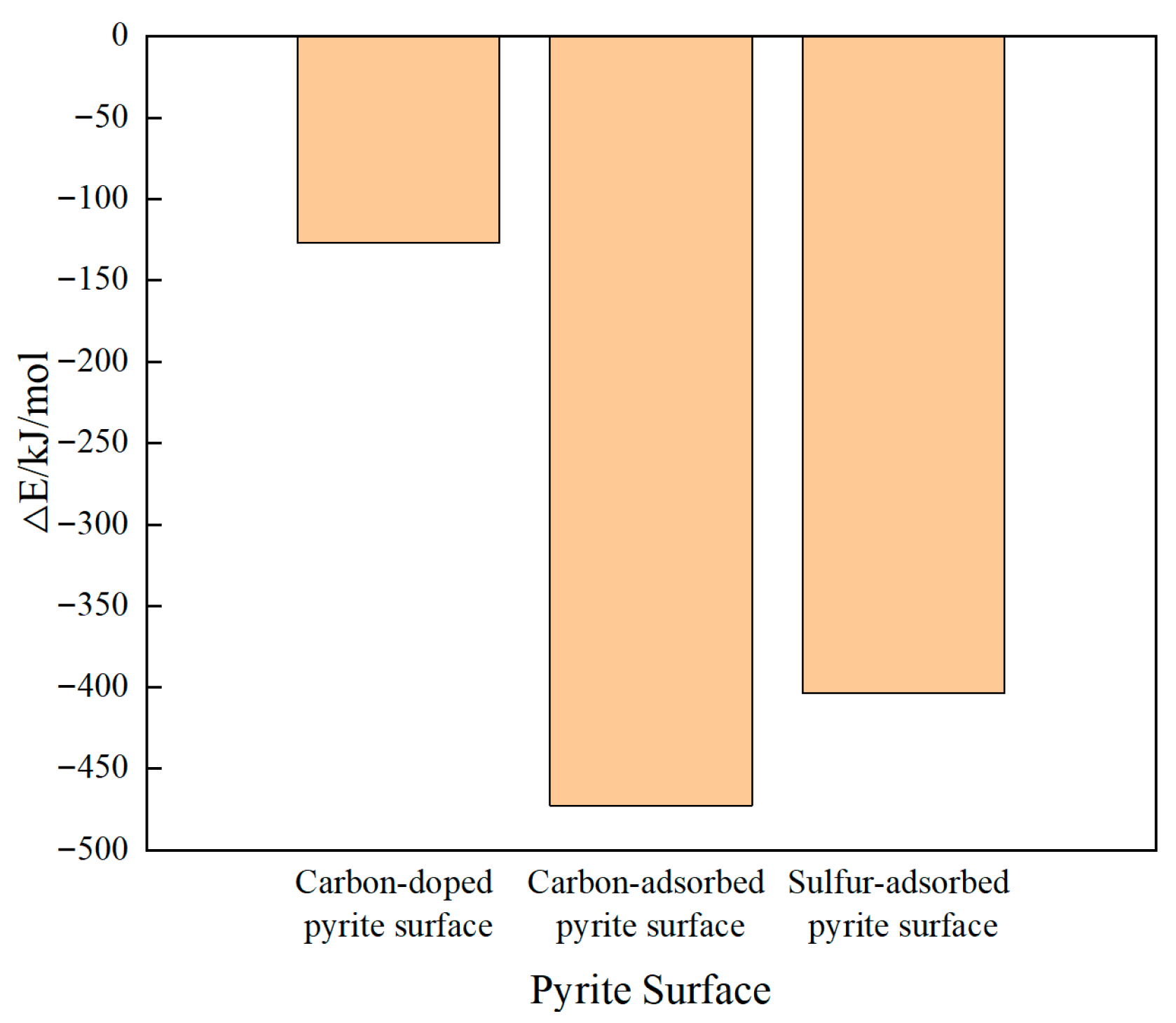
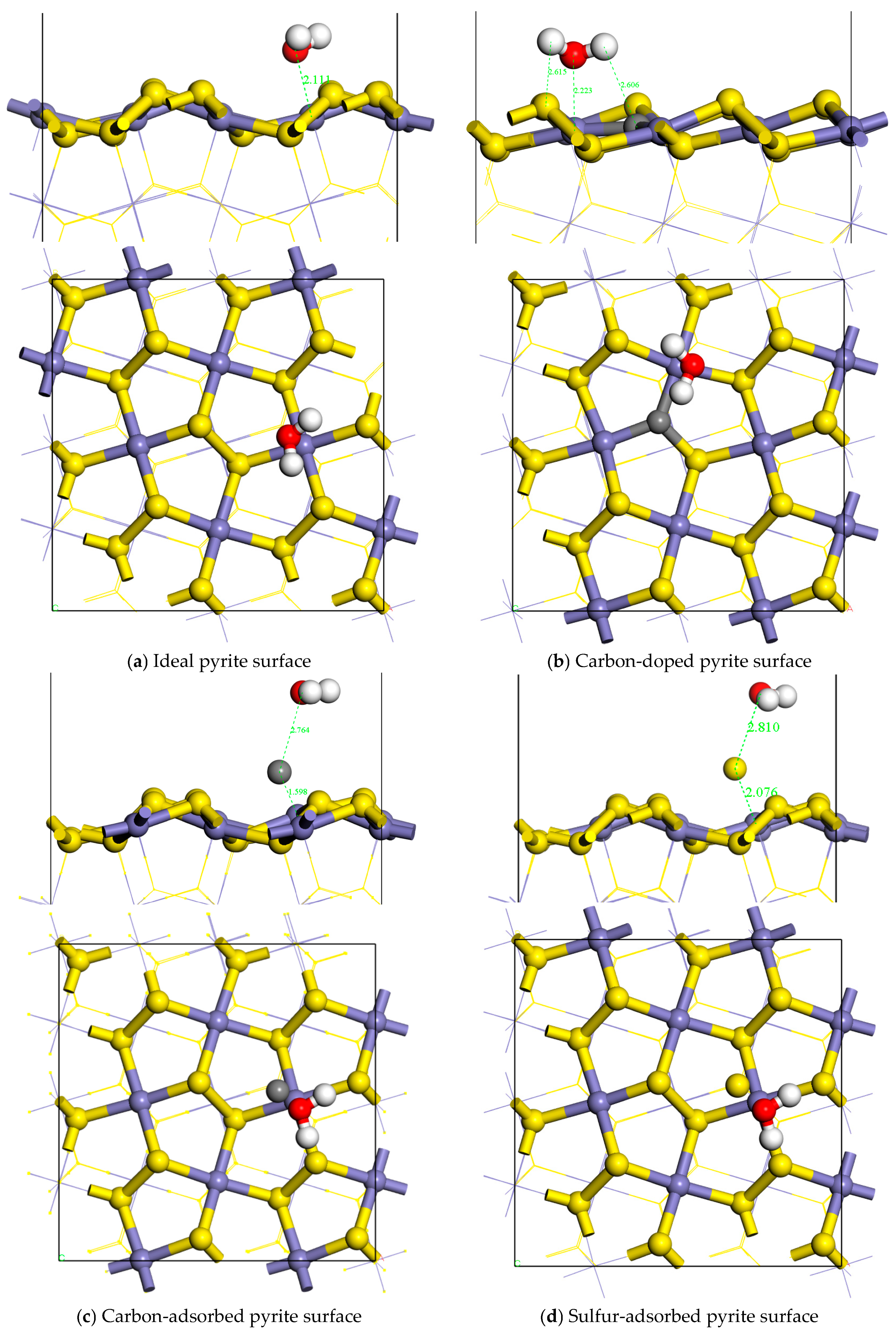
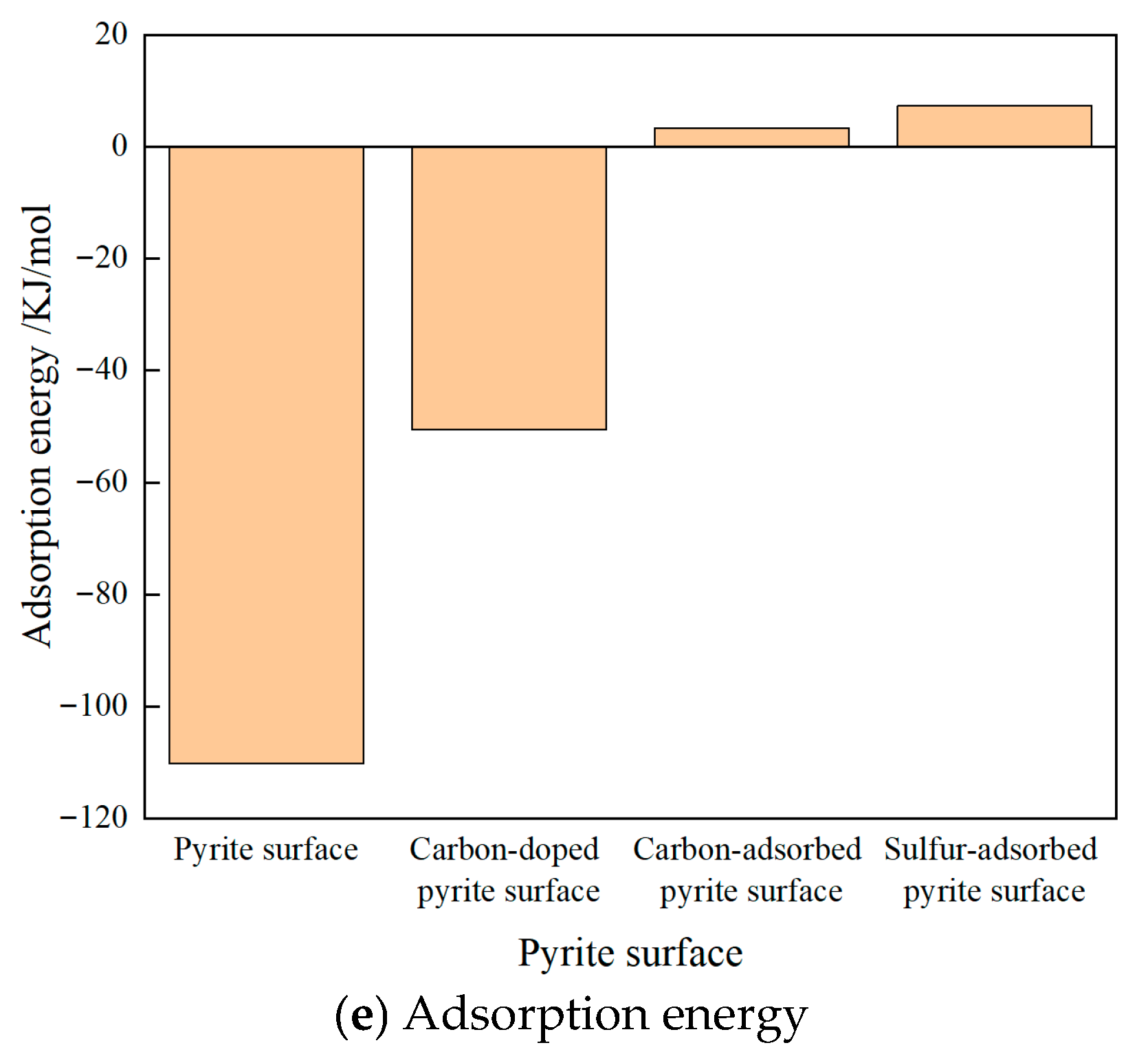
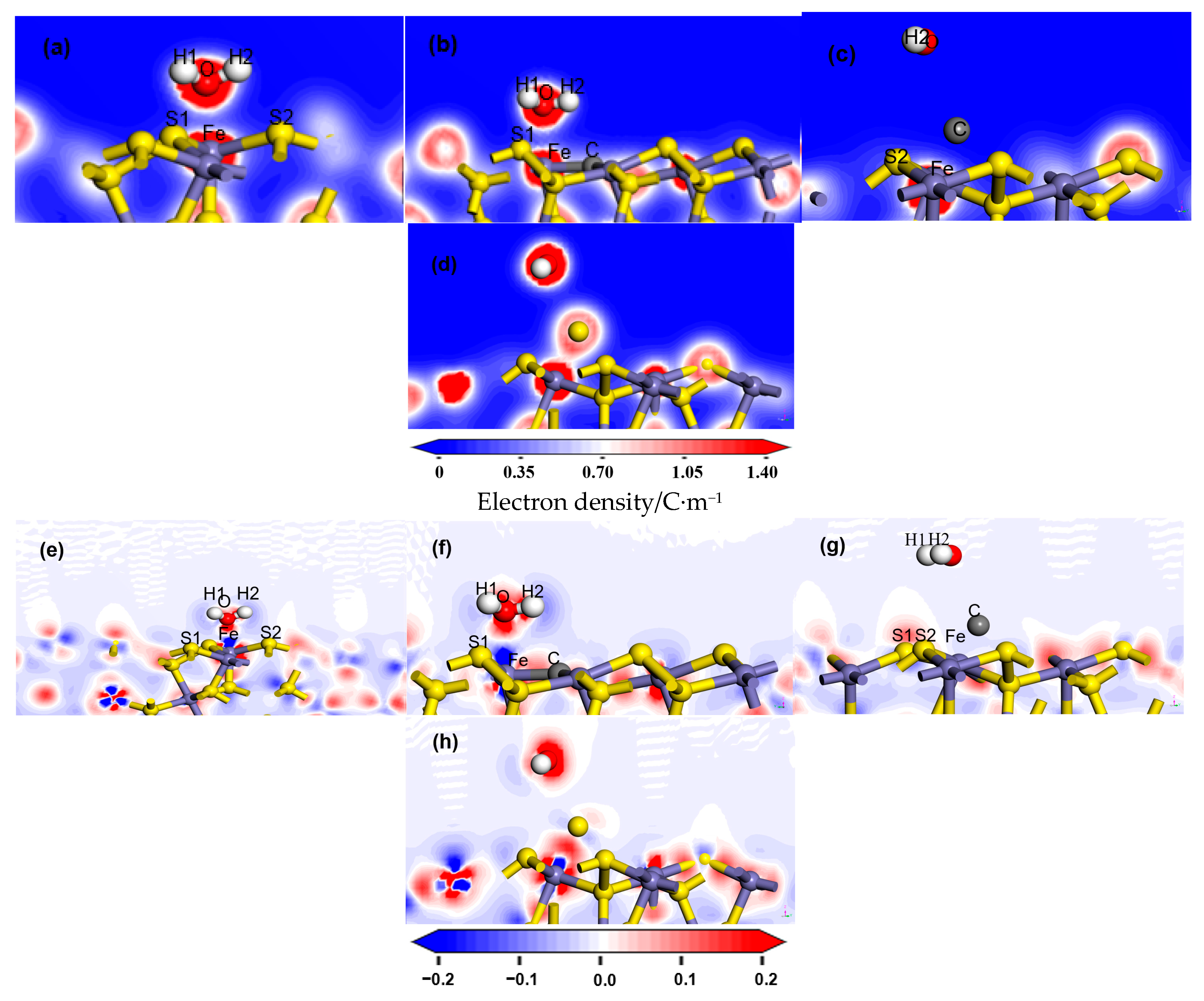
3.1. Adsorption Configurations and Adsorption Energies
3.2. Analysis of Bonding
3.3. The Charge Transfer
