Efficiency of Hydrogen Sulfide Removal from Biogas Using a Laboratory-Scale Biofilter Packed with Biochar, Cellular Concrete Waste, or Polyurethane Foam: A COMSOL Simulation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Operation Conditions of the Pilot-Scale Biofilter
2.2. Physicochemical Properties of Biochar, CLC Waste, and PUF
2.3. Mathematical Modeling of the Experiment
- Negligible turbulence: Large turbulence was assumed negligible based on experimental data indicating a laminar flow pattern (with Reynolds number between 0.2 and 0.5 for full-scale operations) in typical biofilters.
- Homogeneous filter material: The composition of the filter material, including porosity and water content, was assumed to be homogeneous.
- Zero initial H2S concentration: It was assumed that the initial concentration of hydrogen sulfide (H2S) in the biofilter was zero.
- Homogeneous biomass distribution: Biomass distribution and density within the biofilter were assumed to be homogeneous.
- Inlet biogas: H2S concentrations ranged from 100 to 2000 ppm.
- Outlet biogas: Zero-flux boundary conditions were applied to ensure mass conservation.
- Diffusive flux coupled with reactive consumption were modeled at the material interface.
- The experimentally measured surface area, porosity, and functional group data were incorporated into the adsorption parameters.
- Microbial activity coefficients were linked to qPCR-derived population densities of sulfur-oxidizing bacteria (SOBs), particularly Thiobacillus.
- The COMSOL Multiphysics platform simulated the H2S removal efficiency under varying flow rates, concentrations, and packing configurations. Using input parameters from the experimental results, a 3D model of the biofilter was constructed.
- Mesh sensitivity analysis ensured computational accuracy while minimizing processing time.
3. Results
3.1. Biofilter Performance
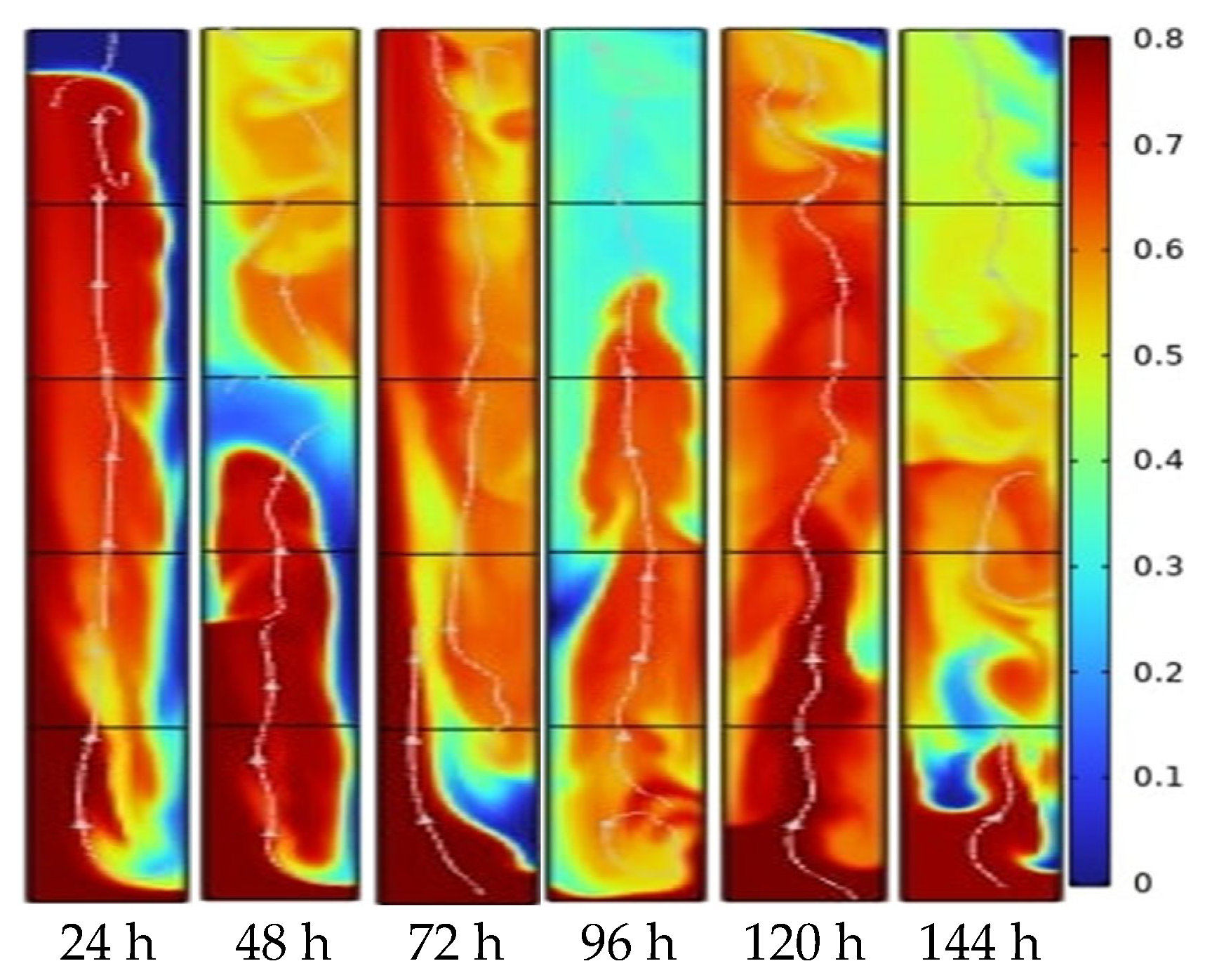
3.2. Simulation in COMSOL
4. Discussion
4.1. Results of Experimental Analysis
- The unmodified biochar demonstrated an H2S removal efficiency of 80.00%, which is relatively high and highlights its inherent adsorption capabilities and suitability as a packing material for biofilters.
- KOH-activated biochar achieved a significantly higher efficiency of 92.00%, showcasing the impact of chemical activation with potassium hydroxide. The increased efficiency can be attributed to the enhanced surface area, pore structure, and chemical reactivity introduced by the activation process, facilitating greater adsorption and microbial activity.
- Unmodified CLC waste: Its moderate removal efficiency of 60.00% indicates that while CLC waste can function as a biofilter material, its unmodified form has limitations in H2S adsorption and microbial support.
