Clarification of Copper Sulfide Precipitates by Polymeric Microfiltration Membranes
Abstract
1. Introduction
2. Materials and Methods
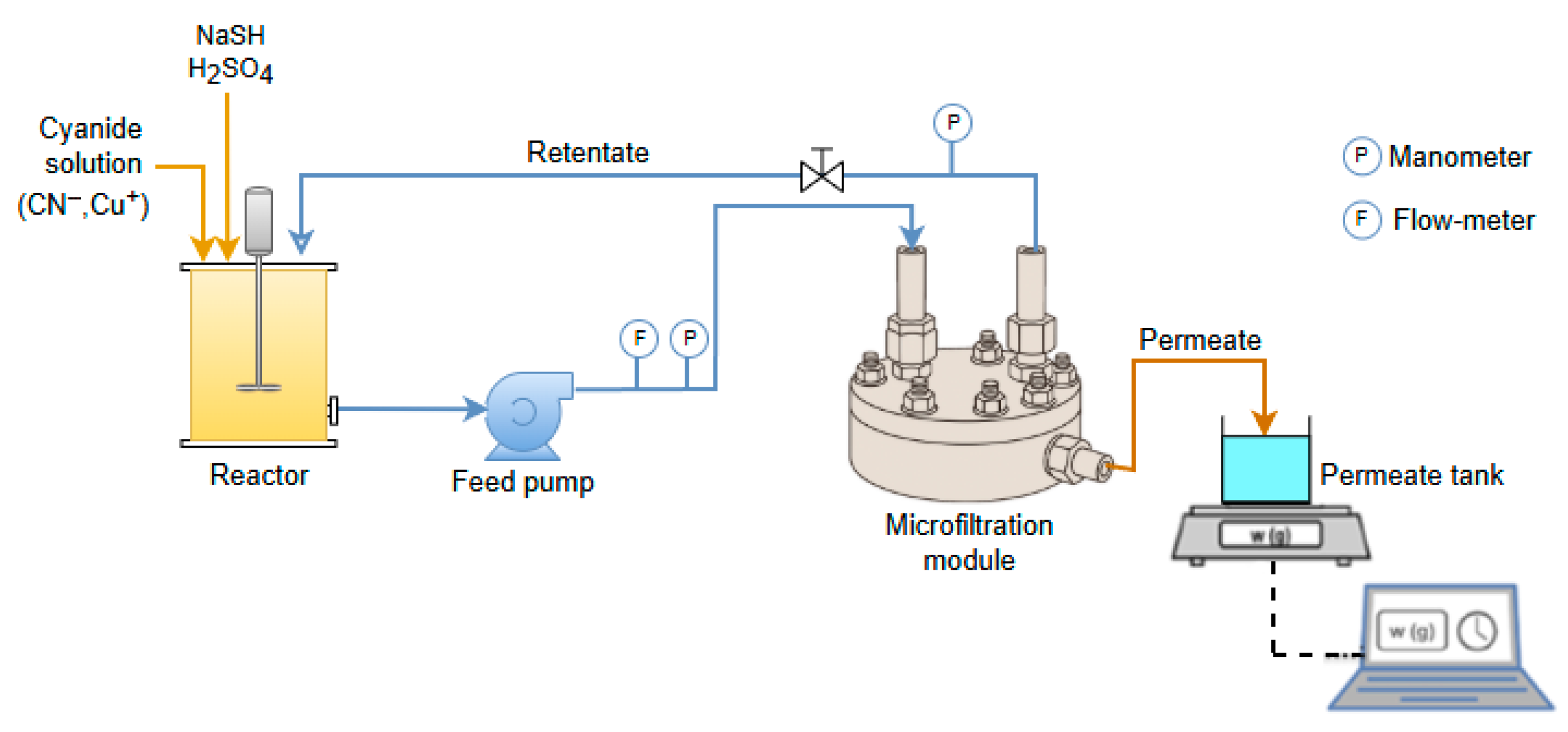
2.1. Experimental Set-Up and Procedure Under Batch Concentration Configuration
2.2. Microfiltration Performance
2.3. Determination of Critical Transmembrane Pressure (CTMP) and Limiting Flux
2.4. Membrane Fouling Analysis
2.5. Feasibility Tests for Membrane Recovery
2.6. Statistical Evaluation and Model Validation
2.7. Capital and Operational Cost Comparison
3. Results
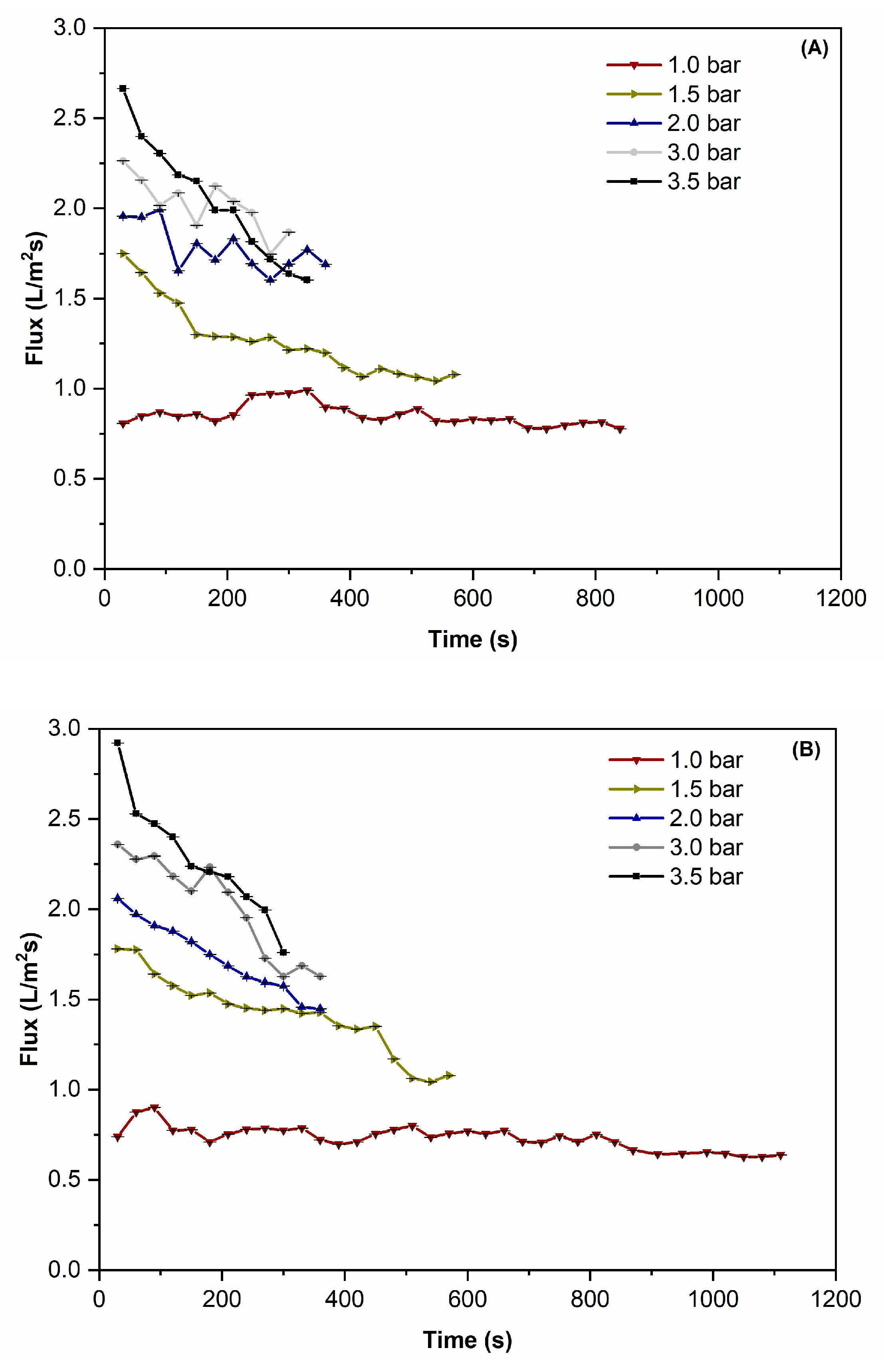
3.1. Microfiltration Performance
3.2. CTMP and Limiting Flux
3.3. Assessment of Membrane Fouling Mechanisms
3.4. Membrane Permeability and Recovery Analysis
3.5. Capital and Operational Cost Comparison of Polymeric and Ceramic Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Acid mine drainage |
| CA | Cellulose acetate |
| CAPEX | Capital expenditures |
| CTMP | Critical transmembrane pressure |
| FSF | Polysulfone |
| K-S | Kolmogorov–Smirnov |
| MF | Microfiltration |
| OPEX | Operational expenditures |
| PVDF | Polyvinylidene fluoride |
| R2 | Coefficient of determination |
| RMSPE | Root mean square percentage error |
| SART | Sulphidization, acidification, recycling, and thickening |
| SDG | Sustainable development goal |
| S-W | Shapiro–Wilk |
| TMP | Transmembrane pressure |
| UF | Ultrafiltration |
