Recent Advances in the Fabrication of Intelligent Packaging for Food Preservation: A Review
Abstract
1. Introduction
2. Brief Background and Types of Food Packaging Systems
3. Intelligent Packaging
3.1. Time–Temperature Indicators (TTIs)
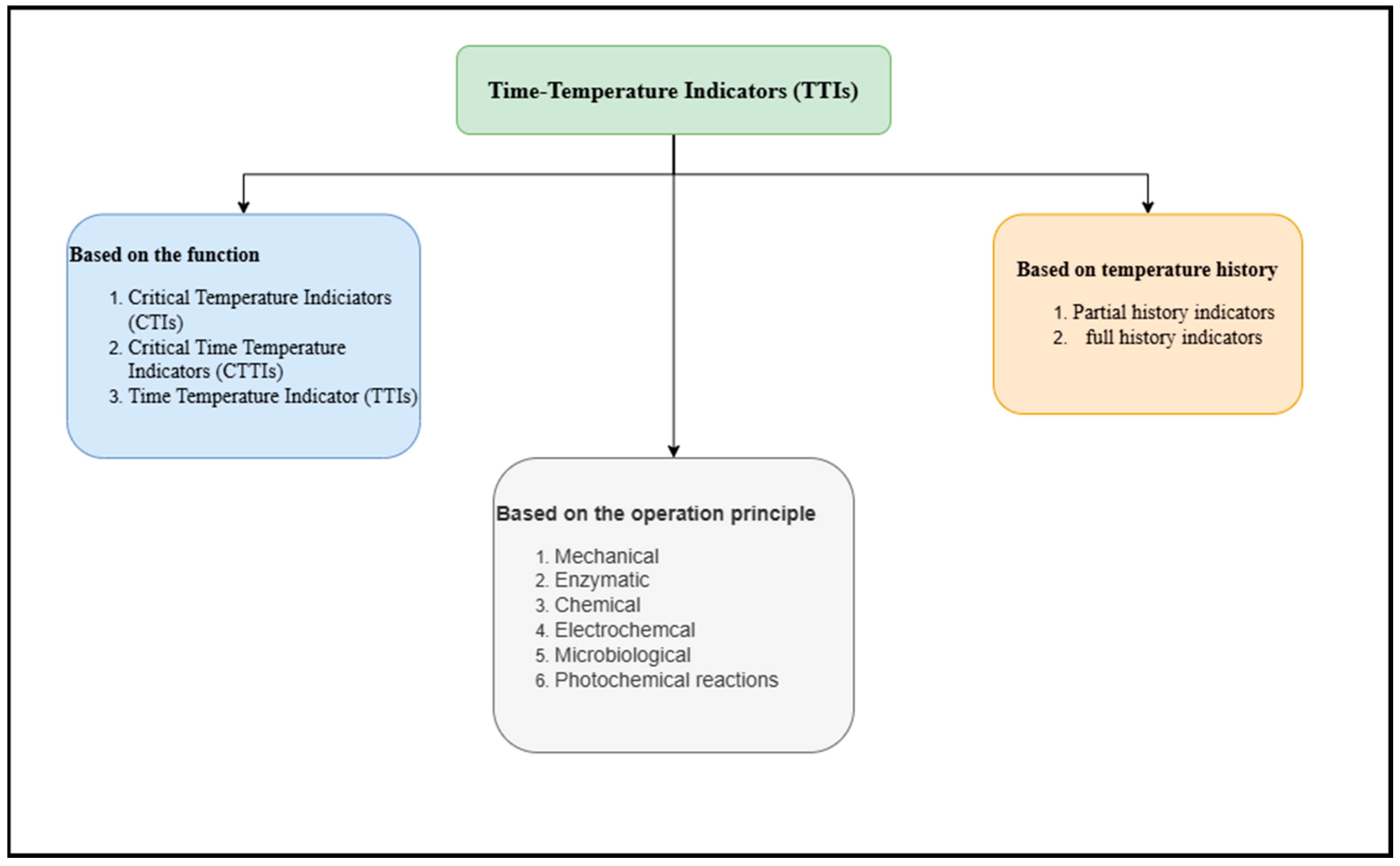
| Company Name | Application | Principle | Website |
|---|---|---|---|
| 3M™ MonitorMark® | Bakery, drinks, and meat | Monitors temperature sensitivity rather than the quality of the item via an adhesive-based pad that is simple to affix to secondary containers. The pad contains esters of blue fatty acid within a carrier material. Once the carrier experiences a phase shift because of exposure to temperatures exceeding the reaction temperature, the dye stays within the pad; consequently, as the dye disperses across a wick, the reaction is calculated based on how far the dye traveled down the track. | (www.3m.com) |
| OnVu™ | Milk products, meat, and fish. | It is made of photochromic ink that operates on benzylpyridines that are turned dark blue under ultraviolet (UV) light. Then, irrespective of the outside temperature increases, benzylpyridines gradually become lighter as time passes. | (www.packworld.com) |
| WarmMark® | Transportation, storage, and processing | Clear evidence of temperature fluctuations that resulted in a pass or fail. It consists of a pad of blotting paper that was colored red. | (www.deltatrak.com) |
| OliTec™ | Fresh goods | It has multiple layers and can track the deterioration patterns of food items under storage circumstances. | (www.oli-tec.com) |
| Cold Chain iToken™ | Supply chain | Barcode scanners can detect the positive “ON” signal from an easy pull-tab operation. | (www.deltatrak.com) |
| Insignia Deli Intelligent Labels™ | Chilled goods | The rate of hue shift increases as the pre-calibrated temperature setting shifts or fluctuates. | (www.insigniatechnologies.com) |
| TOPCRYO | Cold chain | Device can track whether the cold chain is being followed, and the microbial label turns red from green. | (www.cryolog.com) |
| TempDot® | Meat and seafood | The indicator window, which validates activation, allows the labels to be sent and kept at any temperature. | (www.deltatrak.com) |
| Smart dot | Frozen goods and bakery items | When subjected to heat, the indicator turns from green to red. | (www.evigence.com) |
| FreshCode® | Chicken products | The indicator’s white core has smart ink embedded in it. This records the release of flammable gases made when chicken is spoiled in packing with the changed environment. | (www.freshcodelabel.com) |
3.2. Freshness Indicators
| Commercial Identifier | Application | Principle | Description of How They Function | Website |
|---|---|---|---|---|
| Raflatac | Poultry | It works by breaking down cysteine within a hydrogen sulfide reaction using a nanolayer comprising silver or silver nanoparticles. | During the packaging, the indication appears opaque light brown; however, as silver sulfide forms, the coating’s color changes to translucent. | (www.upmraflatac.com) |
| Food fresh™ | Meat | It consists of an irreversible duration tracking self-adhesive label indicator that may be programmed to expire after a specified “consume within” period of time, which can be anything from just a couple of days to several months. | A permeable barrier is present within the indicator, whereby a colored liquid moves at a predetermined speed. | (www.vanprob.com) |
| RipeSense® | Fruit | It can identify gases or fragrance compounds that are part of ripening, such as ethylene. | The label starts off red, changes to orange, and then to yellow when the fruit ripens. | (www.ripesense.co.nz) |
3.3. Biosensors and Gas Sensors
| Commercial Name | Application | Purpose | Indicating Point | References |
|---|---|---|---|---|
| Ageless Eye® | Meat | Monitoring oxygen gas. | Transition from pink to a blue hue or purple. | [21,42,56] |
| Tell-Tab™ | All products | Identifying oxygen gas inside the packaging. | Transition from pink to blue or purple. | [40,51] |
| Shelf Life Guard | Meat | Monitoring of air in the package surroundings. | Transition from colorless to blue. | [18] |
4. Current Fabrication Approaches for Intelligent Packaging
4.1. Nanotechnology
| Type of Material | Company and Brand | Form | Utilization Product | Function |
|---|---|---|---|---|
| Cerium oxide | Mitsubishi Gas Chiyoda-ku, Japan Chemical Inc., OMAC® Imperm®. | Film | Produce responses and hot-fill fish and meat items | O2 scavenger |
| Iron oxidation | Clariant Ltd., Swaziland, Mutten, OxyGuard®. | Film and sachets | Snacks that are fried | O2 scavenger |
| Time–temperature indicator (TTIs) based on Ph-indicating dye, lipase, and enzyme | Mitsubishi Gas Chemical Inc., Japan, Chiyoda-ku, Ageless®. | Stickers | Eggplant, strawberries | CO2 scavenger |
| Nanoencapsulation (titanium dioxide) | Carnation Breakfast Essential, Switzerland, Vevey, Carnation Instant Food. | Powder | Milk-based goods in powder form | Anticaking |
| Composite of nylon 6-nanoclay | Honeywell International Inc., USA, Phoenix, AZ, Aegis HFX Resin and OXCE Resin. | Nylon resin barriers | PET bottles containing beer and flavored alcoholic beverages | O2 scavenger |
| Nanosilver | Addmaster Limited, USA, Monrovia, CA, Biomaster. | Spray and bag | Vegetables and fruits | Antimicrobial properties |
| Adjusting color in response to aromatic chemicals (sensor) | Ripesense Limited, New Zealand, Tauranga, RipeSense™. | Stickers | Fruits | Freshness indicators |
4.1.1. Nanocomposite Compounds for Improved Barrier Properties
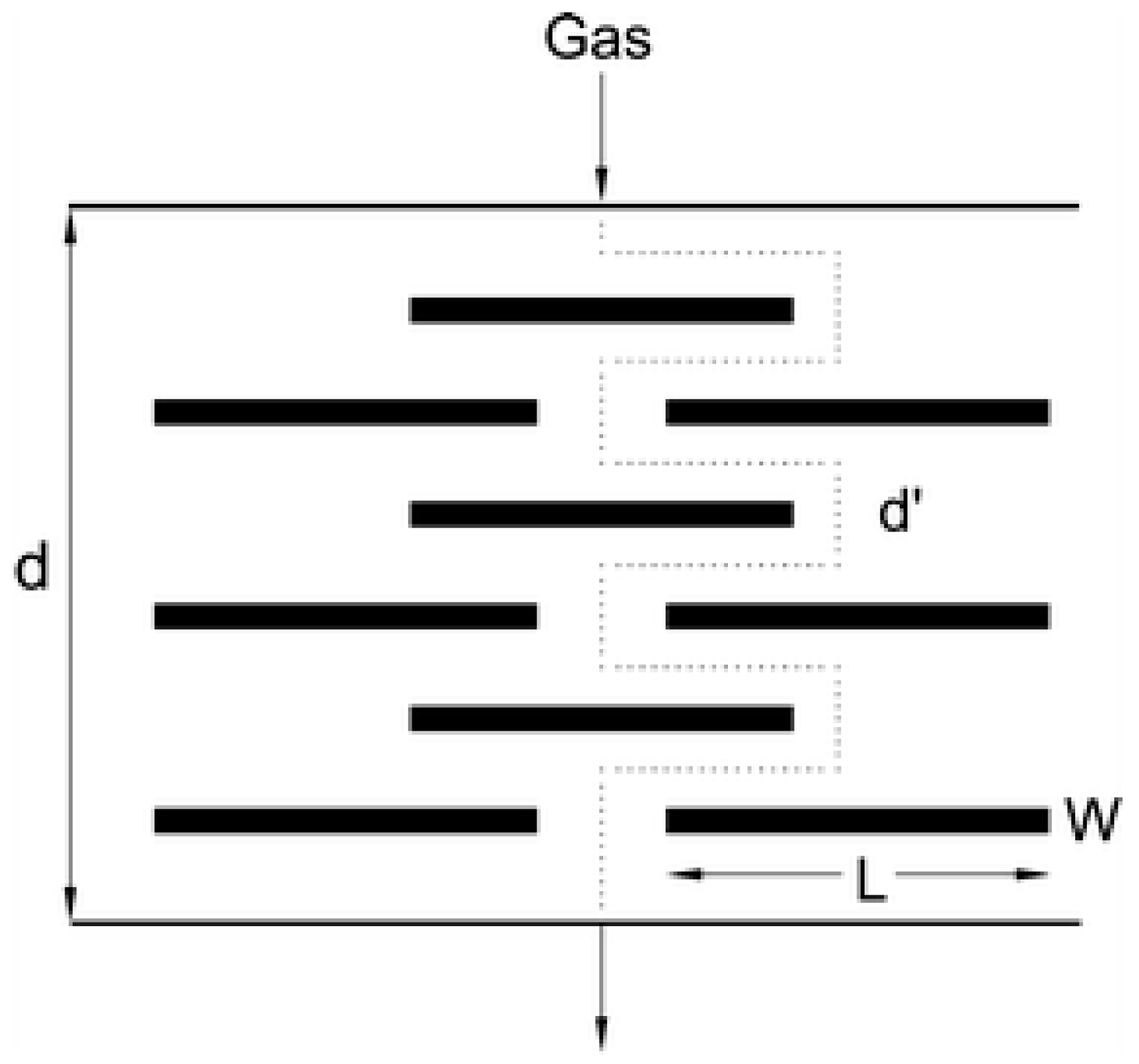
4.1.2. Nanosensors
4.2. Three-Dimensional Printing
4.3. Microfluidics

| Target Analyte | Device Type | Key Discovery | Limitations | Regulatory Challenges | Ref. |
|---|---|---|---|---|---|
| Glutamate | Microfluidic paper-based sensor | Throughout the interval of 5 × 10−6 mol/L to 10−2 mol/L, a straight correlation of 0.99 was found across the hue intensities and the logarithmic of glutamate levels. The outcomes from this gadget were in line with a readily available conventional assay kit. | - Hue intensity-based detection may struggle with complex or pigmented food samples. | - Requires validation against international glutamate standards; colorimetric methods need cross-verification. | [158] |
| Lead and mercury ions | Aptamer-based microfluidic sensor | According to the study results, mercury ions had a detection limit of 0.70 ppb, while lead ions of 0.53 ppb. The sensor demonstrated strong sensitivity to mercury and lead when additional ions like cobalt, magnesium, calcium, and copper ions were present in competing assays. | - Potential interference from other ions in complex samples. | - Heavy metal detection limits vary globally; must comply with international guidelines. | [159] |
| Adulterants of tadalafil and sildenafil | Hybrid microfluidic device made of polyvinyl chloride, nitrocellulose, PDMS, and elastomers | Low cut-off levels ranging from 2.0 to 9.0 ng/mL and low detection limits of 0.027–0.066 ng/mL are possible with the suggested approach. The gadget was extremely sensitive, precise, and highly accurate. The evaluation used mass spectrometry with liquid chromatography, and the outcomes were well concordant. | - Low detection limits may not suit large sample volumes; high production costs due to material diversity. | - Must meet pharmaceutical adulterant detection standards; requires reliability across food types. | [160] |
| Salmonella species and Listeria monocytogenes. | Microfluidic PDMS-PCR technology | It was noted that Listeria monocytogenes, along with Salmonella species, both passed the screening threshold of 103 and 104 CFU/mL. Both the selected and designated pathogens had acquisition efficiencies of more than 70%. | - Struggles with contamination below 103–104 CFU/mL; 70% acquisition efficiency may be insufficient. | - Needs to meet strict accuracy benchmarks; PCR methods require extensive validation for reproducibility. | [161] |
| Aflatoxin B1 | A paper-based microfluidic system that uses a colorimetric test based on aptamers | The instrument demonstrated an excellent detection span from 1 pM to 1 M alongside a maximum limit of up to 10 nM. This might be employed to quickly identify contaminants in food. | - Challenges with complex food matrices or extreme environmental conditions. | - Must comply with sensitivity and repeatability requirements under standardized aflatoxin detection protocols. | [162] |
4.4. Electromagnetic Technologies
4.4.1. Radio-Frequency Identification (RFID)
4.4.2. Microwave
5. Advances in Materials for Intelligent Packaging
5.1. Bio-Based Nanomaterials
5.2. Edible Films and Coatings
Plant Wastes as Viable Alternative Materials for Food Packaging
| Biologically Active Substances | Sources | Extraction Method | Ref. |
|---|---|---|---|
| Anthocyanin | Sweet potato/potato | PSP (50 g) was mixed in a 40% ethanol solution (500 mL) and stirred at (60 °C for 6 h). The resulting mixture was then filtered and concentrated at (50 °C). The extract was subjected to freeze drying under vacuum. | [235] |
| Red radish | Radish underwent cleaning, cutting, and vacuum drying at 65 °C. Ground and incorporated into an 80% ethanol solution, the mixture was stirred at 35 °C for 6 h. After filtration, the supernatant was concentrated at 45 °C in the dark. The extract was freeze-dried, nitrogen-sealed, and stored in dark bottles at 4 °C. | [236] | |
| Mulberry | Ground mulberry (100 g) was extracted using an 80% ethanol and 1% HCl solution at 4 °C for 24 h. Centrifuged at 10,000× g for 30 min, concentrated the supernatant at 50 °C, and vacuum-dried. | [237] | |
| Red cabbage | The red cabbage was crushed and blended with 85% ethanol. The pH was then adjusted to 2 using 1 M of HCl, and the mixture was kept in darkness for 24 h at 4 °C. Centrifuged at 4000 rpm, filtered and neutralized the solution to a pH of 7 using 2M of NaOH. Concentrated the mixture at 40 °C, and then freeze-dried it at 4 °C. | [238] | |
