CO2 to Methanol Conversion: A Bibliometric Analysis with Insights into Reaction Mechanisms, and Recent Advances in Catalytic Conversion
Abstract
1. Introduction
2. Search Strategy and Bibliometric Evaluation Technique
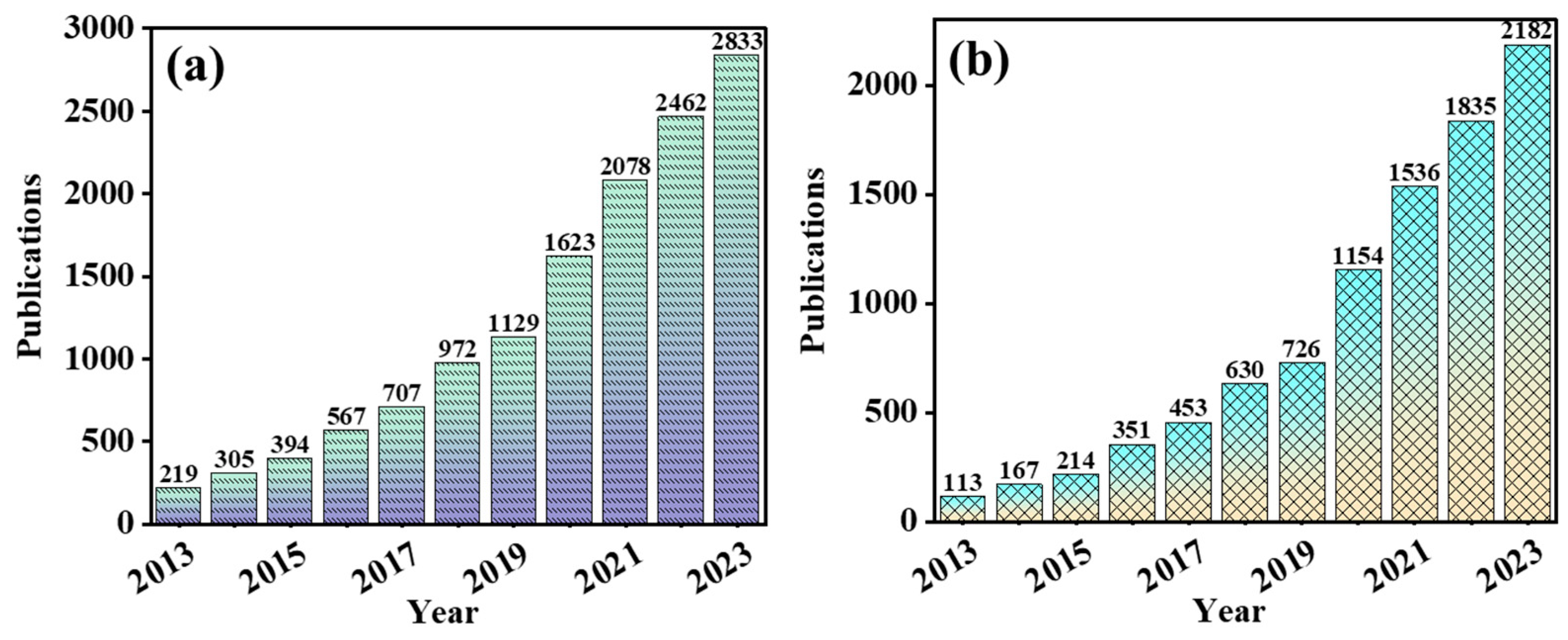
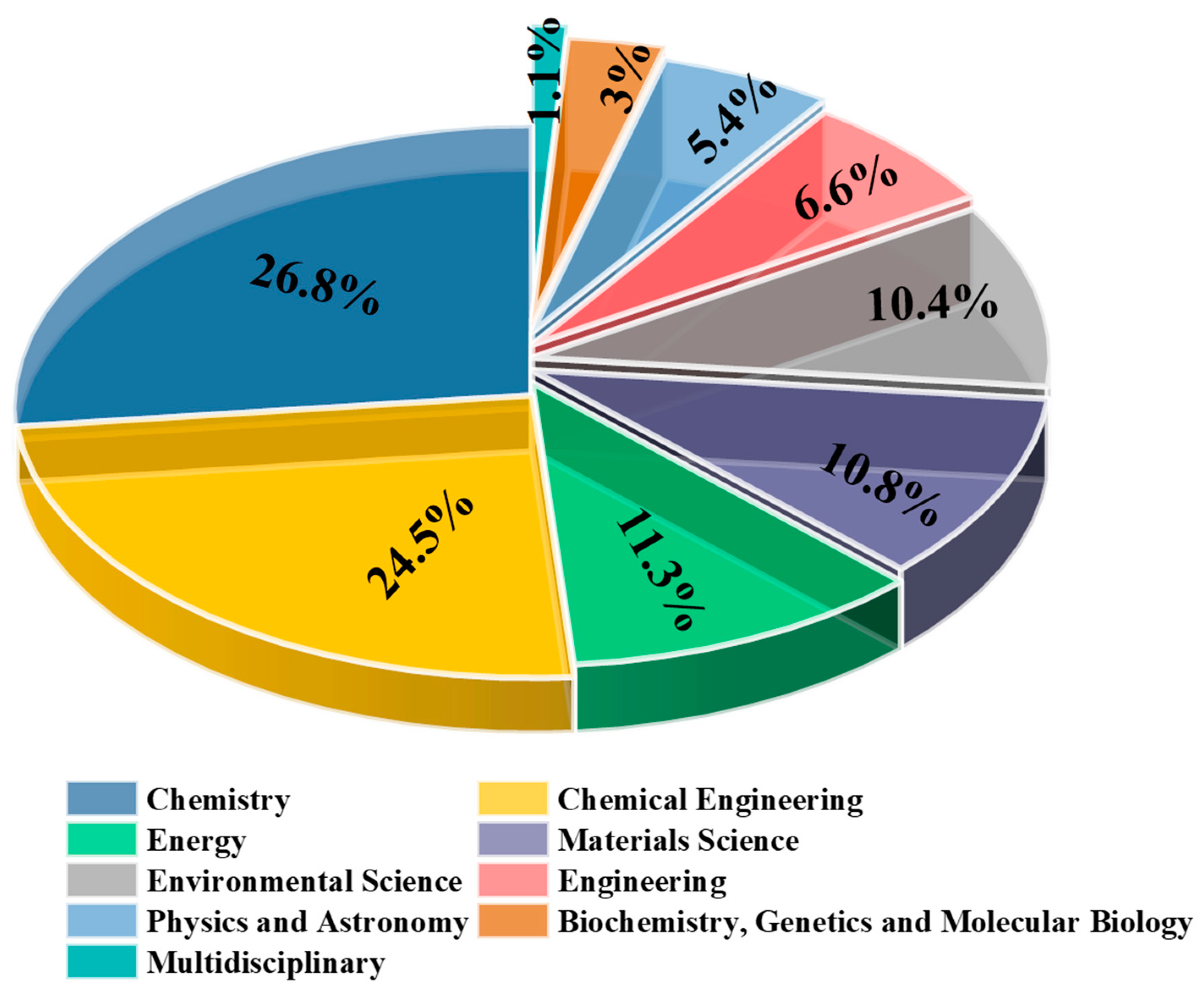
2.1. Publication Trends and Top Research Fields
2.1.1. Publication Trend Analysis
2.1.2. Research Areas
2.2. Top Countries
2.3. Top Journals
2.4. Top Keywords
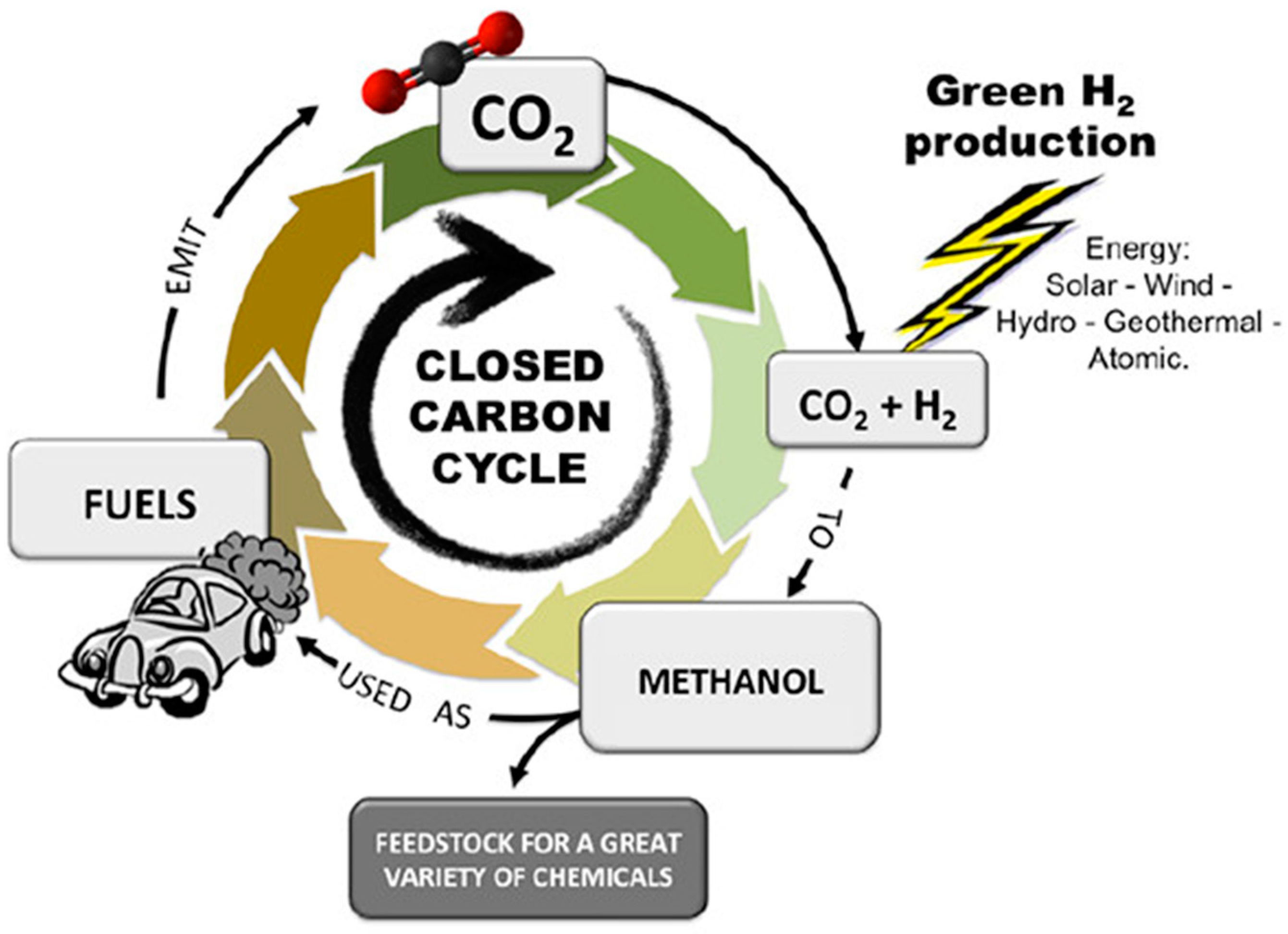
3. CO2 Conversion to Methanol
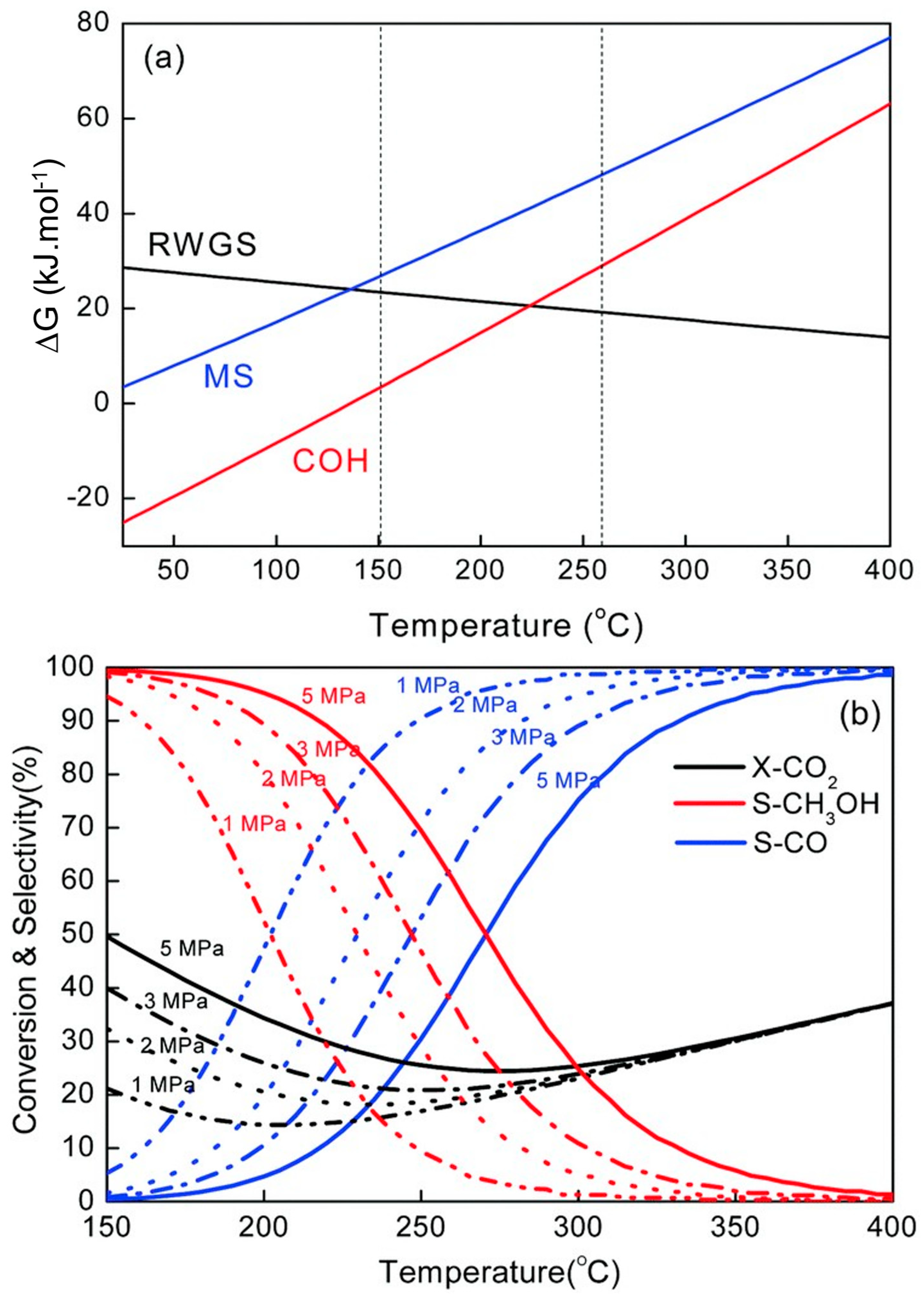
3.1. Thermodynamics and Reaction Mechanism of CO2 Conversion to Methanol
3.2. Recent Advances in Cu-Based Catalyst for CO2 Hydrogenation to Methanol
3.2.1. Role of Supports or Support Effect in Cu-Based Catalysts
3.2.2. Role of Promoters in Cu-Based Catalysts
| Catalysts | T (°C) | P (MPa) | Gas Composition | Space Velocity (mL·g−1 h−1) | CO2 Conversion (%) | CH3OH Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu-ZrO2 | 230 | 1 | CO2/H2/N2 = 1/3/1 | 50,000 | 1.6 | 72.2 | [101] |
| Cu/ZrO2 | 260 | 3 | CO2/H2/N2 = 23/69/8 | 6000 | 17.1 | 58.5 | [102] |
| Cu-Zn-Zr/CuBr2 | 250 | 5 | CO2/H2/N2 = 23/69/8 | 3000 | 10.7 | 97.1 | [103] |
| Cu/TiO2-600 | 240 | 3 | CO2/H2/N2 = 1/3/1 | 3600 | 45.2 | 55.5 | [18] |
| Cu/TiO2-500 | 300 | 4 | CO2/H2/N2 = 1/3/1 | 10,000 | 12.5 | 43.3 | [14] |
| Cu-Ca0.8La0.2TiO3 | 300 | 3 | CO2/H2/N2 = 6/18/1 | 3000 | 22.5 | 58.5 | [19] |
| 0.5% Cu-Zn-Zr | 290 | 4.5 | CO2/H2 = 1/3 | 10,800 | 9.5 | 76 | [104] |
| Cu-Zn-Al-Ce | 250 | 3 | CO2/H2 = 1/3 | 12,000 | 14.2 | 37.8 | [105] |
| 20% ZnO-ZrO2 | 320 | 2 | CO2/H2 = 24/72 | 24,000 | 6.4 | 78.5 | [63] |
| inverse-ZrO2/Cu | 220 | 3 | CO2/H2 = 1/3 | 48,000 | <5 | ~70 | [65] |
| Cu@ZnO-1.0 | 240 | 3 | CO2/H2 = 1/3 | 12,000 | 19.6 | 76.9 | [61] |
| CuO/Ce0.4 Zr0.6 O2 | 220 | 3 | CO2/H2 = 1/3 | 10,000 | 7 | 96.4 | [66] |
| Cu-Zn-Al-K | 240 | 3 | CO2/H2 = 1/4 | 2400 | 14 | 96 | [106] |
| Cu-ZnO-A12O3 | 260 | 3.6 | CO2/H2 = 1/10 | 18,000 | 65.8 | 77.3 | [107] |
| Cu-ZnO-A12O3 | 280 | 4 | CO2/H2 = 1/3 | 10,000 | 65.3 | 91.9 | [108] |
| Cu-ZnO | 250 | 3 | CO2/H2 = 1/3 | 18,000 | 2.3 | 100 | [109] |
| Cu-ZnO-ZrO2 | 200 | 1 | CO2/H2/N2 = 3/9/1 | 4000 | 5.8 | 55.2 | [110] |
| Cu-ZnO-ZrO2 | 230 | 3 | - | 3000 | 15.2 | 35.1 | [111] |
| Cu-ZnO-ZrO2/MgO | 220 | 3 | CO2/H2/N2 = 23/69/8 | 18,000 | 7.3 | 71.8 | [100] |
| Cu-ZnO-ZrO2 | 240 | 3 | CO2/H2 = 1/3 | 3600 | 12.1 | 54.1 | [112] |
| Cu/ZrO2 | 280 | 3 | CO2/H2 = 1/3 | 7200 | 12 | 32 | [113] |
| 30Cu-ZnO-ZrO2 | 280 | 5 | - | 10,000 | 23 | 33 | [114] |
| Fe-Cu-ZnO-ZrO2 | 250 | 3 | CO2/H2 = 1/4 | 60,000 | 18.7 | 53.8 | [115] |
| Cu-Ga2O3-ZrO2 | 250 | 2 | CO2/H2 = 1/3 | 2500 | 13.7 | 75.6 | [116] |
| 7.7 Ga203/IE/Cu/ZrO2 | 250 | 3 | CO2/H2 = 22/75 | 20,000 | 1.3 | 74 | [117] |
| Cu/Al2O3 | 280 | 9.5 | CO2/H2 = 1/4 | 12,000 | 30 | 80 | [118] |
| LaO0.8Zr0.2Cu0.7Zn0.3Ox | 250 | 5 | CO2/H2 = 1/3 | 3600 | 12.6 | 52.5 | [119] |
| 30Cu-ZnO-ZrO2 | 280 | 5 | - | 10,000 | 21 | 34 | [120] |
| Cu-ZnO-ZrO20-Al2O3 | 240 | 3 | CO2/H2/N2 = 23.5/64.5/12 | 2400 | 21.8 | 51 | [78] |
3.2.3. Role of Alloy in Cu-Based Catalysts
4. Conclusions
5. Challenges and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Memon, M.A.; Jiang, Y.; Hassan, M.A.; Ajmal, M.; Wang, H.; Liu, Y. Heterogeneous Catalysts for Carbon Dioxide Methanation: A View on Catalytic Performance. Catalysts 2023, 13, 1514. [Google Scholar] [CrossRef]
- Memon, M.A.; Zhou, W.; Ajmal, M.; Afzal; Jiang, Y.; Zhang, C.; Zhang, J.; Liu, Y. Ni–CaZrO3 with perovskite phase loaded on ZrO2 for CO2 methanation. Int. J. Hydrogen Energy 2024, 92, 1202–1213. [Google Scholar] [CrossRef]