References
- Gebreslassie, G.; Desta, H.G.; Dong, Y.; Zheng, X.; Zhao, M.; Lin, B. Advanced Membrane-Based High-Value Metal Recovery from Wastewater. Water Res. 2024, 265, 122122. [Google Scholar] [CrossRef]
- Chalaris, M.; Gkika, D.A.; Tolkou, A.K.; Kyzas, G.Z. Advancements and Sustainable Strategies for the Treatment and Management of Wastewaters from Metallurgical Industries: An Overview. Environ. Sci. Pollut. Res. 2023, 30, 119627–119653. [Google Scholar] [CrossRef] [PubMed]
- Dashtban Kenari, S.L.; Mortazavi, S.; Mosadeghsedghi, S.; Atallah, C.; Volchek, K. Advancing Ceramic Membrane Technology for Sustainable Treatment of Mining Discharge: Challenges and Future Directions. Membranes 2025, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, H.; Xie, Y.; Cai, H.; Dang, Z.; Lu, G. Ascorbic Acid-Induced Digenite (Cu9S5) Formation: A Strategy to Enhance Sulfidation Efficiency for Copper Recovery from Acidic Wastewater. Water Res. 2025, 281, 123703. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Li, X.; Chen, L.; Mao, H.; Meng, X.; Huhe, T.; Zhou, Z. Designing Anti-Fouling PVDF Membranes by Synergizing Cu2+ and Sodium Lignosulphonate Additives. Sep. Purif. Technol. 2024, 330, 125554. [Google Scholar] [CrossRef]