| Purple potato | Blended 25 g of PSP powder with a 500 mL solution of ethanol acidified. Utilized ultrasound assistance for extraction under the conditions of 270 watts, 50 degrees Celsius, and 30 min, filtered and evaporated (45 °C). The extract was subjected to freeze drying under vacuum conditions. | [237] | |
| Betalains | Cactus pears | Fruits were juiced and soaked in a 2 L, 60% ethanol solution at 4 °C for 12 h. Centrifuged at 10,000× g for 30 min, then concentrated. The extract underwent purification and vacuum drying. | [239] |
| Dragon fruit peel | Minced peels (300 g) underwent double extraction using 30% ethanol solution (800 mL each time) at 4 °C overnight. The extract was undergoing filtration and centrifugation at 8000× g for 15 min at 4 °C. The sample underwent purification, concentration, and vacuum drying. | [240] | |
| Amaranthus leaf | The leaves were isolated, cleansed, and dehydrated in a 45 °C hot air oven. The powder was mixed with distilled water and 0.1M of citric acid (1:10 ratio) and stirred at 150 rpm for 18 h. Centrifuged at 12,100× g for 30 min. The supernatant was filtered to yield the extract. | [241] | |
| Chlorophyll | Spinach | Spinach underwent a 15 min blanching process and was then high-speed crushed for 4 min. A solution in water was kept in a plasma freezer at −30 °C. | [242] |
| Green tea and pu-erh tea | Combined 2 g of tea powder with 200 mL of distilled water, subjected to controlled conditions (90 °C, 20 min), and then filtered. | [243] | |
| Chlorophyll and Carotenoids | Green tea and basil | Basil leaves were steeped in 100 mL of distilled water and heated at 100 °C for 40 min. The samples were cooled, filtered, and then preserved in opaque containers. | [244] |
| Tannins | Cashew nut testa | Testa underwent milling at 500 rpm for 1 h, followed by the addition of MilliQ water at a 1:10 ratio. The blend was mixed and placed in a water bath at 37 °C for one hour. Centrifuged at 10,000× g for 10 min at 4 °C. Filtered supernatant underwent freeze drying. | [245] |
| Pomegranate peel | Pomegranates (500 g) were cleaned, and their peel and flesh were separated. The skin and pulp were separately ground and extracted at 4 °C for 24 h using a 1% HCl ethanol solution. The samples underwent centrifugation at 8000× g for 20 min, followed by evaporation at 50 °C and vacuum drying. | [246] | |
| Curcumin | Turmeric residue | Turmeric residue was acquired using the Soxhlet method at 47 °C for 3 h. An ethanol/isopropanol blend served as the solvent. The leftover material underwent soaking, grinding, sifting, and drying.The liquid portion was centrifuged and then dried to yield turmeric flour. | [247] |
5.3. Smart Polymers
5.3.1. Temperature-Responsive Polymers for Temperature Indicators
5.3.2. pH-Sensitive Polymers for Spoilage Detection

6. Applications of Intelligent Packaging for the Maintenance of Food Quality
6.1. Fresh Produce
| Ethylene Scavengers | Application Form | Type of Fresh Produce | Advantages | Ref. |
|---|---|---|---|---|
| TiO2 | Chitosan film | Tomato | Slowed down the maturation of tomatoes stored at 25 °C and 50% relative humidity, resulting in alterations in their quality. | [298] |
| Silica gel | Not specified | Pointed gourd | Minimized spoilage and lowered the disease index by extending the preservation period for up to 8 days with reduced chlorophyll content loss when stored at temperatures between 29.4 and 33.2 °C and a relative humidity of 68–73%. | [299] |
| Zeolite (clenoptelolite) | Filter | Peach | Decreased firmness and weight loss were observed, along with a delayed rise in pH. The overall appearance remained in good condition, and a minor impact on both SSC (soluble solid content) and TA (total acidity) was noted when stored at a temperature of 0 °C for a duration of 36 days. | [300] |
| Potassium permanganate | Not specified | Guava | The guava’s shelf life, when kept at 4 °C with 85% relative humidity, was prolonged to 32 days. During this time, it maintained a significant level of phenol and ascorbic acid content without experiencing any negative effects from growth. | [301] |
| Vermiculite | Sachet | Sapodilla | A slower reduction in pulp firmness and vitamin C breakdown was observed when stored at 25 °C (with 54% relative humidity) for a duration of 5 days. | [302] |
| Zeolite | Filter | Iceberg, lettuce | Reduced the loss of firmness in texture and minimized alterations in color. Delayed the decrease in weight, pH levels, and the browning of tissues over a period of 21 days. | [303] |
| Vermiculite | Sachet | Baby banana | There was a delay in the yellowing of the peel, a slower increase in soluble solid content (SSC), a decrease in titratable acidity (TA), the reduced loss of firmness and weight, and a minimized increase in the SSC/TA ratio during storage at 18 °C for 16 days at a relative humidity of 70–80%. | [304] |
| Nano-ZnO | A polyvinylchloride film that has been treated with nano-sized zinc oxide | Fresh-cut apple | The decrease in fruit decay, a decrease in ethylene production, the preservation of °Brix and titratable acidity, and the suppression of enzyme activity. | [305] |
| Halloysite nanotube | Low-density polyethylene film | Tomato, strawberry, and banana | The extended durability of bananas and tomatoes enclosed in films and stored at a temperature of 4 °C. | [306] |
| Zeolite infused with KMnO4 | high-density polyethylene HDPE films | Kiwifruit | Increased firmness and elevated vitamin C levels, with no specified shelf life. | [307] |
| Silver nanoparticles, titanium dioxide nanoparticles, and kaolin nanoparticles. | polyethylene film | Strawberry | Enhancements in quality were observed, with physiological, sensory, and physicochemical attributes showing improvements. Specifically, nano-packaging led to reduced malondialdehyde and anthocyanin content, while normal packing maintained these parameters at their original levels. | [308] |
| Kaolin, nano-Ag and nano-TiO2 | polyethylene film | Chinese jujube | The use of nano-packaging results in several advantages, including improvements in the physical and sensory characteristics of the fruit, the prevention of fruit softening, reduced weight loss, the prevention of browning, and the control of climatic changes. Additionally, it effectively manages ethylene levels, maintaining a maximum ethylene content of 17.6 μL/kg h for the control group on the third day and 9.2 μL/kg h for the nano-packaging group on the sixth day of storage. | [305] |
| Alumino-silicate and zeolite-based materials | Low-density polyethylene films | Small pieces of broccoli | Improvement of overall quality and increase in shelf life up to 20 d at 4 °C. | [309] |
6.2. Seafood
| Application | Mode of Action | Type of Packaging Material | Properties Evaluated | Ref. |
|---|---|---|---|---|
| Fish | pH (TVB-N) | Carboxymethyl cellulose/starch | Light barrier, film thickness, and TS | [235] |
| Pork | pH (TVB-N) | Agar/potato starch | - | [310] |
| Shrimp | pH (TVB-N) | Starch/PVA | Thermal stability, film thickness, and light barrier | [311] |
| Fish | pH (TVB-N) | Chitosan/corn Starch | Light barrier and thermal stability | [268] |
| Pork | pH (TVB-N) | Chitosan/PVA | Mechanical properties (EAB and TS) | [312] |
| Poultry | CO2 | Ethyl cellulose | Maintained in a cold storage environment for several weeks | [86] |
| Fish | pH (TVB-N) | Starch/PVA | EAB and film thickness | [313] |
| Pork | pH (TVB-N) | Chitosan/starch/PVA | Antioxidant and TS | [314] |
| Shrimp | pH (TVB-N) | Chitosan | Film thickness | [315] |
| Chicken | pH (TVB-N) | Agarose solution | [316] | |
| Pork | pH (TVB-N) | k-carrageenan | Film thickness, light barrier, hydrophobic | [232] |
| Fish | Time Temperature | Cellulose-based | - | [317] |
| Shrimp | pH (TVB-N) | Cellulose | [318] | |
| Seafood | pH (TVB-N) | Chitosan/chitin | Antioxidant and UV barrier | [223] |
| Shrimp and Hairtail | pH (TVB-N) | Gelatin/oxidized | Antioxidant, water and oxygen barrier, UV barrier | [222] |
| Fish | pH (TVB-N) | Tara gum | Water and oxygen barrier | [319] |
| Shrimp | pH (TVB-N) | Fish gelatin | UV–vis barrier, mechanical properties (EAB and TS), water vapor barrier, antioxidant | [320] |
| Shrimp | pH (TVB-N) | Cellulose-based paper | - | [321] |
| Lard (Pork) | pH (TVB-N) | k-carrageenan | Antioxidant, light Barrier, oxygen Barrier, mechanical Properties (EAB and TS), thermal stability | [322] |
| Pork | pH (TVB-N) | Chitosan | Antioxidant, film thickness, light barrier, EAB | [323] |
| Pork | pH (TVB-N) | Starch | Thermal stability, antioxidant, water barrier, film thickness, UV–vis light, TS | [233] |
| Pork | pH (TVB-N) | Cassava starch | Antioxidant, water barrier, UV–vis light, film thickness, TS | [324] |
| Chicken | pH (TVB-N) | Sago powder | Film thickness, low water solubility | [87] |
| Fish/Chicken | pH (TVB-N) | Gelatin/PVA | Film thickness, antibacterial, oxygen, and water barrier, antioxidant, TS | [241] |
| Fish | pH (TVB–N) | Glucomannan/ PVA | - | [234] |
| Shrimp | pH (TVB-N) | Starch/PVA | Water barrier, film thickness, antimicrobial, mechanical properties (EAB and TS), antioxidant | [240] |
| Shrimp | pH (TVB-N) | Chitosan/PVA | Water barrier, film thickness, mechanical properties (EAB and TS), antimicrobial, UV–vis light barrier, antioxidant | [239] |
| Pork and shrimp | pH (TVB-N) | k-carrageenan | Light barrier, TS, moisture and oxygen barrier | [325] |
| Shrimp/meat | pH (TVB-N) | Chitin | Light barrier, TS, antioxidant, water barrier | [223] |
| Shrimp | pH (TVB-N) | Pectin powder/ Glycerol | Antioxidant, water barrier, film thickness, antimicrobial, thermal stability | [326] |
| Shrimp | pH (TVB-N) | Tara gum/PVA | Thermal stability, EAB, water barrier | [319] |
6.3. Meat
7. Challenges of Intelligent Packaging in Food Packaging
7.1. Identification and Tracking Network
7.2. Smartphone-Compatible Sensors
8. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prosekov, A.Y.; Ivanova, S.A. Critical Review Food Security: The Challenge of the Present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- UN Hunger Numbers Stubbornly High for Three Consecutive Years as Global Crises Deepen: UN Report. Available online: https://www.wfp.org/news/hunger-numbers-stubbornly-high-three-consecutive-years-global-crises-deepen-un-report (accessed on 28 August 2024).
