Optimization and Application of Bio-Enzyme-Enhanced Gel-Breaking Technology in Fracturing Fluids for Tight Sandstone Gas in the Linxing Block, Ordos Basin
Abstract
:1. Introduction
2. Understanding of the Linxing Block Reservoir
2.1. Analysis of Core Samples
- (1)
- Experimental samples and instruments
- (2)
- Experimental methods
- (1)
- Gas permeability
- (2)
- Quantitative analysis of whole-rock X-ray diffraction of sedimentary rock
- (3)
- Quantitative analysis of X-ray diffraction of clay minerals
2.2. Formation Temperature Statistics
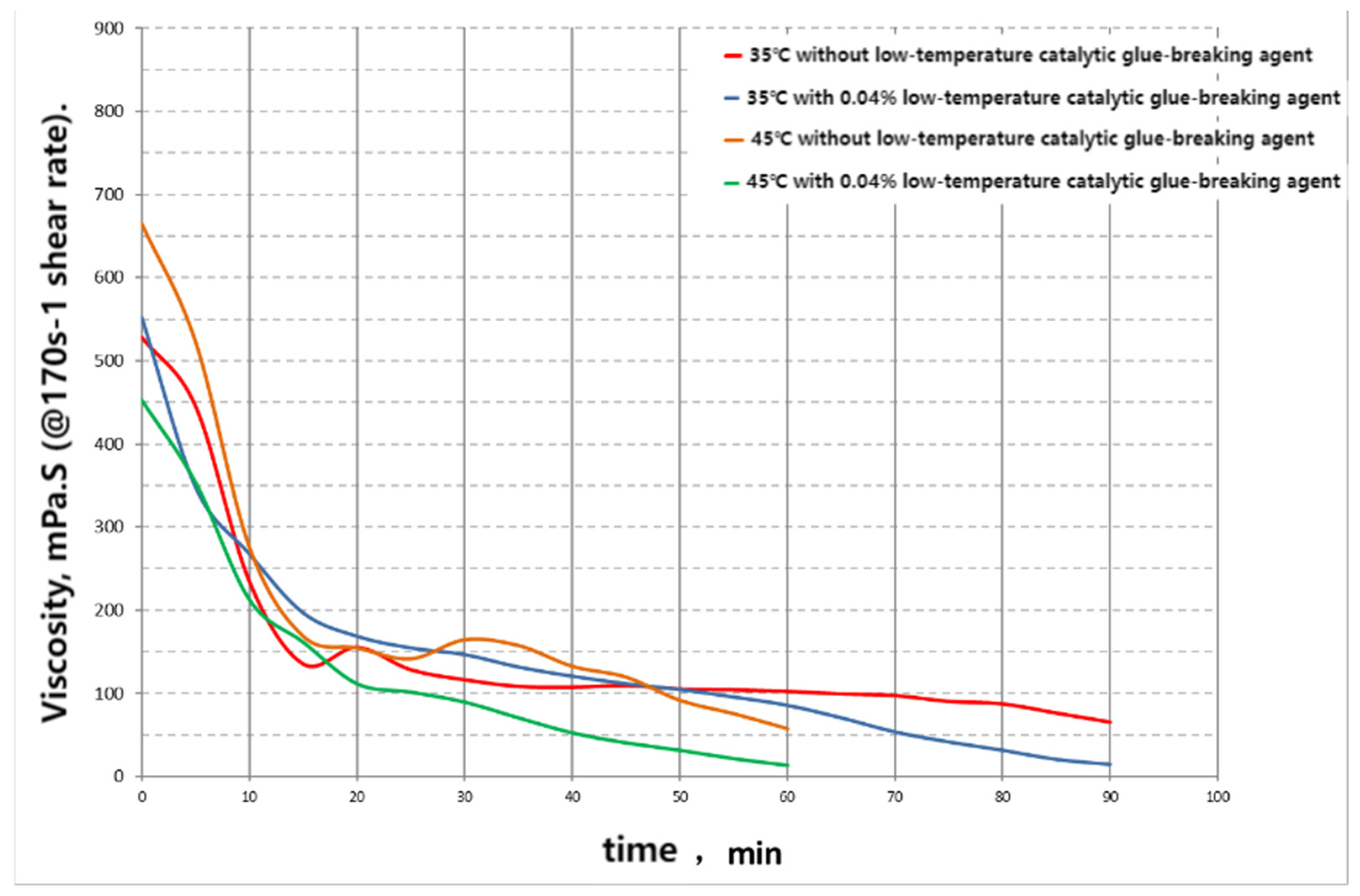