- Kumar, M.; Ajay Kumar, P.V.; Pugazhenthi, G.; Pakshirajan, K. Recovery and Purification of Copper Sulfide Nanoparticles from Acid Mine Drainage by Biological Sulfate Reduction and Microfiltration Using Low-Cost Ceramic Membrane. Clean Technol. Environ. Policy 2023, 25, 1309–1322. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Banik, S.; Das, S. Recovery of Copper from Acid Mine Drainage Using Advanced Recovery Techniques. In Metal Value Recovery from Industrial Waste Using Advanced Physicochemical Treatment Technologies; Elsevier: Amsterdam, The Netherlands, 2025; pp. 141–166. ISBN 978-0-443-21884-2. [Google Scholar]
- Kumar, M.; Pakshirajan, K. Continuous Removal and Recovery of Metals from Wastewater Using Inverse Fluidized Bed Sulfidogenic Bioreactor. J. Clean. Prod. 2021, 284, 124769. [Google Scholar] [CrossRef]
- Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. [Google Scholar] [CrossRef]
- Estay, H.; Barros, L.; Troncoso, E. Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks. Minerals 2021, 11, 1385. [Google Scholar] [CrossRef]
- Podobińska-Staniec, M.; Wiktor-Sułkowska, A.; Kustra, A.; Lorenc-Szot, S. Copper as a Critical Resource in the Energy Transition. Energies 2025, 18, 969. [Google Scholar] [CrossRef]
- International Renewable Energy Agency; Norwegian Institute of International Affairs. Critical Materials for Renewable Energy Improving Data Governance; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2024. [Google Scholar]
- U.S. Department of the Interior; U.S. Geological Survey. Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025.
