Development of an LC-MS Method for the Quantitative Determination of Six Organic Acids Produced by Bacterial Fermentation In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Organic Acid Extraction
2.3. LC-MS
2.4. Standard Curves
2.5. Accuracy and Precision
2.6. Fermentation Medium
2.7. Measuring Individual Variation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Organic Acid Extraction
3.2. Stability of Organic Acid Extraction
3.3. Method Validation
3.4. Ability to Determinate Natural Organic Acids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Ma, X.; Mei, H.; Chang, X.; He, P.; Sun, L.; Xiao, H.; Wang, S.; Li, R. Association between gut microbiota and short-chain fatty acids in children with obesity. Sci. Rep. 2025, 15, 483. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, Y.; Xue, G.; Han, L.; Jia, H.; Wang, Z.; Zhang, J.; Liu, P.; Yang, C.; Zhou, Y. Role of short-chain fatty acids in host physiology. Anim. Models Exp. Med. 2024, 7, 641–652. [Google Scholar] [CrossRef]
- Fang, S.; Qin, T.; Yu, T.; Zhang, G. Improvement of the gut microbiota in vivo by a short-chain fatty acids-producing strain Lactococcus garvieae CF11. Processes 2022, 10, 604. [Google Scholar] [CrossRef]
- Lindström, C.; Holst, O.; Hellstrand, P.; Öste, R.; Andersson, K.E. Evaluation of commercial microbial hydrocolloids concerning their effects on plasma lipids and caecal formation of SCFA in mice. Food Hydrocoll. 2012, 28, 367–372. [Google Scholar] [CrossRef]
- Beylot, M. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br. J. Nutr. 2005, 93, 163–168. [Google Scholar] [CrossRef]
- Valérie, M.; Carole, G.; Emile, L.; Edgard, D. Research Communication: Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer protein in Caco-2 cells. J. Nutr. 2003, 133, 2180–2183. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on conolic function. Aliment. Pharmacol. Ther. 2008, 27, 327–347. [Google Scholar] [CrossRef]
- Vernia, P.; Latella, G.; Magliocca, F.M.; Caprilli, R. Fecal organic anions in diarrhoea1 diseases. Scand. J. Gastroenterol. 1987, 22, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Pajari, A.M. Dietary fiber-a scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 9979. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H.; Hong-Curtiss, J. Probiotics and functional foods in gastrointestinal disorders. Curr. Treat Options Gastroenterol. 2002, 5, 311–321. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef]