- Fe2CO3-impregnated CLC waste: The modification with iron carbonate significantly improved the efficiency to 75.00%. This enhancement could be linked to the catalytic activity provided by Fe2CO3, which likely accelerated the oxidation of H2S as well as improved the surface chemistry of the material.
- PUF exhibited the lowest efficiency of 48.00%, reflecting its limited capability for H2S adsorption or microbial colonization without further modification. While PUF is valuable as a structural material for supporting biofilms, it lacks the chemical properties required for significant H2S removal, emphasizing the need for additional treatment or blending with other materials.
4.2. Results of Simulation Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkhatib, I.; Khalifa, O.; Bahamon, D.; Abu-Zahra, M.R.; Vega, L.F. Sustainability criteria as a game changer in the search for hybrid solvents for CO2 and H2S removal. Sep. Purif. Technol. 2021, 277, 119516. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, Y.; Chen, H.; Liu, Y.; Huang, R.; Pan, J. Biochars derived from by-products of microalgae pyrolysis for sorption of gaseous H2S. J. Environ. Chem. Eng. 2022, 10, 107370. [Google Scholar] [CrossRef]
- Bu, H.; Carvalho, G.; Huang, C.; Sharma, K.R.; Yuan, Z.; Song, Y.; Bond, P.; Keller, J.; Yu, M.; Jiang, G. Evaluation of continuous and intermittent trickling strategies for the removal of hydrogen sulfide in a biotrickling filter. Chemosphere 2021, 291, 132723. [Google Scholar] [CrossRef] [PubMed]
- Ćwiertniewicz-Wojciechowska, C.; Cema, G.; Ziembińska-Buczyńska, A. Sewage sludge pretreatment: Current status and future prospects. Environ. Sci. Pollut. Res. 2023, 30, 88313–88330. [Google Scholar] [CrossRef]
- Konkol, D.; Popiela, E.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Moustakas, K.; Opaliński, S.; Korczyński, M.; Krowiak, A.W. Recent innovations in various methods of harmful gases conversion and its mechanism in poultry farms. Environ. Res. 2022, 214, 113825. [Google Scholar] [CrossRef]
- Nhut, H.H.; Thanh, V.T.; Le, L.T. Removal of H2S in biogas using biotrickling filter: Recent development. Process Saf. Environ. Prot. 2020, 144, 297–309. [Google Scholar] [CrossRef]
- De Souza, F.M.; Kahol, P.K.; Gupta, K.R. Introduction to Polyurethane Chemistry. Am. Chem. Soc. 2021, 1380, 1–24. [Google Scholar]
- Ghimire, A.; Gyawali, R.; Lens, P.N.L.; Lohani, S.P. Technologies for removal of hydrogen sulfide (H2S) from biogas. In Emerging Technologies and Biological Systems for Biogas Upgrading; Elsevier: Amsterdam, The Netherlands, 2021; Volume 11. [Google Scholar]