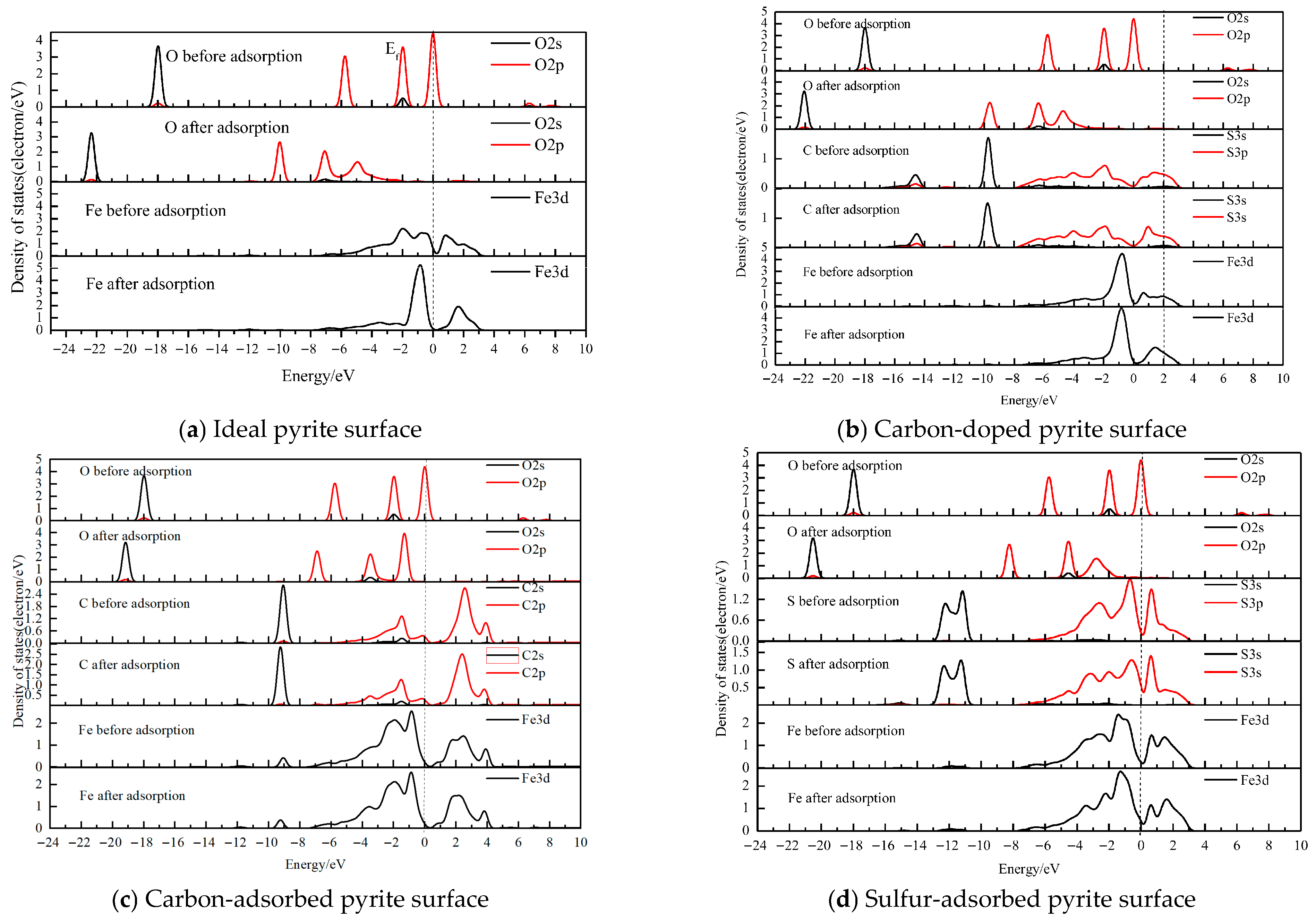
3.4. The Density of States (DOS)
4. Conclusions
- (1)
- Under the same impurity atom doping or adsorption concentration, the pyrite surface with adsorbed sulfur was hydrophobic. The pyrite surface with adsorbed carbons was nearly hydrophobic. The pyrite surface with doped carbons was weakly hydrophilic. The ideal pyrite surface was strongly hydrophilic. Macroscopically, the overall hydrophobicity of the surface of coal-bearing pyrite covered with sulfur is greater than that of coal-bearing pyrite containing co-growth carbon and even greater than that of coal-bearing pyrite doped with carbon atoms.
- (2)
- In the future, coal slime flotation desulfurization can consider separating clean coal from ash and sulfur in the shortest possible time while meeting the ash content requirements of clean coal. In a short period of time, the degree of oxidation on the surface of pyrite is relatively weak, which is not enough to become hydrophobic, and then the clean coal fraction is high as the clean coal slime floats up.