- Bloemen, J.G.; Venema, K.; Poll, M.C.; Damink, S.W.M.O.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and the liver in humans measured at survey. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J. Lactic acid can be easily and precisely determined by reversed-phase high performance liquid chromatography with pre-column derivatization. J. Anim. Feed Sci. 2008, 17, 117–121. [Google Scholar] [CrossRef]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microb. 2005, 71, 8383–8389. [Google Scholar] [CrossRef]
- Kotani, A.; Miyaguchi, Y.; Kohama, M.; Ohtsuka, T.; Shiratori, T.; Kusu, F. Determination of short-chain fatty acids in rat and human feces by high-performance liquid chromatography with electrochemical detection. Anal. Sci. 2009, 25, 1007–1011. [Google Scholar] [CrossRef]
- Gomez, E.; Tuohy, K.M.; Gibson, G.R.; Klinder, A.; Costabile, A. In vitro evaluation of the fermentation properties and potential prebiotic activity of Agave fructans. J. Appl. Microbiol. 2010, 108, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Rao, A.V.; Wolever, T.M. Different substrates and methane producing status affect short-chain fatty acid profiles produced by in vitro fermentation of human faeces. J. Nutr. 2000, 130, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef]
- Horspool, L.J.I.; McKellar, Q.A. Determination of short-chain fatty acids in equine caecal liquor by ion exchange high performance liquid chromatography after solid phase extraction. Biomed. Chromatogr. 1991, 5, 202–206. [Google Scholar] [CrossRef]
- Richardson, A.J.; Galder, A.G.; Stewart, C.S.; Smith, A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas-chromatography. Lett. Appl. Microbiol. 1989, 9, 5–8. [Google Scholar] [CrossRef]
