Development of a Chestnut Shell Bio-Adsorbent for Cationic Pollutants: Encapsulation in an Alginate Carrier for Application in a Flow System
Abstract
1. Introduction
2. Materials and Methods
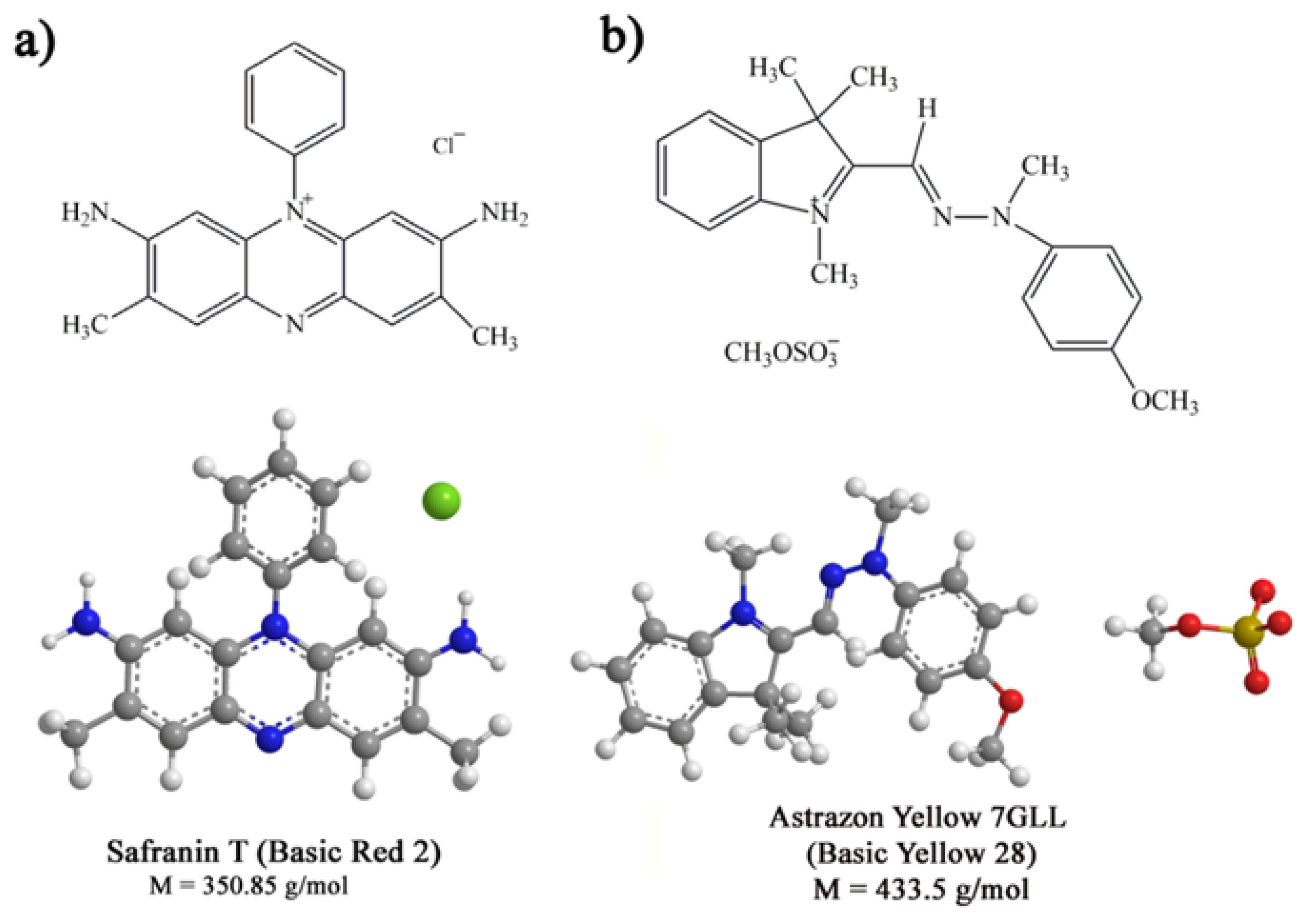
2.1. Chemicals and Materials
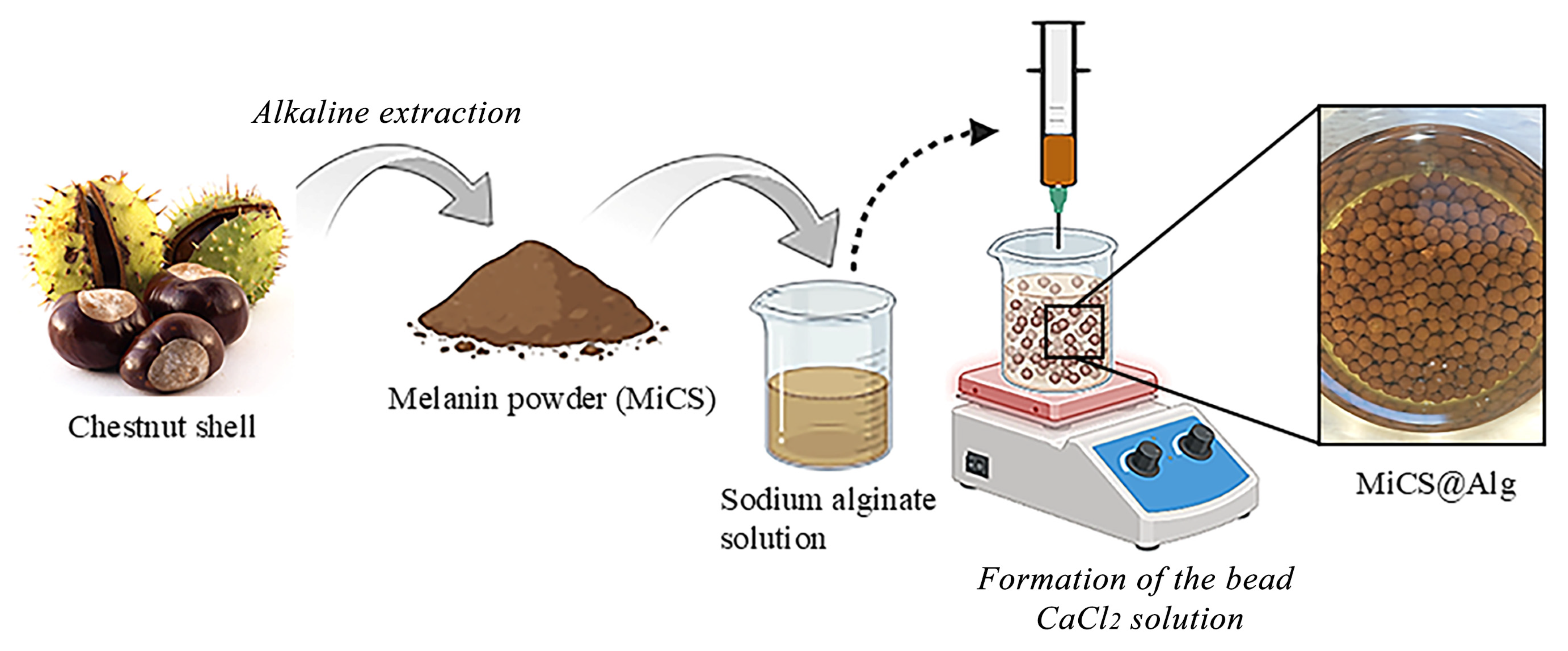
2.2. Extraction of Melanin from Integument Chestnut Shell (MiCS)
2.3. Preparation of MiCS Encapsulated in Alginate Particles (MiCS@Alg)
2.4. Characterization and Analysis
2.5. Adsorption Experiments
2.6. Desorption Experiments
3. Results
3.1. Characterization of Materials
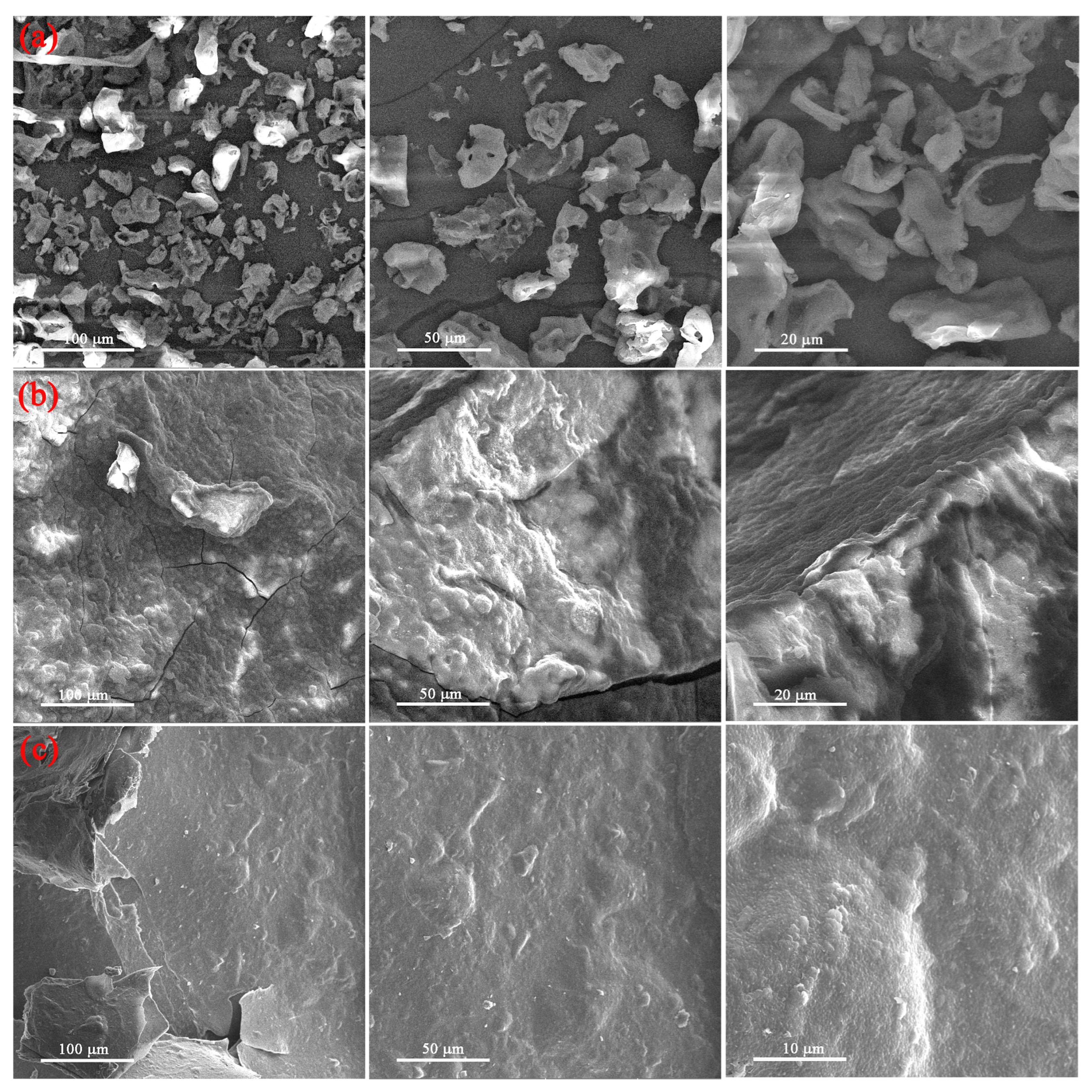
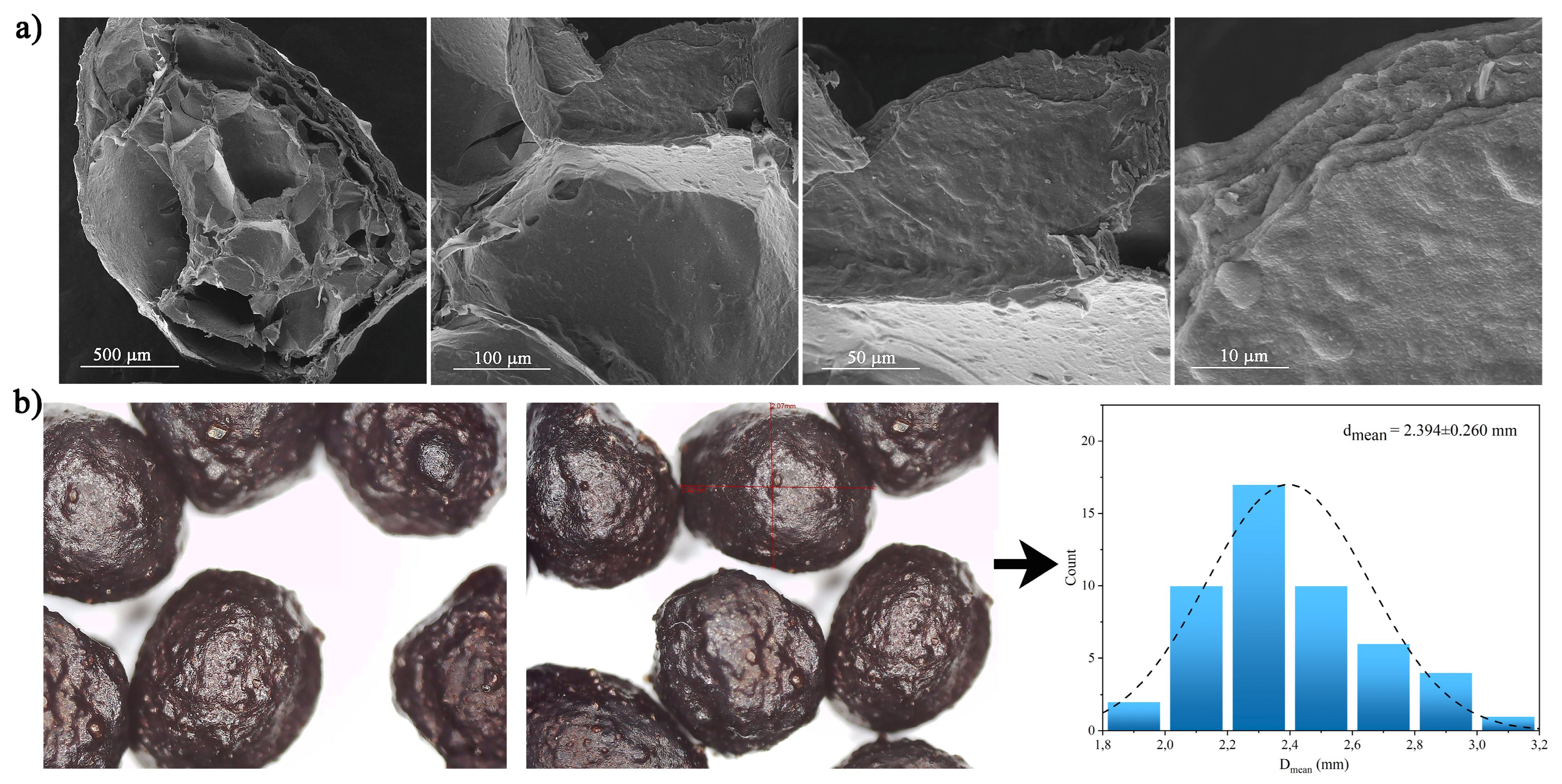
3.1.1. SEM Analysis
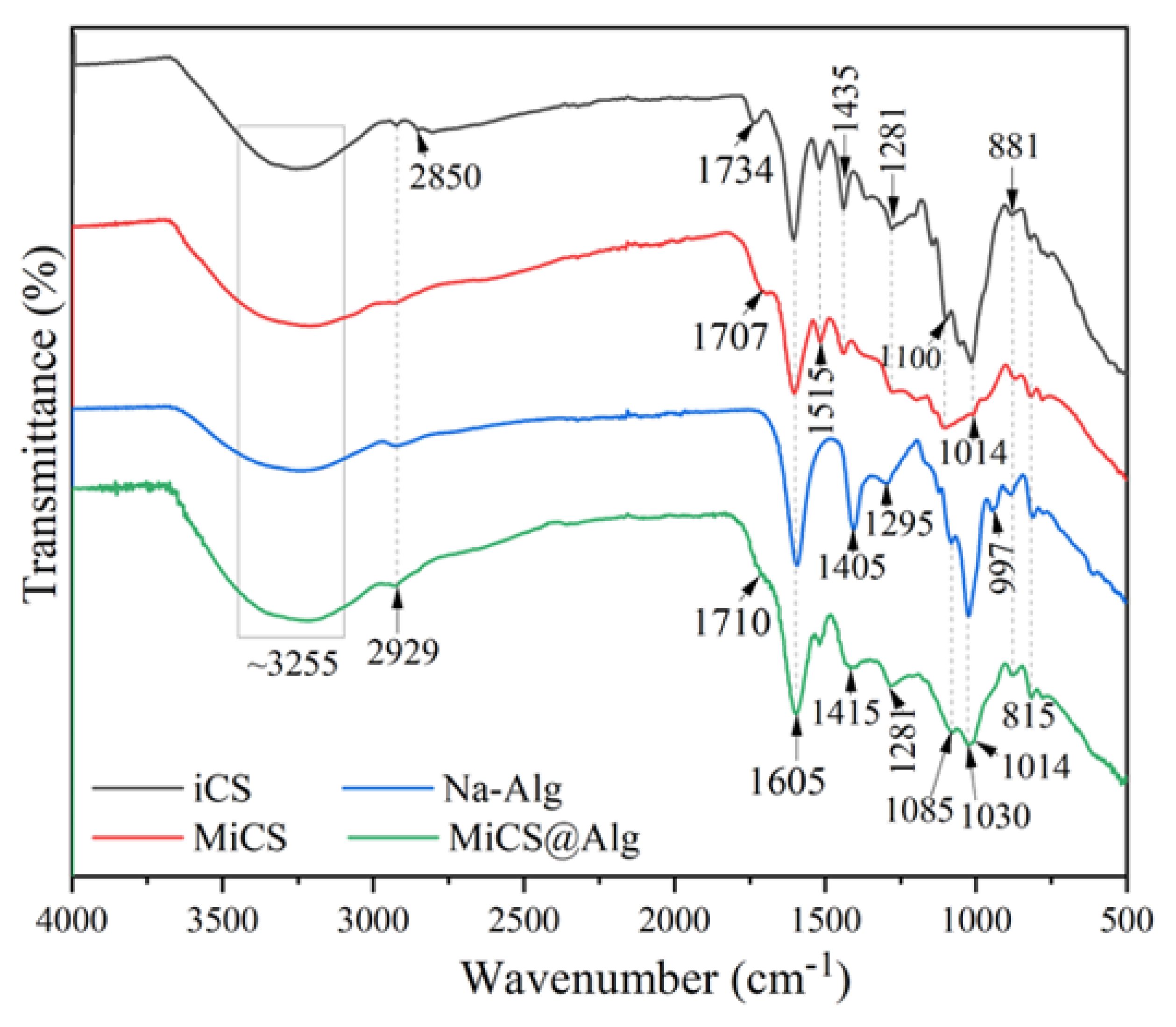
3.1.2. FTIR Analysis
3.1.3. UV-Vis Analysis
3.1.4. DLS and ZETA Analysis
3.2. Elemental Analysis
3.3. Batch Adsorption Experiments
3.3.1. Adsorption Isotherm Study
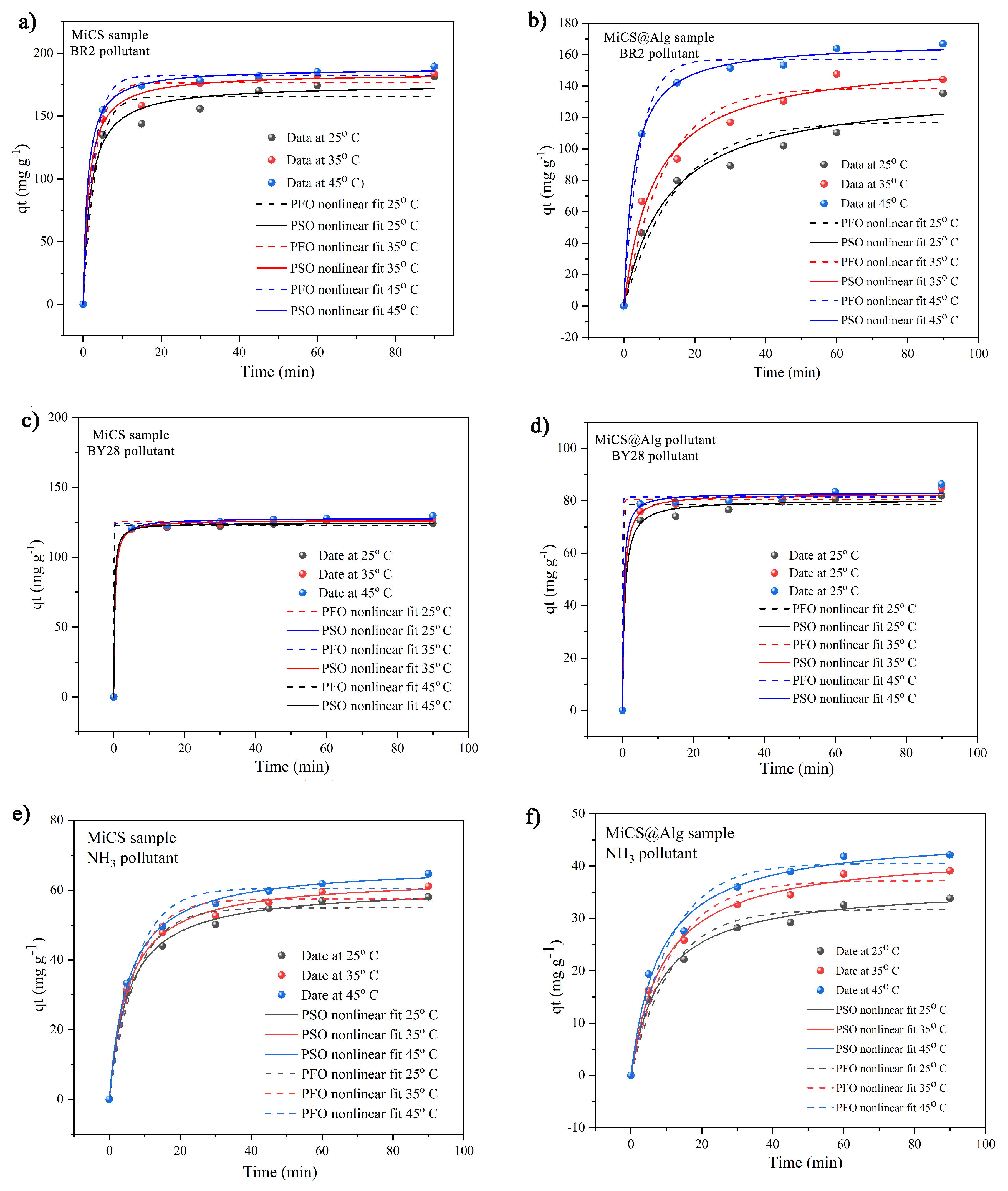
3.3.2. Adsorption Kinetics
3.3.3. Thermodynamic Study and Adsorption Mechanism
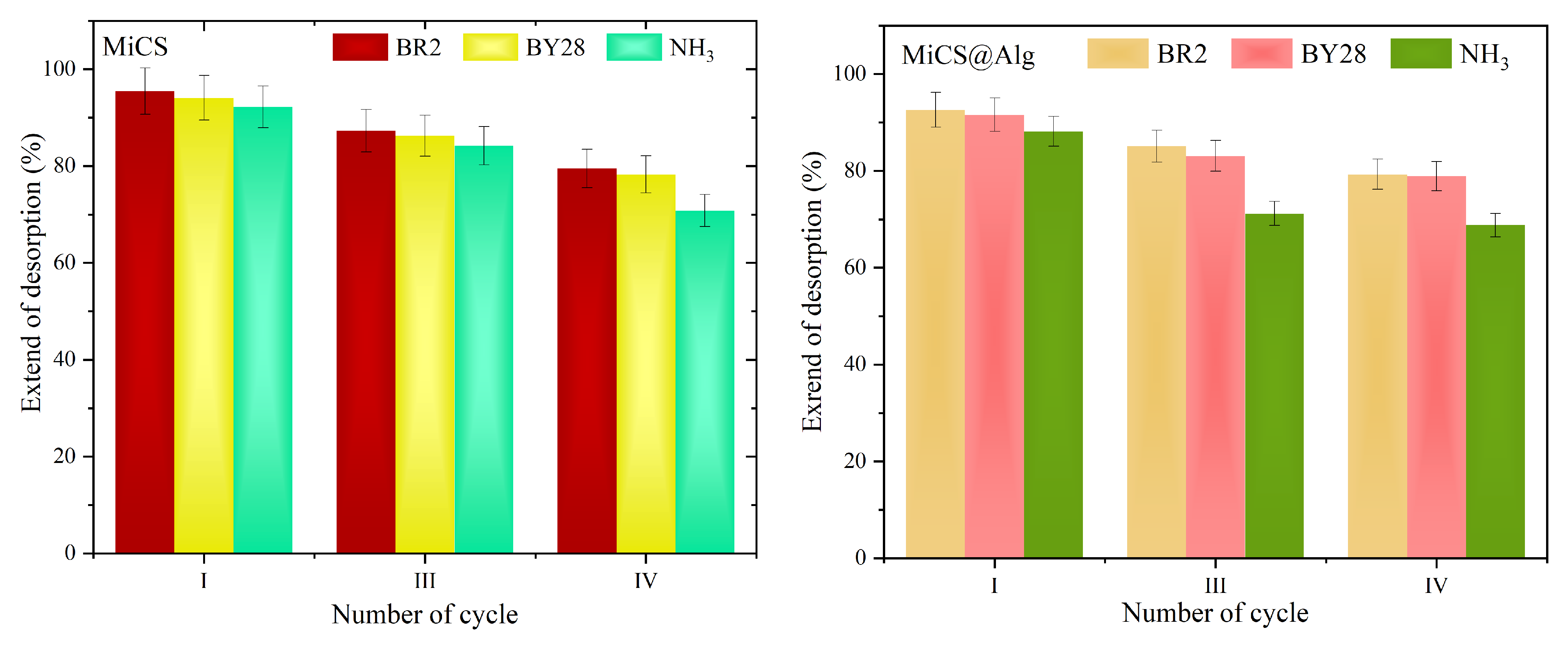
3.4. Desorption Study
3.5. Bed Column Study
3.6. Comparative Overview of Adsorption Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, P.-H.; Liu, P.; Sarkar, B.; Mukhopadhyay, R.; Yang, Q.-Y.; Tzou, Y.-M.; Zhong, B.; Li, X.; Owens, G. Unravelling the Mechanism of Amitriptyline Removal from Water by Natural Montmorillonite through Batch Adsorption, Molecular Simulation and Adsorbent Characterization Studies. J. Colloid Interface Sci. 2021, 598, 379–387. [Google Scholar] [CrossRef]
- Knežević, N.; Milanović, J.; Veličković, Z.; Milošević, M.; Vuksanović, M.M.; Onjia, A.; Marinković, A. A Closed Cycle of Sustainable Development: Effective Removal and Desorption of Lead and Dyes Using an Oxidized Cellulose Membrane. J. Ind. Eng. Chem. 2023, 126, 520–536. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF); World Health Organization (WHO). Joint Monitoring Programme for Water Supply, Sanitation and Hygiene; WHO: Geneva, Switzerland; UNICEF: New York, NY, USA, 2023; ISBN 9789280654769. [Google Scholar]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- da Gama, B.M.V.; Silanpää, M.; Selvasembian, R.; de Farias Silva, C.E.; Meili, L. Effective Adsorptive Removal of a Cationic Dye from Aqueous Solutions Using a Biosorbent Derived from Sargassum sp. Water Pract. Technol. 2024, 19, 263–280. [Google Scholar] [CrossRef]
