Online Monitoring of Faulty Gases (O3, NO2, CO) in Substation Secondary Equipment Based on Cr-Doped BN Sensor: Insights from Density Functional Theory
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Target Gases and Doping Models
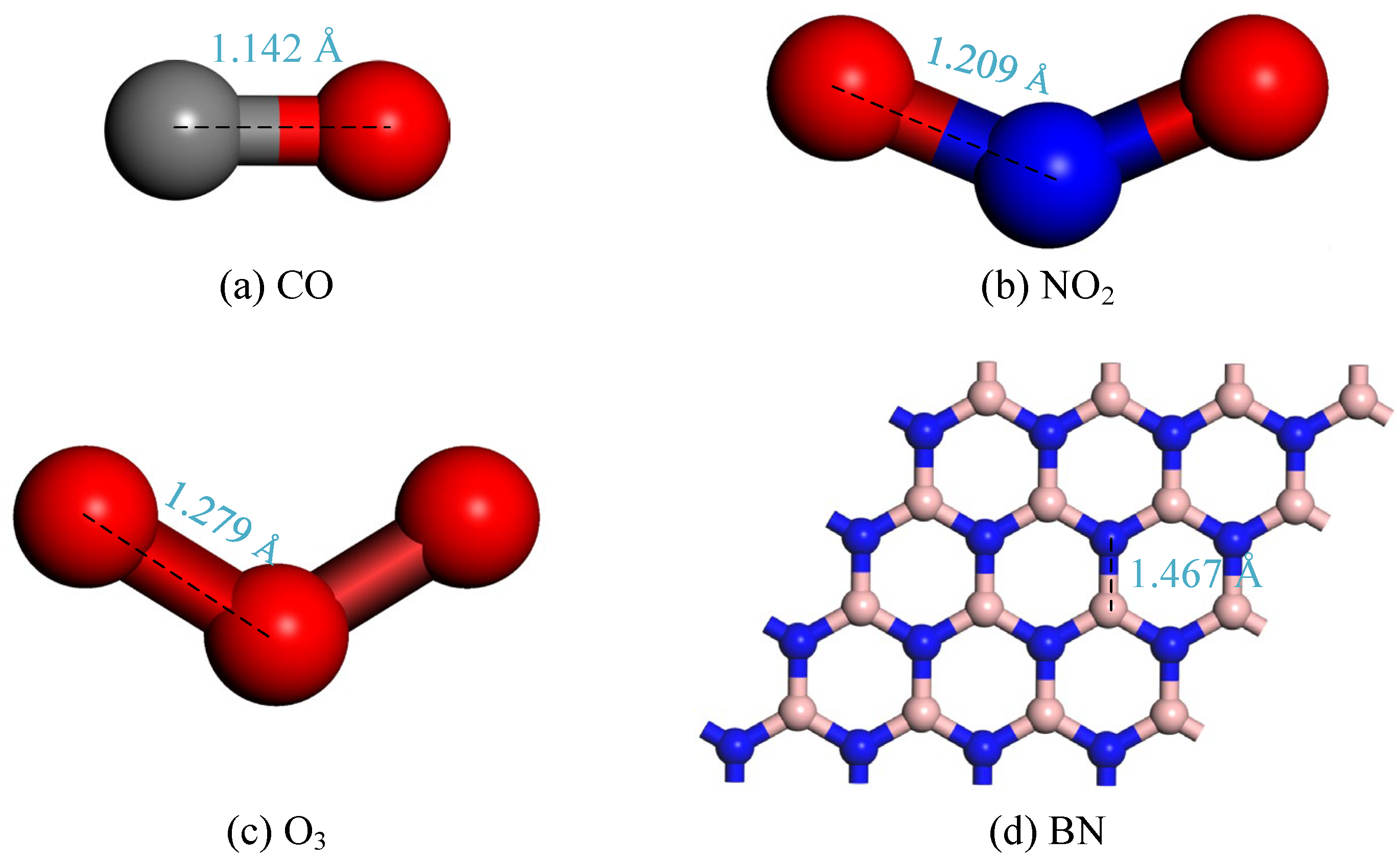
3.1.1. Geometric Structures of Target Gases and Doping Models
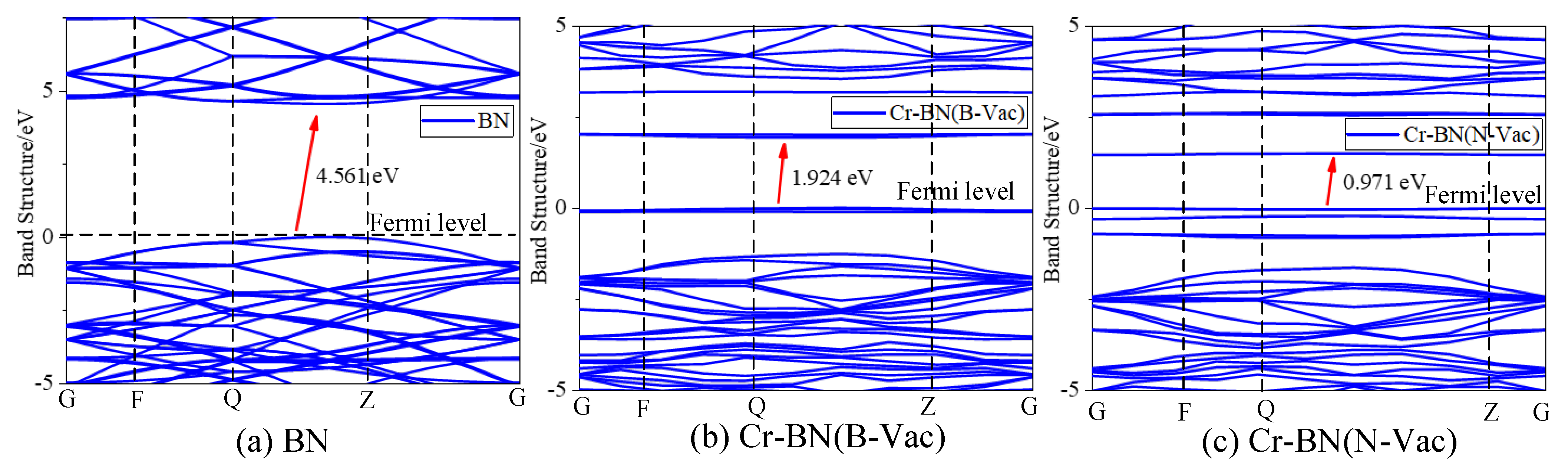
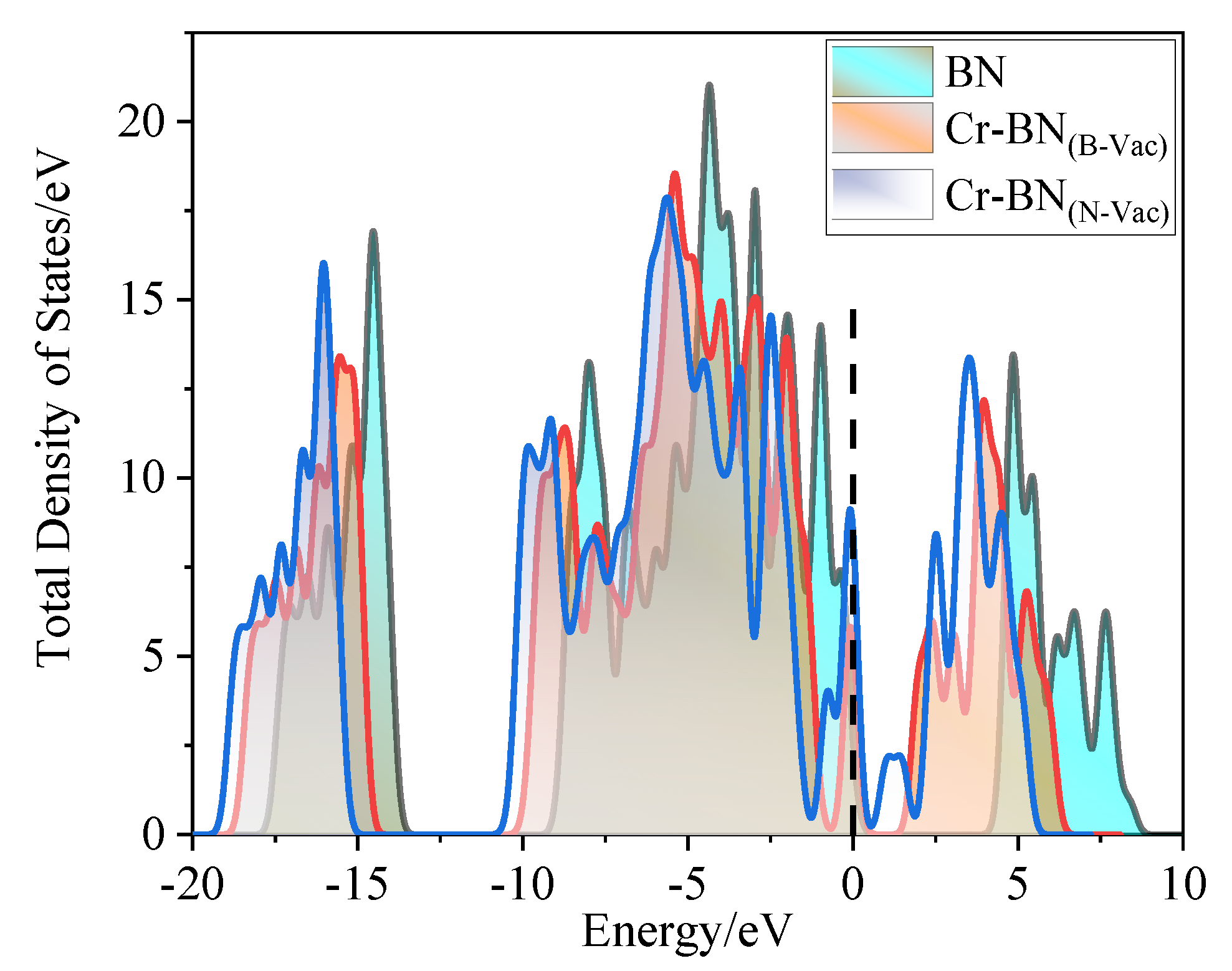