- Liu, R.; Song, P.; Memon, M.A.; Liu, P.; Liu, Y. Manganese Oxide Decorated Co Nanoparticles on CaTiO3 for Higher Alcohols Synthesis from Syngas. Ind. Eng. Chem. Res. 2024, 63, 16111–16123. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, L.; Li, S.; Zhou, Z.; Sun, Y. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels. ACS Cent. Sci. 2020, 6, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Durga Devi, A.; Pushpavanam, S.; Singh, N.; Verma, J.; Kaur, M.P.; Roy, S.C. Enhanced methane yield by photoreduction of CO2 at moderate temperature and pressure using Pt coated, graphene oxide wrapped TiO2 nanotubes. Results Eng. 2022, 14, 100441. [Google Scholar] [CrossRef]
- Cubeiro, M.L.; Morales, H.; Goldwasser, M.R.; Pérez-Zurita, M.J.; González-Jiménez, F.; Urbina De N, C. Hydrogenation of carbon oxides over Fe/Al2O3 catalysts. Appl. Catal. A Gen. 1999, 189, 87–97. [Google Scholar] [CrossRef]
- Sakurai, H.; Ueda, A.; Kobayashi, T.; Haruta, M. Low-temperature water-gas shift reaction over gold deposited on TiO2. Chem. Commun. 1997, 271–272. [Google Scholar] [CrossRef]
- Gao, P.; Dang, S.; Li, S.; Bu, X.; Liu, Z.; Qiu, M.; Yang, C.; Wang, H.; Zhong, L.; Han, Y.; et al. Direct Production of Lower Olefins from CO2 Conversion via Bifunctional Catalysis. ACS Catal. 2018, 8, 571–578. [Google Scholar] [CrossRef]
- Studt, F.; Sharafutdinov, I.; Abild-Pedersen, F.; Elkjær, C.F.; Hummelshøj, J.S.; Dahl, S.; Chorkendorff, I.; Nørskov, J.K. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.A.; Abdullah, A.Z.; Mohamed, A.R. Recent development in catalytic technologies for methanol synthesis from renewable sources: A critical review. Renew. Sustain. Energy Rev. 2015, 44, 508–518. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Olah, G.A. Towards oil independence through renewable methanol chemistry. Angew. Chemie—Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Etim, U.J.; Song, Y.; Gazit, O.M.; Zhong, Z. Oxygen vacancies in Cu/TiO2 boost strong metal-support interaction and CO2 hydrogenation to methanol. J. Catal. 2022, 413, 284–296. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wang, L.; Liu, Z. Transition metal catalysts for CO2 hydrogenation: Mechanistic insights and performance trends. Catal. Today 2023, 423, 101–116. [Google Scholar] [CrossRef]
- Sun, J.; Fujimoto, K. Economic feasibility of Cu-based catalysts for industrial-scale CO2-to-methanol synthesis. Ind. Eng. Chem. Res. 2017, 56, 6060–6069. [Google Scholar] [CrossRef]
- Gao, B.; Wen, Z.; Wang, Y.; Chen, D.; Yang, B.; Ishihara, T.; Guo, L. Recent Advances in Alloy Catalysts for CO2 Hydrogenation to Methanol. ChemCatChem 2024, 16, e202400814. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Ma, P.; Chang, K.; Zhao, Z.; Xie, Z. Lattice-confined Cu-TiO2 catalysts with improved thermal stability for CO2 hydrogenation. ACS Sustain. Chem. Eng. 2023, 11, 18112–18122. [Google Scholar] [CrossRef]
- Zhou, W.; Memon, M.A.; Niu, M.; Fan, X.; Jiang, Y.; Zhang, X.; Liu, Y. Active pairs of Cu0/Cu+ for CO2 hydrogenation. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Xie, X.; Li, C.; Lu, Z.; Wang, Y.; Yang, W.; Chen, M.; Li, W. Noble metal modified copper-exchanged mordenite zeolite (Cu-ex-MOR) catalysts for catalyzing the methane efficient gas-phase synthesis methanol. Energy 2024, 300, 131595. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, D.; Wang, M.; Ma, D. Understanding and Application of Strong Metal-Support Interactions in Conversion of CO2 to Methanol: A Review. Energy Fuels 2021, 35, 19012–19023. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Stacchiola, D.J.; Senanayake, S.D.; White, M.G.; Chen, J.G. Hydrogenation of CO2 to Methanol: Importance of Metal-Oxide and Metal-Carbide Interfaces in the Activation of CO2. Acs Catal. 2015, 5, 6696–6706. [Google Scholar] [CrossRef]
- Bowker, M. Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Sneeden, R.P.A. Copper-zinc oxide-alumina methanol catalysts revisited. Catal. Today 1987, 2, 1–124. [Google Scholar] [CrossRef]