- (3)
- Some coal pyrite samples from different regions can be selected and prepared by grinding in an oxygen-free and water-free environment, and others can be prepared at room temperature and placed for a period of time. And then conduct contact angle experiments on the samples so they can be compared and determined to compare the strength of their hydrophobicity.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, G. Mineral Processing, 3rd ed.; China University of Mining and Technology Press: Xuzhou, China, 2012; pp. 413–414. ISBN 978-7-81070-361-1. [Google Scholar]
- Shao, X.; Tang, Y.; Wang, P. The ESCA study of coal pyrite and pyrite. Anal. Util. Coal Qual. 1994, 2, 9–18. [Google Scholar]
- Yu, J. Study on the Physical and Chemical Characteristics of Coal-Pyrite and Its Inhibitors. Ph.D. Thesis, China University of Mining and Technology (Beijing), Beijing, China, 2013. [Google Scholar]
- Backley, A.; Woods, N.R. The surface oxidation of pyrite. App. Surf. Sci. 1987, 27, 437–452. [Google Scholar] [CrossRef]
- Yoon, R.H.; Lagno, M.L.; Luttrell, G.H. On the hydrophobicity of coal pyrite. In Proceedings of the Processing and Utilization of High Sulfur Coals IV, Idaho Falls, ID, USA, 26–30 August 1991; Dugan, P.R., Quigley, D.R., Attia, Y.A., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 241–253. [Google Scholar]
- Zhu, X.; Wadsworth, M.E.; Bodily, D.M. Surface properties of mineral and coal pyrite after electrochemical alteration. In Proceedings of the Processing and Utilization of High Sulfur Coals IV, Idaho Falls, ID, USA, 26–30 August 1991; Dugan, P.R., Quigley, D.R., Attia, Y.A., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 205–222. [Google Scholar]
- Xi, P.; Liu, W.; Han, Y. Study on the mechanism of coal pyrite crystal lattice defects and floatability. J. China Coal Soc. 2016, 41, 997–1003. [Google Scholar]
- Xi, P.; Ma, R.; Liu, W. Research on the Effect of Carbon Defects on the Hydrophilicity of Coal Pyrite Surface from the Insight of Quantum Chemistry. Molecules 2019, 24, 2285. [Google Scholar] [CrossRef] [PubMed]
- Xi, P.; Wang, D.; Liu, W. DFT Study into the Influence of Carbon Material on the Hydrophobicity of a Coal Pyrite Surface. Molecules 2019, 24, 3534. [Google Scholar] [CrossRef]
- Xi, P.; Liu, W.; Yang, Z. Quantum chemistry investigation on influence of sulfur atom adsorption in sulfur material to the coal pyrite hydrophobicity. J. China Coal Soc. 2017, 42, 1290–1296. [Google Scholar]
- Xi, P.; Shi, C.; Yan, P. DFT study on influence of sulfur on the hydrophobicity of pyrite surfaces in the process of oxidation. App. Sur. Sci. 2019, 466, 964–969. [Google Scholar] [CrossRef]
- Fu, X.; Niu, Z.; Peng, C. Quantitative synergistic adsorption affinity of Ca(II) and sodium oleate to predict the surface reactivity of hematite and quartz. Sep. Purif. Technol. 2025, 360, 131196. [Google Scholar] [CrossRef]