- Bahsaine, K.; Benzeid, H.; Zari, N.; Qaiss, A.e.K.; Bouhfid, R. Biochar-Alginate Beads Derived from Argan Nutshells for Effective Methylene Blue Removal: A Sustainable Approach to Wastewater Treatment. Int. J. Biol. Macromol. 2024, 282, 136853. [Google Scholar] [CrossRef]
- Sadiq, M.U.; Shah, A.; Nisar, J.; Shah, I. Photoelectrocatalytic Detection and Degradation Studies of a Hazardous Textile Dye Safranin T. Nanomaterials 2023, 13, 2218. [Google Scholar] [CrossRef] [PubMed]
- Budak, T.B. Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent. Molecules 2023, 28, 6639. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, R.; Karthikeyan, S.; Mandal, A.B.; Sekaran, G. Adsorption of Ammonium Ion by Coconut Shell-Activated Carbon from Aqueous Solution: Kinetic, Isotherm, and Thermodynamic Studies. Environ. Sci. Pollut. Res. 2013, 20, 533–542. [Google Scholar] [CrossRef]
- Han, B.; Butterly, C.; Zhang, W.; He, J.; Chen, D. Adsorbent Materials for Ammonium and Ammonia Removal: A Review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, C.; Nie, J. Removal of Ammonia Nitrogen from Wastewater Using Modified Activated Sludge. Pol. J. Environ. Stud. 2016, 25, 419–425. [Google Scholar] [CrossRef]
- Liu, Z.-F.; Liu, Z.-J.; Qie, L.-M.; Yao, Z.-Y. Effects of Melanin Extraction on Biosorption Behavior of Chestnut Shells Towards Methylene Blue. Water Conserv. Sci. Eng. 2021, 6, 163–173. [Google Scholar] [CrossRef]
- Khalla, D.; Belguidoum, K.; Nacef, M.; Boukour, M.; Chelaghmia, M.L.; Khelifi, O.; Selaimia, R.; Bengourna, N.; Affoune, A.M.; Amira-Guebailia, H. Competitive Adsorption of Quinary Heavy Metal Ions onto Chestnut Shell Activated Carbon. Mater. Chem. Phys. 2024, 323, 129646. [Google Scholar] [CrossRef]
- Çitlacifci, H.; Pekgözlü, A.K.; Gülsoy, S.K. Characterization of Cheestnut Shell. Bartın Univ. Int. J. Nat. Appl. Sci. 2022, 5, 145–150. [Google Scholar] [CrossRef]