- Studt, F.; Behrens, M.; Kunkes, E.L.; Thomas, N.; Zander, S.; Tarasov, A.; Schumann, J.; Frei, E.; Varley, J.B.; Abild-Pedersen, F.; et al. The Mechanism of CO and CO2 Hydrogenation to Methanol over Cu-Based Catalysts. ChemCatChem 2015, 7, 1105–1111. [Google Scholar] [CrossRef]
- Murthy, P.S.; Liang, W.; Jiang, Y.; Huang, J. Cu-Based Nanocatalysts for CO2 Hydrogenation to Methanol. Energy and Fuels 2021, 35, 8558–8584. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ramirez, P.; Piscina, D.; Toyir, J.; Homs, N. CO2 hydrogenation to methanol over CuZnGa catalysts prepared using microwave-assisted methods. Catal. Today 2015, 242, 193–199. [Google Scholar] [CrossRef]
- Yang, Y.; White, M.G.; Liu, P. Theoretical Study of Methanol Synthesis from CO2 Hydrogenation on Metal-Doped Cu(111) Surfaces. J. Phys. Chem. C 2011, 116, 248–256. [Google Scholar] [CrossRef]
- Park, N.; Park, M.-J.; Lee, Y.-J.; Ha, K.-S.; Jun, K.-W. Kinetic modeling of methanol synthesis over commercial catalysts based on three-site adsorption. Fuel Process. Technol. 2014, 125, 139–147. [Google Scholar] [CrossRef]
- Cui, X.; Kær, S.K. A comparative study on three reactor types for methanol synthesis from syngas and CO2. Chem. Eng. J. 2020, 393, 124632. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Liu, X.; Hua, K.; Shao, Z.; Wei, B.; Huang, C.; Wang, H.; Sun, Y. A Short Review of Recent Advances in Direct CO2 Hydrogenation to Alcohols. Top. Catal. 2021, 64, 371–394. [Google Scholar] [CrossRef]
- Azhari, N.J.; Erika, D.; Mardiana, S.; Ilmi, T.; Gunawan, M.L.; Makertihartha, I.G.B.N.; Kadja, G.T.M. Methanol synthesis from CO2: A mechanistic overview. Results Eng. 2022, 16, 100711. [Google Scholar] [CrossRef]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Ilias, S.; Bhan, A. Mechanism of the Catalytic Conversion of Methanol to Hydrocarbons. ACS Catal. 2012, 3, 18–31. [Google Scholar] [CrossRef]
- Sehested, J. Industrial and scientific directions of methanol catalyst development. J. Catal. 2019, 371, 368–375. [Google Scholar] [CrossRef]
- Behrens, M.; Armbrüster, M. Methanol Steam Reforming. In Catalysis for Alternative Energy Generation; Springer: New York, NY, USA, 2012; pp. 175–235. ISBN 9781461403449. [Google Scholar]
- Mahyuddin, M.H.; Shiota, Y.; Yoshizawa, K. Methane selective oxidation to methanol by metal-exchanged zeolites: A review of active sites and their reactivity. Catal. Sci. Technol. 2019, 9, 1744–1768. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Rodemerck, U.; Holeňa, M.; Wagner, E.; Smejkal, Q.; Barkschat, A.; Baerns, M. Catalyst Development for CO2 Hydrogenation to Fuels. ChemCatChem 2013, 5, 1948–1955. [Google Scholar] [CrossRef]
- Koh, M.K.; Khavarian, M.; Chai, S.P.; Mohamed, A.R. The morphological impact of siliceous porous carriers on copper-catalysts for selective direct CO2 hydrogenation to methanol. Int. J. Hydrogen Energy 2018, 43, 9334–9342. [Google Scholar] [CrossRef]
- Koh, M.K.; Wong, Y.J.; Chai, S.P.; Mohamed, A.R. Carbon dioxide hydrogenation to methanol over multi-functional catalyst: Effects of reactants adsorption and metal-oxide(s) interfacial area. J. Ind. Eng. Chem. 2018, 62, 156–165. [Google Scholar] [CrossRef]
- Li, M.M.; Zeng, Z.; Liao, F.; Hong, X.; Chi, S.; Tsang, E. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu / ZnO catalysts. J. Catal. 2016, 343, 157–167. [Google Scholar] [CrossRef]
- Lucci, F.R.; Marcinkowski, M.D.; Lawton, T.J.; Sykes, E.C.H. H2 Activation and Spillover on Catalytically Relevant Pt–Cu Single Atom Alloys. J. Phys. Chem. C 2015, 119, 24351–24357. [Google Scholar] [CrossRef]
- Hu, B.; Yin, Y.; Liu, G.; Chen, S.; Hong, X.; Tsang, S.C.E. Hydrogen spillover enabled active Cu sites for methanol synthesis from CO2 hydrogenation over Pd doped CuZn catalysts. J. Catal. 2018, 359, 17–26. [Google Scholar] [CrossRef]