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Dawam, M.; Gobara, M.; Oraby, H.; Zorainy, M.Y.; Nabil, I.M. Advances in Membrane Technologies for Heavy Metal Removal from Polluted Water: A Comprehensive Review. Water Air Soil Pollut. 2025, 236, 461. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Dobre, T.; Isopencu, G.O.; Bdaiwi Ahmed, S.; Deleanu, I.M. Heavy Metal Pollution and Solutions for Its Control: General Aspects with a Focus on Cobalt Removal and Recovery from Aqueous Systems. ChemEngineering 2024, 8, 118. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Recent Progress in Removal of Heavy Metals from Wastewater: A Comprehensive Review. Chemosphere 2023, 335, 139077. [Google Scholar] [CrossRef] [PubMed]
- Sole, K.C.; Prinsloo, A.; Hardwick, E. Recovery of copper from Chilean mine waste waters. In Proceedings of the Mining Meets Water—Conflicts and Solutions, Leipzig, Germany, 11–15 July 2016. [Google Scholar]
- Lewis, A.E. Review of Metal Sulphide Precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Menzel, K.; Barros, L.; García, A.; Ruby-Figueroa, R.; Estay, H. Metal Sulfide Precipitation Coupled with Membrane Filtration Process for Recovering Copper from Acid Mine Drainage. Sep. Purif. Technol. 2021, 270, 118721. [Google Scholar] [CrossRef]
- Estay, H.; Gim-Krumm, M.; Seriche, G.; Quilaqueo, M.; Barros, L.; Ruby-Figueroa, R.; Romero, J.; Troncoso, E. Optimizing the SART Process: A Critical Assessment of Its Design Criteria. Miner. Eng. 2020, 146, 106116. [Google Scholar] [CrossRef]
- Mokone, T.P.; Van Hille, R.P.; Lewis, A.E. Metal Sulphides from Wastewater: Assessing the Impact of Supersaturation Control Strategies. Water Res. 2012, 46, 2088–2100. [Google Scholar] [CrossRef] [PubMed]
- Mokone, T.P.; Lewis, A.E.; Van Hille, R.P. Effect of Post-Precipitation Conditions on Surface Properties of Colloidal Metal Sulphide Precipitates. Hydrometallurgy 2012, 119–120, 55–66. [Google Scholar] [CrossRef]
- Barros, L.; Gim-Krumm, M.; Seriche, G.; Quilaqueo, M.; Castillo, C.; Ihle, C.F.; Ruby-Figueroa, R.; Estay, H. In-Situ and Real-Time Aggregation Size Evolution of Copper Sulfide Precipitates Using Focused Beam Reflectance Measurement (FBRM). Powder Technol. 2021, 380, 205–218. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Xu, X.; Zhou, S. Bioleaching of Different Copper Sulfides by Acidithiobacillus Ferrooxidans and Its Adsorption on Minerals. Hydrometallurgy 2013, 140, 42–47. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Xu, X.; Zhang, Y.; Gao, Y.; Zhou, S. Comparative Study on the Bioleaching, Biosorption and Passivation of Copper Sulfide Minerals. Int. Biodeterior. Biodegrad. 2013, 84, 29–34. [Google Scholar] [CrossRef]
- Estay, H.; Ruby-Figueroa, R.; Gim-Krumm, M.; Seriche, G.; Quilaqueo, M.; Díaz-Quezada, S.; Cortés, I.; Barros, L. Changing the Conventional Clarification Method in Metal Sulfide Precipitation by a Membrane-Based Filtration Process. J. Mater. Res. Technol. 2021, 11, 693–709. [Google Scholar] [CrossRef]
- Bertan, A.S.; Cremasco, M.A. Multi-Steps Microfiltration of Micelles from Fermentation of Streptomyces Tsukubaensis and Its Impact on Proteins Retention and Tacrolimus Yield. Food Bioprod. Process. 2024, 148, 208–217. [Google Scholar] [CrossRef]
- Estay, H.; Ruby-Figueroa, R.; Quilaqueo, M.; Seriche, G.; Cortés, I.; Gim-Krumm, M.; Barros, L. Enhancing the Effectiveness of Copper and Cyanide Recovery in Gold Cyanidation: A New Integrated Membrane Process. Hydrometallurgy 2021, 202, 105606. [Google Scholar] [CrossRef]