3. Comparison of the Application of Conventional Gel-Breakers and Bio-Enzymatic Gel-Breakers in the Pre-Existing Period
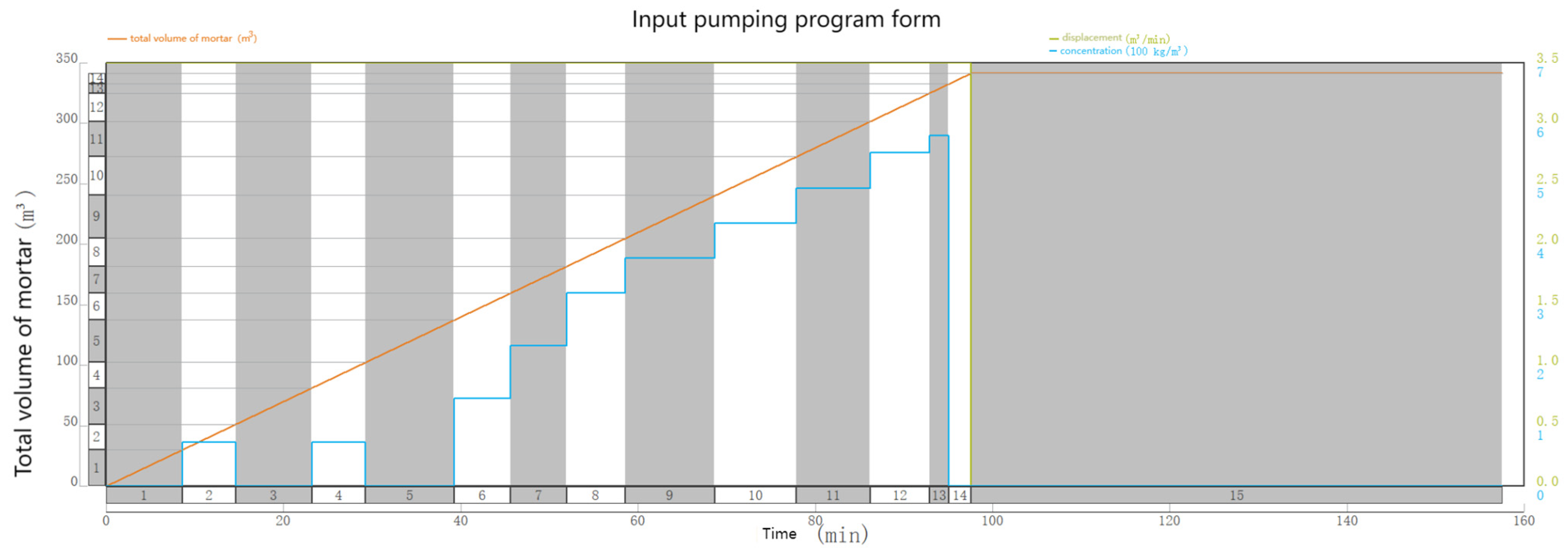
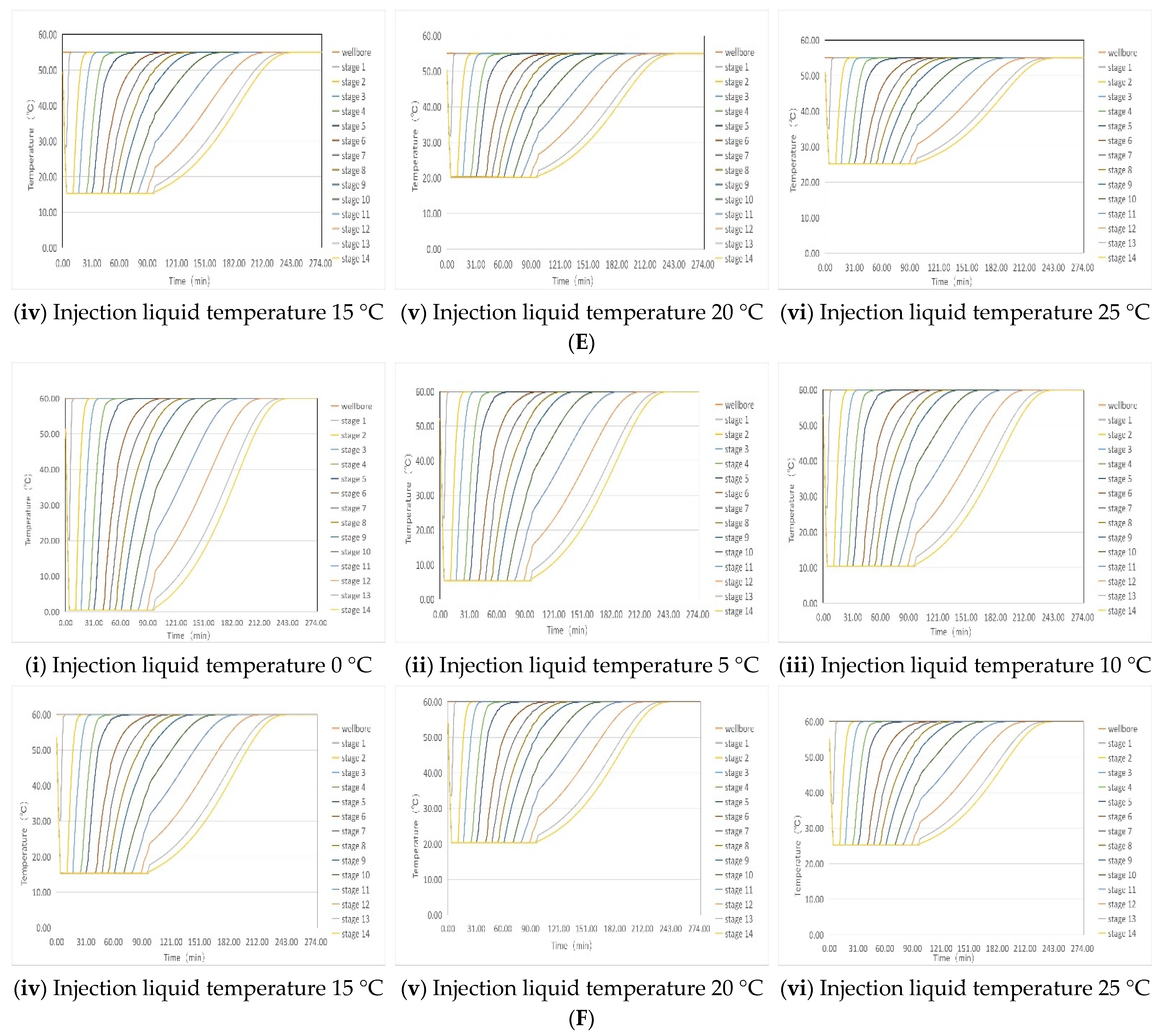
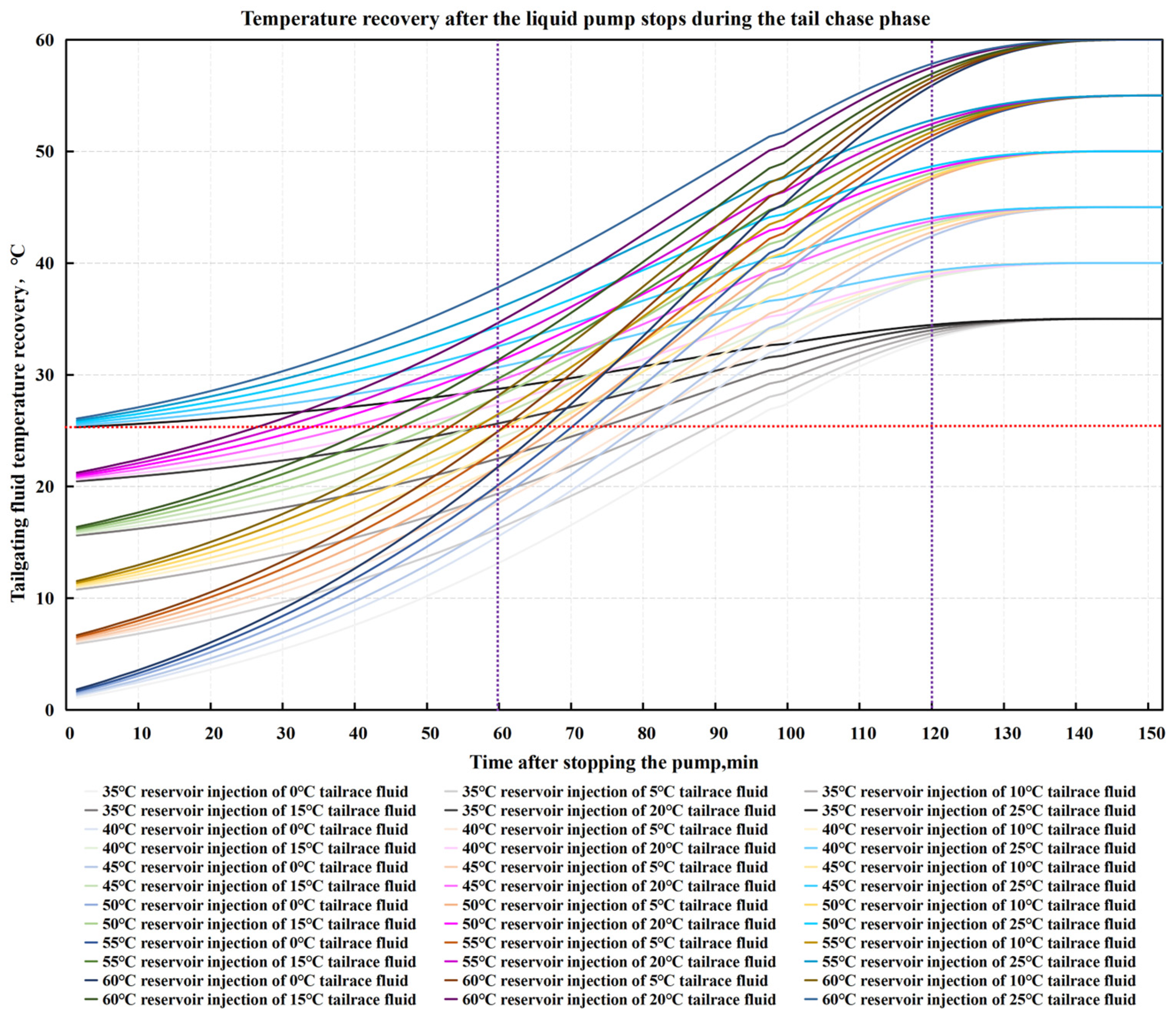
4. Optimization of Gum Breaking Procedure Based on Simulation of Temperature Recovery of Incoming Fluids
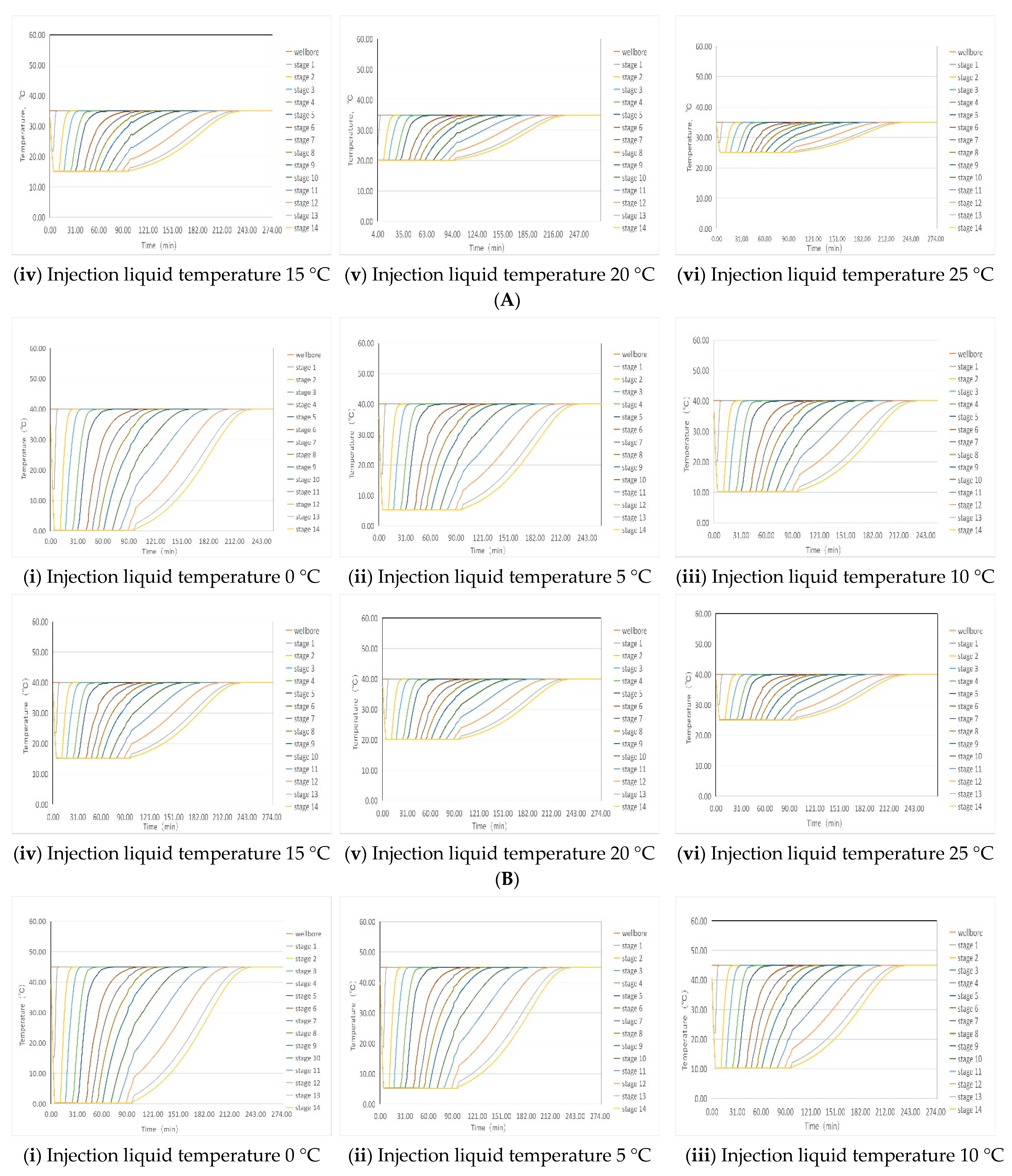
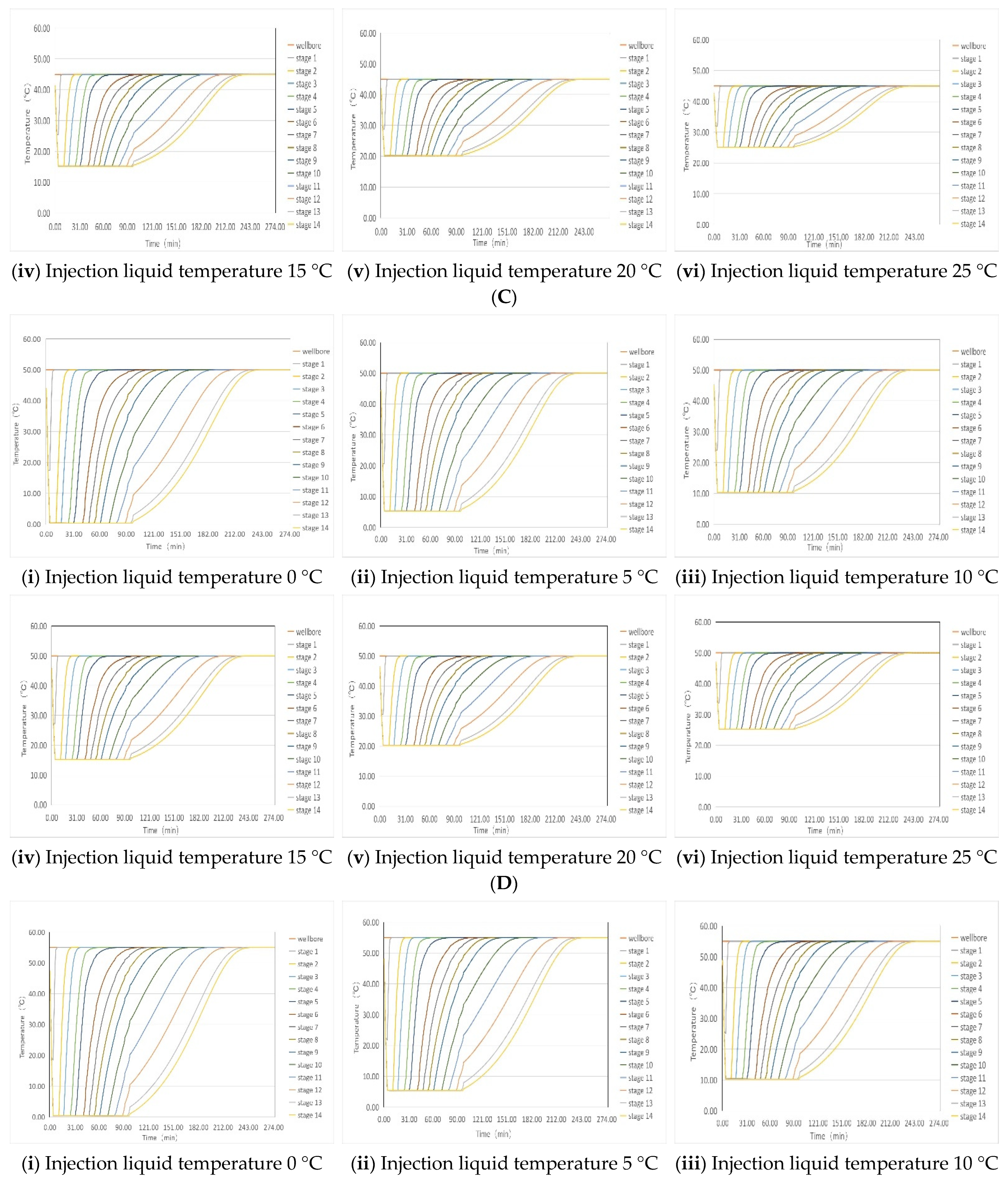
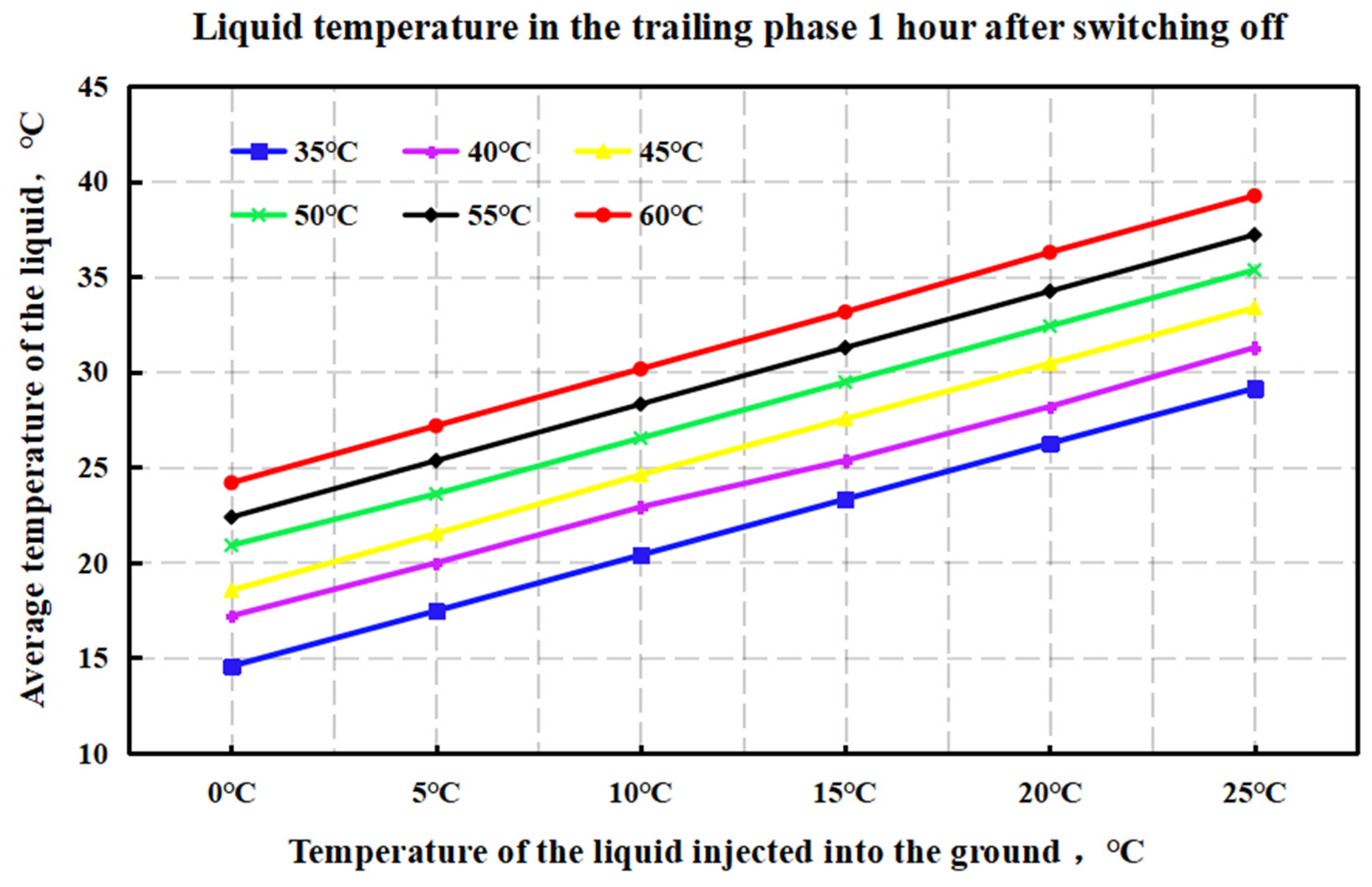
4.1. Simulation of Liquid Temperature Recovery
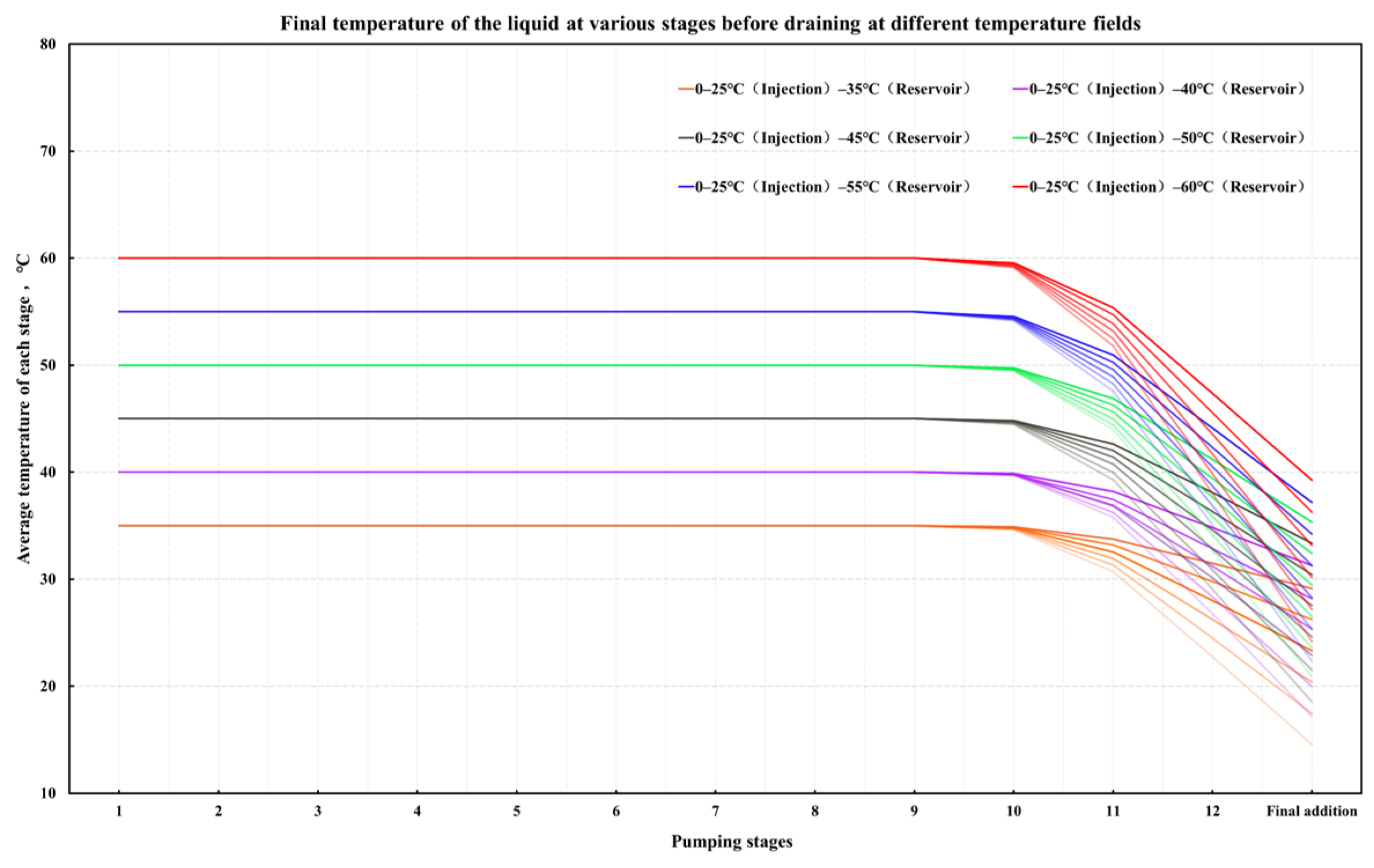
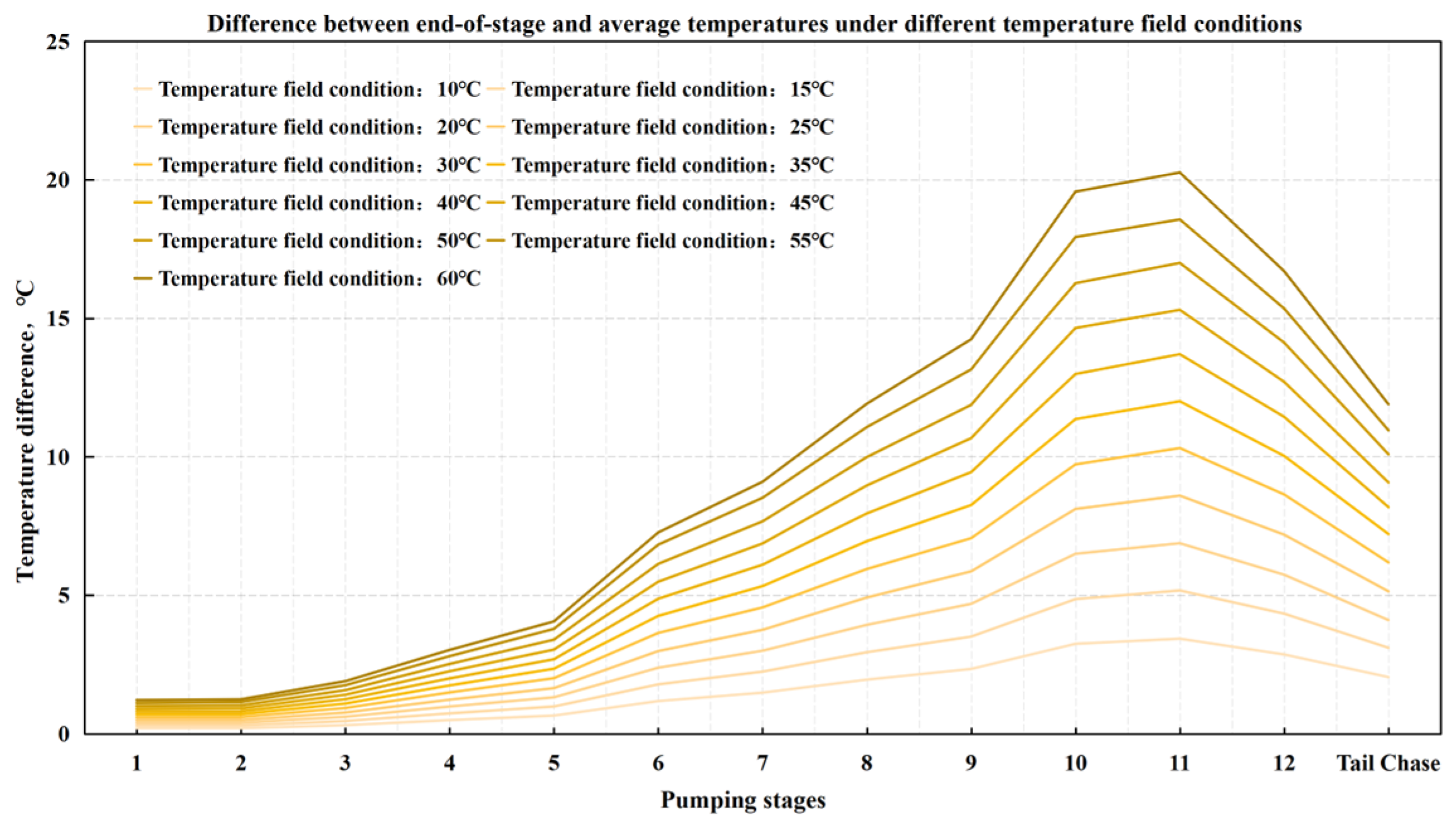
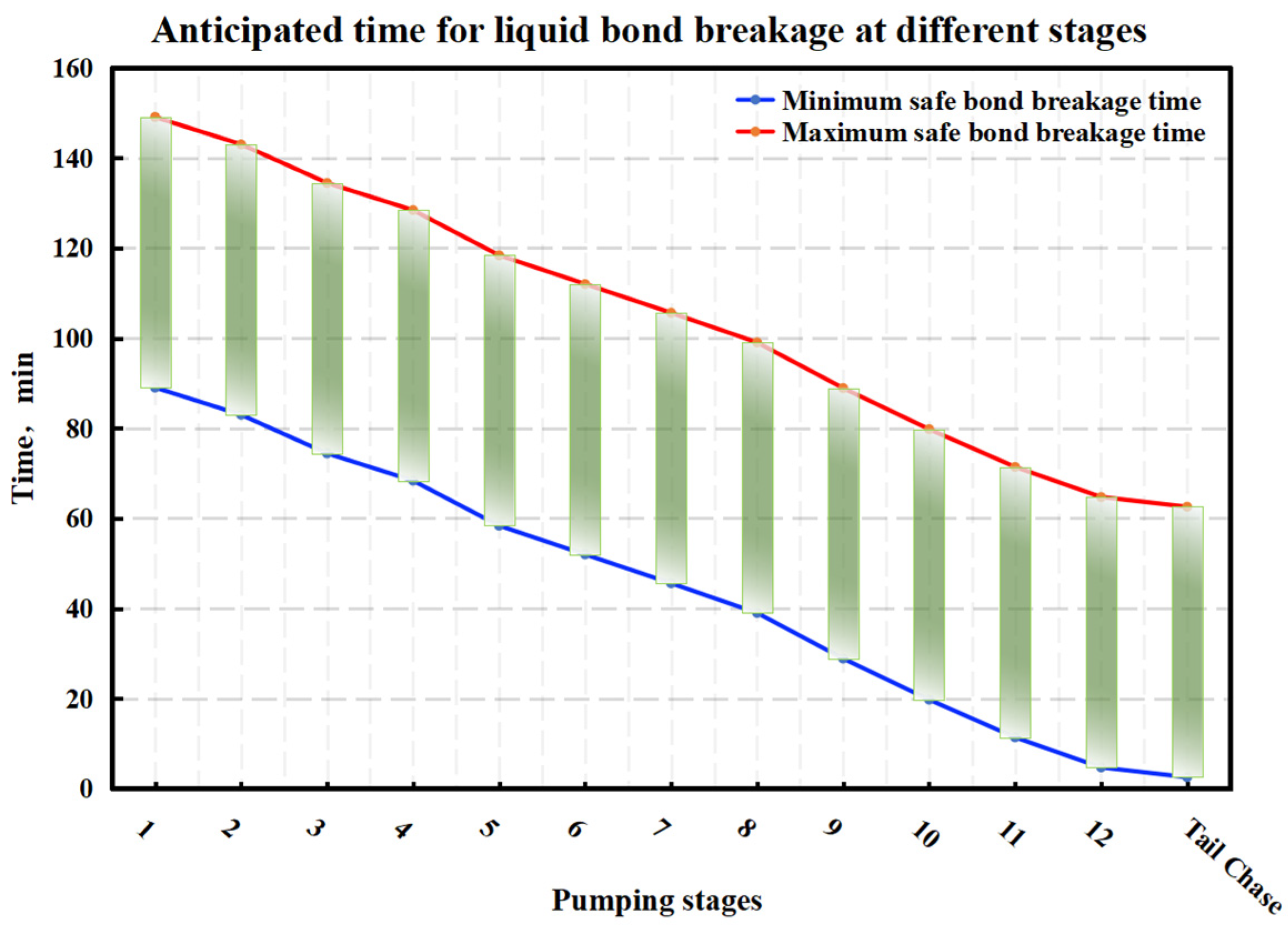
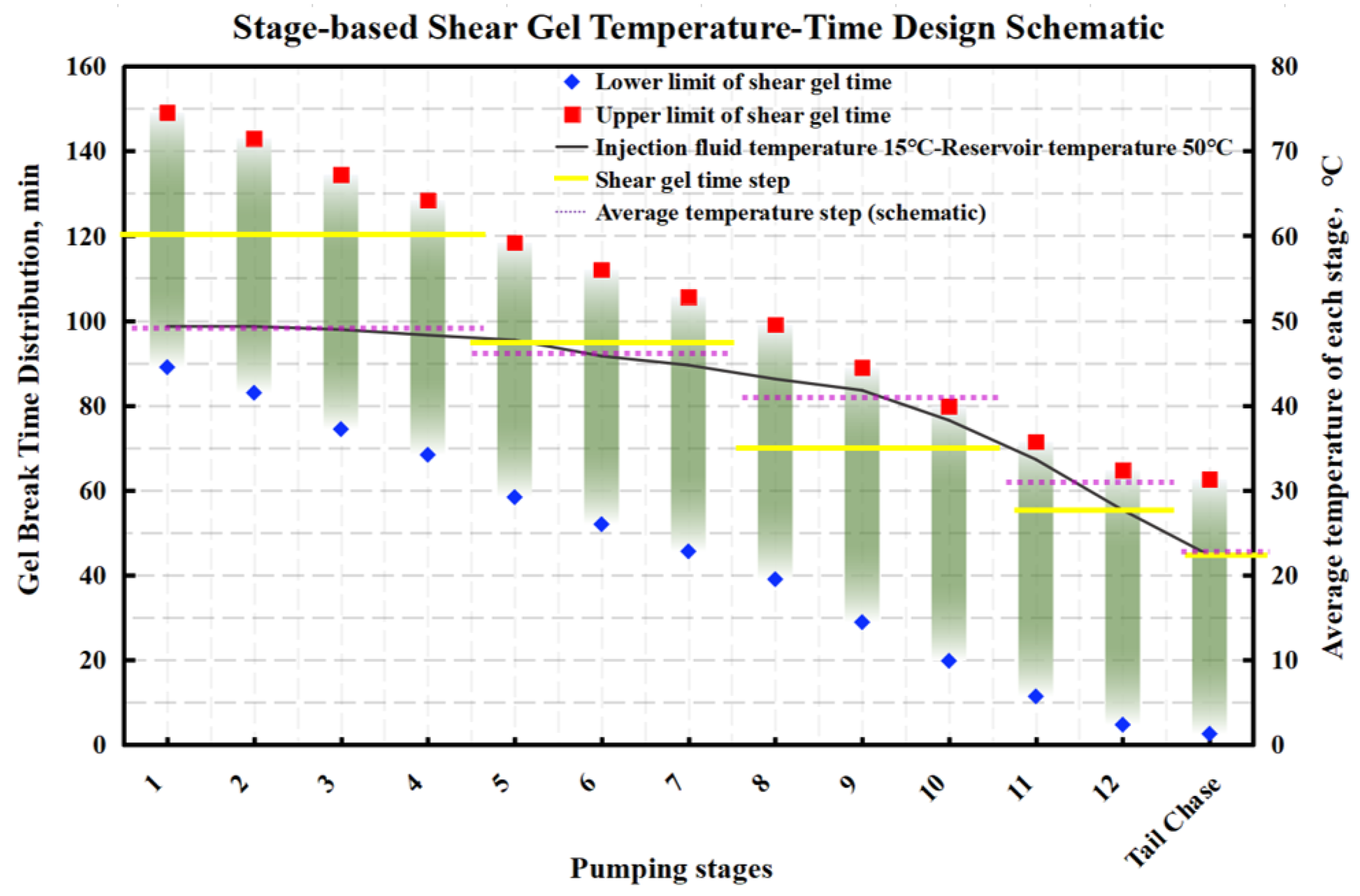