- G20 Task Force for Global Alliance against Hunger and Poverty. Social Protection and the Way Forward to Eliminate Poverty and Ensure Human Dignity and Adequate Nutritious Food for All; G20 Task Force for Global Alliance against Hunger and Poverty: New York, NY, USA, 2024. [Google Scholar]
- Charles Boliko, M. FAO and the Situation of Food Security and Nutrition in the World. J. Nutr. Sci. Vitaminol. 2019, 65, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Riley, M.; Stockmann, R.; Bennett, L.; Kahl, A.; Lockett, T.; Osmond, M.; Sanguansri, P.; Stonehouse, W.; Zajac, I.; et al. Role of Food Processing in Food and Nutrition Security. Trends Food Sci. Technol. 2016, 56, 115–125. [Google Scholar] [CrossRef]
- Ma, J.; Sun, D.W.; Qu, J.H.; Pu, H. Prediction of Textural Changes in Grass Carp Fillets as Affected by Vacuum Freeze Drying Using Hyperspectral Imaging Based on Integrated Group Wavelengths. LWT-Food Sci. Technol. 2017, 82, 377–385. [Google Scholar] [CrossRef]
- Pu, Y.Y.; Sun, D.W. Combined Hot-Air and Microwave-Vacuum Drying for Improving Drying Uniformity of Mango Slices Based on Hyperspectral Imaging Visualisation of Moisture Content Distribution. Biosyst. Eng. 2017, 156, 108–119. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, D.W.; Cheng, W. Development of Simplified Models for Nondestructive Hyperspectral Imaging Monitoring of TVB-N Contents in Cured Meat during Drying Process. J. Food Eng. 2017, 192, 53–60. [Google Scholar] [CrossRef]
- Demirdöven, A.; Baysal, T. The Use of Ultrasound and Combined Technologies in Food Preservation. Food Rev. Int. 2008, 25, 1–11. [Google Scholar] [CrossRef]
- Khan, H.; Flint, S.; Yu, P.L. Enterocins in Food Preservation. Int. J. Food Microbiol. 2010, 141, 1–10. [Google Scholar] [CrossRef]
- Kiani, H.; Sun, D.W.; Delgado, A.; Zhang, Z. Investigation of the Effect of Power Ultrasound on the Nucleation of Water during Freezing of Agar Gel Samples in Tubing Vials. Ultrason. Sonochem. 2012, 19, 576–581. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Pu, H. Combining the Genetic Algorithm and Successive Projection Algorithm for the Selection of Feature Wavelengths to Evaluate Exudative Characteristics in Frozen–Thawed Fish Muscle. Food Chem. 2016, 197, 855–863. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, D.W.; Zhu, Z.; Zhang, Z. Emerging Techniques for Assisting and Accelerating Food Freezing Processes: A Review of Recent Research Progresses. Crit. Rev. Food Sci. Nutr. 2017, 57, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Lung, H.M.; Yang, B.B.; Wang, C.Y. Responses of Microorganisms to High Hydrostatic Pressure Processing. Food Control 2014, 40, 250–259. [Google Scholar] [CrossRef]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-Pressure Processing—Effects on Microbial Food Safety and Food Quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nabi, B.G.; Mukhtar, K.; Arshad, R.N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M.U.; Walayat, N.; Nawaz, A.; Inam-Ur-raheem, M.; Aadil, R.M. High-Pressure Processing for Sustainable Food Supply. Sustainability 2021, 13, 13908. [Google Scholar] [CrossRef]
- Martindale, W. The Potential of Food Preservation to Reduce Food Waste. Proc. Nutr. Soc. 2017, 76, 28–33. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An Overview of the Intelligent Packaging Technologies in the Food Sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Kohli, D.; Loushigam, G.; Richa, R.; Bajad, R. Recent Advances in Food Packaging for Shelf Life of Food. Adv. Sci. Technol. Innov. 2024, Part F 2519, 273–284. [Google Scholar] [CrossRef]
- Han, J.H. New Technologies in Food Packaging: Overiew. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2005; pp. 3–11. [Google Scholar]
- Sohail, M.; Sun, D.W.; Zhu, Z. Recent Developments in Intelligent Packaging for Enhancing Food Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Kerry, J.P. New Packaging Technologies, Materials and Formats for Fast-Moving Consumer Products. In Innovations in Food Packaging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 549–584. [Google Scholar]
- Mirza Alizadeh, A.; Masoomian, M.; Shakooie, M.; Zabihzadeh Khajavi, M.; Farhoodi, M. Trends and Applications of Intelligent Packaging in Dairy Products: A Review. Crit. Rev. Food Sci. Nutr. 2021, 62, 383–397. [Google Scholar] [CrossRef]
- Kuswandi, B. Jumina Active and Intelligent Packaging, Safety, and Quality Controls. In Fresh-Cut Fruits and Vegetables: Technologies and Mechanisms for Safety Control; Academic Press: Cambridge, MA, USA, 2020; pp. 243–294. [Google Scholar]
- Kuswandi, B.; Murdyaningsih, E.A. Simple on Package Indicator Label for Monitoring of Grape Ripening Process Using Colorimetric PH Sensor. J. Food Meas. Charact. 2017, 11, 2180–2194. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon Dioxide Sensors for Intelligent Food Packaging Applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Lu, M.; Shiau, Y.; Wong, J.; Lin, R.; Kravis, H.; Blackmon, T.; Pakzad, T.; Jen, T.; Cheng, A.; Chang, J.; et al. Milk Spoilage: Methods and Practices of Detecting Milk Quality. Food Nutr. Sci. 2013, 4, 113–123. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Saha, N.C. Food Packaging: Concepts and Its Significance. In Food Packaging; Springer Nature: Singapore, 2022; pp. 1–45. [Google Scholar]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S.; Singh, R.; Dutt, S.; Jammu, J.; et al. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green Synthesis of Nanoparticles from Biodegradable Waste Extracts and Their Applications: A Critical Review. Nanotechnol. Environ. Eng. 2022, 8, 377–397. [Google Scholar] [CrossRef]
- Stoma, M.; Dudziak, A. Eastern Poland Consumer Awareness of Innovative Active and Intelligent Packaging in the Food Industry: Exploratory Studies. Sustainability 2022, 14, 13691. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Mthiyane, D.M.N.; Onwudiwe, D.C.; Babalola, O.O. Harnessing the Known and Unknown Impact of Nanotechnology on Enhancing Food Security and Reducing Postharvest Losses: Constraints and Future Prospects. Agronomy 2022, 12, 1657. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A Review on Active Packaging for Quality and Safety of Foods: Current Trends, Applications, Prospects and Challenges. Food Packag. Shelf. Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- de Abreu, D.A.P.; Cruz, J.M.; Losada, P.P. Active and Intelligent Packaging for the Food Industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Suppakul, P. Active and Intelligent Packaging: The Indication of Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef] [PubMed]
- Kruijf, D.D.; Beest, V.V.; Rijk, R.; Sipiläinen-Malm, T.; Losada, P.P.; Meulenaer, D.D. Active and Intelligent Packaging: Applications and Regulatory Aspects. Food Addit. Contam. 2002, 19, 144–162. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A Critical Review on Intelligent and Active Packaging in the Food Industry: Research and Development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Realini, C.E.; Marcos, B. Active and Intelligent Packaging Systems for a Modern Society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent Food Packaging: The next Generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Jayus; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart Packaging: Sensors for Monitoring of Food Quality and Safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Schaefer, D.; Cheung, W.M. Smart Packaging: Opportunities and Challenges. Procedia CIRP 2018, 72, 1022–1027. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Yu, Z.; Ye, C.; Pan, L.; Song, Y. Principle, Development and Application of Time–Temperature Indicators for Packaging. Packag. Technol. Sci. 2023, 36, 833–853. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Göransson, M.; Nilsson, F.; Jevinger, Å. Temperature Performance and Food Shelf-Life Accuracy in Cold Food Supply Chains—Insights from Multiple Field Studies. Food Control 2018, 86, 332–341. [Google Scholar] [CrossRef]
- Kumar, J.; Konala, A.; Gaikwad, K.K. Recent Developments in Intelligent Packaging Systems for Food Processing Industry: A Review Development of Bio-Based Phase Change Material for Application in Food Packaging View Project Development of Plant Based Edible Ink for Printing and Sustainable Packaging Application View Project. J. Food Proc. Technol. 2021, 12, 895. [Google Scholar]
- Singh, S.; Omre, P.K.; Gaikwad, K. Sensory Evaluation of Optimized and Stabilized Sugarcane Juice. Int. J. Eng. Res. Gen. Sci. 2014, 2, 637–648. [Google Scholar]
- Mehauden, K.; Cox, P.W.; Bakalis, S.; Simmons, M.J.H.; Tucker, G.S.; Fryer, P.J. A Novel Method to Evaluate the Applicability of Time Temperature Integrators to Different Temperature Profiles. Innov. Food Sci. Emerg. Technol. 2007, 8, 507–514. [Google Scholar] [CrossRef]
- Dobrucka, R.; Cierpiszewski, R. Active and Intelligent Packaging Food-Research and Development-a Review. Pol. J. Food Nutr. Sci. 2014, 64, 7–153. [Google Scholar] [CrossRef]
- Han, J.H. A Review of Food Packaging Technologies and Innovations. In Innovations in Food Packaging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 3–12. [Google Scholar]
- Robertson, G.L. Food Packaging Principles and Practice; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Corradini, M.G. Shelf Life of Food Products: From Open Labeling to Real-Time Measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef]
- Fu, B.; Labuza, T.P. Considerations for the Application of Time-Temperature Integrators in Food Distribution. J. Food Distrib. Res. 1992, 23, 9–18. [Google Scholar]
- Albrecht, A.; Ibald, R.; Raab, V.; Reichstein, W.; Haarer, D.; Kreyenschmidt, J. Implementation of Time Temperature Indicators to Improve Temperature Monitoring and Support Dynamic Shelf Life in Meat Supply Chains. J. Packag. Technol. Res 2019, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and Intelligent Packaging in Meat Industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Rossaint, S.; Kreyenschmidt, J. Intelligent Label—A New Way to Support Food Waste Reduction. In Proceedings of the Institution of Civil Engineers-Waste and Resource Management 2015; Thomas Telford Ltd.: London, UK, 2015; Volume 168, pp. 63–71. [Google Scholar] [CrossRef]
- Thyberg, K.L.; Tonjes, D.J. Drivers of Food Waste and Their Implications for Sustainable Policy Development. Resour. Conserv. Recycl. 2016, 106, 110–123. [Google Scholar] [CrossRef]