- Tursunov, O.; Kustov, L.; Kustov, A. A Brief Review of Carbon Dioxide Hydrogenation to Methanol Over Copper and Iron Based Catalysts. Oil Gas Sci. Technol.—Rev. d’IFP Energies Nouv. 2017, 72, 30. [Google Scholar] [CrossRef]
- Gesmanee, S.; Koo-amornpattana, W. Catalytic hydrogenation of CO2 for methanol production in fixed-bed reactor using Cu-Zn supported on gamma-Al 2 O 3. Energy Procedia 2017, 138, 739–744. [Google Scholar] [CrossRef]
- Grabow, L.C.; Mavrikakis, M. Mechanism of Methanol Synthesis on Cu through CO2 and CO Hydrogenation. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Cao, H.S.; Li, S.; Pan, Y.X.; Zhang, X.B.; Luo, Z.H. Exploring the reaction mechanism and kinetic properties of CO2 hydrogenation to methanol on Cu/CeO2. Chem. Eng. Sci. 2025, 304, 120990. [Google Scholar] [CrossRef]
- Toyir, J.; Ramírez De La Piscina, P.; Fierro, J.L.G.; Homs, N. Highly effective conversion of CO2 to methanol over supported and promoted copper-based catalysts: Influence of support and promoter. Appl. Catal. B Environ. 2001, 29, 207–215. [Google Scholar] [CrossRef]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-based catalysts for Fischer-Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tong, Y.; Huang, J.; Zhu, J.; Fang, X.; Xu, J.; Wang, X. Insights into CO2 methanation mechanism on cubic ZrO2 supported Ni catalyst via a combination of experiments and DFT calculations. Fuel 2021, 283, 118867. [Google Scholar] [CrossRef]
- Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjær, C.F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 2016, 352, 969–974. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Ramírez, P.J.; Gutiérrez, R.A.; Stacchiola, D.J.; Viñes, F.; Liu, P.; Illas, F.; Rodriguez, J.A. The conversion of CO2 to methanol on orthorhombic β-Mo2C and Cu/β-Mo2C catalysts: Mechanism for admetal induced change in the selectivity and activity. Catal. Sci. Technol. 2016, 6, 6766–6777. [Google Scholar] [CrossRef]
- Kondrat, S.A.; Smith, P.J.; Wells, P.P.; Chater, P.A.; Carter, J.H.; Morgan, D.J.; Fiordaliso, E.M.; Wagner, J.B.; Davies, T.E.; Lu, L.; et al. Stable amorphous georgeite as a precursor to a high-activity catalyst. Nature 2016, 531, 83–87. [Google Scholar] [CrossRef]
- Behrens, M.; Girgsdies, F. Structural Effects of Cu/Zn Substitution in the Malachite–Rosasite System. Zeitschrift für Anorg. und Allg. Chemie 2010, 636, 919–927. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Sha, F.; Tang, S.; Wang, J.; Li, C. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure. J. Catal. 2021, 396, 242–250. [Google Scholar] [CrossRef]
- Guo, X.; Mao, D.; Lu, G.; Wang, S.; Wu, G. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared via a route of solid-state reaction. Catal. Commun. 2011, 12, 1095–1098. [Google Scholar] [CrossRef]
- Wu, C.; Lin, L.; Liu, J.; Zhang, J.; Zhang, F.; Zhou, T.; Rui, N.; Yao, S.; Deng, Y.; Yang, F.; et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 2020, 11, 5767. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. Effect of the nature of copper species on methanol synthesis from CO2 hydrogenation reaction over CuO/Ce0.4Zr0.6O2 catalyst. Mol. Catal. 2020, 493, 111105. [Google Scholar] [CrossRef]
- Singh, R.; Tripathi, K.; Pant, K.K. Investigating the role of oxygen vacancies and basic site density in tuning methanol selectivity over Cu/CeO2 catalyst during CO2 hydrogenation. Fuel 2021, 303, 121289. [Google Scholar] [CrossRef]
- Gothe, M.L.; Pérez-Sanz, F.J.; Braga, A.H.; Borges, L.R.; Abreu, T.F.; Bazito, R.C.; Gonçalves, R.V.; Rossi, L.M.; Vidinha, P. Selective CO2 hydrogenation into methanol in a supercritical flow process. J. CO2 Util. 2020, 40, 101195. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, Y.; Wang, L.; Li, Y.; Fang, Z.; Xu, Y.; Liu, B.; Liu, X. Dependence of copper particle size and interface on methanol and CO formation in CO2 hydrogenation over Cu@ZnO catalysts. Catal. Sci. Technol. 2022, 12, 551–564. [Google Scholar] [CrossRef]