- Shang, G.; Liu, L.; Chen, P.; Shen, G.; Li, Q. Kinetics and the mass transfer mechanism of hydrogen sulfide removal by biochar derived from rice hull. J. Air Waste Manag. Assoc. 2016, 133, 495–499. [Google Scholar] [CrossRef]
- Haosagul, S.; Prommeenate, P.; Hobbs, G.; Pisutpaisal, N. Sulfur-oxidizing bacteria in full-scale biogas cleanup system of ethanol industry. Renew. Energy 2020, 150, 965–972. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, J.; Jin, Z.; Yang, S.; Shen, L. Enhancing the removal of H2S from biogas through refluxing of outlet gas in biological bubble-column. Bioresour. Technol. 2020, 299, 122621. [Google Scholar] [CrossRef]
- Juntarachat, N.; Onthong, U. Removal of hydrogen sulfide from biogas using banana peel and banana empty fruit bunch biochars as alternative adsorbents. Biomass Convers. Biorefinery 2022, 14, 27077–27088. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, J.; Yin, L.; Liu, H.; Zuo, W.; Tian, Y. Relationship between heavy metal consolidation and H2S removal by biochar from microwave pyrolysis of municipal sludge: Effect and mechanism. Environ. Sci. Pollut. Res. 2021, 28, 27694–27702. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Zhou, X.; Hu, J.; Sun, Z.; Yao, J.; Chen, D.; Wang, J. Simultaneous removal of carbon disulfide and hydrogen sulfide from viscose fibre waste gas with a biotrickling filter in pilot scale. J. Clean. Prod. 2019, 230, 21–28. [Google Scholar] [CrossRef]
- Khan, M.U.; EnLee, J.T.; Bashir, M.A.; Dissanayake, P.D.; WahTong, Y.S.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Kulawong, S.; Artkla, R.; Sriprapakhan, P.; Maneechot, P. Biogas purification by adsorption of hydrogen sulfide on NaX and Ag-exchanged NaX zeolites. Biomass Bioenergy 2022, 159, 106417. [Google Scholar] [CrossRef]
- Cortés, J.J.G.; Almenglo, F.; Ramírez, M.; Cantero, D. Simultaneous removal of ammonium from landfill leachate and hydrogen sulfide from biogas using a novel two-stage oxic-anoxic system. Sci. Total Environ. 2021, 750, 141664. [Google Scholar] [CrossRef]
- Kirchmann, H.; Börjesson, G.; Kätterer, T.; Cohen, Y. From agricultural use of sewage sludge to nutrient extraction: A soil science outlook. Ambio 2017, 46, 143–154. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Adsorption properties of biochars obtained by KOH activation. Environ. Manag. 2023, 30, 167–183. [Google Scholar] [CrossRef]
- Lee, J.T.E.; Ok, Y.S.; Song, S.; Dissanayake, P.D.; Tian, H.; Tio, Z.K.; Cui, R.; Lim, E.Y.; Jong, M.C.; Hoy, S.H.; et al. Biochar utilisation in the anaerobic digestion of food waste for the creation of a circular economy via biogas upgrading and digestate treatment. Bioresour. Technol. 2021, 333, 125190. [Google Scholar] [CrossRef]