- Buisman, M.E.; Haijema, R.; Bloemhof-Ruwaard, J.M. Discounting and Dynamic Shelf Life to Reduce Fresh Food Waste at Retailers. Int. J. Prod. Econ. 2019, 209, 274–284. [Google Scholar] [CrossRef]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, Current and Potential Utilisation of Active and Intelligent Packaging Systems for Meat and Muscle-Based Products: A Review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Maryska, C.; Jayus; Abdullah, A.; Heng, L.Y. Real Time On-Package Freshness Indicator for Guavas Packaging. J. Food Meas. Charact. 2013, 7, 29–39. [Google Scholar] [CrossRef]
- Kerry, J.; Butler, P. Smart Packaging Technologies for Fast Moving Consumer Goods. In Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–340. [Google Scholar]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan Biobased and Intelligent Films: Monitoring PH Variations. LWT Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart Packaging Systems for Food Applications: A Review. J. Food Sci. Technol. 2015, 52, 6125. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Puligundla, P.; Ko, S. Proof-of-Concept Study of Chitosan-Based Carbon Dioxide Indicator for Food Packaging Applications. Food Chem. 2012, 135, 2170–2174. [Google Scholar] [CrossRef]
- Lee, K.; Ko, S. Proof-of-Concept Study of a Whey Protein Isolate Based Carbon Dioxide Indicator to Measure the Shelf-Life of Packaged Foods. Food Sci. Biotechnol. 2014, 23, 115–120. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent Packaging: Trends and Applications in Food Systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with PH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring Some Colour to Your Package: Freshness Indicators Based on Anthocyanin Extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Tichoniuk, M.; Radomska, N.; Cierpiszewski, R. The Application of Natural Dyes in Food Freshness Indicators Designed for Intelligent Packaging. Stud. Oeconomica Posnaniensia 2017, 5, 19–34. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active Chitosan/PVA Films with Anthocyanins from Brassica Oleraceae (Red Cabbage) as Time–Temperature Indicators for Application in Intelligent Food Packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total Volatile Basic Nitrogen (TVB-N) and Its Role in Meat Spoilage: A Review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Dudnyk, I.; Janeček, E.R.; Vaucher-Joset, J.; Stellacci, F. Edible Sensors for Meat and Seafood Freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Kaewprachu, P.; Rawdkuen, S.; Kaewprachu, P. Valorization of Food Processing By-Products as Smart Food Packaging Materials and Its Application. In Food Preservation and Waste Exploitation; IntechOpen: London, UK, 2019. [Google Scholar]
- Shi, S.; Xie, Y.; Wang, G.; Luo, Y. Metabolite-Based Biosensors for Natural Product Discovery and Overproduction. Curr. Opin. Biotechnol. 2022, 75, 102699. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N. Intelligent Packaging to Improve Shelf Life. In Food Quality and Shelf Life; Academic Press: Cambridge, MA, USA, 2019; pp. 261–279. [Google Scholar]
- Kalakonda, S.N.; Bammidi, R.; Edubilli, H.; Medapati, S.; Boyina, S.L.D.; Prasad, V.V.S.R. Biosensors – An Insight into the Electrochemical and Optical Biosensors. Int. J. Pharm. Investig. 2023, 13, 402–412. [Google Scholar] [CrossRef]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Intelligent Packaging: Concepts and Applications. J. Food Sci. 2005, 70, R1–R10. [Google Scholar] [CrossRef]
- Pavelková, A.; Pavelková, I.A. Intelligent Packaging as Device for Monitoring of Risk Factors in Food. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 282–292. [Google Scholar]
- Meng, X.; Kim, S.; Puligundla, P.; Ko, S. Carbon Dioxide and Oxygen Gas Sensors-Possible Application for Monitoring Quality, Freshness, and Safety of Agricultural and Food Products with Emphasis on Importance of Analytical Signals and Their Transformation. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 723–733. [Google Scholar] [CrossRef]
- Vu, C.H.T.; Won, K. Novel Water-Resistant UV-Activated Oxygen Indicator for Intelligent Food Packaging. Food Chem. 2013, 140, 52–56. [Google Scholar] [CrossRef]
- Won, K.; Jang, N.Y.; Jeon, J. A Natural Component-Based Oxygen Indicator with In-Pack Activation for Intelligent Food Packaging. J. Agric. Food Chem. 2016, 64, 9675–9679. [Google Scholar] [CrossRef] [PubMed]
- Raudienė, E.; Rušinskas, D.; Balčiūnas, G.; Juodeikienė, G.; Gailius, D. Carbon Dioxide Respiration Rates in Wheat at Various Temperatures and Moisture Contents. Mapan J. Metrol. Soc. India 2017, 32, 51–58. [Google Scholar] [CrossRef]
- Dewi, F.R.; Powell, S.M.; Stanley, R.A.; Dirpan, A.; Milijasevic, J.B.; Milijasevic, M.; Djordjevic, V. Modified Atmosphere Packaging of Fish—An Impact on Shelf Life. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012028. [Google Scholar] [CrossRef]
- Saliu, F.; Della Pergola, R. Carbon Dioxide Colorimetric Indicators for Food Packaging Application: Applicability of Anthocyanin and Poly-Lysine Mixtures. Sens. Actuators B Chem. 2018, 258, 1117–1124. [Google Scholar] [CrossRef]
- Ahmad, A.N.; Abdullah Lim, S.; Navaranjan, N. Development of Sago (Metroxylon sagu)-Based Colorimetric Indicator Incorporated with Butterfly Pea (Clitoria ternatea) Anthocyanin for Intelligent Food Packaging. J. Food Saf. 2020, 40, e12807. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Anthocyanin-A Natural Dye for Smart Food Packaging Systems. Artic. J. Packag. Sci. Technol. 2018, 24, 167–180. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Fabrication of Cellulose Nanofiber-Based Functional Color Indicator Film Incorporated with Shikonin Extracted from Lithospermum Erythrorhizon Root. Food Hydrocoll. 2021, 114, 106566. [Google Scholar] [CrossRef]
- Gupta, R.K.; El Gawad, F.A.; Ali, E.A.E.; Karunanithi, S.; Yugiani, P.; Srivastav, P.P. Nanotechnology: Current Applications and Future Scope in Food Packaging Systems. Meas. Food 2024, 13, 100131. [Google Scholar] [CrossRef]
- Rao, M.M.V.; Mohammad, N.; Banerjee, S.; Khanna, P.K. Synthesis and Food Packaging Application of Silver Nano-Particles: A Review. Hybrid Adv. 2024, 6, 100230. [Google Scholar] [CrossRef]
- Ray, R.; Prabhu, A.; Prasad, D.; Garlapati, V.; Aminabhavi, T.M.; Mani, N.K.; Simal-Gandara, J. Paper-Based Microfluidic Devices for Food Adulterants: Cost-Effective Technological Monitoring Systems. Food Chem. 2022, 390, 133173. [Google Scholar] [CrossRef]
- Tracey, C.T.; Predeina, A.L.; Krivoshapkina, E.F.; Kumacheva, E. A 3D Printing Approach to Intelligent Food Packaging. Trends Food Sci. Technol. 2022, 127, 87–98. [Google Scholar] [CrossRef]
- Zuo, J.; Feng, J.; Gameiro, M.G.; Tian, Y.; Liang, J.; Wang, Y.; Ding, J.; He, Q. RFID-Based Sensing in Smart Packaging for Food Applications: A Review. Future Foods 2022, 6, 100198. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.C.; Sidhu, M.S. Review of RFID and IoT Integration in Supply Chain Management. Oper. Res. Perspect. 2022, 9, 100229. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Fawole, O.A. Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review. Biomolecules 2023, 13, 1092. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, S.; Jaiswal, A.K. A Review on Nanomaterials and Nanohybrids Based Bio-Nanocomposites for Food Packaging. Food Chem. 2022, 376, 131912. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Kim, J.T.; Rhim, J.W.; Nandi, D.; Parameswaranpillai, J.; Siengchin, S. Recent Innovations in Bionanocomposites-Based Food Packaging Films—A Comprehensive Review. Food Packag. Shelf Life 2022, 33, 100877. [Google Scholar] [CrossRef]
- Din, M.I.; Ghaffar, T.; Najeeb, J.; Hussain, Z.; Khalid, R.; Zahid, H. Potential Perspectives of Biodegradable Plastics for Food Packaging Application-Review of Properties and Recent Developments. Food Addit. Contam. Part A 2020, 37, 665–680. [Google Scholar] [CrossRef]
- Wyser, Y.; Adams, M.; Avella, M.; Carlander, D.; Garcia, L.; Pieper, G.; Rennen, M.; Schuermans, J.; Weiss, J. Outlook and Challenges of Nanotechnologies for Food Packaging. Packag. Technol. Sci. 2016, 29, 615–648. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Nanostructured Materials for Food Applications: Spectroscopy, Microscopy and Physical Properties. Bioengineering 2019, 6, 26. [Google Scholar] [CrossRef]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent Advances and Challenges on Applications of Nanotechnology in Food Packaging. A Literature Review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current Progress in Production of Biopolymeric Materials Based on Cellulose, Cellulose Nanofibers, and Cellulose Derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Vitrolife Private Equity Investment Committee Paper—Company Valuation in the Context of Private Equity—ProQuest. Available online: https://www.proquest.com/openview/32a8bebe579003b30f0df4cae3ec468e/1?pq-origsite=gscholar&cbl=2026366&diss=y (accessed on 14 January 2025).
- Smart Packaging Market Size, Share|Growth Report [2032]. Available online: https://www.fortunebusinessinsights.com/smart-packaging-market-109166 (accessed on 14 January 2025).
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Hakim, L.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Nano Clays and Its Composites for Food Packaging Applications. Int. Nano Lett. 2023, 13, 131–153. [Google Scholar] [CrossRef]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent Advances in Biopolymers and Biopolymer-Based Nanocomposites for Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Nielsen, L.E. Models for the Permeability of Filled Polymer Systems. J. Macromol. Sci.—Chem. 1967, 1, 929–942. [Google Scholar] [CrossRef]
- Farhoodi, M. Nanocomposite Materials for Food Packaging Applications: Characterization and Safety Evaluation. Food Eng. Rev. 2015, 8, 35–51. [Google Scholar] [CrossRef]
- Adame, D.; Beall, G.W. Direct Measurement of the Constrained Polymer Region in Polyamide/Clay Nanocomposites and the Implications for Gas Diffusion. Appl. Clay Sci. 2009, 42, 545–552. [Google Scholar] [CrossRef]
- Mirzadeh, A.; Kokabi, M. The Effect of Composition and Draw-down Ratio on Morphology and Oxygen Permeability of Polypropylene Nanocomposite Blown Films. Eur. Polym. J. 2007, 43, 3757–3765. [Google Scholar] [CrossRef]
- Beall, G.W. New Conceptual Model for Interpreting Nanocomposite Behavior. 2000. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=72.%09Beall%2C+G.+W.+%282000%29.+New+conceptu-al+model+for+interpreting+nanocomposite+behavior.+Polymer%E2%80%93clay+nanocomposites%2C+267-279.&btnG= (accessed on 8 November 2023).