4.2. Optimization of the Stepped Gel Breaking Procedure
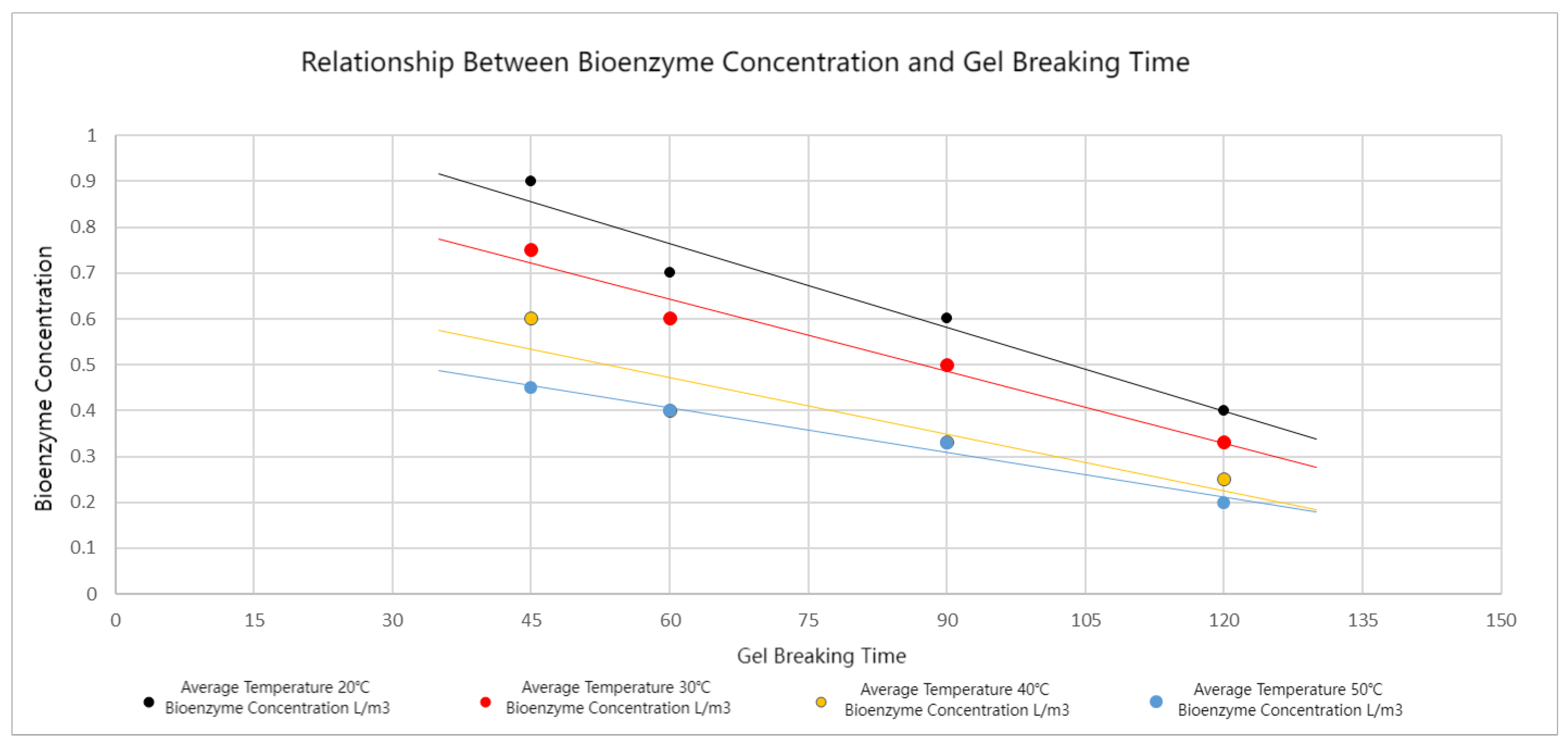
5. Optimized Application of Bio-Enzyme-Enhanced Gel-Breaking Technology
6. Suggestions for Optimizing Pump Downtime for Winter Construction
7. Conclusions
- (1)
- Bio-enzyme-enhanced gel-breaking technology for low-temperature reservoirs (35–60 °C): Preliminary studies indicate that bio-enzyme-enhanced gel-breaking technology significantly improves fracture conductivity and production in low-temperature reservoirs. However, during the initial design, the impact of temperature variations on the fracturing