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage sludge for sustainable agriculture: Contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Agric. 2018, 5, 10. [Google Scholar] [CrossRef]
- Mohammadi, K.; Vaiskunaite, R. Analysis and evaluation of the biogas purification technologies from H2S. In Environmental Protection Technology and Management VGTU; VGTU: Vilnius, Lithuania, 2023; Volume 15, pp. 1–9. [Google Scholar]
- Januševičius, T.; Mažeikienė, A.; Stepova, K.; Danila, V.; Paliulis, D. The Removal of Phosphorus from Wastewater Using a Sewage Sludge Biochar: A Column Study. Water 2024, 16, 1104. [Google Scholar] [CrossRef]
- Mitchell, K.; Beesley, L.; Šípek, V.; Trakal, L. Chapter 3—Biochar and its potential to increase water, trace element, and nutrient retention in soils. Biochar Agric. Achiev. Sustain. Dev. Goals 2022, 36, 8–20. [Google Scholar]
- Bagheri, M.; Bauer, T.; Burgman, L.E.; Wetterlund, E. Fifty years of sewage sludge management research: Mapping researchers’ motivations and concerns. J. Environ. Manag. 2023, 325, 116412. [Google Scholar] [CrossRef]
- Danila, V.; Zagorskis, A.; Januševičius, T. Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review. Processes 2022, 10, 1304. [Google Scholar] [CrossRef]
- Paulionyte, J.; Vaiskunaite, R.; Mazeikiene, A. Evaluation of sewage sludge biochar use in wastewater treatment from phosphate. In Environmental Protection Technology and Management VGTU; VGTU: Vilnius, Lithuania, 2022. [Google Scholar]
- Zhang, Y.; Kawasaki, Y.; Oshita, K.; Takaoka, M.; Minami, D.; Inoue, G.; Tanaka, T. Economic assessment of biogas purification systems for removal of both H2S and siloxane from biogas. Renew. Energy 2021, 168, 119–130. [Google Scholar] [CrossRef]
- Sugurbekova, G.; Nagyzbekkyzy, E.; Sarsenova, A.; Danlybayeva, G.; Anuarbekova, S.; Kudaibergenova, R.; Frochot, C.; Acherar, S.; Zhatkanbayev, Y.; Moldagulova, N. Sewage Sludge Management and Application in the Form of Sustainable Fertilizer. Sustainability 2023, 15, 6112. [Google Scholar] [CrossRef]
- Gaga, Y.; Benmessaoud, S.; Kara, M.; Assouguem, A.; Al-Ghamdi, A.A.; Al-Hemaid, F.M.; Elshikh, M.S.; Ullah, R.; Banach, A.; Bahhou, J. New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment. Water 2022, 14, 3319. [Google Scholar] [CrossRef]
- Styszko, K.; Durak, J.; Kończak, B.; Głodniok, M. The impact of sewage sludge processing on the safety of its use. Sci. Rep. 2022, 12, 12227. [Google Scholar] [CrossRef]
- Torres, R.A.; Marín, D.; Rodero, M.D.R.; Pascual, C.; Sanchez, A.G.; Crespo, I.G.; Lebrero, R.; Torre, R.M. Biogas treatment for H2S, CO2, and other contaminants removal. In From Biofiltration to Promising Options in Gaseous Fluxes Biotreatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–176. [Google Scholar]