- Sisay, E.J.; Al-Tayawi, A.N.; László, Z.; Kertész, S. Recent Advances in Organic Fouling Control and Mitigation Strategies in Membrane Separation Processes: A Review. Sustainability 2023, 15, 13389. [Google Scholar] [CrossRef]
- Barros, L.; Piaggio, G.; Quilaqueo, M.; Seriche, G.; Pérez, K.; Barraza, B.; Romero, J.; Ruby-Figueroa, R.; Estay, H. Analysis of Membrane Fouling during Microfiltration of Copper Sulfide Precipitates. Sep. Purif. Technol. 2025, 354, 129165. [Google Scholar] [CrossRef]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Muthukumar, N.; Syed Ali, S.M.Y.; Murali, S.; Seetharaman, S.A. Fabrication of Polysulfone-Cellulose Acetate Based TiO2 @SiO2-CuS and Fe3O4 @XG Membrane and Its Application in Wastewater Treatment. Desalination Water Treat. 2024, 320, 100636. [Google Scholar] [CrossRef]
- Filipponi, A.; Masi, G.; Bandini, S.; Bignozzi, M.C. Preparation and Characterization of Metakaolin-Based Geopolymer Membrane Supports by Facile Pressed One-Part Route. Ceram. Int. 2023, 49, 6834–6842. [Google Scholar] [CrossRef]
- Shah, P.; Murthy, C.N. Studies on the Porosity Control of MWCNT/Polysulfone Composite Membrane and Its Effect on Metal Removal. J. Membr. Sci. 2013, 437, 90–98. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Nik Sulaiman, N.M.; Aroua, M.K.; Hashim, N.A. Effects of Alkaline Environments at Mild Conditions on the Stability of PVDF Membrane: An Experimental Study. Ind. Eng. Chem. Res. 2013, 52, 15874–15882. [Google Scholar] [CrossRef]
- Al-Obeidani, S.K.S.; Al-Hinai, H.; Goosen, M.F.A.; Sablani, S.; Taniguchi, Y.; Okamura, H. Chemical Cleaning of Oil Contaminated Polyethylene Hollow Fiber Microfiltration Membranes. J. Membr. Sci. 2008, 307, 299–308. [Google Scholar] [CrossRef]
- Seriche, G.; Quilaqueo, M.; Barros, L.; Gim-Krumm, M.; Cortés, I.; Troncoso, E.; Ruby-Figueroa, R.; Estay, H. Integrated Membrane Process Coupled with Metal Sulfide Precipitation to Recover Zinc and Cyanide. Minerals 2022, 12, 229. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical Flux Concept for Microfiltration Fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Astudillo-Castro, C.L. Limiting Flux and Critical Transmembrane Pressure Determination Using an Exponential Model: The Effect of Concentration Factor, Temperature, and Cross-Flow Velocity during Casein Micelle Concentration by Microfiltration. Ind. Eng. Chem. Res. 2015, 54, 414–425. [Google Scholar] [CrossRef]
- Hermia, J. Constant Pressure Blocking Filtration Laws-Application to Power-Law Non-Newtonian Fluids. Trans IChemE 1982, 60, 183–187. [Google Scholar]
- Vincent Vela, M.C.; Rodríguez, E.B.; Álvarez Blanco, S.; Lora García, J. Validation of Dynamic Models to Predict Flux Decline in the Ultrafiltration of Macromolecules. Desalination 2007, 204, 344–350. [Google Scholar] [CrossRef]
- Song, L. Flux Decline in Crossflow Microfiltration and Ultrafiltration: Mechanisms and Modeling of Membrane Fouling. J. Membr. Sci. 1998, 139, 183–200. [Google Scholar] [CrossRef]
- Menon, S.; Bansode, K.; Nandi, S.; Kalyanraman, V. Impact of Cleaning Agents on Properties of Tubular Polyvinylidene Fluoride (PVDF) Membrane. Mater. Today Process 2021, 47, 1466–1471. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Su, Q.; Wen, G.; Huang, T. Effects of Hydrogen Peroxide and Sodium Hypochlorite Aging on Properties and Performance of Polyethersulfone Ultrafiltration Membrane. Int. J. Environ. Res. Public Health 2019, 16, 3972. [Google Scholar] [CrossRef]