- Pompei, A.; Cordisco, L.; Raimondi, S.; Amaretti, A.; Pagnoni, U.M.; Matteuzzi, D.; Rossi, M. In vitro comparison of the prebiotic effects of two inulin-type fructans. Anaerobe 2008, 14, 280–286. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Zijlstra, R.T.; Mosenthin, R.; Gänzle, M.G. Dietary calcium phosphate content and oat β-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. Microbiol. Ecol. 2011, 75, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Asta, M.D.; Rio, D.D.; Mangia, A.; Musci, M.; Scazzina, F. Development of a headspace solid-phase microextraction gas chromatography-mass spectrometric method for the determination of shot-chain fatty acids from intestinal fermentation. Food Chem. 2011, 129, 200–205. [Google Scholar] [CrossRef]
- Mills, G.A.; Walker, V.; Mughal, H. Headspace solid-phase microextraction with 1-pyrenyldiazomethane in-fibre derivatisation for analysis of faecal short-chain fatty acids. J. Chromatogr. B 1999, 730, 113–122. [Google Scholar] [CrossRef]
- Preter, V.D.; Staeyen, G.V.; Esser, D.; Rutgeers, P.; Verbeke, K. Development of a screening method to determine the pattern of fermentation metabolites in faecal samples using on-line purge-and-trap gas chromatographic-mass spectrometric analysis. J. Chromatogr. A 2009, 1216, 1476–1483. [Google Scholar] [CrossRef]
- Garcia, A.; Olmo, B.; Lopez-Gonzalvez, A.; Cornejo, L.; Rupérez, F.J.; Barbas, C. Capillary electrophoresis for short chain organic acids in faeces. J. Pharmaceut. Biomed. Anal. 2007, 46, 356–361. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Martínez-Cruz, O. Quantification of organic acids in fermented shrimp waste by HPLC. Food Technol. Biotechnol. 2008, 46, 456–460. [Google Scholar] [CrossRef]
- Pettinella, C.; Lee, S.H.; Cipollone, F.; Blair, I.A. Targed quantitative analysis of fatty acids in atherosclerotic plaques by high sensitivity liquid chromatography/tandem mass spectrometry. J. Chromatogr. B 2007, 850, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.S.; Davies, N.W. Improved detection of polyunsaturated fatty acids as phenacyl esters using liquid chromatography-ion trap mass spectrometry. J. Microbiol. Methods 2002, 50, 103–113. [Google Scholar] [CrossRef]