- Paudyal, H.; Pangeni, B.; Inoue, K.; Kawakita, H.; Ohto, K.; Ghimire, K.N.; Alam, S. Preparation of Novel Alginate Based Anion Exchanger from Ulva Japonica and Its Application for the Removal of Trace Concentrations of Fluoride from Water. Bioresour. Technol. 2013, 148, 221–227. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Chen, H.; Chi, T.; Lu, J.; Shen, X.; Xie, F.; Wang, P.; Zhang, Z. Highly Efficient and Regenerable Amine-Impregnated Adsorbents: Mechanistic Insights into Glycerol Modification for Enhanced Direct Air Capture. Chem. Eng. J. 2025, 520, 166450. [Google Scholar] [CrossRef]
- Filatova, A.V.; Azimova, L.B.; Djurabaev, D.T.; Radjabov, O.I.; Otajonov, A.Y.; Yakubova, R.A. Technology for Obtaining Melanin from the Shells of Horse Chestnut (Aesculus hippocastanum L.), Studying Its Composition. BIO Web Conf. 2024, 113, 03001. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, W.; Ma, K.; Tian, M. Fermentative Production of Melanin by the Fungus Auricularia auricula Using Wheat Bran Extract as Major Nutrient Source. Food Sci. Technol. Res. 2017, 23, 23–29. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible Core-Shell/Bead-like Alginate@PEI with Exceptional Adsorption Capacity, Recycling Performance toward Batch and Column Sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Jovanović, A.; Stevanović, M.; Barudžija, T.; Cvijetić, I.; Lazarević, S.; Tomašević, A.; Marinković, A. Advanced Technology for Photocatalytic Degradation of Thiophanate-Methyl: Degradation Pathways, DFT Calculations and Embryotoxic Potential. Process Saf. Environ. Prot. 2023, 178, 423–443. [Google Scholar] [CrossRef]
- Mahvi, H.; Bazrafshan, E.; Jahed, G.R. Evaluation of COD Determination by ISO, 6060 Method, Comparing with Standard Method (5220, B). Pak. J. Biol. Sci. 2005, 8, 892–894. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Hardieka, A.M.; Budak, T.B. Investigation of Removing Basic Yellow 28 and Basic Blue 3 Dyes from Water Using Mulberry Leaves (Morus nigra L.) and Assessment of Ultrasonic Effects. Molecules 2025, 30, 3539. [Google Scholar] [CrossRef]
- Yao, Z.; Qi, J.; Wang, L. Isolation, Fractionation and Characterization of Melanin-like Pigments from Chestnut (Castanea mollissima) Shells. J. Food Sci. 2012, 77, C671–C676. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.G.; Younger, R.; Poe, C.; Farmer, P.J.; Szpoganicz, B. Studies on Synthetic and Natural Melanin and Its Affinity for Fe(III) Ion. Bioinorg. Chem. Appl. 2012, 2012, 712840. [Google Scholar] [CrossRef]