fluid’s heat exchange with the reservoir was not fully considered. The temperature at the end of each stage before flowback was not precisely determined, leaving room for optimization of the bio-enzyme concentrations to better align with the dynamic temperature changes, thus improving overall fracturing performance.
- (2)
- Numerical simulation of fluid temperature variation: Using Meyer Fracturing Software, numerical simulations revealed that the fluid’s average temperature in the later pumping stages deviates from both the reservoir and endpoint fluid temperatures. This provides a more accurate picture of temperature variation, allowing for precise optimization of the biomass breaker concentration at each stage. Tailoring the enzyme concentration to the actual temperature behavior enhances the gel-breaking procedure, improving its field applicability and efficiency.
- (3)
- Stepwise optimization of fluid stages: To optimize bio-enzyme gel-breaking, the fracturing process is divided into steps based on the overlap of the expected breakage times and similar average temperatures between adjacent fluid stages. Experimental studies led to the creation of a concentration template for bio-enzyme breakers. This template allows for efficient gel-breaking across stages, improving fracture inflow capacity and minimizing reservoir damage.
- (4)
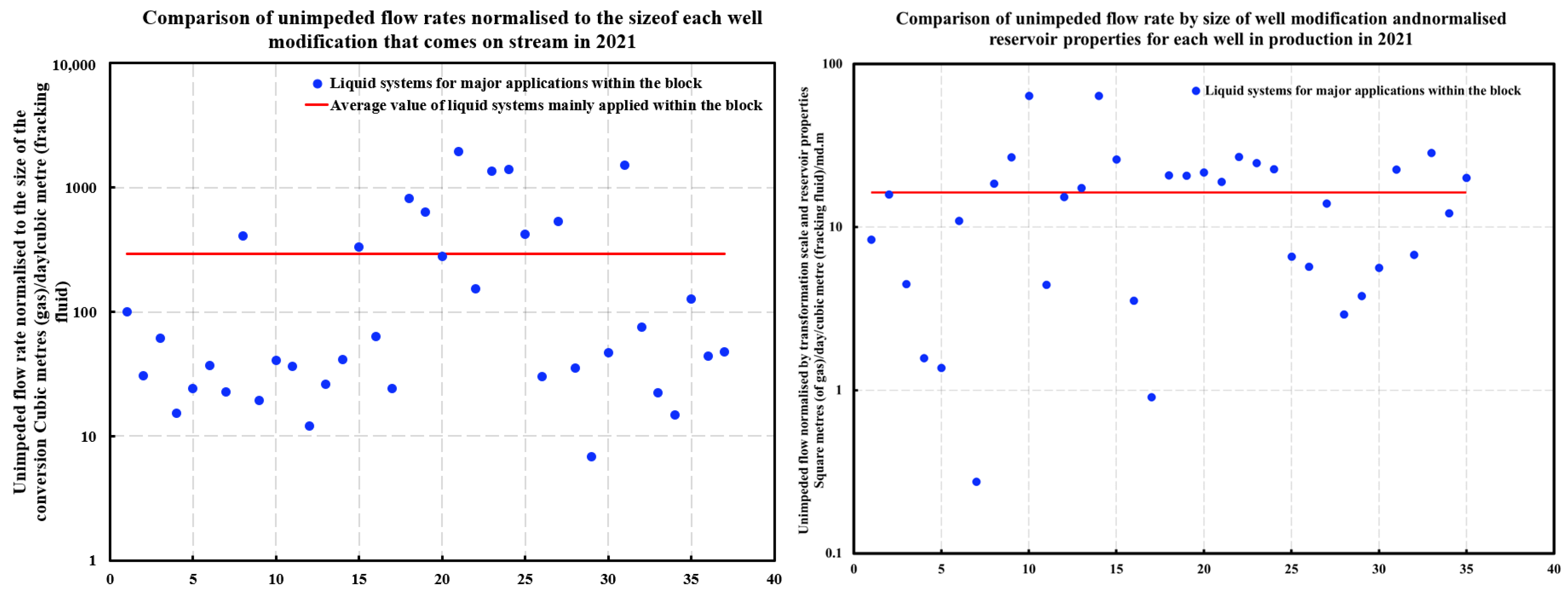
- Application and results of optimized technology: In 2021, the optimized bio-enzyme gel-breaking technology was applied in 40 wells, significantly improving the gel breaking efficiency and fracture cleaning. These wells showed higher production rates compared to earlier wells using non-optimized technology, achieving better results without a substantial increase in construction costs. The optimization proved both cost-effective and beneficial in enhancing reservoir productivity.