- Karlsson, A.; Karlsson, O. Enantiomeric separation of amino alcohols using Z-L-Glu-L-Pro or Z-L-Glu-D-Pro as chiral counter ions and Hypercarb as the solid phase. Chirality 1997, 9, 650–655. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, C.; Newburg, D.S. Quantification of neutral human milk oligosaccharides by graphitic carbon high-performance liquid chromatography with tandem mass spectrometry. Ana. Biochem. 2013, 433, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Barroso, B.; Dijkstra, R.; Geerts, M.; Lagerwerf, F.; Veelen, P.; Ru, A. On-line high-performance liquid chromatography/mass spectrometric characterization of native oligosaccharides from glycoproteins. Rapid Commun. Mass Spectrom. 2002, 16, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef] [PubMed]
- Pabst, M.; Bondili, J.S.; Stadlmann, J.; Mach, L.; Altman, F. Mass + retention time = structure: A strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans. Anal. Chem. 2007, 79, 5051–5057. [Google Scholar] [CrossRef]
- Robinson, S.; Bergström, E.; Seymour, M.; Thomas-Oates, J. Screening of underivatized oligosaccharides extracted from the stems of Triticumaestivum using porous graphitized carbon liquid chromatography-mass spectrometry. Anal. Chem. 2007, 79, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.T.; Papac, D.I. The use of a porous graphitic carbon column for desalting hydrophilic peptides prior to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Biochem. 1999, 273, 179–185. [Google Scholar] [CrossRef]
- Xing, J.; Apedo, A.; Tymiak, A.; Zhao, N. Liquid chromatographic analysis of nucleosides and their mono-, di-, and triphosphates using porous graphitic carbon stationary phase coupled with electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1599–1606. [Google Scholar] [CrossRef]
- Helali, N.; Monser, L. Stability indicating method for famotidine in pharmaceuticals using porous graphic carbon coulumn. J. Sep. Sci. 2008, 31, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Duncan, C.J.; Brisson, J.M. Porous graphic carbon chromatography/tandem mass spectrometric determination of cytarabine in mouse plasma. Rapid Commun. Mass Spectrom. 2007, 21, 629–634. [Google Scholar] [CrossRef]