3.1.2. Electronic Performance Analysis of Doping Process
3.2. Adsorption and Electronic Performance Analysis of Adsorption Process
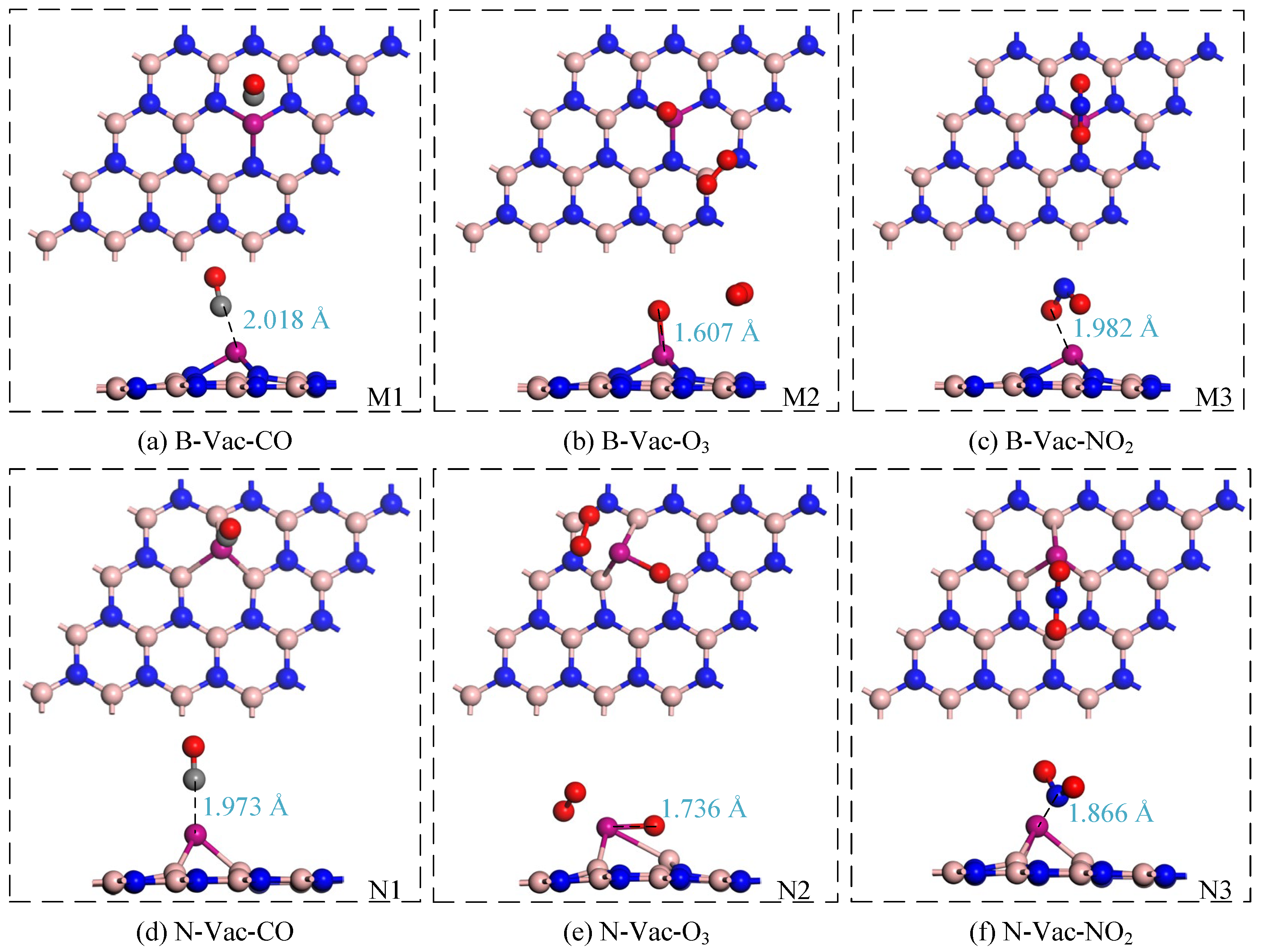
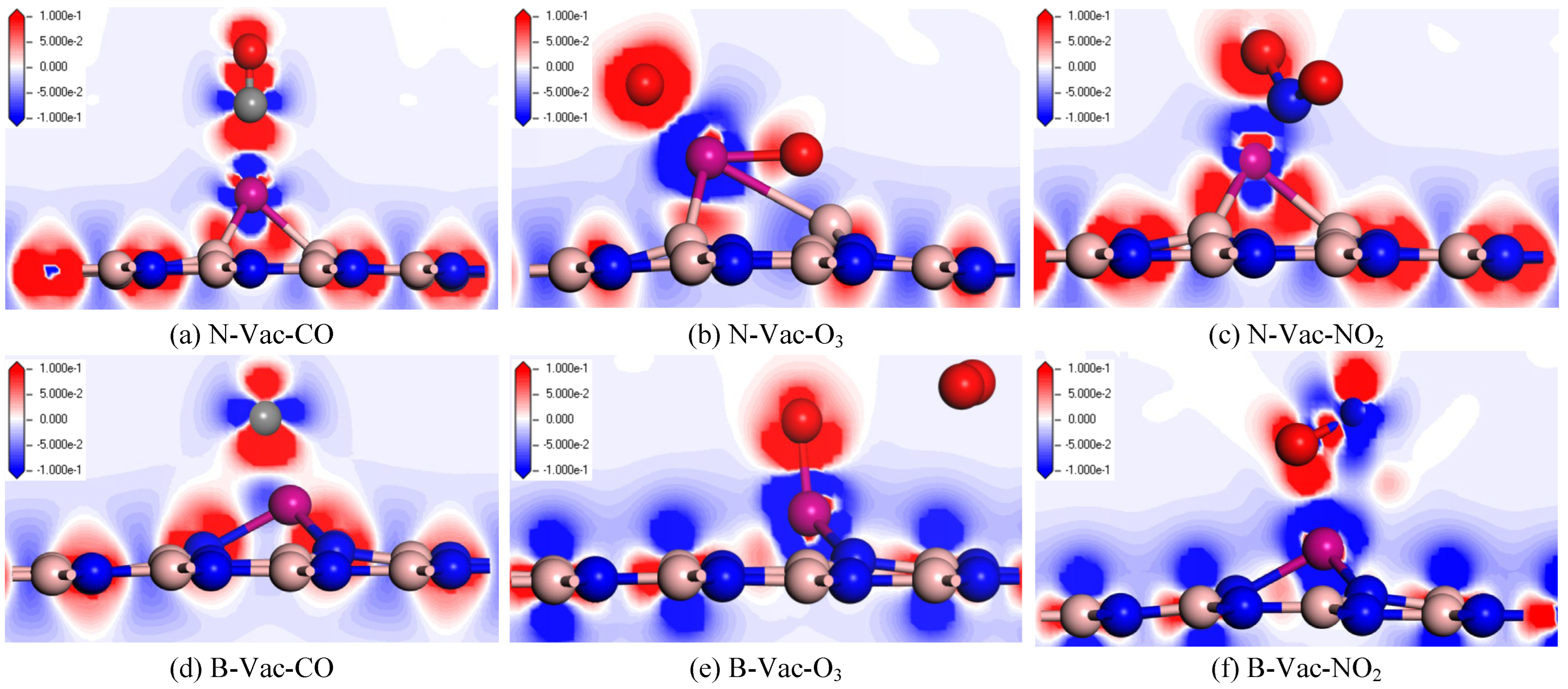