- Yano, K.; Usuki, A.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis and Properties of Polyimide–Clay Hybrid. J. Polym. Sci. A Polym. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Novel PET Nanocomposites of Interest in Food Packaging Applications and Comparative Barrier Performance With Biopolyester Nanocomposites. J. Plast. Film. Sheeting 2007, 23, 133–148. [Google Scholar] [CrossRef]
- Ozcalik, O.; Tihminlioglu, F. Barrier Properties of Corn Zein Nanocomposite Coated Polypropylene Films for Food Packaging Applications. J. Food Eng. 2013, 114, 505–513. [Google Scholar] [CrossRef]
- Park, H.M.; Li, X.; Jin, C.Z.; Park, C.Y.; Cho, W.J.; Ha, C.S. Preparation and Properties of Biodegradable Thermoplastic Starch/Clay Hybrids. Macromol. Mater. Eng. 2002, 287, 553–558. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S.; Herald, T.J. Barrier and Mechanical Properties of Starch-Clay Nanocomposite Films. Cereal Chem. 2008, 85, 433–439. [Google Scholar] [CrossRef]
- Abdullahi, N.; Nura, A. Advances in Food Packaging Technology-A Review. J. Postharvest Technol. 2018, 6, 55–64. [Google Scholar]
- Omanović-Mikličanin, E.; Maksimović, M. Nanosensors Applications in Agriculture and Food Industry. Bull. Chem. Technol. Bosnia Herzeg. 2016, 47, 59–70. [Google Scholar]
- Joyner, J.J.; Kumar, D.V. Nanosensors and their applications in food analysis: A review. Int. J. Sci. Technoledge 2015, 3, 80. [Google Scholar]
- Caon, T.; Martelli, S.M.; Fakhouri, F.M. New Trends in the Food Industry: Application of Nanosensors in Food Packaging. In Nanobiosensors; Academic Press: Cambridge, MA, USA, 2017; pp. 773–804. [Google Scholar]
- Mohammadpour, Z.; Naghib, S.M. Smart Nanosensors for Intelligent Packaging. In Nanosensors for Smart Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 323–346. [Google Scholar]
- Pavase, T.R.; Lin, H.; Shaikh, Q.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent Advances of Conjugated Polymer (CP) Nanocomposite-Based Chemical Sensors and Their Applications in Food Spoilage Detection: A Comprehensive Review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, R.; Qian, J. Printed Electronics Based on Inorganic Conductive Nanomaterials and Their Applications in Intelligent Food Packaging. RSC Adv. 2019, 9, 29154–29172. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nanomaterial-Based Optical Indicators: Promise, Opportunities, and Challenges in the Development of Colorimetric Systems for Intelligent Packaging. Nano Res. 2019, 12, 489–500. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Ul Haq, M.I. 3D Printing—A Review of Processes, Materials and Applications in Industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Su, W.; Cook, B.S.; Fang, Y.; Tentzeris, M.M. Fully Inkjet-Printed Microfluidics: A Solution to Low-Cost Rapid Three-Dimensional Microfluidics Fabrication with Numerous Electrical and Sensing Applications. Scientific 2016, 6, 35111. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Alomgir Hossen, M.; Dai, J.; Qin, W.; Liu, Y. Facile Fabrication of Sandwich-like Anthocyanin/Chitosan/Lemongrass Essential Oil Films via 3D Printing for Intelligent Evaluation of Pork Freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, J.; Tang, S.; Wu, Z.; Wang, X. 3D-Printed Nanocellulose-Based Cushioning–Antibacterial Dual-Function Food Packaging Aerogel. Molecules 2021, 26, 3543. [Google Scholar] [CrossRef]
- Zhai, X.; Sun, Y.; Cen, S.; Wang, X.; Zhang, J.; Yang, Z.; Li, Y.; Wang, X.; Zhou, C.; Arslan, M.; et al. Anthocyanins-Encapsulated 3D-Printable Bigels: A Colorimetric and Leaching-Resistant Volatile Amines Sensor for Intelligent Food Packaging. Food Hydrocoll. 2022, 133, 107989. [Google Scholar] [CrossRef]
- Chaiya, N.; Daranarong, D.; Worajittiphon, P.; Somsunan, R.; Meepowpan, P.; Tuantranont, A.; Rakbamrung, N.; Topham, P.D.; Tighe, B.J.; Mahomed, A.; et al. 3D-Printed PLA/PEO Blend as Biodegradable Substrate Coating with CoCl2 for Colorimetric Humidity Detection. Food Packag. Shelf Life 2022, 32, 100829. [Google Scholar] [CrossRef]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent Development in Silver-Based Ink for Flexible Electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Rab, S. Role of Additive Manufacturing Applications towards Environmental Sustainability. Adv. Ind. Eng. Polym. Res. 2021, 4, 312–322. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Tahir, A.U.; Majeed, Y. Printing the Future of Food: The Physics Perspective on 3D Food Printing. Food Phys. 2024, 1, 100003. [Google Scholar] [CrossRef]
- Brown, A.C.; Conradie, P.; De Beer, D. Development of a Stereolithography (STL) Input and Computer Numerical Control (CNC) Output Algorithm for an Entry-Level 3-D Printer. S. Afr. J. Ind. Eng. 2014, 25, 39–47. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Yan, L.; Huang, D.; Lin, L. Extrusion-Based Food Printing for Digitalized Food Design and Nutrition Control. J. Food Eng. 2018, 220, 1–11. [Google Scholar] [CrossRef]
- Oropallo, W.; Piegl, L.A. Ten Challenges in 3D Printing. Eng. Comput. 2016, 32, 135–148. [Google Scholar] [CrossRef]
- Li, X.; You, B.; Shum, H.C.; Chen, C.H. Future Foods: Design, Fabrication and Production through Microfluidics. Biomaterials 2022, 287, 121631. [Google Scholar] [CrossRef]
- Chen, K.H.; Liu, C.C.; Lu, S.Y.; Chen, S.J.; Sheu, F.; Fu, L.M. Rapid Microfluidic Analysis Detection System for Sodium Dehydroacetate in Foods. Chem. Eng. J. 2022, 427, 131530. [Google Scholar] [CrossRef]
- Schroën, K.; Wu, L.; Corstens, M. Food-Grade Microgel Capsules Tailored for Anti-Obesity Strategies through Microfluidic Preparation. Curr. Opin. Food Sci. 2022, 45, 100816. [Google Scholar] [CrossRef]
- Pou, K.R.J.; Raghavan, V.; Packirisamy, M. Applications of Microfluidic Technology in Food Sector: A Bibliometric Analysis. COLLNET J. Scientometr. Inf. Manag. 2021, 15, 259–285. [Google Scholar]
- Atalay, Y.T.; Vermeir, S.; Witters, D.; Vergauwe, N.; Verbruggen, B.; Verboven, P.; Nicolaï, B.M.; Lammertyn, J. Microfluidic Analytical Systems for Food Analysis. Trends Food Sci. Technol. 2011, 22, 386–404. [Google Scholar] [CrossRef]
- Ozhikandathil, J.; Badilescu, S.; Packirisamy, M. A Brief Review on Microfluidic Platforms for Hormones Detection. J. Neural. Transm. 2017, 124, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering Flows in Small Devices: Microfluidics Toward a Lab-on-a-Chip. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Pou, K.R.J.; Raghavan, V.; Packirisamy, M. Microfluidics in Smart Packaging of Foods. Food Res. Int. 2022, 161, 111873. [Google Scholar] [CrossRef]
- Mu, R.; Bu, N.; Pang, J.; Wang, L.; Zhang, Y. Recent Trends of Microfluidics in Food Science and Technology: Fabrications and Applications. Foods 2022, 11, 3727. [Google Scholar] [CrossRef]
- Mu, R.J.; Ni, Y.; Wang, L.; Yuan, Y.; Yan, Z.; Pang, J.; Chen, S. Fabrication of Ordered Konjac Glucomannan Microfiber Arrays via Facile Microfluidic Spinning Method. Mater. Lett. 2017, 196, 410–413. [Google Scholar] [CrossRef]
- Lin, W.; Ni, Y.; Liu, D.; Yao, Y.; Pang, J. Robust Microfluidic Construction of Konjac Glucomannan-Based Micro-Films for Active Food Packaging. Int. J. Biol. Macromol. 2019, 137, 982–991. [Google Scholar] [CrossRef]
- Shao, C.; Chi, J.; Shang, L.; Fan, Q.; Ye, F. Droplet Microfluidics-Based Biomedical Microcarriers. Acta Biomater. 2022, 138, 21–33. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Luo, Z.; Zhao, G.; Panhwar, F.; Akbar, M.F.; Shu, Z. Well-Designed Microcapsules Fabricated Using Droplet-Based Microfluidic Technique for Controlled Drug Release. J. Drug Deliv. Sci. Technol. 2017, 39, 379–384. [Google Scholar] [CrossRef]
- Danchana, K.; Iwasaki, H.; Ochiai, K.; Namba, H.; Kaneta, T. Determination of Glutamate Using Paper-Based Microfluidic Devices with Colorimetric Detection for Food Samples. Microchem. J. 2022, 179, 107513. [Google Scholar] [CrossRef]
- Huang, W.H.; Mai, V.P.; Wu, R.Y.; Yeh, K.L.; Yang, R.J. A Microfluidic Aptamer-Based Sensor for Detection of Mercury(II) and Lead(II) Ions in Water. Micromachines 2021, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Shen, Y.; Jiang, Z.; Zhao, Y.; Liang, Z.; Liu, Y.; Shen, X.; Li, X.; Xu, Z.; Lei, H. An Ultrasensitive Microfluidic Chip-Based Immunoassay for Multiplex Determination of 11 PDE-5 Inhibitors in Adulterated Health Foods. Sens. Actuators B Chem. 2022, 358, 131450. [Google Scholar] [CrossRef]
- Azinheiro, S.; Kant, K.; Shahbazi, M.A.; Garrido-Maestu, A.; Prado, M.; Dieguez, L. A Smart Microfluidic Platform for Rapid Multiplexed Detection of Foodborne Pathogens. Food Control 2020, 114, 107242. [Google Scholar] [CrossRef]
- Kasoju, A.; Shrikrishna, N.S.; Shahdeo, D.; Khan, A.A.; Alanazi, A.M.; Gandhi, S. Microfluidic Paper Device for Rapid Detection of Aflatoxin B1 Using an Aptamer Based Colorimetric Assay. RSC Adv. 2020, 10, 11843–11850. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Bhat, R. Electromagnetic Technologies in Food Science. In Electromagnetic Technologies in Food Science; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1–424. [Google Scholar]
- Ibrahim, H.H.; Singh, M.J.; Al-Bawri, S.S.; Ibrahim, S.K.; Islam, M.T.; Alzamil, A.; Islam, M.S. Radio Frequency Energy Harvesting Technologies: A Comprehensive Review on Designing, Methodologies, and Potential Applications. Sensors 2022, 22, 4144. [Google Scholar] [CrossRef]
- Tajima, M. Strategic Value of RFID in Supply Chain Management. J. Purch. Supply Manag. 2007, 13, 261–273. [Google Scholar] [CrossRef]
- Hong, I.-H.; Dang, J.-F.; Tsai, Y.-H.; Liu, C.-S.; Lee, W.-T.; Wang, M.-L.; Chen, P.-C. An RFID Application in the Food Supply Chain: A Case Study of Convenience Stores in Taiwan. J. Food Eng. 2011, 106, 119–126. [Google Scholar] [CrossRef]
- Todorovic, V.; Neag, M.; Lazarevic, M. On the Usage of RFID Tags for Tracking and Monitoring of Shipped Perishable Goods. Procedia Eng. 2014, 69, 1345–1349. [Google Scholar] [CrossRef]
- Jones, P.; Clarke-Hill, C.; Shears, P.; Comfort, D.; Hillier, D. Radio Frequency Identification in the UK: Opportunities and Challenges. Int. J. Retail. Distrib. Manag. 2004, 32, 164–171. [Google Scholar] [CrossRef]
- Sarac, A.; Absi, N.; Dauzere-Pérès, S. A Literature Review on the Impact of RFID Technologies on Supply Chain Management. Int. J. Prod. Econ. 2010, 128, 77–95. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Lunadei, L. The Role of RFID in Agriculture: Applications, Limitations and Challenges. Comput. Electron. Agric. 2011, 79, 42–50. [Google Scholar] [CrossRef]
- Jedermann, R.; Ruiz-Garcia, L.; Lang, W. Spatial Temperature Profiling by Semi-Passive RFID Loggers for Perishable Food Transportation. Comput. Electron. Agric. 2009, 65, 145–154. [Google Scholar] [CrossRef]
- Eom, K.H.; Kim, M.C.; Lee, S.; Lee, C.W. The Vegetable Freshness Monitoring System Using RFID with Oxygen and Carbon Dioxide Sensor. Int. J. Distrib. Sens. Netw. 2012, 8, 472986. [Google Scholar] [CrossRef]
- Martínez-Olmos, A.; Fernández-Salmerón, J.; Lopez-Ruiz, N.; Rivadeneyra Torres, A.; Capitan-Vallvey, L.F.; Palma, A.J. Screen Printed Flexible Radiofrequency Identification Tag for Oxygen Monitoring. Anal. Chem. 2013, 85, 11098–11105. [Google Scholar] [CrossRef]
- Amin, E.M.; Karmakar, N.C.; Jensen, B.W. Fully Printable Chipless RFID Multi-Parameter Sensor. Sens. Actuators A Phys. 2016, 248, 223–232. [Google Scholar] [CrossRef]
- Xanthakis, E.; Valdramidis, V.P. Impact of Heating Operations on the Microbial Ecology of Foods. In Modeling the Microbial Ecology of Foods: Quantitative Microbiology in Food Processinge; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 117–141. [Google Scholar]
- Silberbauer, A.; Schmid, M. Packaging Concepts for Ready-to-Eat Food: Recent Progress. J. Packag. Technol. Res. 2017, 1, 113–126. [Google Scholar] [CrossRef]
- Gallo, T.; Lorence, M.; Abbaoui, M. Passive Microwave Packaging Forms. In Development of Packaging and Products for Use in Microwave Ovens, 2nd ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2020; pp. 249–260. [Google Scholar]
- Thanakkasaranee, S.; Sadeghi, K.; Seo, J. Packaging Materials and Technologies for Microwave Applications: A Review1. Crit. Rev. Food Sci. Nutr. 2023, 63, 6464–6483. [Google Scholar] [CrossRef]
- Galdi, M.R.; Olivieri, R.; Liguori, L.; Albanese, D.; Di Matteo, M.; Di Maio, L. PET Based Nanocomposite Films for Microwave Packaging Applications. AIP Conf. Proc. 2015, 1695, 020059. [Google Scholar]