- Wu, J.; Jiang, X.; Jin, Z.; Yang, S.; Zhang, J. The performance and microbial community in a slightly alkaline biotrickling filter for the removal of high concentration H2S from biogas. Chemosphere 2020, 249, 126127. [Google Scholar] [CrossRef]
- Watsuntorn, W.; Khanongnuch, R.; Chulalaksananuku, W.; Rene, E.R.; Lens, P.N.L. Resilient performance of an anoxic biotrickling filter for hydrogen sulfide removal from a biogas mimic: Steady, transient state and neural network evaluation. J. Clean. Prod. 2020, 249, 119351. [Google Scholar] [CrossRef]
- Wang, S.; Nam, H.; Lee, D.; Nam, H. H2S gas adsorption study using copper impregnated on KOH activated carbon from coffee residue for indoor air purification. J. Environ. Chem. Eng. 2022, 10, 108797. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Ma, C.; Qiao, W.; Wang, J.; Ling, L. Functionalization of activated carbon fiber mat with bimetallic active sites for NH3 and H2S adsorption at room temperature. Sep. Purif. Technol. 2022, 303, 122335. [Google Scholar] [CrossRef]
- Appala, V.N.S.G.; Pandhare, N.N.; Bajpai, S. Mathematical Models for Optimization of Anaerobic Digestion and Biogas Production. In Zero Waste Biorifinery; Springer Nature Singapore: Singapore, 2022; pp. 21–36. [Google Scholar]
- Ying, S.; Kong, X.; Cai, Z.; Man, Z.; Xin, Y.; Liu, D. Interactions and microbial variations in a biotrickling filter treating low concentrations of hydrogen sulfide and ammonia. Chemosphere 2020, 255, 126931. [Google Scholar] [CrossRef]
- Zhu, H.L.; Papurello, D.; Gandiglio, M.; Lanzini, A.; Akpinar, I.; Shearing, P.R.; Manos, G.; Brett, D.J.L.; Zhang, Y.S. Study of H2S Removal Capability from Simulated Biogas by Using Waste-Derived Adsorbent Materials. Processes 2020, 8, 1030. [Google Scholar] [CrossRef]
- Zhang, X.; Lawan, I.; Danhassan, U.A.; He, Y.; Qi, R.; Wu, A.; Sheng, K.; Lin, H. Advances in technologies for in situ desulfurization of biogas. Adv. Bioenergy 2022, 7, 99–137. [Google Scholar]
- Su, J.J.; Hong, Y.Y. Removal of hydrogen sulfide using a photocatalytic livestock biogas desulfurizer. Renew. Energy 2020, 149, 181–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Oshita, K.; Kusakabe, T.; Takaoka, M.; Kawasaki, Y.; Minami, D.; Tanaka, T. Simultaneous removal of siloxanes and H2S from biogas using an aerobic biotrickling filter. J. Hazard. Mater. 2020, 391, 122187. [Google Scholar] [CrossRef]