3.2.1. Adsorption Performance Analysis of Adsorption Process
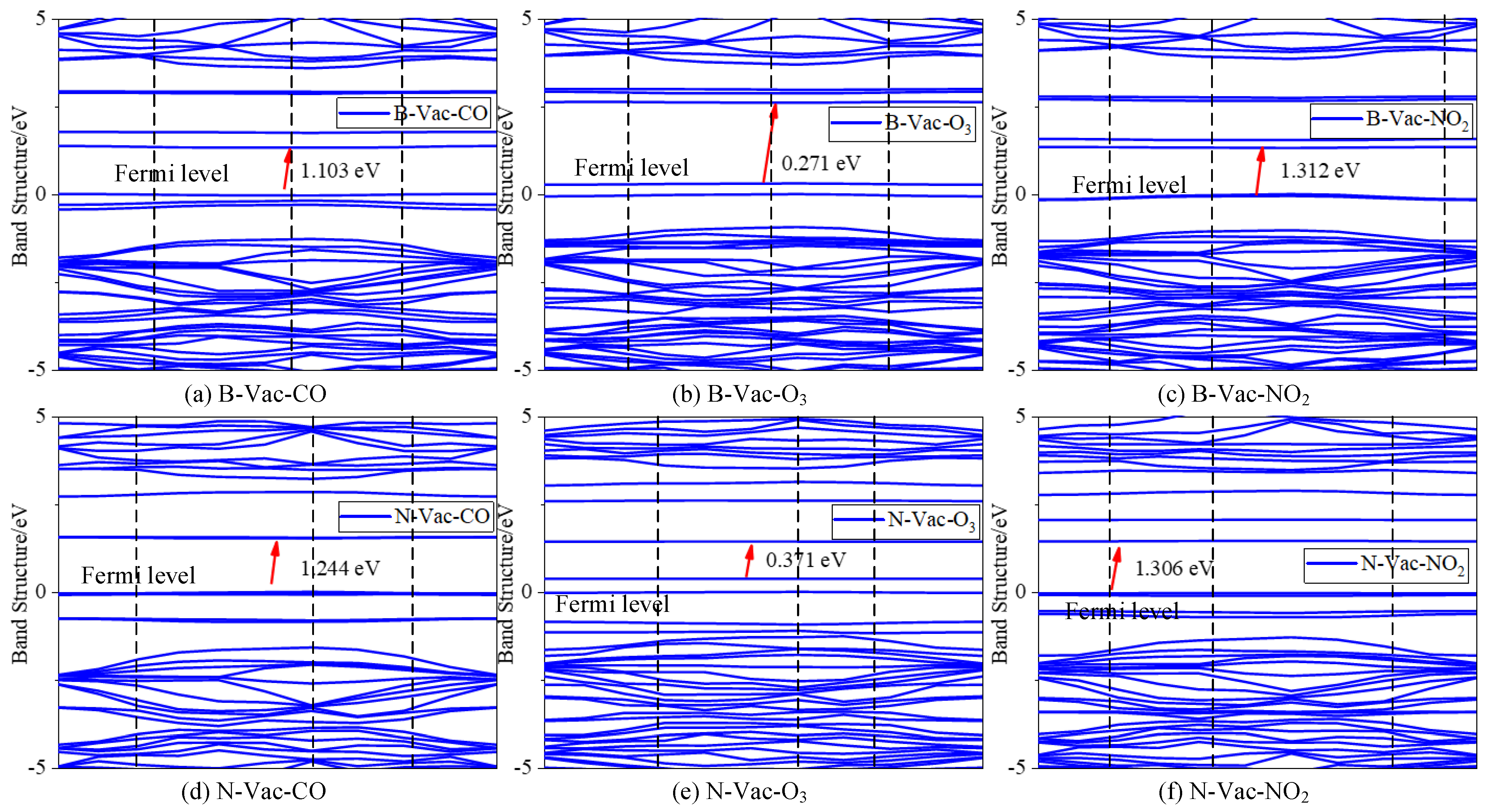
3.2.2. Electronic Performance Analysis of Adsorption Process
4. Conclusions
- The incorporation of metallic chromium (Cr) doping substantially boosts the native sensitivity of BN nanosheets to fault gases found in the secondary equipment of substations, offering an abundance of active adsorption sites for gases.