- Liem, L.K.; Choong, L.H.; Woo, K.T. Porous graphitic carbon shows promise for the rapid screening partial DPD deficiency in lymphocyte dihydropyrimidine dehydrogenase in Chinese, Indian, and Malay in Singapore by using semi-automated HPLC-radioassay. Clin. Biochem. 2002, 35, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hauwe, O.V.D.; Perez, J.C.; Claereboudt, J.; Peteghem, C.V. Simultaneous determination of betamethasone and dexamethasone residues in bovine liver by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 857–861. [Google Scholar] [CrossRef]
- Vial, J.; Hennion, M.C.; Fernandez-Alba, A.; Agüera, A. Use of porous graphic carbon coupled with mass detection for the analysis of polar phenolic compounds by liquid chromatography. J. Chromatogr. A 2001, 937, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Yu, Z.; He, Y.; Yong, Q.; Newburg, D.S. Relative fermentation of oligosaccharides from human milk and plants by gut microbes. Eur. Food Res. Technol. 2017, 243, 133–146. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Li, W.; Wang, K.; Xue, X.; Naumovski, N.; Peng, W. Rapid purification and quantification of intestinal and fecal short-chain fatty acids by solid-phase extraction using Bond Elut Plexa. Separations 2024, 11, 270. [Google Scholar] [CrossRef]
- Wang, S.; Feng, R.; Kong, L.; Zhou, R.; Hu, F.; Sun, S.; Chen, G.; Tao, F.; Liu, K. An UHPLC-QE-Orbitrap-MS method for accurate quantification of short-chain fatty acids in serum from an older Chinese population. Chromatographia 2024, 87, 125–136. [Google Scholar] [CrossRef]
- Li, P.K.T.; Tian, N.; Zhou, S. #695 Modulation of probiotics on intestinal flora and related short-chain fatty acids in patients with peritoneal dialysis. Nephrol. Dial. Transpl. 2025, 39 (Suppl. S1), gfae069-0909-695. [Google Scholar] [CrossRef]
- Jensen, C.; Sommersten, C.H.; Laupsa-Borge, J.; Storås, I.; Valeur, J.; Mellgren, G.; Dierkes, J.; Dankel, S.N.; Lied, G.A. Quality and quantity of carbohydrates, faecal short-chain fatty acids and gastrointestinal symptoms—Results from a randomised, controlled trial (CARBFUNC). Clin. Nutr. 2025, 44, 54–64. [Google Scholar] [CrossRef]