- Lam, E.; Corral-Pérez, J.J.; Larmier, K.; Noh, G.; Wolf, P.; Comas-Vives, A.; Urakawa, A.; Copéret, C. CO2 Hydrogenation on Cu/Al2O3: Role of the Metal/Support Interface in Driving Activity and Selectivity of a Bifunctional Catalyst. Angew. Chemie Int. Ed. 2019, 58, 13989–13996. [Google Scholar] [CrossRef] [PubMed]
- Klier, K. Methanol Synthesis. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1982; pp. 243–313. ISBN 9780120078318. [Google Scholar]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong Interactions in Supported-Metal Catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, Y.; Guo, H.; Tu, X. Atmospheric Pressure and Room Temperature Synthesis of Methanol through Plasma-Catalytic Hydrogenation of CO2. ACS Catal. 2017, 8, 90–100. [Google Scholar] [CrossRef]
- Liao, F.; Huang, Y.; Ge, J.; Zheng, W.; Tedsree, K.; Collier, P.; Hong, X.; Tsang, S.C. Morphology-Dependent Interactions of ZnO with Cu Nanoparticles at the Materials’ Interface in Selective Hydrogenation of CO2 to CH3OH. Angew. Chemie Int. Ed. 2011, 50, 2162–2165. [Google Scholar] [CrossRef]
- Meunier, F.C. Mixing Copper Nanoparticles and ZnO Nanocrystals: A Route towards Understanding the Hydrogenation of CO2 to Methanol? Angew. Chem. Int. Ed. 2011, 50, 4053–4054. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.-J.; Tsang, S.C.E. Bimetallic catalysts for green methanol production via CO2 and renewable hydrogen: A mini-review and prospects. Catal. Sci. Technol. 2018, 8, 3450–3464. [Google Scholar] [CrossRef]
- Ling, X.; Wang, G.; Han, J.; Wang, L.; Yu, J.; Mao, D. Solvent effects on the preparation of CuO-ZnO-ZrO2-Al2O3 catalyst by citrate complexing method for CO2 hydrogenation to methanol. Fuel 2025, 382, 133653. [Google Scholar] [CrossRef]
- Samson, K.; Śliwa, M.; Socha, R.P.; Góra-Marek, K.; Mucha, D.; Rutkowska-Zbik, D.; Paul, J.-F.; Ruggiero-Mikołajczyk, M.; Grabowski, R.; Słoczyński, J. Influence of ZrO2 Structure and Copper Electronic State on Activity of Cu/ZrO2 Catalysts in Methanol Synthesis from CO2. ACS Catal. 2014, 4, 3730–3741. [Google Scholar] [CrossRef]
- Conrad, F.; Massue, C.; Kühl, S.; Kunkes, E.; Girgsdies, F.; Kasatkin, I.; Zhang, B.; Friedrich, M.; Luo, Y.; Armbrüster, M.; et al. Microwave-hydrothermal synthesis and characterization of nanostructured copper substituted ZnM2O4 (M = Al, Ga) spinels as precursors for thermally stable Cu catalysts. Nanoscale 2012, 4, 2018. [Google Scholar] [CrossRef]
- Liu, T.; Xu, D.; Wu, D.; Liu, G.; Hong, X. Spinel ZnFe2O4 Regulates Copper Sites for CO2 Hydrogenation to Methanol. ACS Sustain. Chem. Eng. 2021, 9, 4033–4041. [Google Scholar] [CrossRef]
- Kühl, S.; Schumann, J.; Kasatkin, I.; Hävecker, M.; Schlögl, R.; Behrens, M. Ternary and quaternary Cr or Ga-containing ex-LDH catalysts—Influence of the additional oxides onto the microstructure and activity of Cu/ZnAl2O4 catalysts. Catal. Today 2015, 246, 92–100. [Google Scholar] [CrossRef]
- Conrad, F.; Zhou, Y.; Yulikov, M.; Hametner, K.; Weyeneth, S.; Jeschke, G.; Günther, D.; Grunwaldt, J.; Patzke, G.R. Microwave-Hydrothermal Synthesis of Nanostructured Zinc-Copper Gallates. Eur. J. Inorg. Chem. 2010, 2010, 2036–2043. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal-Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Taylor, J.M.; Mitsuka, Y.; Ogiwara, N.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kitagawa, H. Charge transfer dependence on CO2 hydrogenation activity to methanol in Cu nanoparticles covered with metal-organic framework systems. Chem. Sci. 2019, 10, 3289–3294. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Pasupulety, N.; Driss, H.; Abobakor, Y.; Ahmed, A.; Daous, M.A.; Petrov, L. Applied Catalysis A: General Studies on Au / Cu–Zn–Al catalyst for methanol synthesis from CO2. Applied Catal. A Gen. 2015, 504, 308–318. [Google Scholar] [CrossRef]