- Haldimann, M.; Blanc, A.; Dudler, V. Exposure to Antimony from Polyethylene Terephthalate (PET) Trays Used in Ready-to-Eat Meals. Food Addit. Contam. 2007, 24, 860–868. [Google Scholar] [CrossRef]
- Çiğil, A.B.; Cankurtaran, H.; Kahraman, M.V. Photo-Crosslinked Thiol-Ene Based Hybrid Polymeric Sensor for Humidity Detection. React. Funct. Polym. 2017, 114, 75–85. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Wu, Y.; Wang, X.; Wu, D. Design and Fabrication of PH-Responsive Microencapsulated Phase Change Materials for Multipurpose Applications. React. Funct. Polym. 2019, 140, 111–123. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.L.; Martínez-Máñez, R. Recent Advances on Intelligent Packaging as Tools to Reduce Food Waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Kumari, S.; Debbarma, R.; Nasrin, N.; Khan, T.; Taj, S.; Bhuyan, T. Recent Advances in Packaging Materials for Food Products. Food Bioeng. 2024, 3, 236–249. [Google Scholar] [CrossRef]
- Bhatlawande, A.R.; Ghatge, P.U.; Shinde, G.U.; Anushree, R.K.; Patil, S.D. Unlocking the Future of Smart Food Packaging: Biosensors, IoT, and Nano Materials. Food Sci. Biotechnol. 2024, 33, 1075. [Google Scholar] [CrossRef]
- Sarmah, J.K.; Ali, A.A.; Saikia, R.; Dey, R.R.; Dutta, R.R. Functionalized Carbon Nanostructures for Smart Packaging. In Handbook of Functionalized Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2024; pp. 2463–2493. [Google Scholar]
- Shao, P.; Liu, L.; Yu, J.; Lin, Y.; Gao, H.; Chen, H.; Sun, P. An Overview of Intelligent Freshness Indicator Packaging for Food Quality and Safety Monitoring. Trends Food Sci. Technol. 2021, 118, 285–296. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent Fundamental Advances and Emerging Biological and Biomimicking Applications. Adv. Mater. 2021, 33, 2004349. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent Advances in Nanocellulose-Based Different Biomaterials: Types, Properties, and Emerging Applications. J. Mater. Res. Technol. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. Biopolymers from Agriculture Waste and By-Products. In Biopolymers: Recent Updates, Challenges and Opportunities; Springer International Publishing: Cham, Switzerland, 2022; pp. 111–128. [Google Scholar]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Swielam, E.M.; Hussien, Z.M.; Hasanin, M.S. Design, Characterizations, and Antimicrobial Activity of Sustainable Home Furnishing-Based Waste Fabric Treated Using Biobased Nanocomposite. Bioresour. Bioprocess. 2024, 11, 75. [Google Scholar] [CrossRef]
- Weng, S.; Marcet, I.; Rendueles, M.; Díaz, M. Insect-Derived Materials for Food Packaging-A Review. Food Packag. Shelf Life 2023, 38, 101097. [Google Scholar] [CrossRef]
- Yong, Y.; Ahmad, H.N.; Gu, Y.; Zhu, X.; Wen, Y.; Guo, L.; Zhu, J. The Synergistic Effect of Polyphenols and Polypeptides for Plant-Based Bioplastic Film—Enhanced UV Resistance, Antioxidant and Antibacterial Performance. Food Chem. 2024, 460, 140746. [Google Scholar] [CrossRef]
- Laranjeiro, F.; Rotander, A.; López-Ibáñez, S.; Vilas, A.; Södergren Seilitz, F.; Clérandeau, C.; Sampalo, M.; Rial, D.; Bellas, J.; Cachot, J.; et al. Comparative Assessment of the Acute Toxicity of Commercial Bio-Based Polymer Leachates on Marine Plankton. Sci. Total Environ. 2024, 946, 174403. [Google Scholar] [CrossRef]
- Lindh, A.; Wijayarathna, E.R.K.B.; Ciftci, G.C.; Syed, S.; Bashir, T.; Kadi, N.; Zamani, A. Dry Gel Spinning of Fungal Hydrogels for the Development of Renewable Yarns from Food Waste. Fungal Biol. Biotechnol. 2024, 11, 9. [Google Scholar] [CrossRef]
- Vidaurre, N.A.M.; Vargas-Carpintero, R.; Wagner, M.; Lask, J.; Lewandowski, I. Social Aspects in the Assessment of Biobased Value Chains. Sustainability 2020, 12, 9843. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; Ghani, A.A. Expanding Policy for Biodegradable Plastic Products and Market Dynamics of Bio-Based Plastics: Challenges and Opportunities. Sustainability 2021, 13, 6170. [Google Scholar] [CrossRef]
- D’Almeida, A.P.; de Albuquerque, T.L. Innovations in Food Packaging: From Bio-Based Materials to Smart Packaging Systems. Processes 2024, 12, 2085. [Google Scholar] [CrossRef]
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer Chitosan: Potential Sources, Extraction Methods, and Emerging Applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Anushikha; Gaikwad, K.K. Lignin as a UV Blocking, Antioxidant, and Antimicrobial Agent for Food Packaging Applications. Biomass Convers. Biorefin. 2024, 14, 16755–16767. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart Gelatin Films Prepared Using Red Cabbage (Brassica oleracea L.) Extracts as Solvent. Food Hydrocoll. 2019, 89, 674–681. [Google Scholar] [CrossRef]
- Pereira, P.F.M.; Picciani, P.H.S.; Calado, V.M.A.; Tonon, R.V. Gelatin-Based Nanobiocomposite Films as Sensitive Layers for Monitoring Relative Humidity in Food Packaging. Food Bioproc. Tech. 2020, 13, 1063–1073. [Google Scholar] [CrossRef]
- Tshamisane, M.; Adeyemi, J.O.; Fawole, O.A. Fabrication of Active and Intelligent Bio-Based Edible Films Using Encapsulated Beetroot Powders for Smart Packaging. Mater. Adv. 2025, 6, 1051–1066. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of Active Fish Gelatin Films with Anthocyanins by Compression Molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and Characterization of PH-Indicator Films Based on Cassava Starch and Blueberry Residue by Thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A Colorimetric Hydrogen Sulfide Sensor Based on Gellan Gum-Silver Nanoparticles Bionanocomposite for Monitoring of Meat Spoilage in Intelligent Packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Manuspiya, H. Influence of Hydrogen Sulfide Gas Concentrations on LOD and LOQ of Thermal Spray Coated Hybrid-Bacterial Cellulose Film for Intelligent Meat Label. Carbohydr. Polym. 2021, 254, 117442. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic Flexible Films Waste Management—A State of Art Review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The Effect of Plasticizers on the Functional Properties of Biodegradable Gelatin-Based Film: A Review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Mkhari, T.; Kaseke, T.; Fawole, O.A. Encapsulation of Betalain-Rich Extract from Beetroot Postharvest Waste Using a Binary Blend of Gum Arabic and Maltodextrin to Promote a Food Circular Bioeconomy. Front Nutr. 2023, 10, 1235372. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Jayus; Larasati, T.S.; Abdullah, A.; Heng, L.Y. Real-Time Monitoring of Shrimp Spoilage Using On-Package Sticker Sensor Based on Natural Dye of Curcumin. Food Anal. Methods 2012, 5, 881–889. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Alam, T.; Talwar, N. Recent Advances in Active Packaging of Agri-Food Products: A Review. J. Postharvest Technol. 2019, 7, 33–62. [Google Scholar]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible Coatings to Incorporate Active Ingredients to Fresh-Cut Fruits: A Review. Trends Food Sci. Technol. 2009, 20, 438–447. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and Intelligent Biodegradable Packaging Films Using Food and Food Waste-Derived Bioactive Compounds: A Review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound Phenolic Compounds and Antioxidant Properties of Whole Grain and Bran of White, Red and Black Rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent Gelatin/Oxidized Chitin Nanocrystals Nanocomposite Films Containing Black Rice Bran Anthocyanins for Fish Freshness Monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an Intelligent Film Based on Chitosan/Oxidized Chitin Nanocrystals Incorporating Black Rice Bran Anthocyanins for Seafood Spoilage Monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, P.; Zhu, Y.; Liu, X.; Hu, X.; Chen, F. Blueberry Anthocyanins Extract Inhibits Acrylamide-Induced Diverse Toxicity in Mice by Preventing Oxidative Stress and Cytochrome P450 2E1 Activation. J. Funct. Foods 2015, 14, 95–101. [Google Scholar] [CrossRef]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and Characterization of Cassava Starch Films Incorporated with Blueberry Pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Menegotto, J.; Pavoni, F.; Spada, J.C.; Tessaro, I.C. Influence of Blueberry and Jaboticaba Agroindustrial Residue Particle Size on Color Change of Corn Starch Based Films Submitted to Different PH Values Solutions. J. Renew. Mater. 2019, 7, 235. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and Evaluation of a Novel Antioxidant and PH Indicator Film Based on Chitosan and Food Waste Sources of Antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Wang, L.; Xue, J.; Zhang, Y. Preparation and Characterization of Curcumin Loaded Caseinate/Zein Nanocomposite Film Using PH-Driven Method. Ind. Crops Prod. 2019, 130, 71–80. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Guerra Dias, A.R.; da Rosa Zavareze, E. PH-Sensitive Films Containing Anthocyanins Extracted from Black Bean Seed Coat and Red Cabbage. LWT 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a Visual PH-Sensing Film Based on Tara Gum Incorporating Cellulose and Extracts from Grape Skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Chi, W.; Cao, L.; Sun, G.; Meng, F.; Zhang, C.; Li, J.; Wang, L. Developing a Highly PH-Sensitive ĸ-Carrageenan-Based Intelligent Film Incorporating Grape Skin Powder via a Cleaner Process. J. Clean. Prod. 2020, 244, 118862. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and Characterization of Antioxidant, Antimicrobial and PH-Sensitive Films Based on Chitosan, Silver Nanoparticles and Purple Corn Extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Ardiyansyah; Apriliyanti, M.W.; Wahyono, A.; Fatoni, M.; Poerwanto, B.; Suryaningsih, W. The Potency of Betacyanins Extract from a Peel of Dragon Fruits as a Source of Colourimetric Indicator to Develop Intelligent Packaging for Fish Freshness Monitoring. IOP Conf. Ser. Earth Environ. Sci. 2018, 207, 012038. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and Characterization of Indicator Films from Carboxymethyl-Cellulose/Starch and Purple Sweet Potato (Ipomoea Batatas (L.) Lam) Anthocyanins for Monitoring Fish Freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M.; et al. Natural Biomaterial-Based Edible and PH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef]
- Li, Y.; Ying, Y.; Zhou, Y.; Ge, Y.; Yuan, C.; Wu, C.; Hu, Y. A PH-Indicating Intelligent Packaging Composed of Chitosan-Purple Potato Extractions Strength by Surface-Deacetylated Chitin Nanofibers. Int. J. Biol. Macromol. 2019, 127, 376–384. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel Konjac Glucomannan Films with Oxidized Chitin Nanocrystals Immobilized Red Cabbage Anthocyanins for Intelligent Food Packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of Antioxidant, Antimicrobial and Ammonia-Sensitive Films Based on Quaternary Ammonium Chitosan, Polyvinyl Alcohol and Betalains-Rich Cactus Pears (Opuntia ficus-indica) Extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of Active and Intelligent Packaging by Incorporating Betalains from Red Pitaya (Hylocereus polyrhizus) Peel into Starch/Polyvinyl Alcohol Films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of Active/Intelligent Food Packaging Film Containing Amaranthus Leaf Extract for Shelf Life Extension of Chicken/Fish during Chilled Storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Ditchfield, C.; Tadini, C.C. Development and Evaluation of a Novel PH Indicator Biodegradable Film Based on Cassava Starch. J. Appl. Polym. Sci. 2011, 120, 1069–1079. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and Active Furcellaran-Gelatin Films Containing Green or Pu-Erh Tea Extracts: Characterization, Antioxidant and Antimicrobial Potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and Smart Biodegradable Packaging Based on Starch and Natural Extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.L.; Cui, X.; Chai, K.F.; Zhao, G.; Chen, W.N. Interfacial Assembly of a Cashew Nut (Anacardium occidentale) Testa Extract onto a Cellulose-Based Film from Sugarcane Bagasse to Produce an Active Packaging Film with PH-Triggered Release Mechanism. Food Bioproc. Tech. 2020, 13, 501–510. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the Structural, Physical and Functional Properties of κ-Carrageenan Films Incorporated with Pomegranate Flesh and Peel Extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Maniglia, B.C.; De Paula, R.L.; Domingos, J.R.; Tapia-Blácido, D.R. Turmeric Dye Extraction Residue for Use in Bioactive Film Production: Optimization of Turmeric Film Plasticized with Glycerol. LWT—Food Sci. Technol. 2015, 64, 1187–1195. [Google Scholar] [CrossRef]