- Jaochico, A.; Sangaraju, D.; Shahidi-Latham, S.K. A rapid derivatization based LC–MS/MS method for quantitation of short chain fatty acids in human plasma and urine. Bioanalysis 2019, 11, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Cao, H. Fast quantification of short chain fatty acids and ketone bodies by liquid chromatography-tandem mass spectrometry after facile derivatization coupled with liquid-liquid extraction. J. Chromatogr. B 2018, 1083, 137–145. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Wang, J.; Wang, J.S. Afatoxin B1 disrupts gut-microbial metabolisms of short-chain fatty acids, longchain fatty acids, and bile acids in male F344 rats. Toxicol. Sci. 2018, 164, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Wang, C.; Guo, L.X.; Zheng, Y.F.; Hu, W.H.; Dong, T.T.X.; Wang, T.J.; Tsim, K.W.K. Simultaneous determination of short-chain fatty acids in human feces by HPLC with ultraviolet detection following chemical derivatization and solid-phase extraction segmental elution. J. Sep. Sci. 2019, 42, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
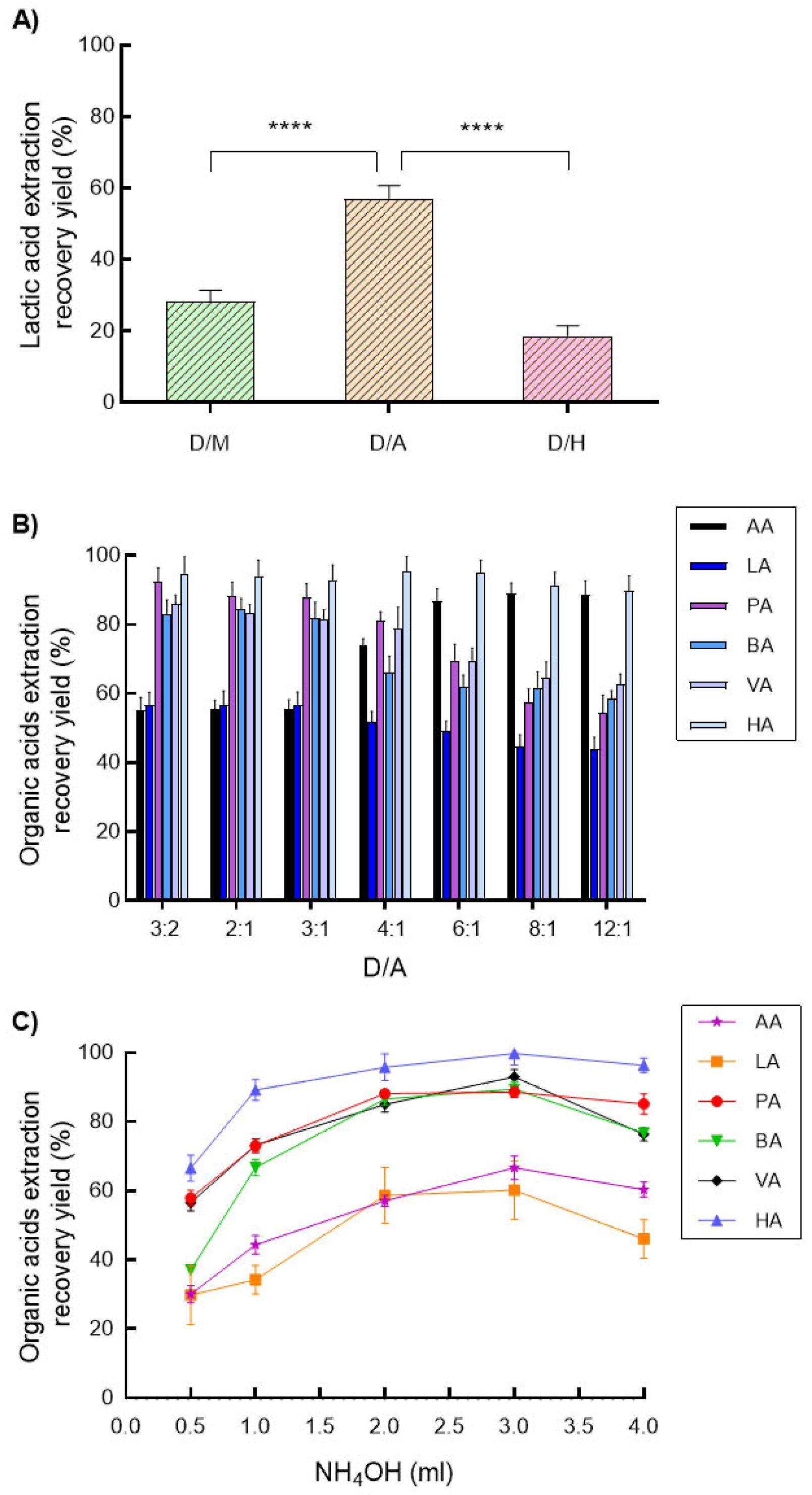
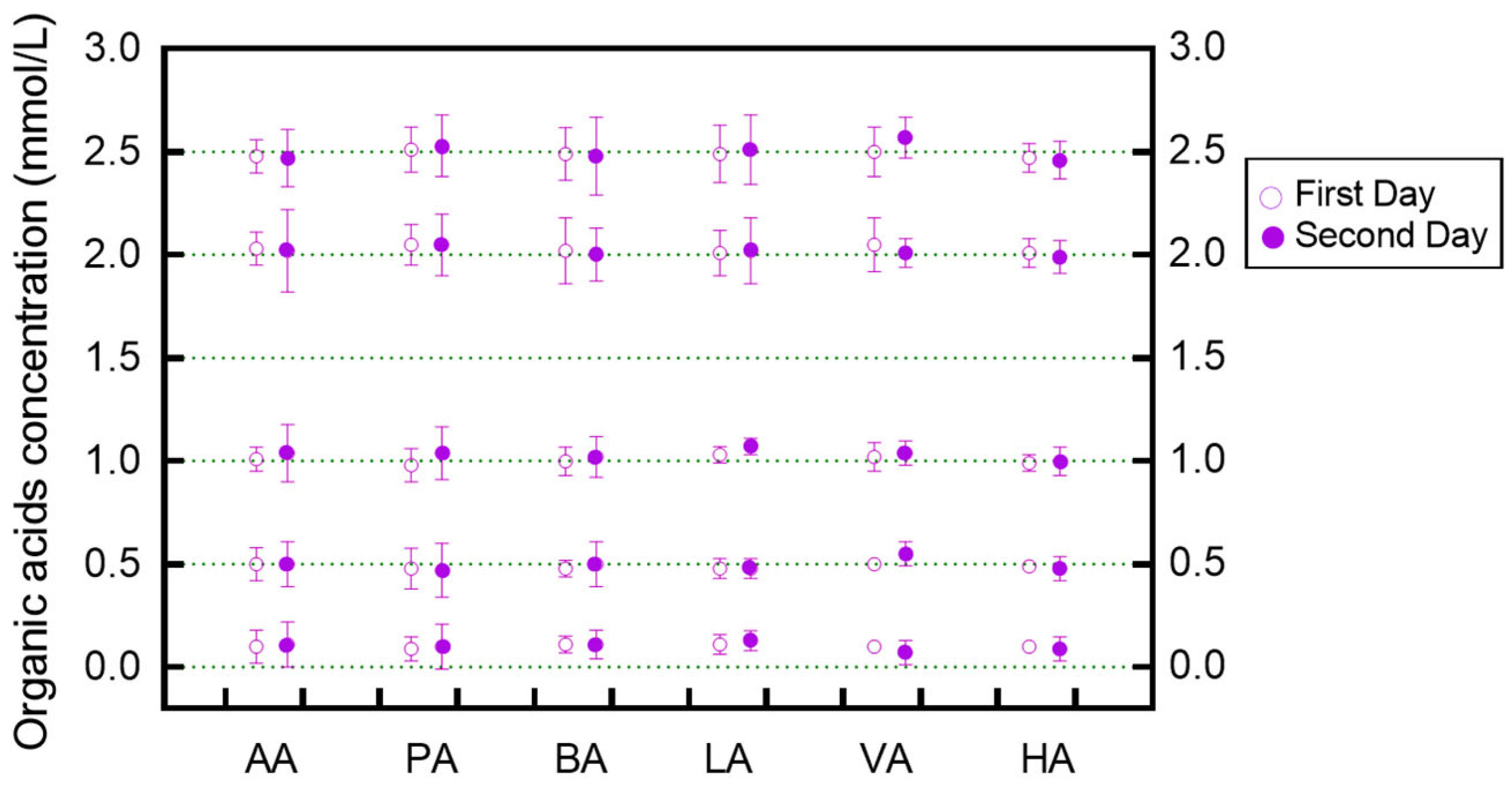

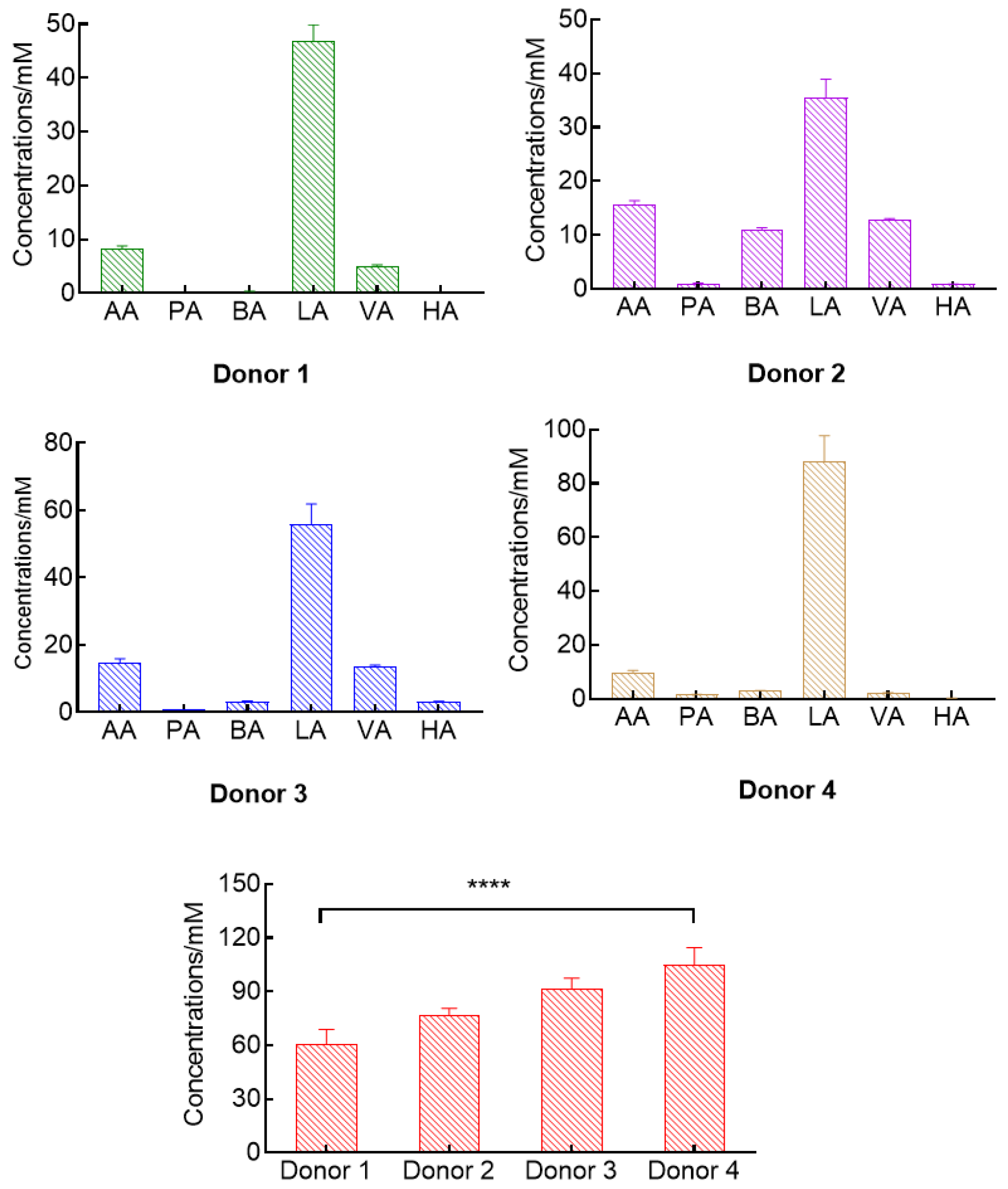
| Organic Acids | Regression Equations * | Coefficient of Determinations (R2) | Dynamic Ranges (mM) |
|---|---|---|---|
| Acetic acid | Y = 15,093X + 11,348 | 0.9993 | 0.01–10.0 |
| Propionic acid | Y = 288,086X + 60,179 | 0.9980 | 0.01–5.0 |
| Butyric acid | Y = 145,822X + 45,833 | 0.9993 | 0.01–10.0 |
| Lactic acid | Y = 3688X + 28,466 | 0.9913 | 0.01–20.0 |
| Valeric acid | Y = 62,334X + 17,018 | 0.9970 | 0.01–5.0 |
| Hexanoic acid | Y = 130,755X + 11,194 | 0.9993 | 0.01–5.0 |
| Organic Acids | 0.1 mM | 0.5 mM | 1.0 mM | 2.5 mM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measured 1 (mM) | CV 2 (%) | Rec 3 (%) | Measured (mM) | CV (%) | Rec (%) | Measured (mM) | CV (%) | Rec (%) | Measured (mM) | CV (%) | Rec (%) | |
| Acetic acid | 0.10 ± 0.01 | 10 | 99 | 0.55 ± 0.04 | 7 | 109 | 1.01 ± 0.09 | 8 | 101 | 2.66 ± 0.09 | 3 | 106 |
| Propionic acid | 0.09 ± 0.01 | 6 | 94 | 0.51 ± 0.03 | 6 | 102 | 0.97 ± 0.06 | 6 | 97 | 2.63 ± 0.14 | 5 | 105 |
| Butyric acid | 0.10 ± 0.01 | 11 | 97 | 0.54 ± 0.05 | 10 | 108 | 1.00 ± 0.05 | 5 | 100 | 2.52 ± 0.12 | 4 | 100 |
| Lactic acid | 0.09 ± 0.01 | 6 | 92 | 0.51 ± 0.04 | 8 | 102 | 1.02 ± 0.08 | 8 | 102 | 2.47 ± 0.13 | 5 | 99 |
| Valeric acid | 0.11 ± 0.01 | 5 | 107 | 0.50 ± 0.03 | 7 | 101 | 1.04 ± 0.05 | 4 | 104 | 2.57 ± 0.05 | 2 | 103 |