- Popovic, A.L.; Rusmirovic, J.D.; Velickovic, Z.; Kovacevic, T.; Jovanovic, A.; Cvijetic, I.; Marinkovic, A.D. Kinetics and Column Adsorption Study of Diclofenac and Heavy-Metal Ions Removal by Amino-Functionalized Lignin Microspheres. J. Ind. Eng. Chem. 2021, 93, 302–314. [Google Scholar] [CrossRef]
- Sun, X.-F.; Sun, R.; Sun, J.-X. Acetylation of Rice Straw with or without Catalysts and Its Characterization as a Natural Sorbent in Oil Spill Cleanup. J. Agric. Food Chem. 2002, 50, 6428–6433. [Google Scholar] [CrossRef] [PubMed]
- Kocer, S.; Utku Copur, O.; Ece Tamer, C.; Suna, S.; Kayahan, S.; Uysal, E.; Cavus, S.; Akman, O. Optimization and Characterization of Chestnut Shell Pigment Extract Obtained Microwave Assisted Extraction by Response Surface Methodology. Food Chem. 2024, 443, 138424. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Achour, R.; Hassoune, H.; Lachehab, A.; Qaiss, A.e.K.; Bouhfid, R. Highly Synergic Adsorption/Photocatalytic Efficiency of Alginate/Bentonite Impregnated TiO2 Beads for Wastewater Treatment. J. Photochem. Photobiol. A Chem. 2021, 412, 113215. [Google Scholar] [CrossRef]
- Ballard, M.; Shafiee, A.; Grage, E.; DeMarco, M.; Atala, A.; Ghadiri, E. Inkjet Printing of Synthesized Melanin Nanoparticles as a Biocompatible Matrix for Pharmacologic Agents. Nanomaterials 2020, 10, 1840. [Google Scholar] [CrossRef]
- Rajagopal, K.; Kathiravan, G.; Karthikeyan, S. Extraction and Characterization of Melanin from Phomopsis: A Phellophytic Fungi Isolated from Azadirachta Indica A. Juss. Afr. J. Microbiol. Res. 2011, 5, 762–766. [Google Scholar] [CrossRef]
- Aqlinia, M.; Astuti, R.I.; Prastya, M.E.; Wahyudi, A.T. Antioxidant Potential of Melanin Pigment from Marine Sponge-Associated Actinomycete Micromonospora sp. J. Appl. Pharm. Sci. 2025, 15, 212–224. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Filipkowska, U.; Kuczajowska-Zadrożna, M.; Jóźwiak, T.; Szymczyk, P.; Kaczyński, A. Adsorption of Basic Yellow 28 (BY 28) and Acid Yellow 23 (AY 23) Dyes onto Chitin. Prog. Chem. Appl. Chitin Deriv. 2015, XX, 34–42. [Google Scholar] [CrossRef]