- Zheng, J.; Lebedev, K.; Wu, S.; Huang, C.; Ayvall, T.; Wu, T.S.; Li, Y.; Ho, P.L.; Soo, Y.L.; Kirkland, A.; et al. High Loading of Transition Metal Single Atoms on Chalcogenide Catalysts. J. Am. Chem. Soc. 2021, 143, 7979–7990. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Wang, K.; Ding, F.; Jia, S.; Liu, G.; Tan, L. Investigation on the promotional role of Ga2O3 on the CuO–ZnO/HZSM-5 catalyst for CO2 hydrogenation. RSC Adv. 2021, 11, 14426–14433. [Google Scholar] [CrossRef]
- Schumann, J.; Eichelbaum, M.; Lunkenbein, T.; Thomas, N.; Consuelo, M.; Galva, A.; Schlo, R.; Behrens, M. Promoting Strong Metal Support Interaction: Doping ZnO for Enhanced Activity of Cu/ZnO:M (M = Al, Ga, Mg) Catalysts. ACS Catal. 2015, 5, 3260–3270. [Google Scholar] [CrossRef]
- Sharma, S.K.; Paul, B.; Pal, R.S.; Bhanja, P.; Banerjee, A.; Samanta, C.; Bal, R. Influence of Indium as a Promoter on the Stability and Selectivity of the Nanocrystalline Cu/CeO2 Catalyst for CO2 Hydrogenation to Methanol. ACS Appl. Mater. Interfaces 2021, 13, 28201–28213. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mao, D.; Lu, G.; Wang, S.; Wu, G. The influence of la doping on the catalytic behavior of Cu/ZrO2 for methanol synthesis from CO2 hydrogenation. J. Mol. Catal. A Chem. 2011, 345, 60–68. [Google Scholar] [CrossRef]
- Ban, H.; Li, C.; Asami, K.; Fujimoto, K. Influence of rare-earth elements (La, Ce, Nd and Pr) on the performance of Cu/Zn/Zr catalyst for CH3OH synthesis from CO2. Catal. Commun. 2014, 54, 50–54. [Google Scholar] [CrossRef]
- Kourtelesis, M.; Kousi, K.; Kondarides, D.I. CO2 Hydrogenation to Methanol over La2O3-Promoted CuO/ZnO/Al2O3 Catalysts: A Kinetic and Mechanistic Study. Catalysts 2020, 10, 183. [Google Scholar] [CrossRef]
- Jeong, E.; Hee, Y.; Lee, D.; Moon, D.; Lee, K. Hydrogenation of CO2 to methanol over Pd–Cu/CeO2 catalysts. Mol. Catal. 2023, 434, 146–153. [Google Scholar] [CrossRef]
- Luk, H.T.; Mondelli, C.; Ferré, D.C.; Stewart, J.A.; Pérez-Ramírez, J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 2017, 46, 1358–1426. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M.; Lawes, N.; Gow, I.; Hayward, J.; Esquius, J.R.; Richards, N.; Smith, L.R.; Slater, T.J.A.; Davies, T.E.; Dummer, N.F.; et al. The Critical Role of βpdZn Alloy in Pd/ZnO Catalysts for the Hydrogenation of Carbon Dioxide to Methanol. ACS Catal. 2022, 12, 5371–5379. [Google Scholar] [CrossRef]
- Martin, O.; Mondelli, C.; Curulla-ferre, D.; Drouilly, C.; Hauert, R.; Pe, J. Zinc-Rich Copper Catalysts Promoted by Gold for Methanol Synthesis. ACS Catal. 2015, 5, 5607–5616. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.; Li, D.; Ning, F.; Wang, Q.; Liu, W.; Zhang, W.; Cui, Y.; Zhang, J.; Liu, C. Mg enhanced the performance of Cu/ZnO/ZrO2 for CO2 hydrogenation to methanol and the mechanism investigation. Mol. Catal. 2024, 558, 114008. [Google Scholar] [CrossRef]
- Oshima, K.; Honma, Y.; Kinoshita, K.; Gao, Z.; Honma, T.; Tada, S.; Satokawa, S. Mechanochemical Effect in Mixing Sponge Copper with Amorphous ZrO2 Creates Effective Active Sites for Methanol Synthesis by CO2 Hydrogenation. J. Phys. Chem. C 2021, 125, 8155–8162. [Google Scholar] [CrossRef]
- Dai, W.; Meng, X.; Xu, B.; Zhao, R.; Jin, D.; Xu, F.; Yang, D.; Xin, Z. Effect of Reflux Time on the Performance of the Cu/ZrO2 Catalyst for CO2 Hydrogenation to Methanol. ACS Appl. Energy Mater. 2023, 6, 9417–9426. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Song, F.; Zhang, Q.; Yang, G.; Zhang, M.; Wang, X.; Xie, H.; Tan, Y. Induced high selectivity methanol formation during CO2 hydrogenation over a CuBr2-modified CuZnZr catalyst. J. Catal. 2020, 389, 47–59. [Google Scholar] [CrossRef]