- Morgado, M.F.; Cervantes, A.T.; Sánchez, A.G.; Lebrero, R.; Muñoz, R. Integral (VOCs, CO2, mercaptans and H2S) photosynthetic biogas upgrading using innovative biogas and digestate supply strategies. Chem. Eng. J. 2018, 354, 363–369. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, Y.; Huan, C.; Shuai, Y.; Liu, Y.; Xu, L.; Ji, G.; Yan, Z. Anoxic biodesulfurization using biogas digestion slurry in biotrickling filters. J. Clean. Prod. 2019, 224, 88–99. [Google Scholar] [CrossRef]
- Vikrant, K.; Kailasa, S.K.; Tsang, D.C.; Lee, S.S.; Kumar, P.; Giri, B.S.; Singh, R.S.; Kim, K.H. Biofiltration of hydrogen sulfide: Trends and challenges. J. Clean. Prod. 2018, 187, 131–147. [Google Scholar] [CrossRef]
- Vaiskunaite, R. Cleaning of H2S from polluted air using peat biofilter. In Environmental Protection Technology and Management VGTU; VGTU: Lietuvos, Lithuania, 2022; Volume 12, pp. 1–5. [Google Scholar]
- Vaiskunaite, R. Using Biofilter Packed with Different Wood Waste Charges for Purification of Air Contaminated with Benzene. In Environmental Protection Technology and Management VGTU; VGTU: Vilnius, Lithuania, 2020; Volume 11. [Google Scholar]
- Shi, M.; Xiong, W.; Zhang, X.; Ji, J.; Hu, X.; Tu, Z.; Wu, Y. Highly efficient and selective H2S capture by task-specific deep eutectic solvents through chemical dual-site absorption. Sep. Purif. Technol. 2022, 283, 120167. [Google Scholar] [CrossRef]
- Pepper, I.L.; Brusseau, M.L. Chapter 2—Physical-Chemical Characteristics of Soils and the Subsurface. In Environmental and Pollution Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 9–22. [Google Scholar]
- Poser, M.; Silva, L.R.D.E.; Peu, P.; Couvert, A.; Dumont, É. Cellular concrete waste: An efficient new way for H2S removal. Sep. Purif. Technol. 2023, 309, 123014. [Google Scholar] [CrossRef]
- Petrauskaite, E.; Vaiskunaite, R.; Blumberga, D.; Ivanovs, K. Experimental study of droplet biofilter packed with green sphagnum to clean air from volatile organic compounds. Energy Procedia 2017, 128, 373–378. [Google Scholar] [CrossRef]
- Mamet, S.D.; Jimmo, A.; Conway, A.; Teymurazyan, A.; Talebitaher, A.; Papandreou, Z.; Chang, Y.F.; Shannon, W.; Peak, D.; Siciliano, S.D. Soil Buffering Capacity Can Be Used To Optimize Biostimulation of Psychrotrophic Hydrocarbon Remediation. Environ. Sci. Technol. 2021, 14, 9864–9875. [Google Scholar] [CrossRef] [PubMed]
- Zagorskis, A.; Vaiskunaite, R. Modelling of a biofiltration process of volatile organic compound mixtures in a biofilter. Agric. Environ. Biotechnol. 2016, 306, 1217–1222. [Google Scholar]
- Khanongnuch, R. Hydrogen sulfide removal from synthetic biogas using anoxic biofilm reactors. Mater. Sci. Environ. Eng. 2019, 6, 479–498. [Google Scholar]
- Khanongnuch, R.; Di Capua, F.; Lakaniemi, A.M.; Rene, E.R.; Lens, P.N. Transient–state operation of an anoxic biotrickling filter for H2S removal. J. Hazard. Mater. 2019, 377, 42–51. [Google Scholar] [CrossRef]