- Hu, Q.H.; Qiao, S.Z.; Haghseresht, F.; Wilson, M.A.; Lu, G.Q. Adsorption Study for Removal of Basic Red Dye Using Bentonite. Ind. Eng. Chem. Res. 2006, 45, 733–738. [Google Scholar] [CrossRef]
- Gubitosa, J.; Cignolo, D.; Lotito, S.; Fini, P.; Milella, A.; Perrotta, A.; Cosma, P.; Rizzi, V. Physical and Chemical Parameters Driving the Direct Blue-78 Adsorption from Water Using Chitosan/ZnO Hybrid Sponges Engineered via Atomic Layer Deposition. ACS Omega 2025, 10, 33897–33909. [Google Scholar] [CrossRef]
- Bugarčić, M.; Lopičić, Z.; Šoštarić, T.; Marinković, A.; Rusmirovic, J.D.; Milošević, D.; Milivojević, M. Vermiculite Enriched by Fe(III) Oxides as a Novel Adsorbent for Toxic Metals Removal. J. Environ. Chem. Eng. 2021, 9, 106020. [Google Scholar] [CrossRef]
- Rahimi, S.M.; Ramavandi, B.; Moslehi, M.H.; Rahiminia, M.; Nasseh, N. Application of CuFe2O4/CuS as a New Green Magnetic Nanocomposite in Adsorption of Tetracycline from Aqueous Solutions: Mathematical Models of Thermodynamics, Isotherms, and Kinetics. Appl. Water Sci. 2025, 15, 6. [Google Scholar] [CrossRef]
- Knežević, N.; Vuksanović, M.M.; Banjanac, K.; Pantić, K.; Veličković, Z.; Cvijetić, I.; Marinković, A.; Milošević, M. Cationic Waste Hemp Fibers-Based Membrane: Case Study of Anionic Pollutants Removal through Environmentally Friendly Processes. J. Environ. Manag. 2024, 371, 123174. [Google Scholar] [CrossRef]
- Momcilovic, M.; Purenovic, M.; Miljkovic, M.; Bojic, A.; Randjelovic, M. Adsorption of Cationic Dye Methylene Blue onto Activated Carbon Obtained from Horse Chestnut Kernel. Hem. Ind. 2011, 65, 123–129. [Google Scholar] [CrossRef]
- Soldatkina, L.; Yanar, M. Equilibrium, Kinetic, and Thermodynamic Studies of Cationic Dyes Adsorption on Corn Stalks Modified by Citric Acid. Colloids Interfaces 2021, 5, 52. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, M. Adsorption of Methylene Blue on Chestnut Shell-Based Activated Carbon: Calculation of Thermodynamic Parameters for Solid–Liquid Interface Adsorption. Catalysts 2022, 12, 813. [Google Scholar] [CrossRef]