- Peng, C.; Fu, X.; Niu, Z. Protonation behavior study of the active sites on typical sulfide minerals surface using surface complexation model. Colloids Surf. A. 2025, 170, 136307. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, S.; Hao, J. Flotation separation of chalcopyrite from pyrite using mineral fulvic acid as selective depressant under weakly alkaline conditions. Trans. Nonferrous Met. Soc. China 2025, 35, 313–325. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, L.; Cao, Y.; Xie, J.; Wang, X.; Qiao, Y.; Zhang, L.; Sun, W. Separation of chalcopyrite from pentlandite with sodium persulfate depressant: Flotation behavior and mechanism. Trans. Nonferrous Met. Soc. China 2025, 1–13. [Google Scholar]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrated and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phy. Rev. B Condens. Matter Mater. Phys. 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Zhao, C. Influence of external electronic field on the electronic structure and optical properties of pyrite. RSC Adv. 2017, 7, 56676–56681. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 4, 7892–7895. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B Solid State 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. “Special points for Brillouin-zone integrations”—A reply. Phys. Rev. B Solid State 1977, 16, 1748–1749. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.L.; Zhou, J. Interactions between kaolinite Al-OH surface and sodium hexametaphosphate. App. Surf. Sci. 2016, 387, 759–765. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.L.; Chen, J.H. DFT simulation of the adsorption of sodium silicate species on kaolinite surfaces. App. Surf. Sci. 2016, 370, 403–409. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, W.; Zhang, H. DFT study of the adsorption of 3-chloro-2-hydroxypropyl trimethylammonium chloride on montmorillonite surfaces in solution. App. Surf. Sci. 2018, 436, 58–65. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO-MO molecular wave functions. IV. bonding and antibonding in LCAO and Valence-bond theories. J. Chem. Phy. 1955, 23, 2343–2346. [Google Scholar] [CrossRef]
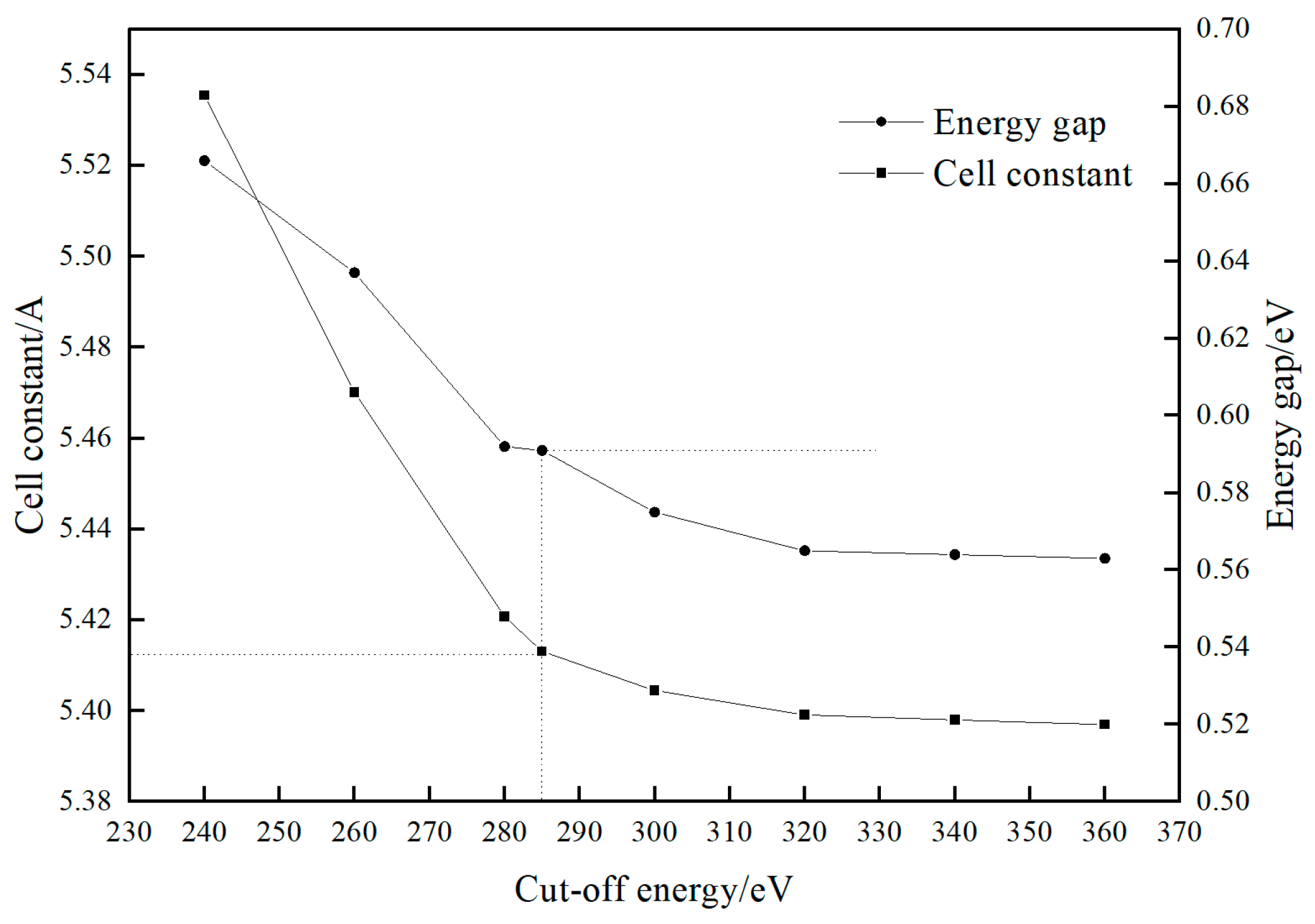

| Exchange-Correlation Functionals | Lattice Parameters/Å | Bandwidth/eV | Cell Volume/Å3 | Energy/eV |
|---|---|---|---|---|
| GGA-PBE | 5.413 | 0.591 | 158.612 | −5691.699 |
| GGA-RPBE | 5.473 | 0.673 | 163.926 | −5695.789 |
| GGA-PW91 | 5.416 | 0.603 | 158.847 | −5702.04 |
| GGA-WC | 5.338 | 0.449 | 152.098 | −5683.08 |
| GGA-PBESOL | 5.326 | 0.409 | 151.047 | −5670.969 |
| LDA-CA-PZ | 5.272 | 0.402 | 146.462 | −5679.188 |
| Experimental value | 5.417 | 0.950 | ||
| Initial value | 5.428 | 159.935 |
| Adsorption Model | Bond | Population | Length |
|---|---|---|---|