- Irani, V.; Tavasoli, A.; Vahidi, M. Preparation of amine functionalized reduced graphene oxide/methyl diethanolamine nanofluid and its application for improving the CO2 and H2S absorption. J. Colloid Interface Sci. 2018, 28, 57–67. [Google Scholar] [CrossRef]
- Hasanzadeh, V.; Alipour, V.; Goodarzi, B.; Rahmanian, O.; Dindarloo, K.; Khosravi, R.; Heidari, M. Experimental and kinetic analysis of H2S removal in a Polyurethane foam/ Palm fiber-based Biofilter with no pH control. Iran. J. Sci. Technol. Trans. Civ. Eng. 2018, 43, 781–789. [Google Scholar] [CrossRef]


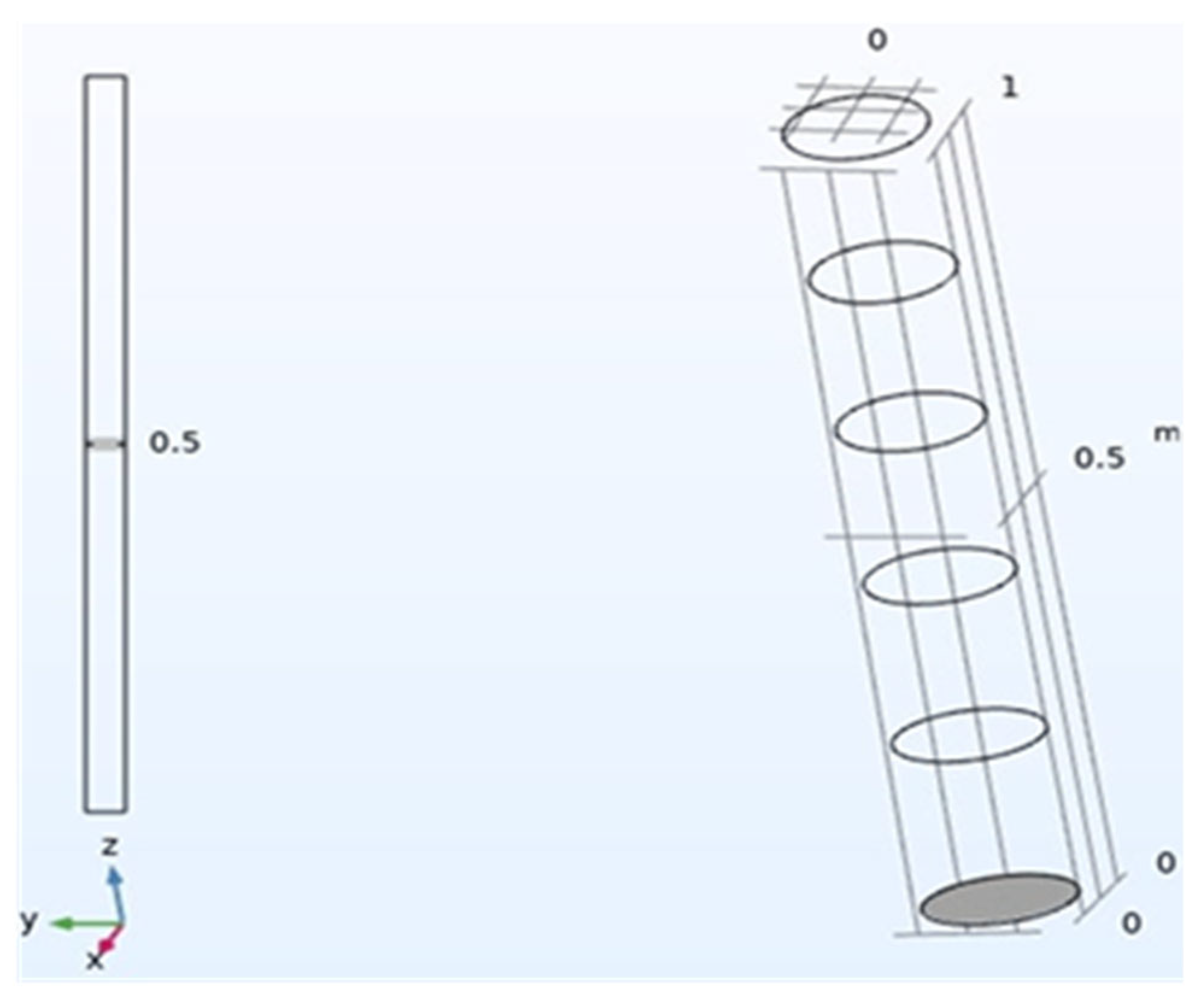




| Component | CH4 | CO2 | H2S | H2O | N2 | O2 | NH3 | VOCs |
|---|---|---|---|---|---|---|---|---|
| Range (% by volume) | 60.10% | 40.50% | 0.006–0.01% (60–100 ppm) | ~1.50–5.20% | 0–0.90% | 0–0.50% | Trace (<0.10%) | Trace |
| Packing Material | Initial H2S Concentration in Balloon | Bottom Stage (After 3 Days) | 1st Stage | 2nd Stage | 3rd Stage | 4th Stage | 5th Stage |
|---|---|---|---|---|---|---|---|
| Biochar | 90 | 90 | 20 | 10 | 10 | 10 | 80 |
| CLC waste | 100 | 30 | 10 | 10 | 10 | 10 | 20 |
| PUF | 60 | 50 | 10 | 10 | 10 | 10 | 50 |
| Packing Material | Initial H2S Concentration in Balloon | Bottom Stage (After 6 Days) | 1st Stage | 2nd Stage | 3rd Stage | 4th Stage | 5th Stage |
| Biochar | 90 | 20 | 10 | 10 | 0 | 0 | 0 |
| CLC waste | 100 | 10 | 10 | 10 | 0 | 10 | 10 |
| PUF | 60 | 20 | 10 | 10 | 0 | 40 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, K.; Vaiškūnaitė, R.; Zigmontienė, A. Efficiency of Hydrogen Sulfide Removal from Biogas Using a Laboratory-Scale Biofilter Packed with Biochar, Cellular Concrete Waste, or Polyurethane Foam: A COMSOL Simulation Study. Processes 2025, 13, 329. https://doi.org/10.3390/pr13020329
Mohammadi K, Vaiškūnaitė R, Zigmontienė A. Efficiency of Hydrogen Sulfide Removal from Biogas Using a Laboratory-Scale Biofilter Packed with Biochar, Cellular Concrete Waste, or Polyurethane Foam: A COMSOL Simulation Study. Processes. 2025; 13(2):329. https://doi.org/10.3390/pr13020329
Chicago/Turabian StyleMohammadi, Kamyab, Rasa Vaiškūnaitė, and Ausra Zigmontienė. 2025. "Efficiency of Hydrogen Sulfide Removal from Biogas Using a Laboratory-Scale Biofilter Packed with Biochar, Cellular Concrete Waste, or Polyurethane Foam: A COMSOL Simulation Study" Processes 13, no. 2: 329. https://doi.org/10.3390/pr13020329
APA StyleMohammadi, K., Vaiškūnaitė, R., & Zigmontienė, A. (2025). Efficiency of Hydrogen Sulfide Removal from Biogas Using a Laboratory-Scale Biofilter Packed with Biochar, Cellular Concrete Waste, or Polyurethane Foam: A COMSOL Simulation Study. Processes, 13(2), 329. https://doi.org/10.3390/pr13020329