- An, J.; Nhung, N.T.H.; Ding, Y.; Chen, H.; He, C.; Wang, X.; Fujita, T. Chestnut Shell-Activated Carbon Mixed with Pyrolytic Snail Shells for Methylene Blue Adsorption. Materials 2022, 15, 8227. [Google Scholar] [CrossRef]
- Rial, A.; Pimentel, C.H.; Gómez-Díaz, D.; Freire, M.S.; González-Álvarez, J. Evaluation of Almond Shell Activated Carbon for Dye (Methylene Blue and Malachite Green) Removal by Experimental and Simulation Studies. Materials 2024, 17, 6077. [Google Scholar] [CrossRef]
- Geçgel, Ü.; Üner, O.; Gökara, G.; Bayrak, Y. Adsorption of Cationic Dyes on Activated Carbon Obtained from Waste Elaeagnus Stone. Adsorpt. Sci. Technol. 2016, 34, 512–525. [Google Scholar] [CrossRef]
- Koromilas, N.D.; Anastasopoulos, C.; Oikonomou, E.K.; Kallitsis, J.K. Preparation of Porous Polymeric Membranes Based on a Pyridine Containing Aromatic Polyether Sulfone. Polymers 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Batinić, P.; Jovanović, A.; Stojković, D.; Čutović, N.; Cvijetić, I.; Gašić, U.; Carević, T.; Zengin, G.; Marinković, A.; Marković, T. A Novel Source of Biologically Active Compounds—The Leaves of Serbian Herbaceous Peonies. Saudi Pharm. J. 2024, 32, 102090. [Google Scholar] [CrossRef]
- FAO/IAEA. 2000. Available online: https://www.scienceopen.com/book?vid=e26b5125-3459-484c-bda9-fe5fa58477e2 (accessed on 1 October 2025).
- Durán-Lara, E.F.; López-Cortés, X.A.; Castro, R.I.; Avila-Salas, F.; González-Nilo, F.D.; Laurie, V.F.; Santos, L.S. Experimental and Theoretical Binding Affinity between Polyvinylpolypyrrolidone and Selected Phenolic Compounds from Food Matrices. Food Chem. 2015, 168, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Mertoglu Elmas, G.; Yilgor, N. Chemical and Thermal Characterizations of Pinus Sylvestris and Pinus Pinaster. BioResources 2020, 15, 3604–3620. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439853818. [Google Scholar]
- TAPPI. *T 222 om-06: Acid-Insoluble Lignin in Wood and Pulp*; TAPPI Press: Atlanta, GA, USA, 2006. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–6. [Google Scholar]
- ASTM D-1166–84; American Society for Testing and Materials (ASTM). Standard Test Method for Methoxyl Groups in Wood and Related Materials. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2013.