- (5)
- Impact of extreme weather and low-temperature reservoirs: Increasing fracturing in low-temperature reservoirs and extreme weather conditions raises the likelihood of insufficient fluid temperature recovery within one hour of shut-in. Extending the shut-in time allows better fluid temperature recovery, enhancing bio-enzyme reactivity, improving guar gum breakdown, and reducing reservoir damage, thereby ensuring fracture conductivity and optimizing long-term production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Jia, A.; Tan, J.; He, D. Tight sand gas development technologies and practices in China. Pet. Explor. Dev. 2012, 39, 572–579. [Google Scholar] [CrossRef]
- Zou, C.; Yang, Z.; Zhu, R.; Zhang, G.; Hou, L.; Wu, S.; Tao, S.; Yuan, X.; Dong, D.; Wang, Y.; et al. Progress in China’s unconventional oil & gas exploration and development and theoretical technologies. Acta Geol. Sin. 2015, 89, 979–1007. [Google Scholar]
- Zou, C.; Zhang, G.; Tao, S.; Hu, S.; Li, X.; Li, J.; Dong, D.; Zhu, R.; Yuan, X.; Hou, L.; et al. Geological features, major discoveries and unconventional petroleum geology in the global petroleum exploration. Pet. Explor. Dev. 2010, 37, 129–145. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.; Xu, C.; Bian, C.; Wang, Z.; Gao, X. Reservoir-forming mechanism and enrichment conditions of the extensive Xujiahe Formation gas reservoirs, central Sichuan Basin. Pet. Explor. Dev. 2010, 37, 146–157. [Google Scholar] [CrossRef]
- Tang, H.; Yu, Y.; Sun, Q. Progress, Challenges, and Strategies for China’s Natural Gas Industry Under Carbon-Neutrality Goals. Processes 2024, 12, 1683. [Google Scholar] [CrossRef]
- Zhao, W.; Bian, C.; Xu, C.; Wang, H.; Wang, T.; Shi, Z. Assessment on gas accumulation potential and favorable plays within the Xu-1, 3 and 5 Members of Xujiahe Formation in Sichuan Basin. Pet. Explor. Dev. 2011, 38, 385–393. [Google Scholar] [CrossRef]
- Guo, Q.; Li, J.; Chen, N.; Hu, J.; Xie, H.; Gao, X. Modeling of the tight sandstone gas accumulation for the Xujiahe Formation, Hechuan-Tongnan Area, Sichuan Basin. Pet. Explor. Dev. 2011, 38, 409–417. [Google Scholar] [CrossRef]
- Li, W.; Qin, S.; Hu, G.; Gong, Y. Accumulation of water-soluble gas by degasification: One of important mechanisms of large gas accumulations in the Xujiahe Formation, Sichuan Basin. Pet. Explor. Dev. 2011, 38, 662–670. [Google Scholar] [CrossRef]
- Wu, L. Low temperature gel breaking mechanism and development application of water-based fracturing fluid. Prog. Fine Petrochem. Ind. 2018, 19, 13–15. [Google Scholar]
- Li, J.; Wang, W.; Wang, J. Study and application of low-temperature fracturing fluids gel-breaking technique. Spec. Oil Gas Reserv. 2009, 16, 72–75. [Google Scholar]
- Wei, D.; Chen, H.; Zhu, D. Study and application of super low-temperature fracturing fluids in Yanchuan area. Pet. Drill. Tech. 2000, 28, 41–43. [Google Scholar]
- Wang, W.; Zhang, S.; Wen, H.; Zhang, H.; Sun, G. Study and application of super low-temperature fracturing fluids in shallow horizontal well. Oilfield Chem. 2012, 29, 155–158. [Google Scholar]
- Chen, Z.; Wang, Y.; Yang, Q.; Du, C.; Duan, Z. Research on Fracturing Optimization Technology for Low Permeability and Low Temperature Water Sensitive Shallow Gas Reservoirs. Nat. Gas Ind. 2006, 26, 99–101. [Google Scholar]
- Ren, Z.; Sun, H.; Qin, L. Research on ultra-low temperature gel breaking system of hydroxypropyl guar gum fracturing fluid. Oilfield Chem. 1995, 12, 234–236. [Google Scholar]
- Wang, M.; Zhang, J.; Yang, Y.; Zhou, J. The effect of different additives on the low-temperature gel breaking performance of hydroxypropyl guanidine gum fracturing fluid. J. Xi’an Pet. Univ. (Nat. Sci. Ed.) 2006, 21, 65–68. [Google Scholar]
- Yi, X.; Lu, Y.; Ma, H. Adaptability evaluation of fracturing fluid system in low-permeability reservoirs of Tuha Oilfield. Drill. Prod. Technol. 2006, 29, 84–86. [Google Scholar]
- Lu, Y. New Trends in the Development of Fracturing Fluid Technology Abroad in the 1990s. Pet. Nat. Gas Chem. Ind. 1998, 27, 115–118. [Google Scholar]
- Deng, H.; Tao, P. Application of low-temperature frac fluids in the shallow formation of South E area. Spec. Oil Gas Reserv. 2016, 23, 142–145. [Google Scholar]
- Zheng, K.; Wang, Y.; Yuan, W. Low temperature breaking fracturing fluid system application in Xinjiang oilfield. Xinjiang Pet. Technol. 2008, 18, 31–32. [Google Scholar]
- He, X.; Li, J.; Li, N.; Zhe, H. Ammonium persulfate and enzyme technology comparative study gel breaking fracturing. Petrochem. Ind. Appl. 2012, 31, 81–83. [Google Scholar]
- Li, J. Research and Application of New Biological Enzymes. Pet. Mach. Manuf. 2008, 37, 90–92. [Google Scholar]
- Guan, B.; Liu, J. Biocatalytic Gel Breaking Technology for Fracturing in Changqing Oil and Gas Field and Its Application. Oilfield Chem. 2008, 25, 89–91. [Google Scholar]
- Wang, L. Research and application of SUN_Y100 biological enzyme fracturing gel breaking technology. J. Shengli Oilfield Staff. Univ. 2009, 23, 33–39. [Google Scholar]
- Li, B.; Zhang, H.; Zhang, Z.; Zhao, J. Optimization and Application of Low Temperature Fracturing Fluid for Tight Sandstone Gas in Linxing Shenfu Block, Ordos Basin. Pet. Drill. Prod. Technol. 2019, 41, 7. [Google Scholar]
- Hu, K.; Yi, S.; Deng, Y. The current situation and prospect of biological enzyme gel breaker. Sci. Technol. Advis. Guide 2007, 21, 159. [Google Scholar]
- Wu, J. Low-temperature fracturing fluid gel breaking technology for stimulation technology transformation in shallow gas reservoirs. Drill. Prod. Technol. 2000, 23, 79–81. [Google Scholar]
- Han, J.; Chen, H.; Wang, Y. New type of low-temperature gel breaker activator for fracturing fluid. Pet. Drill. Prod. Technol. 2001, 23, 821. [Google Scholar]
- Qi, Y.; Yang, C. Research and Application of Linxing Dense Gas Pressure Fracturing Liquid Synchronous Gel Breaking Technology. Chem. Eng. Equip. 2019, 11, 181–182. [Google Scholar]
- Yin, Y.; Huang, S.; Ye, H. Research and application of low-temperature gel breaking technology using biological enzymes. Petrochem. Technol. 2018, 11, 118–119. [Google Scholar]
- Li, H.; Cheng, Y.; Liu, Z.; Zhao, M. Physical mutagenesis and enzymatic properties of bacteria producing biological enzyme breakers. Chem. Eng. 2018, 8, 14–17. [Google Scholar]
- Chang, Y. Indoor performance evaluation and field application of biological enzyme breaker. Inn. Mong. Petrochem. 2019, 45, 28–30. [Google Scholar]
- Guan, B.; Liang, L.; Yang, W.; Liu, Y. Research and application of biological enzyme breaker. In Proceedings of the 2018 National Natural Gas Academic Annual Conference (04 Engineering Technology), Fuzhou, China, 15–16 November 2018. [Google Scholar]
- Wang, Z. Indoor evaluation of biological enzyme breaker and its application analysis in oilfield fracturing. China Pet. Chem. Stand. Qual. 2020, 21, 3–5. [Google Scholar]
- Bao, M.; Yin, Y.; Zhang, Y. Research on the on-site application process of biological enzyme gel breaking. Neijiang Technol. 2019, 5, 19. [Google Scholar]
- Zhao, X.; Cao, G.; Zhang, X.; Zhang, L.; Lei, T.; Han, F. Study on the Control Method of Gel Breaking Speed of Biological Enzyme Fracturing Fluid. Contemp. Chem. Ind. 2018, 47, 454–457. [Google Scholar]
- Cao, G.; Feng, Y.; Lin, J.; Chen, Z.; Ding, M.; Jiang, Y.; Wang, W. Research progress on bio-enzyme preparations for polymer plugging in oil fields. J. Nanjing Univ. Technol. (Nat. Sci. Ed.) 2024, 46, 131–140. [Google Scholar]
- SY/T 5336-2006; Core Analysis Method. Petroleum Industry Press: Beijing, China, 2006.