- Jingcheng, L.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Ramakrishna, S.; Ghosh, R. Intelligent Polymers, Fibers and Applications. Polymers 2021, 13, 1427. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-Responsive Polymers: Biomedical Applications and Challenges for Clinical Translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of Polymeric Nanoparticles for Biomedical Delivery Applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Safian, M.T.U.; Umar, K.; Parveen, T.; Yaqoob, A.A.; Ibrahim, M.N.M. Biomedical Applications of Smart Polymer Composites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Woodhead Publishing: Sawston, UK, 2021; pp. 183–204. [Google Scholar]
- Chauhan, G.S. Evaluation of Nanogels as Supports for Enzyme Immobilization. Polym. Int. 2014, 63, 1889–1894. [Google Scholar] [CrossRef]
- Rzaev, Z.M.O.; Dinçer, S.; Pişkin, E. Functional Copolymers of N-Isopropylacrylamide for Bioengineering Applications. Prog. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Güney, O.; Serin, E. Stimuli-Responsive Molecularly Imprinted Hybrid Polymer Gel as a Potential System for Controlled Release. J. Appl. Polym. Sci. 2016, 133, 4. [Google Scholar] [CrossRef]
- Thambi, T.; Lee, D.S. Stimuli-Responsive Polymersomes for Cancer Therapy. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications: Volume 2: Advanced Nanocarriers for TherapeuticsI; Woodhead Publishing: Sawston, UK, 2019; pp. 413–438. [Google Scholar]
- Yang, M.Y.; Tan, L.; Wu, H.X.; Liu, C.J.; Zhuo, R.X. Dual-Stimuli-Responsive Polymer-Coated Mesoporous Silica Nanoparticles Used for Controlled Drug Delivery. J. Appl. Polym. Sci. 2015, 132, 32. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Jerković, I.; Koncar, V.; Cochrane, C.; Kelly, F.M.; Soulat, D.; Legrand, X. Conductive Polymers for Smart Textile Applications. J. Ind. Text. 2018, 48, 612–642. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-Responsive Polymers and Their Applications. Polym. Chem. 2016, 8, 127–143. [Google Scholar] [CrossRef]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart Monitoring of Gas/Temperature Changes within Food Packaging Based on Natural Colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef]
- Du, H.; Sun, X.; Chong, X.; Yang, M.; Zhu, Z.; Wen, Y. A Review on Smart Active Packaging Systems for Food Preservation: Applications and Future Trends. Trends Food Sci. Technol. 2023, 141, 104200. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef]
- Dias, C.; Ribeiro, T.; Rodrigues, A.C.; Ferrante, A.; Vasconcelos, M.W.; Pintado, M. Improving the Ripening Process after 1-MCP Application: Implications and Strategies. Trends Food Sci. Technol. 2021, 113, 382–396. [Google Scholar] [CrossRef]
- Ju, G.; Liu, X.; Li, R.; Li, M.; Qin, Z.; Yin, X. Temperature-Controlled Release of Curcumin from Thermosensitive PVA/CurM Nanofibrous Membranes with Antibacterial Activity. Colloid Polym. Sci. 2021, 299, 1955–1966. [Google Scholar] [CrossRef]
- Xia, S.; Fang, D.; Shi, C.; Wang, J.; Lyu, L.; Wu, W.; Lu, T.; Song, Y.; Guo, Y.; Huang, C.; et al. Preparation of a Thermosensitive Nanofibre Membrane for Blackberry Preservation. Food Chem. 2023, 415, 135752. [Google Scholar] [CrossRef] [PubMed]
- Douaki, A.; Tran, T.N.; Suarato, G.; Bertolacci, L.; Petti, L.; Lugli, P.; Papadopoulou, E.L.; Athanassiou, A. Thermo-Responsive Nanofibers for on-Demand Biocompound Delivery Platform. Chem. Eng. J. 2022, 445, 136744. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, A.; Sameen, D.E.; Ahmed, S.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Recent Advances in the Fabrication of PH-Sensitive Indicators Films and Their Application for Food Quality Evaluation. Crit. Rev. Food Sci. Nutr. 2023, 63, 1102–1118. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/Corn Starch Blend Films with Extract from Brassica oleraceae (Red Cabbage) as a Visual Indicator of Fish Deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and Characterization of Intelligent Starch/PVA Films for Simultaneous Colorimetric Indication and Antimicrobial Activity for Food Packaging Applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, S.; Chen, X. A Visual PH Sensing Film Using Natural Dyes from Bauhinia blakeana Dunn. Sens. Actuators B Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Golasz, L.B.; da Silva, J.; da Silva, S.B. Film with Anthocyanins as an Indicator of Chilled Pork Deterioration. Food Sci. Technol. 2013, 33, 155–162. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Liu, L.; Cui, Z.; Zhong, Y. Recent Advances in PH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness. Foods 2022, 11, 1884. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.W.; Jafari, S.M. PH-Sensitive (Halochromic) Smart Packaging Films Based on Natural Food Colorants for the Monitoring of Food Quality and Safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Suslick, K.S.; Rakow, N.A.; Sen, A. Colorimetric Sensor Arrays for Molecular Recognition. Tetrahedron 2004, 60, 11133–11138. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a Colorimetric PH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica oleraceae) Extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of Anthocyanin-Rich Purple and Black Eggplant Extracts on the Physical, Antioxidant and PH-Sensitive Properties of Chitosan Film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A Novel PH-Sensing Indicator Based on Bacterial Cellulose Nanofibers and Black Carrot Anthocyanins for Monitoring Fish Freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of PH Indicator and Antimicrobial Cellulose Nanofibre Packaging Film Based on Purple Sweet Potato Anthocyanin and Oregano Essential Oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart Edible Films Based on Gelatin and Curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent PH-Sensitive Indicator Based on Starch-Cellulose and Alizarin Dye to Track Freshness of Rainbow Trout Fillet. Int. J. Biol. Macromol. 2019, 132, 157–165. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Yousefi, S.; Heydari, M.; Seyedfatehi, F.; Jafarzadeh, S.; Mohammadi, R.; Rouhi, M.; Gara-vand, F. Application of Red Cabbage Anthocyanins as PH-Sensitive Pigments in Smart Food Packaging and Sensors. Polymers 2022, 14, 1629. [Google Scholar] [CrossRef]
- Ntuli, V.; Sibanda, T.; Elegbeleye, J.A.; Mugadza, D.T.; Seifu, E.; Buys, E.M. Dairy Production: Microbial Safety of Raw Milk and Processed Milk Products. In Present Knowledge in Food Safety: A Risk-Based Approach Through the Food Chain; Academic Press: Cambridge, MA, USA, 2022; pp. 439–454. [Google Scholar]
- Ebrahimi Tirtashi, F.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/Chitosan PH-Responsive Indicator Incorporated with Carrot Anthocyanins for Intelligent Food Packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef]
- Zaragozá, P.; Fuentes, A.; Ruiz-Rico, M.; Vivancos, J.L.; Fernández-Segovia, I.; Ros-Lis, J.V.; Barat, J.M.; Martínez-Máñez, R. Development of a Colorimetric Sensor Array for Squid Spoilage Assessment. Food Chem. 2015, 175, 315–321. [Google Scholar] [CrossRef]
- Fernandes, P. Enzymes in Fish and Seafood Processing. Front. Bioeng. Biotechnol. 2016, 4, 205363. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.; Xiao, X.; Liu, Y.; Zheng, X. Application of Microbial TTIs as Smart Label for Food Quality: Response Mechanism, Application and Research Trends. Trends Food Sci. Technol. 2016, 51, 12–23. [Google Scholar] [CrossRef]
- Fazial, F.F.; Tan, L.L.; Zubairi, S.I. Bienzymatic Creatine Biosensor Based on Reflectance Measurement for Real-Time Monitoring of Fish Freshness. Sens. Actuators B Chem. 2018, 269, 36–45. [Google Scholar] [CrossRef]
- Chow, C.F.; Ho, P.Y.; Sun, D.; Lu, Y.J.; Wong, W.L.; Tang, Q.; Gong, C. Bin Development of Sensitive and Selective Food Sensors Using New Re(I)-Pt(II) Bimetallic Complexes to Detect Volatile Biogenic Sulfides Formed by Meat Spoilage. Food Chem. 2017, 216, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Koskela, J.; Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Pulkkinen, P.; Tenhu, H.; Nieminen, T.; Kilpelä, A.; Peltonen, J. Monitoring the Quality of Raw Poultry by Detecting Hydrogen Sulfide with Printed Sensors. Sens. Actuators B Chem. 2015, 218, 89–96. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-Package Dual Sensors Label Based on PH Indicators for Real-Time Monitoring of Beef Freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Moazami Goodarzi, M.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Development of an Easy-to-Use Colorimetric PH Label with Starch and Carrot Anthocyanins for Milk Shelf Life Assessment. Int. J. Biol. Macromol. 2020, 153, 240–247. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food Packaging’s Materials: A Food Safety Perspective. Saudi. J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh Fruits and Vegetables—An Overview on Applied Methodologies to Improve Its Quality and Safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene Scavengers for Active Packaging of Fresh Food Produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Singh, S.; Lee, M.; Gaikwad, K.K.; Lee, Y.S. Antibacterial and Amine Scavenging Properties of Silver–Silica Composite for Post-Harvest Storage of Fresh Fish. Food Bioprod. Process. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium Permanganate-Based Ethylene Scavengers for Fresh Horticultural Produce as an Active Packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active Packaging from Chitosan-Titanium Dioxide Nanocomposite Film for Prolonging Storage Life of Tomato Fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Bailen, G.; Guillen, F.; Castillo, S.; Zapata, P.J.; Serrano, M.; Valero, D.; Martínez-Romero, D. Use of a Palladium Catalyst to Improve the Capacity of Activated Carbon to Absorb Ethylene, and Its Effect on Tomato Ripening. Span. J. Agric. Res. 2007, 5, 579–586. [Google Scholar] [CrossRef]
- Emadpour, M.; Ghareyazie, B.; Kalaj, Y.R.; Entesari, M.; Bouzari, N. Effect of the Potassium Permanganate Coated Zeolite Nanoparticles on the Quality Characteristic and Shelf Life of Peach and Nectarine. J. Agric. Technol. 2015, 11, 1263–1273. [Google Scholar]
- Murmu, S.B.; Mishra, H.N. Selection of the Best Active Modified Atmosphere Packaging with Ethylene and Moisture Scavengers to Maintain Quality of Guava during Low-Temperature Storage. Food Chem. 2018, 253, 55–62. [Google Scholar] [CrossRef] [PubMed]
- De Souza Freitas, W.E.; Almeida, M.L.B.; De Morais, P.L.D.; Da Curnha Moura, A.K.; Sales, R. Potassium Permanganate Effects on the Quality and Post-Harvest Conservation of Sapodilla (Manilkara zapota (L.) P.Royen) Fruits under Modified Atmosphere. Acta Agron. 2017, 66, 331–337. [Google Scholar] [CrossRef]
- Rezaei, Y. Effect of the Removal of Ethylen Hormone by Potassium Permangenate Coated Zeolite Nanoparticles on the Increased Quality and Quantity of Storage of Iceberg Lettuce (Lactuca sativa L.) and Chinese Cabbage (Brassica pekinensis). J. Agric. Sci. Nat. Resour. 2017, 15, 188–197. [Google Scholar]
- García, J.C.; Balaguera-López, H.E.; Herrera, A.O. Conservación Del Fruto de Banano Bocadillo (Musa AA Simmonds) Con La Aplicación de Permanganato de Potasio (KMnO4). Rev. Colomb. De Cienc. Hortícolas 2013, 6, 161–171. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Jiang, Y.; Ding, Y.; Yun, J.; Tang, Y.; Zhang, P. Effect of Nano-ZnO-Coated Active Packaging on Quality of Fresh-Cut ‘Fuji’ Apple. Int. J. Food Sci. Technol. 2011, 46, 1947–1955. [Google Scholar] [CrossRef]
- Tas, C.E.; Hendessi, S.; Baysal, M.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Halloysite Nanotubes/Polyethylene Nanocomposites for Active Food Packaging Materials with Ethylene Scavenging and Gas Barrier Properties. Food Bioproc. Tech. 2017, 10, 789–798. [Google Scholar] [CrossRef]
- Küçük, V. Investigation of the Effect of Packaging with Zeolite on the Quality Characteristics of Some Fruits and Vegetables to Extend Their Shelf Life; Ege University, Institute of Science, Department of Food Engineering: İzmir, Turkey, 2006; Volume 185. [Google Scholar]
- Yang, F.M.; Li, H.M.; Li, F.; Xin, Z.H.; Zhao, L.Y.; Zheng, Y.H.; Hu, Q.H. Effect of Nano-Packing on Preservation Quality of Fresh Strawberry (Fragaria ananassa Duch. Cv Fengxiang) during Storage at 4 °C. J. Food Sci. 2010, 75, C236–C240. [Google Scholar] [CrossRef]