- Xu, D.; Hong, X.; Liu, G. Highly dispersed metal doping to ZnZr oxide catalyst for CO2 hydrogenation to methanol: Insight into hydrogen spillover. J. Catal. 2021, 393, 207–214. [Google Scholar] [CrossRef]
- Mureddu, M.; Lai, S.; Atzori, L.; Rombi, E.; Ferrara, F.; Pettinau, A.; Cutrufello, M.G. Ex-ldh-based catalysts for CO2 conversion to methanol and dimethyl ether. Catalysts 2021, 11, 615. [Google Scholar] [CrossRef]
- Pasupulety, N.; Al-Zahrani, A.A.; Daous, M.A.; Podila, S.; Driss, H. A study on highly active Cu-Zn-Al-K catalyst for CO2 hydrogenation to methanol. Arab. J. Chem. 2021, 14, 102951. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products. J. Catal. 2014, 309, 66–70. [Google Scholar] [CrossRef]
- Gaikwad, R.; Bansode, A.; Urakawa, A. High-pressure advantages in stoichiometric hydrogenation of carbon dioxide to methanol. J. Catal. 2016, 343, 127–132. [Google Scholar] [CrossRef]
- Le Valant, A.; Comminges, C.; Tisseraud, C.; Canaff, C.; Pinard, L.; Pouilloux, Y. The Cu–ZnO synergy in methanol synthesis from CO2, Part 1: Origin of active site explained by experimental studies and a sphere contact quantification model on Cu + ZnO mechanical mixtures. J. Catal. 2015, 324, 41–49. [Google Scholar] [CrossRef]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, characterization and activity pattern of Cu–ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Raudaskoski, R.; Niemelä, M.V.; Keiski, R.L. The effect of ageing time on co-precipitated Cu/ZnO/ZrO2 catalysts used in methanol synthesis from CO2 and H2. Top. Catal. 2007, 45, 57–60. [Google Scholar] [CrossRef]
- Frei, E.; Schaadt, A.; Ludwig, T.; Hillebrecht, H.; Krossing, I. The Influence of the Precipitation/Ageing Temperature on a Cu/ZnO/ZrO2 Catalyst for Methanol Synthesis from H2 and CO2. ChemCatChem 2014, 6, 1721–1730. [Google Scholar] [CrossRef]
- Witoon, T.; Chalorngtham, J.; Dumrongbunditkul, P.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to methanol over Cu/ZrO 2 catalysts: Effects of zirconia phases. Chem. Eng. J. 2016, 293, 327–336. [Google Scholar] [CrossRef]
- Angelo, L.; Kobl, K.; Tejada, L.M.M.; Zimmermann, Y.; Parkhomenko, K.; Roger, A.-C. Study of CuZnMOx oxides (M = Al, Zr, Ce, CeZr) for the catalytic hydrogenation of CO2 into methanol. Comptes Rendus. Chim. 2015, 18, 250–260. [Google Scholar] [CrossRef]
- Kanuri, S.; Singh, S.A.; Dinda, S. Prominence of Fe on Cu/ZnO/ZrO2 catalyst for methanol synthesis from CO2: Material preparation, performance demonstration, and kinetic analysis. Chem. Eng. Sci. 2024, 286, 119661. [Google Scholar] [CrossRef]
- Liu, X.M.; Yan, Z.F.; Lu, G.Q. Role of nanosized zirconia on the properties of Cu/Ga2O 3/ZrO2 catalysts for methanol synthesis. Chinese J. Chem. 2006, 24, 172–176. [Google Scholar] [CrossRef]
- Fornero, E.L.; Sanguineti, P.B.; Chiavassa, D.L.; Bonivardi, A.L.; Baltanás, M.A. Performance of ternary Cu–Ga2O3–ZrO2 catalysts in the synthesis of methanol using CO2-rich gas mixtures. Catal. Today 2013, 213, 163–170. [Google Scholar] [CrossRef]
- Tidona, B.; Koppold, C.; Bansode, A.; Urakawa, A.; Rudolf Von Rohr, P. CO2 hydrogenation to methanol at pressures up to 950 bar. J. Supercrit. Fluids 2013, 78, 70–77. [Google Scholar] [CrossRef]
- Zhan, H.; Li, F.; Gao, P.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Methanol synthesis from CO2 hydrogenation over La-M-Cu-Zn-O (M = Y, Ce, Mg, Zr) catalysts derived from perovskite-type precursors. J. Power Sources 2014, 251, 113–121. [Google Scholar] [CrossRef]
- Angelo, L.; Girleanu, M.; Ersen, O.; Serra, C.; Parkhomenko, K.; Roger, A.C. Catalyst synthesis by continuous coprecipitation under micro-fluidic conditions: Application to the preparation of catalysts for methanol synthesis from CO2/H2. Catal. Today 2016, 270, 59–67. [Google Scholar] [CrossRef]