| Ideal pyrite surface | Fe–O | 0.13 | 2.111 |
| H1–S1 | 0.01 | 2.480 | |
| H2–S2 | −0.01 | 2.567 | |
| Carbon-doped pyrite surface | Fe–O | 0.11 | 2.223 |
| H1–S1 | −0.00 | 2.615 | |
| H2–C | −0.01 | 2.606 | |
| Carbon-adsorbed pyrite surface | Fe–C | 0.87 | 1.598 |
| C–O | −0.02 | 2.764 | |
| Sulfur-adsorbed pyrite surface | Fe–S | 0.54 | 2.076 |
| S–O | −0.05 | 2.810 |
| Atomic Label | Adsorption Status | s | p | d | T | Charge/e |
|---|---|---|---|---|---|---|
| Fe | BA | 0.40 | 0.52 | 6.90 | 7.82 | 0.18 |
| AA | 0.32 | 0.42 | 7.11 | 7.85 | 0.15 | |
| O | BA | 1.89 | 5.16 | 0.00 | 7.05 | −1.05 |
| AA | 1.86 | 4.96 | 0.00 | 6.84 | −0.83 |
| Atomic Label | Adsorption Status | s | p | d | T | Charge/e |
|---|---|---|---|---|---|---|
| Fe | BA | 0.33 | 0.37 | 7.08 | 7.78 | 0.22 |
| AA | 0.30 | 0.38 | 7.04 | 7.74 | 0.27 | |
| O | BA | 1.89 | 5.16 | 0.00 | 7.05 | −1.05 |
| AA | 1.87 | 4.98 | 0.00 | 6.84 | −0.84 |
| Atomic Label | Adsorption Status | s | p | d | T | Charge/e |
|---|---|---|---|---|---|---|
| Fe | BA | 0.32 | 0.54 | 7.02 | 7.88 | 0.12 |
| AA | 0.30 | 0.53 | 7.05 | 0.00 | 0.12 | |
| C | BA | 1.82 | 2.28 | 0.00 | 4.12 | −0.15 |
| AA | 1.83 | 2.29 | 0.00 | 4.12 | −0.12 | |
| O | BA | 1.90 | 5.16 | 0.00 | 7.06 | −1.05 |
| AA | 1.89 | 5.10 | 0.00 | 6.99 | −0.99 |
| Atomic Label | Adsorption Status | s | p | d | T | Charge/e |
|---|---|---|---|---|---|---|
| Fe | BA | 0.37 | 0.58 | 6.98 | 7.92 | 0.08 |
| AA | 0.36 | 0.59 | 7.03 | 7.97 | 0.03 | |
| S | BA | 1.91 | 4.29 | 0.00 | 6.21 | −0.21 |
| AA | 1.92 | 4.22 | 0.00 | 6.15 | −0.14 | |
| O | BA | 1.89 | 5.16 | 0.00 | 7.05 | −1.05 |
| AA | 1.89 | 5.09 | 0.00 | 6.98 | −0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, P.; Tang, X.; Sun, F.; Fan, X.; Cong, G.; Zhuo, Q. Comparative Study on the Effect of Carbon Existence Form and Sulfur on the Hydrophilicity of Coal Pyrite Surface Based on the Density Functional Theory. Processes 2025, 13, 3232. https://doi.org/10.3390/pr13103232
Xi P, Tang X, Sun F, Fan X, Cong G, Zhuo Q. Comparative Study on the Effect of Carbon Existence Form and Sulfur on the Hydrophilicity of Coal Pyrite Surface Based on the Density Functional Theory. Processes. 2025; 13(10):3232. https://doi.org/10.3390/pr13103232
Chicago/Turabian StyleXi, Peng, Xiaoyu Tang, Fengling Sun, Xiaoping Fan, Guangpei Cong, and Qiming Zhuo. 2025. "Comparative Study on the Effect of Carbon Existence Form and Sulfur on the Hydrophilicity of Coal Pyrite Surface Based on the Density Functional Theory" Processes 13, no. 10: 3232. https://doi.org/10.3390/pr13103232
APA StyleXi, P., Tang, X., Sun, F., Fan, X., Cong, G., & Zhuo, Q. (2025). Comparative Study on the Effect of Carbon Existence Form and Sulfur on the Hydrophilicity of Coal Pyrite Surface Based on the Density Functional Theory. Processes, 13(10), 3232. https://doi.org/10.3390/pr13103232