- Esturk, O.; Ayhan, Z.; Gokkurt, T. Production and Application of Active Packaging Film with Ethylene Adsorber to Increase the Shelf Life of Broccoli (Brassica oleracea L. Var. Italica). Packag. Technol. Sci. 2014, 27, 179–191. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent PH Indicator Film Composed of Agar/Potato Starch and Anthocyanin Extracts from Purple Sweet Potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, T.S.; Yan, H.; Hu, X.; Ren, T. Novel PH-Sensitive Films Based on Starch/Polyvinyl Alcohol and Food Anthocyanins as a Visual Indicator of Shrimp Deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.V.; Dang, T.H.; Chen, B.H. Synthesis of Intelligent PH Indicative Films from Chitosan/Poly(Vinyl Alcohol)/Anthocyanin Extracted from Red Cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel Colorimetric Films Based on Starch/Polyvinyl Alcohol Incorporated with Roselle Anthocyanins for Fish Freshness Monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.W.; Jiang, C.; Holmes, M. Preparation of an Intelligent PH Film Based on Biodegradable Polymers and Roselle Anthocyanins for Monitoring Pork Freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A Novel Colorimetric Indicator Film Based on Chitosan, Polyvinyl Alcohol and Anthocyanins from Jambolan (Syzygium cumini) Fruit for Monitoring Shrimp Freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef]
- Talukder, S.; Mendiratta, S.K.; Kumar, R.R.; Agrawal, R.K.; Soni, A.; Luke, A.; Chand, S. Jamun Fruit (Syzgium cumini) Skin Extract Based Indicator for Monitoring Chicken Patties Quality during Storage. J. Food Sci. Technol. 2020, 57, 537–548. [Google Scholar] [CrossRef]
- Amiri, R.; Piri, H.; Akbari, M.; Moradi, G. The Fabrication and Kinetic Modeling of a New Time–Temperature Label Based on Paraffin Wax and Black Carrot Anthocyanin for Monitoring Fish Products. Anal. Methods 2020, 12, 544–551. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium amoenum Anthocyanins into Bacterial Cellulose Film: A Novel Colorimetric PH Indicator for Freshness/Spoilage Monitoring of Shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Wang, L. Tara Gum/Polyvinyl Alcohol-Based Colorimetric NH3 Indicator Films Incorporating Curcumin for Intelligent Packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and Characterization of Active and Intelligent Packaging Films Based on Cassava Starch and Anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Listyarini, A.; Sholihah, W.; Imawan, C. A Paper-Based Colorimetric Indicator Label Using Natural Dye for Monitoring Shrimp Spoilage. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012045. [Google Scholar] [CrossRef]
- Sun, G.; Chi, W.; Zhang, C.; Xu, S.; Li, J.; Wang, L. Developing a Green Film with PH-Sensitivity and Antioxidant Activity Based on -Carrageenan and Hydroxypropyl Methylcellulose Incorporating Prunus maackii Juice. Food Hydrocoll. 2019, 94, 345–353. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and PH-Sensitive Films Developed by Incorporating Purple and Black Rice Extracts into Chitosan Matrix. Int. J Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of Active and Intelligent Films Based on Cassava Starch and Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films Based on κ-Carrageenan Incorporated with Curcumin for Freshness Monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. PH-Responsive Pectin-Based Multifunctional Films Incorporated with Curcumin and Sulfur Nanoparticles. Carbohydr. Polym. 2020, 230, 115638. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Nesvadba, P.; Di Natale, C.; Careche, M.; Oehlenschläger, J.; Tryggvadóttir, S.V.; Schubring, R.; Kroeger, M.; Heia, K.; Esaiassen, M.; et al. Multisensor for Fish Quality Determination. Trends Food Sci. Technol. 2004, 15, 86–93. [Google Scholar] [CrossRef]
- Joseph Pellissery, A.; Gopika Vinayamohan, P.; Anne Roshni Amalaradjou, M.; Venkitanarayanan, K. Spoilage Bacteria and Meat Quality. In Meat Quality Analysis; Academic Press: Cambridge, MA, USA, 2020; pp. 307–334. [Google Scholar]
- Liu, Z.; Shaposhnikov, M.; Zhuang, S.; Tu, T.; Wang, H.; Wang, L. Growth and Survival of Common Spoilage and Pathogenic Bacteria in Ground Beef and Plant-Based Meat Analogues. Food Res. Int. 2023, 164, 112408. [Google Scholar] [CrossRef]
- Chmielowiec-Korzeniowska, A.; Trawińska, B.; Tymczyna, L.; Bis-Wencel, H.; Matuszewski, Ł. Microbial Contamination of the Air in Livestock Buildings as a Threat to Human and Animal Health- A Review. Ann. Anim. Sci. 2021, 21, 417–431. [Google Scholar] [CrossRef]
- Shukla, V.; Kandeepan, G.; Vishnuraj, M.R.; Soni, A. Anthocyanins Based Indicator Sensor for Intelligent Packaging Application. Agric. Res. 2016, 5, 205–209. [Google Scholar] [CrossRef]
- Kovačević, D.; Mešić, E.; Užarević, J.; Brozović, M. The Influence of Packaging Visual Design on Consumer Food Product Choices. J. Print Media Technol. Res. 2022, 11, 7–18. [Google Scholar]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef]
- de Jong, A.R.; Boumans, H.; Slaghek, T.; Van Veen, J.; Rijk, R.; Van Zandvoort, M. Active and Intelligent Packaging for Food: Is It the Future? Food Addit. Contam. 2005, 22, 975–979. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and Intelligent Food Packaging: Legal Aspects and Safety Concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Heising, J.K.; Dekker, M.; Bartels, P.V.; (Tiny) Van Boekel, M.A.J.S. Monitoring the Quality of Perishable Foods: Opportunities for Intelligent Packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 645–654. [Google Scholar] [CrossRef]
- Owusu-Apenten, R.; Vieira, E. Food Regulatory Agencies, 5th ed.; Heldman, D.R., Ed.; Springer Nature: Cham, Switzerland, 2023; ISBN 9783030654313. [Google Scholar]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-Based Biosensor and Their Applications: A Review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Penloglou, G.; Basna, A.; Pavlou, A.; Kiparissides, C. Techno-Economic Considerations on Nanocellulose’s Future Progress: A Short Review. Processes 2023, 11, 2312. [Google Scholar] [CrossRef]
- Choudhury, I.A.; Hossain, A.; Bhuiyan, S.H. Issues of Connectivity, Durability, and Reliability of Sensors and Their Applications. Compr. Mater. Process. Thirteen Vol. Set 2014, 13, V13-121–V13-148. [Google Scholar]
- Chaudhary, V.; Gaur, P.; Rustagi, S. Sensors, Society, and Sustainability. Sustain. Mater. Technol. 2024, 40, e00952. [Google Scholar] [CrossRef]
- Greener, J.; Pearson, G.; Cakmak, M. Roll-to-Roll Manufacturing: Process Elements and Recent Advances. In Roll-to-Roll Manufacturing: Process Elements and Recent Advances; Wiley: Hoboken, NJ, USA, 2018; pp. 1–408. [Google Scholar]
- Roth, B.; Søndergaard, R.R.; Krebs, F.C. Roll-to-Roll Printing and Coating Techniques for Manufacturing Large-Area Flexible Organic Electronics. In Handbook of Flexible Organic Electronics: Materials, Manufacturing and Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 171–197. [Google Scholar]
- Htwe, Y.Z.N.; Mariatti, M. Printed Graphene and Hybrid Conductive Inks for Flexible, Stretchable, and Wearable Electronics: Progress, Opportunities, and Challenges. J. Sci. Adv. Mater. Devices 2022, 7, 100435. [Google Scholar] [CrossRef]
- Bhutta, M.N.M.; Ahmad, M. Secure Identification, Traceability and Real-Time Tracking of Agricultural Food Supply during Transportation Using Internet of Things. IEEE Access 2021, 9, 65660–65675. [Google Scholar] [CrossRef]
- Li, P.; Yang, J.; Jiménez-Carvelo, A.M.; Erasmus, S.W. Applications of Food Packaging Quick Response Codes in Information Transmission toward Food Supply Chain Integrity. Trends Food Sci. Technol. 2024, 146, 104384. [Google Scholar] [CrossRef]
- M’hand, M.A.; Boulmakoul, A.; Badir, H.; Lbath, A. A Scalable Real-Time Tracking and Monitoring Architecture for Logistics and Transport in RoRo Terminals. Procedia Comput. Sci. 2019, 151, 218–225. [Google Scholar] [CrossRef]
- Tripathi, G.; Ahad, M.A.; Casalino, G. A Comprehensive Review of Blockchain Technology: Underlying Principles and Historical Background with Future Challenges. Decis. Anal. J. 2023, 9, 100344. [Google Scholar] [CrossRef]
- Singh, A.K.; Deeba, F.; Kumar, M.; Kumari, S.; Wani, S.A.; Paul, T.; Gaur, N.A. Development of Engineered Candida Tropicalis Strain for Efficient Corncob-Based Xylitol-Ethanol Biorefinery. Microb. Cell Fact. 2023, 22, 201. [Google Scholar] [CrossRef]
- Yele, S.; Litoriya, R. Blockchain-Based Secure Dining: Enhancing Safety, Transparency, and Traceability in Food Consumption Environment. Blockchain Res. Appl. 2024, 5, 100187. [Google Scholar] [CrossRef]
- Zhang, Y.; Gupta, V.K.; Karimi, K.; Wang, Y.; Yusoff, M.A.; Vatanparast, H.; Pan, J.; Aghbashlo, M.; Tabatabaei, M.; Rajaei, A. Synergizing Blockchain and Internet of Things for Enhancing Efficiency and Waste Reduction in Sustainable Food Management. Trends Food Sci. Technol. 2025, 156, 104873. [Google Scholar] [CrossRef]
- Dabbene, F.; Gay, P.; Tortia, C. Radio-Frequency Identification Usage in Food Traceability. In Advances in Food Traceability Techniques and Technologies: Improving Quality Throughout the Food Chain; Woodhead Publishing: Sawston, UK, 2016; pp. 67–89. [Google Scholar]
- Qiao, J.; Hao, M.; Guo, M. Design of Meat Product Safety Information Chain Traceability System Based on UHF RFID. Sensors 2023, 23, 3372. [Google Scholar] [CrossRef]
- Singh, A.; Gutub, A.; Nayyar, A.; Khan, M.K. Redefining Food Safety Traceability System through Blockchain: Findings, Challenges and Open Issues. Multimed. Tools Appl. 2023, 82, 21243–21277. [Google Scholar] [CrossRef]
- Geng, Z.; Miao, Y.; Zhang, G.; Liang, X. Colorimetric Biosensor Based on Smartphone: State-of-Art. Sens. Actuators A Phys. 2023, 349, 114056. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Z.; Liu, Q. Smartphone-Based Biosensors for Portable Food Evaluation. Curr. Opin. Food Sci. 2019, 28, 74–81. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and Bioelectronics on Smartphone for Portable Biochemical Detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Mobile Phone-Based Biosensing: An Emerging “Diagnostic and Communication” Technology. Biosens. Bioelectron. 2017, 92, 549–562. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Chen, Z.; Lu, Y.; Low, S.S.; Zhu, L.; Cheng, C.; He, Y.; Chen, Q.; Su, B.; et al. Electrogenerated Chemiluminescence on Smartphone with Graphene Quantum Dots Nanocomposites for Escherichia coli Detection. Sens. Actuators B Chem. 2019, 297, 126811. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-Care Testing Based on Smartphone: The Current State-of-the-Art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Song, Y.; Zeinhom, M.M.A.; Chang, Y.C.; Sheng, L.; Li, H.; Du, D.; Li, L.; Zhu, M.J.; Luo, Y.; et al. Nanozyme-Mediated Dual Immunoassay Integrated with Smartphone for Use in Simultaneous Detection of Pathogens. ACS Appl. Mater. Interfaces 2017, 9, 40671–40680. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mei, Q.; Yu, L.; Ge, H.; Yue, J.; Zhang, K.; Hayat, T.; Alsaedi, A.; Wang, S. Rapid and On-Site Detection of Uranyl Ions via Ratiometric Fluorescence Signals Based on a Smartphone Platform. ACS Appl. Mater. Interfaces 2018, 10, 42225–42232. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Wang, B.; Huang, H.; Jian, D.; Wu, X.; Xue, L.; Wang, S.; Liu, F. On-Site Quantitative Hg2+ Measurements Based on Selective and Sensitive Fluorescence Biosensor and Miniaturized Smartphone Fluorescence Microscope. Biosens. Bioelectron. 2019, 132, 238–247. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkhari, T.; Adeyemi, J.O.; Fawole, O.A. Recent Advances in the Fabrication of Intelligent Packaging for Food Preservation: A Review. Processes 2025, 13, 539. https://doi.org/10.3390/pr13020539
Mkhari T, Adeyemi JO, Fawole OA. Recent Advances in the Fabrication of Intelligent Packaging for Food Preservation: A Review. Processes. 2025; 13(2):539. https://doi.org/10.3390/pr13020539
Chicago/Turabian StyleMkhari, Tshamisane, Jerry O. Adeyemi, and Olaniyi A. Fawole. 2025. "Recent Advances in the Fabrication of Intelligent Packaging for Food Preservation: A Review" Processes 13, no. 2: 539. https://doi.org/10.3390/pr13020539
APA StyleMkhari, T., Adeyemi, J. O., & Fawole, O. A. (2025). Recent Advances in the Fabrication of Intelligent Packaging for Food Preservation: A Review. Processes, 13(2), 539. https://doi.org/10.3390/pr13020539