- Milošević, D.L.; Tomić, N.Z.; Đokić, V.R.; Vidović, M.M.; Veličković, Z.S.; Jančić-Heinemann, R.; Marinković, A.D. Structural and Surface Modification of Highly Ordered Alumina for Enhanced Removal of Pb2+, Cd2+ and Ni2+ from Aqueous Solution. Desalination Water Treat. 2020, 178, 220–239. [Google Scholar] [CrossRef]
- Serrano, L.; Esakkimuthu, E.S.; Marlin, N.; Brochier-Salon, M.C.; Mortha, G.; Bertaud, F. Fast, Easy, and Economical Quantification of Lignin Phenolic Hydroxyl Groups: Comparison with Classical Techniques. Energy Fuels 2018, 32, 5969–5977. [Google Scholar] [CrossRef]
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent Advances and Progress on Melanin: From Source to Application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef] [PubMed]
- Sansinenea, E.; Ortiz, A. Melanin: A Photoprotection for Bacillus Thuringiensis Based Biopesticides. Biotechnol. Lett. 2015, 37, 483–490. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wu, X.; Jin, X.; Zhang, Z.; Zhao, H.; Liu, J.; Huang, Z.; Fang, M.; Min, X. Kinetics and Equilibrium Studies of the Adsorption of Methylene Blue on Euryale Ferox Shell-based Activated Carbon. Micro Nano Lett. 2018, 13, 552–557. [Google Scholar] [CrossRef]
- Doğan, M.; Abak, H.; Alkan, M. Adsorption of Methylene Blue onto Hazelnut Shell: Kinetics, Mechanism and Activation Parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef]
- Selengil, U.; Yildiz, D.; Tan, B. Adsorption of deep red on ac prepared from chestnut shell. Bartın Univ. Int. J. Nat. Appl. Sci. 2023, 6, 189–205. [Google Scholar] [CrossRef]
- Hoc Thang, N.; Sy Khang, D.; Duy Hai, T.; Thi Nga, D.; Dinh Tuan, P. Methylene Blue Adsorption Mechanism of Activated Carbon Synthesised from Cashew Nut Shells. RSC Adv. 2021, 11, 26563–26570. [Google Scholar] [CrossRef]
- Alkhabbas, M.; Al-Ma’abreh, A.M.; Edris, G.; Saleh, T.; Alhmood, H. Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies. Int. J. Environ. Res. Public Health 2023, 20, 3280. [Google Scholar] [CrossRef]
- Küçük, İ.; Önal, Y.; Başar, C. Ceviz Kabuğundan Karbon Dioksit Kullanılarak Aktif Karbon Üretimi ve Metilen Mavisi Adsorpsiyonu. DÜMF Mühendislik Derg. 2020. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, X.; Yang, R.; Cai, D.; Ren, W. Enzymatic Modification of Walnut Shell for High-Efficiency Adsorptive Methylene Blue Removal. Materials 2025, 18, 3434. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Krishni, R.R.; Sata, S.A. A Novel Agricultural Waste Adsorbent for the Removal of Cationic Dye from Aqueous Solutions. J. Hazard. Mater. 2009, 162, 305–311. [Google Scholar] [CrossRef] [PubMed]



| Sample | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| Pollutant | T (°C) | qm (mg g−1) | KL (L mg−1) | R2 | KF (mg g−1) (L mg−1)1/n | n | R2 | |
| MiCS | BR2 | 25 | 291.1 ± 21.5 | 0.2166 ± 0.061 | 0.9635 | 56.21 ± 2.47 | 1.714 ± 0.08 | 0.9912 |
| 35 | 295.7 ± 22.2 | 0.2247 ± 0.064 | 0.9632 | 58.36 ± 2.62 | 1.715 ± 0.08 | 0.9905 | ||
| 45 | 300.2 ± 22.6 | 0.2471 ± 0.075 | 0.9601 | 62.73 ± 2.98 | 1.709 ± 0.08 | 0.9912 | ||
| BY28 | 25 | 192.5 ± 13.4 | 0.1864 ± 0.03 | 0.9829 | 35.67 ± 0.96 | 1.873 ± 0.045 | 0.9974 | |
| 35 | 198.2 ± 13.8 | 0.1942 ± 0.03 | 0.9827 | 37.65 ± 1.36 | 1.882 ± 0.074 | 0.9933 | ||
| 45 | 201.5 ± 14.3 | 0.2154 ± 0.03 | 0.9857 | 40.40 ± 1.26 | 1.895 ± 0.056 | 0.9960 | ||
| NH3 | 25 | 71.26 ± 5.46 | 1.9554 ± 1.15 | 0.7565 | 38.35 ± 3.04 | 3.563 ± 0.501 | 0.9354 | |
| 35 | 71.94 ± 5.52 | 2.0242 ± 1.19 | 0.7558 | 38.98 ± 3.06 | 3.569 ± 0.503 | 0.9361 | ||
| 45 | 73.08 ± 5.85 | 2.2128 ± 1.32 | 0.7519 | 40.28 ± 3.13 | 3.596 ± 0.504 | 0.9361 | ||
| MiCS@Alg | BR2 | 25 | 180.5 ± 9.40 | 0.2351 ± 0.106 | 0.8829 | 43.09 ± 3.14 | 2.175 ± 0.17 | 0.9757 |
| 35 | 184.3 ± 10.5 | 0.2338 ± 0.116 | 0.8654 | 44.37 ± 3.71 | 2.193 ± 0.205 | 0.9674 | ||
| 45 | 189.3 ± 10.8 | 0.2853 ± 0.141 | 0.8741 | 48.91 ± 3.68 | 2.141 ± 0.187 | 0.9755 | ||
| BY28 | 25 | 109.3 ± 8.96 | 0.4981 ± 0.28 | 0.7919 | 39.28 ± 3.33 | 2.736 ± 0.301 | 0.9564 | |
| 35 | 115.2 ± 9.45 | 0.4566 ± 0.27 | 0.7872 | 40.24 ± 3.51 | 2.706 ± 0.304 | 0.9545 | ||
| 45 | 117.1 ± 9.60 | 0.4873 ± 0.30 | 0.7775 | 42.20 ± 3.67 | 2.747 ± 0.316 | 0.9529 | ||
| NH3 | 25 | 49.78 ± 5.15 | 4.1601 ± 2.78 | 0.7809 | 34.24 ± 2.28 | 5.622 ± 1.00 | 0.9309 | |
| 35 | 49.65 ± 5.14 | 4.5621 ± 3.17 | 0.7762 | 34.62 ± 2.24 | 5.726 ± 1.01 | 0.9333 | ||
| 45 | 50.06 ± 4.99 | 4.7934 ± 3.17 | 0.7882 | 35.13 ± 2.23 | 5.807 ± 1.02 | 0.9338 | ||
| Adsorbent | Pollutant | Model Parameters | Pseudo-First | Pseudo-Second |
|---|---|---|---|---|
| MiCS | BR2 | qe (mg g−1) | 165.7 ± 6.00 | 175.3 ± 5.58 |