- Fujitani, T.; Saito, M.; Kanai, Y.; Watanabe, T.; Nakamura, J.; Uchijima, T. Development of an active Ga2O3 supported palladium catalyst for the synthesis of methanol from carbon dioxide and hydrogen. Appl. Catal. A Gen. 1995, 125, L199–L202. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The Active Site of Methanol Synthesis over Cu/ZnO/Al 2 O 3 Industrial Catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Nakamura, J.; Fujitani, T.; Kuld, S.; Helveg, S.; Chorkendorff, I.; Sehested, J. Comment on “Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts”. Science 2017, 357, eaan8074. [Google Scholar] [CrossRef] [PubMed]
- Muhler, M.; Nielsen, L.P.; Törnqvist, E.; Clausen, B.S.; Topsøe, H. Temperature-programmed desorption of H2 as a tool to determine metal surface areas of Cu catalysts. Catal. Letters 1992, 14, 241–249. [Google Scholar] [CrossRef]
- Amann, P.; Klötzer, B.; Degerman, D.; Köpfle, N.; Götsch, T.; Lömker, P.; Rameshan, C.; Ploner, K.; Bikaljevic, D.; Wang, H.Y.; et al. The state of zinc in methanol synthesis over a Zn/ZnO/Cu(211) model catalyst. Science 2022, 376, 603–608. [Google Scholar] [CrossRef]
- Jiang, X.; Koizumi, N.; Guo, X.; Song, C. Bimetallic Pd–Cu catalysts for selective CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2015, 170–171, 173–185. [Google Scholar] [CrossRef]
- Nie, X.; Li, W.; Jiang, X.; Guo, X.; Song, C. Chapter Two—Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 65, pp. 121–233. [Google Scholar]
- Nie, X.; Jiang, X.; Wang, H.; Luo, W.; Janik, M.J.; Chen, Y.; Guo, X.; Song, C. Mechanistic Understanding of Alloy Effect and Water Promotion for Pd-Cu Bimetallic Catalysts in CO2 Hydrogenation to Methanol. ACS Catal. 2018, 8, 4873–4892. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, X.; Boreriboon, N.; Wang, Z.; Song, C.; Cen, K. Effects of supports on bimetallic Pd-Cu catalysts for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 585, 117210. [Google Scholar] [CrossRef]
- Wambach, J.; Baiker, A.; Wokaun, A. CO2 hydrogenation over metal/zirconia catalysts. Phys. Chem. Chem. Phys. 1999, 1, 5071–5080. [Google Scholar] [CrossRef]
- Li, L.; Mao, D.; Yu, J.; Guo, X. Highly selective hydrogenation of CO2 to methanol over CuO–ZnO–ZrO2 catalysts prepared by a surfactant-assisted co-precipitation method. J. Power Sources 2015, 279, 394–404. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F.; et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajnani, S.; Memon, M.A.; Memon, S.A.; Kumar, A.; Mehvish, D.; Ameen, S.; Mukarama; Zhou, W.; Liu, Y. CO2 to Methanol Conversion: A Bibliometric Analysis with Insights into Reaction Mechanisms, and Recent Advances in Catalytic Conversion. Processes 2025, 13, 314. https://doi.org/10.3390/pr13020314
Sajnani S, Memon MA, Memon SA, Kumar A, Mehvish D, Ameen S, Mukarama, Zhou W, Liu Y. CO2 to Methanol Conversion: A Bibliometric Analysis with Insights into Reaction Mechanisms, and Recent Advances in Catalytic Conversion. Processes. 2025; 13(2):314. https://doi.org/10.3390/pr13020314
Chicago/Turabian StyleSajnani, Shahdev, Mazhar Ahmed Memon, Shabir Ahmed Memon, Akash Kumar, Darakhshan Mehvish, Somavia Ameen, Mukarama, Wei Zhou, and Yuan Liu. 2025. "CO2 to Methanol Conversion: A Bibliometric Analysis with Insights into Reaction Mechanisms, and Recent Advances in Catalytic Conversion" Processes 13, no. 2: 314. https://doi.org/10.3390/pr13020314
APA StyleSajnani, S., Memon, M. A., Memon, S. A., Kumar, A., Mehvish, D., Ameen, S., Mukarama, Zhou, W., & Liu, Y. (2025). CO2 to Methanol Conversion: A Bibliometric Analysis with Insights into Reaction Mechanisms, and Recent Advances in Catalytic Conversion. Processes, 13(2), 314. https://doi.org/10.3390/pr13020314