- Jarvis, P.; Carra, I.; Jafari, M.; Judd, S.J. Ceramic vs Polymeric Membrane Implementation for Potable Water Treatment. Water Res. 2022, 215, 118269. [Google Scholar] [CrossRef]
- Park, S.H.; Park, Y.G.; Lim, J.-L.; Kim, S. Evaluation of Ceramic Membrane Applications for Water Treatment Plants with a Life Cycle Cost Analysis. Desalination Water Treat. 2015, 54, 973–978. [Google Scholar] [CrossRef]
- Jarrar, R.; Abbas, M.K.G.; Al-Ejji, M. Environmental Remediation and the Efficacy of Ceramic Membranes in Wastewater Treatment—A Review. Emergent Mater. 2024, 7, 1295–1327. [Google Scholar] [CrossRef]


| Fouling Model (Model-Specific Exponent) | Fouling Rate Constant and Statistical Performance Metrics | Flow Rate, mL/min | |
|---|---|---|---|
| 900 | 1100 | ||
| Complete blocking (n = 2) | K | −1.34 × 10−4 | −2.12 × 10−4 |
| R2 | 0.24 | 0.62 | |
| RMSPE % | 5.70 | 5.32 | |
| K-S test (p-value) | 0.06 | 0.11 | |
| S-W test (p-value) | 3.20 × 10−3 | 4.33 × 10−3 | |
| Standard blocking (n = 1.5) | K | 7.24 × 10−5 | 1.25 × 10−4 |
| R2 | 0.24 | 0.62 | |
| RMSPE % | 5.70 | 5.36 | |
| K-S test (p-value) | 0.44 | 0.73 | |
| S-W test (p-value) | 0.01 | 0.08 | |
| Intermediate blocking (n = 1) | K | 1.57 × 10−4 | 2.94 × 10−4 |
| R2 | 0.25 | 0.63 | |
| RMSPE % | 5.71 | 5.39 | |
| K-S test (p-value) | 0.42 | 0.68 | |
| S-W test (p-value) | 0.01 | 0.07 | |
| Cake formation (n = 0) | K | 3.69 × 10−4 | 8.21 × 10−4 |
| R2 | 0.26 | 0.64 | |
| RMSPE % | 5.73 | 5.48 | |
| K-S test (p-value) | 0.59 | 0.64 | |
| S-W test (p-value) | 0.02 | 0.05 | |
| Inmersion Time, h | Cleaning Solution | Initial Permeability, m3/m2·s·Pa | Post-Test Permeability, m3/m2·s·Pa | % Recovery |
|---|---|---|---|---|
| 1 | NaCN | 1.57 × 10−8 | 8.22 × 10−9 | 52.5 |
| 2 | NaCN | 1.58 × 10−8 | 9.52 × 10−9 | 60.4 |
| 24 | NaCN | 1.61 × 10−8 | 1.01 × 10−8 | 62.7 |
| 1 | HCl + H2O2 | 1.62 × 10−8 | 1.27 × 10−8 | 78.5 |
| 2 | HCl + H2O2 | 1.33 × 10−8 | 1.01 × 10−8 | 76.1 |
| 24 | HCl + H2O2 | 1.41 × 10−8 | 1.35 × 10−8 | 96.1 |
| 1 | HCl + H2O2 + NaCN | 1.26 × 10−8 | 1.08 × 10−8 | 85.4 |
| 2 | HCl + H2O2 + NaCN | 1.60 × 10−8 | 1.43 × 10−8 | 89.7 |
| 24 | HCl + H2O2 + NaCN | 1.50 × 10−8 | 1.55 × 10−8 | 103.0 |
| Type of Membrane | CAPEX Per Capacity, US$/(m3/h) | OPEX Per Capacity, US$/m3 | Reference |
|---|---|---|---|
| Ceramic | 46.1 | 8.6 | [30] |
| Polymeric | 41.5 | 8.4 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilaqueo, M.; Barraza, N.; Barros, L.; Pérez, K.; Ruby-Figueroa, R.; Troncoso, E.; Estay, H. Clarification of Copper Sulfide Precipitates by Polymeric Microfiltration Membranes. Processes 2025, 13, 3292. https://doi.org/10.3390/pr13103292
Quilaqueo M, Barraza N, Barros L, Pérez K, Ruby-Figueroa R, Troncoso E, Estay H. Clarification of Copper Sulfide Precipitates by Polymeric Microfiltration Membranes. Processes. 2025; 13(10):3292. https://doi.org/10.3390/pr13103292
Chicago/Turabian StyleQuilaqueo, Michelle, Nicolás Barraza, Lorena Barros, Karla Pérez, René Ruby-Figueroa, Elizabeth Troncoso, and Humberto Estay. 2025. "Clarification of Copper Sulfide Precipitates by Polymeric Microfiltration Membranes" Processes 13, no. 10: 3292. https://doi.org/10.3390/pr13103292
APA StyleQuilaqueo, M., Barraza, N., Barros, L., Pérez, K., Ruby-Figueroa, R., Troncoso, E., & Estay, H. (2025). Clarification of Copper Sulfide Precipitates by Polymeric Microfiltration Membranes. Processes, 13(10), 3292. https://doi.org/10.3390/pr13103292