| k1 (min−1)/k2 (g mg−1 min−1) | 0.7401 ± 0.196 | 0.0031 ± 0.001 | ||
| R2 | 0.956 | 0.979 | ||
| BY28 | qe (mg g−1) | 125.5 ± 1.33 | 127.9 ± 0.88 | |
| k1 (min−1)/k2 (g mg−1 min−1) | 36.38 ± 0.00 | 2.4812 ± 0.006 | ||
| R2 | 0.995 | 0.999 | ||
| NH3 | qe (mg g−1) | 54.90 ± 1.71 | 60.97 ± 0.855 | |
| k1 (min−1)/k2 (g mg−1 min−1) | 0.135 ± 0.02 | 0.0031 ± 0.0003 | ||
| R2 | 0.975 | 0.997 | ||
| MiCS@Alg | BR2 | qe (mg g−1) | 117.3 ± 6.61 | 138.9 ± 8.6 |
| k1 (min−1)/k2 (g mg−1 min−1) | 0.1565 ± 0.043 | 5.7928 ± 2.077 | ||
| R2 | 0.921 | 0.962 | ||
| BY28 | qe (mg g−1) | 78.45 ± 1.75 | 80.23 ± 1.18 | |
| k1 (min−1)/k2 (g mg−1 min−1) | 10.07 ± 8.38 | 0.0198 ± 0.0065 | ||
| R2 | 0.982 | 0.995 | ||
| NH3 | qe (mg g−1) | 31.70 ± 1.24 | 36.35 ± 0.984 | |
| k1 (min−1)/k2 (g mg−1 min−1) | 0.0906 ± 0.015 | 0.0032 ± 0.0004 | ||
| R2 | 0.969 | 0.993 |
| Kinetic Model | Model Parameters | MiCS | MiCS@Alg | ||||
|---|---|---|---|---|---|---|---|
| BR2 | BY28 | NH3 | BR2 | BY28 | NH3 | ||
| Weber–Morris (Step 1) | kid1 (mg g−1 min−0.5) | 6.385 ± 0.224 | 1.400 ± 0.050 | 1.078 ± 0.038 | 13.25 ± 0.517 | 0.935 ± 0.033 | 1.200 ± 0.042 |
| CBL (mg g−1) | 120.2 | 118.3 | 83.41 | 20.64 | 71.10 | 43.78 | |
| R2 | 0.992 | 0.997 | 0.879 | 0.909 | 0.865 | 0.982 | |
| Weber–Morris (Step 2) | kid2 (mg g−1 min−0.5) | 4.015 ± 0.141 | 1.219 ± 0.043 | 0.6746 ± 0.024 | 12.25 ± 0.480 | 0.8248 ± 0.029 | 0.9287 ± 0.035 |
| CBL (mg g−1) | 143.2 | 115.9 | 87.66 | 18.17 | 74.08 | 45.15 | |
| R2 | 0.999 | 0.887 | 0.879 | 0.982 | 0.992 | 0.973 | |
| Adsorbent | Pollutants | ΔGΘ (kJ mol−1) | ΔHΘ (kJ mol−1) | ΔSΘ (J mol−1 K−1) | R2 | ||
|---|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | |||||
| MiCS | BR2 | −39.33 | −40.78 | −42.43 | 6.78 | 154.5 | 0.941 |
| BY28 | −38.58 | −40.01 | −41.50 | 4.91 | 145.8 | 0.988 | |
| NH3 | −32.59 | −33.74 | −34.95 | 2.63 | 118.1 | 0.959 | |
| MiCS@Alg | BR2 | −39.37 | −40.82 | −42.54 | 7.77 | 157.9 | 0.915 |
| BY28 | −40.14 | −41.52 | −43.05 | 3.23 | 145.4 | 0.869 | |
| NH3 | −33.83 | −35.05 | −36.29 | 2.87 | 123.1 | 0.997 | |
| Model and Parameters | Pollutant | Q (cm3 min−1) | |||
|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | |||
| B-A | KBA (dm3 mg−1 min−1) | BR2 | 0.0360 ± 0.0002 | 0.0703 ± 0.0006 | 0.109 ± 0.0012 |
| qo (mg g−1) | 147.6 ± 0.19 | 122.8 ± 0.25 | 100.1 ± 0.30 | ||
| R2 | 0.999 | 0.999 | 0.993 | ||
| KBA (dm3 mg−1 min−1) | BY28 | 0.0409 ± 0.0005 | 0.0949 ± 0.0023 | 0.138 ± 0.0017 | |
| qo (mg g−1) | 94.71 ± 0.32 | 77.02 ± 0.44 | 61.58 ± 0.26 | ||
| R2 | 0.998 | 0.998 | 0.998 | ||
| KBA (dm3 mg−1 min−1) | NH3 | 0.0665 ± 0.0008 | 0.130 ± 0.0024 | 0.216 ± 0.0047 | |
| qo (mg g−1) | 47.28 ± 0.18 | 43.19 ± 0.28 | 37.51 ± 0.29 | ||
| R2 | 0.999 | 0.999 | 0.998 | ||
| Y-N | kYN (min−1) | BR2 | 0.360 ± 0.0025 | 0.351 ± 0.0032 | 0.344 ± 0.0040 |
| θ (min) | 16.83 ± 0.022 | 13.99 ± 0.050 | 11.42 ± 0.034 | ||
| R2 | 0.999 | 0.999 | 0.999 | ||
| kYN (min−1) | BY28 | 0.409 ± 0.0054 | 0.475 ± 0.0099 | 0.480 ± 0.0001 | |
| θ (min) | 10.80 ± 0.036 | 8.78 ± 0.069 | 7.02 ± 0.0003 | ||
| R2 | 0.999 | 0.999 | 0.999 | ||
| kYN (min−1) | NH3 | 0.665 ± 0.00001 | 0.668 ± 0.012 | 0.719 ± 0.0056 | |
| θ (min) | 5.39 ± 0.00001 | 4.92 ± 0.031 | 4.27 ± 0.032 | ||
| R2 | 0.999 | 0.998 | 0.998 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljnin, A.; Cvijanović, G.; Stojadinović, B.; Milosavljević, M.; Simić, K.; Marinković, A.D.; Knežević, N.Đ. Development of a Chestnut Shell Bio-Adsorbent for Cationic Pollutants: Encapsulation in an Alginate Carrier for Application in a Flow System. Processes 2025, 13, 3314. https://doi.org/10.3390/pr13103314
Aljnin A, Cvijanović G, Stojadinović B, Milosavljević M, Simić K, Marinković AD, Knežević NĐ. Development of a Chestnut Shell Bio-Adsorbent for Cationic Pollutants: Encapsulation in an Alginate Carrier for Application in a Flow System. Processes. 2025; 13(10):3314. https://doi.org/10.3390/pr13103314
Chicago/Turabian StyleAljnin, Atef, Gorica Cvijanović, Bojan Stojadinović, Milutin Milosavljević, Katarina Simić, Aleksandar D. Marinković, and Nataša Đ. Knežević. 2025. "Development of a Chestnut Shell Bio-Adsorbent for Cationic Pollutants: Encapsulation in an Alginate Carrier for Application in a Flow System" Processes 13, no. 10: 3314. https://doi.org/10.3390/pr13103314
APA StyleAljnin, A., Cvijanović, G., Stojadinović, B., Milosavljević, M., Simić, K., Marinković, A. D., & Knežević, N. Đ. (2025). Development of a Chestnut Shell Bio-Adsorbent for Cationic Pollutants: Encapsulation in an Alginate Carrier for Application in a Flow System. Processes, 13(10), 3314. https://doi.org/10.3390/pr13103314