- Gong, Y.; Lei, Y.; Chen, X.; Song, H.; Liu, R. A novel silica aerogel microspheres loaded with ammonium persulfate gel breaker for mid-deep reservoirs. J. Sol-Gel Sci. Technol. 2018, 88, 105–113. [Google Scholar] [CrossRef]




| Core Sample | Length/mm | Diameter/mm | Permeability/10−3 μm2 |
|---|---|---|---|
| A | 60.22 | 25.12 | 72.53 |
| B | 63.96 | 25.24 | 0.0058 |
| C | 43.36 | 25.13 | 0.2807 |
| D | 61.67 | 25.13 | 0.5579 |
| E | 61.33 | 25.16 | 0.2037 |
| F | 44.91 | 25.15 | 0.2154 |
| G | 57.60 | 25.10 | 1.1820 |
| H | 58.58 | 25.09 | 0.1955 |
| Core Sample | Mineral Content/per Cent | ||||||
|---|---|---|---|---|---|---|---|
| Sapphire | Potassium Feldspar | Plagioclase (Rock-Forming Mineral, Type of Feldspar) | Calcite (CaCO3 as Rock-Forming Mineral) | Limonite | Ferric Oxide Fe2O3 | Total Clay Minerals | |
| A | 58.2 | 0.6 | 13.4 | 3.0 | - | 1.1 | 23.7 |
| B | 59.1 | 0.7 | 5.5 | 1.0 | - | - | 33.7 |
| C | 65.4 | - | 22.7 | - | - | - | 11.9 |
| D | 76.5 | - | 2.5 | 2.1 | - | - | 18.9 |
| E | 56.1 | 4.7 | 10.1 | 3.4 | - | 1.3 | 24.4 |
| F | 69.9 | - | - | - | 1.0 | - | 29.1 |
| G | 68.0 | 0.5 | 17.3 | 0.5 | - | - | 13.7 |
| H | 37.2 | 5.1 | 10.7 | 0.8 | - | 1.7 | 44.5 |
| Core Sample | Relative Clay Mineral Content | Mixed Layer Ratio/% | ||||
|---|---|---|---|---|---|---|
| I | I/S | Kao | C | I/S | C/S | |
| A | 6 | 41 | 18 | 11 | 27 | 56 |
| B | 3 | 36 | 40 | 17 | 26 | 56 |
| C | 5 | 35 | 32 | 18 | 27 | 38 |
| D | 4 | 60 | 22 | 10 | 25 | 10 |
| E | 8 | 72 | 8 | 4 | 13 | 19 |
| F | 31 | 67 | 2 | - | 5 | - |
| G | 3 | 47 | 21 | 21 | 7 | 11 |
| H | 3 | 76 | 11 | 7 | 25 | 21 |
| Core Sample | Stratum | Reservoir Thicknesses/m | Average Formation Temperature/°C | Average Formation Pressure/MPa | Average Pressure Coefficient |
|---|---|---|---|---|---|
| A | Tai 2 | 11.3 | 56.9 | 16.1 | 0.88 |
| B | He 8 | 9.8 | 48.5 | 15.53 | 0.95 |
| C | He 7 | 12.0 | 48.7 | 15.44 | 0.96 |
| D | He 6 | 11.4 | 48.4 | 15.82 | 0.97 |
| E | He 4 | 8.0 | 47.5 | 14.3 | 0.96 |
| F | He 2 | 7.8 | 45.5 | 12.8 | 0.97 |
| G | He 1 | 6.9 | 43.2 | 13.94 | 0.94 |
| H | Qian 5 | 2.6 | 42.1 | 12.22 | 0.98 |
| Position | Tai 2 | Tai 1 | He 8 | He 7 | He 6 | He 4 | He 3 | He 2 | He 1 | Qian 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Reservoir temperature | 58.4 | 53.1 | 50.2 | 48.4 | 47.2 | 47.6 | 43.2 | 41.7 | 42.3 | 36.4 |
| 59.6 | 52.7 | 51.4 | 46.6 | 46.4 | 48.1 | 47.8 | 44.6 | 47.4 | 39.2 | |
| 56.4 | 52.6 | 47.7 | 48.9 | 44.6 | 46.4 | 44.7 | 43.3 | |||
| 55.5 | 53.5 | 49.1 | 51.4 | 48.2 | 45 | 47.2 | 45.5 | |||
| 62.1 | 58.5 | 52.1 | 48.1 | 45.4 | 45.1 | 43.6 | ||||
| 57.4 | 57.4 | 50.3 | 50.2 | 46.7 |
| Broken Gel Time/min | Formulation A/(mPa·s) | Formulation B/(mPa·s) | Formulation C/(mPa·s) | Formulation D/(mPa·s) |
|---|---|---|---|---|
| 30 | 58 | 31 | 49 | 29 |
| 45 | 41 | 25 | 35 | 12 |
| 60 | 33 | 19 | 26 | 18 |
| 75 | 26 | 15 | 21 | 6 |
| 90 | 21 | 14 | 18 | 6 |
| 105 | 18 | 13 | 15 | 4 |
| 120 | 16 | 13 | 11 | 3 |
| 150 | 15 | 12 | 9 | 2 |
| 180 | 15 | 12 | 7 | 2 |
| Formulas | Experimental Temperature/°C | Viscosity of Broken Gel (mPa·s) | |||
|---|---|---|---|---|---|
| 45 min | 60 min | 90 min | 120 min | ||
| A | 20 | 4 | 3 | 2 | 1 |
| B | 20 | 7 | 4 | 3 | 1 |
| C | 20 | 10 | 7 | 4 | 3 |
| D | 20 | 13 | 8 | 6 | 4 |
| E | 30 | 4 | 2 | 1 | 1 |
| F | 30 | 7 | 4 | 3 | 1 |
| G | 30 | 9 | 6 | 4 | 2 |
| H | 30 | 15 | 11 | 6 | 4 |
| I | 40 | 4 | 3 | 2 | 1 |
| J | 40 | 7 | 4 | 2 | 2 |
| K | 40 | 11 | 7 | 4 | 3 |
| L | 40 | 18 | 11 | 8 | 4 |
| M | 50 | 4 | 3 | 2 | 1 |
| N | 50 | 7 | 4 | 3 | 2 |
| O | 50 | 10 | 7 | 4 | 2 |
| P | 50 | 18 | 12 | 8 | 4 |
| Testimonials | Reservoir Temperature | ||||||
|---|---|---|---|---|---|---|---|
| Well Closure Time | 35 °C | 40 °C | 45 °C | 50 °C | 55 °C | 60 °C | |
| Temperature of injected liquid | 0 °C | 90 min | 85 min | 80 min | 75 min | 70 min | 65 min |
| 5 °C | 85 min | 80 min | 75 min | 70 min | 65 min | 60 min | |
| 10 °C | 80 min | 70 min | 60 min | 60 min | 60 min | 60 min | |
| 15 °C | 70 min | 60 min | 60 min | 60 min | 60 min | 60 min | |
| 20 °C | 60 min | 60 min | 60 min | 60 min | 60 min | 60 min | |
| 25 °C | 60 min | 60 min | 60 min | 60 min | 60 min | 60 min | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, G.; Yao, W.; Li, Y.; Jing, M.; Lan, T.; Xie, Z.; Du, A. Optimization and Application of Bio-Enzyme-Enhanced Gel-Breaking Technology in Fracturing Fluids for Tight Sandstone Gas in the Linxing Block, Ordos Basin. Processes 2025, 13, 440. https://doi.org/10.3390/pr13020440
Hu J, Wang G, Yao W, Li Y, Jing M, Lan T, Xie Z, Du A. Optimization and Application of Bio-Enzyme-Enhanced Gel-Breaking Technology in Fracturing Fluids for Tight Sandstone Gas in the Linxing Block, Ordos Basin. Processes. 2025; 13(2):440. https://doi.org/10.3390/pr13020440
Chicago/Turabian StyleHu, Jiachen, Gaosheng Wang, Weida Yao, Yu Li, Meiyang Jing, Tian Lan, Zhongxu Xie, and Anxun Du. 2025. "Optimization and Application of Bio-Enzyme-Enhanced Gel-Breaking Technology in Fracturing Fluids for Tight Sandstone Gas in the Linxing Block, Ordos Basin" Processes 13, no. 2: 440. https://doi.org/10.3390/pr13020440
APA StyleHu, J., Wang, G., Yao, W., Li, Y., Jing, M., Lan, T., Xie, Z., & Du, A. (2025). Optimization and Application of Bio-Enzyme-Enhanced Gel-Breaking Technology in Fracturing Fluids for Tight Sandstone Gas in the Linxing Block, Ordos Basin. Processes, 13(2), 440. https://doi.org/10.3390/pr13020440