| Hexanoic acid | 0.10 ± 0.01 | 8 | 101 | 0.55 ± 0.02 | 5 | 111 | 1.06 ± 0.11 | 10 | 106 | 2.58 ± 0.08 | 3 | 103 |
| Organic Acids | Native Organic Acid in Bacterial Fermentation Medium (Dilution, CV%) | Standard Organic Acid at Three Levels [Native + Standards (CV%, Recoveryb%)] * | Recovery Average (%) | ||
|---|---|---|---|---|---|
| 0.5 mM | 1.0 mM | 2.5 mM | |||
| Acetic acid | 0.3 ± 0.1 (1-fold, 15) | 0.7 ± 0.2 (18, 90) | 1.4 ± 0.3 (11, 108) | 2.7 ± 0.4 (14, 96) | 98 |
| Propionic acid | 1.1 ± 0.2 (1-fold, 13) | 1.5 ± 0.1 (7, 94) | 1.8 ± 0.2 (11, 87) | 3.7 ± 0.5 (14, 104) | 95 |
| Butyric acid | 9.0 ± 0.7 (10-fold, 8) | 9.5 ± 0.4 (4, 100) | 10.8 ± 0.6 (6, 108) | 12.3 ± 0.8 (7,107) | 105 |
| Lactic acid | 13.4 ± 0.9 (10-fold, 7) | 14.1 ± 0.5 (4, 101) | 15.0 ± 0.6 (4, 104) | 15.5 ± 0.5 (3, 98) | 101 |
| Valeric acid | 2.0 ± 0.1 (1-fold, 2) | 2.4 ± 0.2 (8, 96) | 3.1 ± 0.4 (12, 103) | 3.9 ± 0.7 (18, 87) | 95 |
| Hexanoic acid | 1.7 ± 0.2 (1-fold, 9) | 2.4 ± 0.2 (8, 110) | 2.6 ± 0.2 (8, 96) | 3.8 ± 0.7 (18, 90) | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Tang, X.; Qu, C.; Zhang, Y.; Zhang, C.; Chu, Q.; Wang, H. Development of an LC-MS Method for the Quantitative Determination of Six Organic Acids Produced by Bacterial Fermentation In Vitro and In Vivo. Processes 2025, 13, 697. https://doi.org/10.3390/pr13030697
Wang J, Tang X, Qu C, Zhang Y, Zhang C, Chu Q, Wang H. Development of an LC-MS Method for the Quantitative Determination of Six Organic Acids Produced by Bacterial Fermentation In Vitro and In Vivo. Processes. 2025; 13(3):697. https://doi.org/10.3390/pr13030697
Chicago/Turabian StyleWang, Jing, Xuxiao Tang, Chao Qu, Yingzhong Zhang, Chaoqun Zhang, Qiulu Chu, and Hao Wang. 2025. "Development of an LC-MS Method for the Quantitative Determination of Six Organic Acids Produced by Bacterial Fermentation In Vitro and In Vivo" Processes 13, no. 3: 697. https://doi.org/10.3390/pr13030697
APA StyleWang, J., Tang, X., Qu, C., Zhang, Y., Zhang, C., Chu, Q., & Wang, H. (2025). Development of an LC-MS Method for the Quantitative Determination of Six Organic Acids Produced by Bacterial Fermentation In Vitro and In Vivo. Processes, 13(3), 697. https://doi.org/10.3390/pr13030697