- The adsorption capabilities of the two Cr-modified BN materials for fault gases in the secondary equipment of substations are ranked as follows: O3 > NO2 > CO. The corresponding adsorption energies are −4.452 eV and −8.577 eV for O3, −2.576 eV and −3.024 eV for NO2, and −1.029 eV and −1.550 eV for CO, respectively. The nature of these adsorptions is characterized as physical–chemical in type.
- After adsorption, various fault gases exhibit different impacts on the electronic properties of the entire system. These variations in adsorption types and strengths lead to the generation of distinct electrical signals, which allows the differentiation of different fault gases.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Li, W.; Gu, Y.; Cui, W.; He, X. Improved Transformerless Inverter with Common-mode Leakage Current Elimination for a Photovoltaic Grid-connected Power System. IEEE Trans. Power Electron. 2012, 27, 752–762. [Google Scholar] [CrossRef]
- Yu, X.; Singh, C. A Practical Approach for Integrated Power System Vulnerability Analysis with Protection Failures. IEEE Trans. Power Syst. PWRS 2004, 19, 1811–1820. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, F.; Xue, Y.; Dong, Z.; Xin, J. Power System Stochastic Economic Dispatch Considering Uncertain Outputs from Plug-in Electric Vehicles and Wind Generators. Autom. Electr. Power Syst. 2010, 34, 22–29. [Google Scholar]
- Jieyu, W.; Zhe, Z.; Xianggen, Y. Study on Condition-based Maintenance of Electrical Secondary Equipment. Relay 2002, 30, 22–24. [Google Scholar]
- Hailing, W.; Rui, W. Status-oriented Maintenance of Secondary Electrical Equipment. Heilongjiang Electr. Power 2005, 1, 78–80. [Google Scholar]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B-Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 Nanotubes in Gas Sensor and Lithium-ion Battery Applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B-Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Chen, C.; Kou, L.; Frauenheim, T. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I–V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.; Tiemann, M. Ordered Mesoporous In2O3: Synthesis by Structure Replication and Application as a Methane Gas Sensor. Adv. Funct. Mater. 2010, 19, 653–661. [Google Scholar] [CrossRef]
- Moriya, R.; Park, S.; Masubuchi, S.; Watanabe, K.; Taniguchi, T.; Machida, T. Probing Many-body Interactions in the Cyclotron Resonance of h-BN/bilayer Graphene/h-BN. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2021, 24, 104. [Google Scholar] [CrossRef]
- Chaouche, A.C.; Lachebi, A.; Hamza, A.; Driz, A.; Miloud, B. First-principles Study of Two-dimensional (2D) AlN/BN and GaN/BN Bilayer Heterostructures. In Proceedings of the Deuxièmes Journées Doctorales de Génie Electrique, Sidi Bel Abbes, Algeria, 4–8 December 2018. [Google Scholar]
- Ramasubramaniam, A.; Naveh, D.; Towe, E. Tunable Band Gaps in Bilayer Graphene-BN Heterostructures. Nano Lett. 2011, 11, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, X.; Niu, M.; Dai, X.; Li, W.; Wang, X.; Zhao, M.; Wang, T.; Tang, W. Modulation of Interfacial Electronic Properties in PbI2 and BN Van Der Waals Heterobilayer via External Electric Field. Appl. Surf. Sci. J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2017, 411, 46–52. [Google Scholar]
- Cobaleda, C.; Pezzini, S.; Diez, E.; Bellani, V. Temperature and Density-dependent Transport Regimes in a h-BN/bilayer Graphene/h-BN Heterostructure. Phys. Rev. B 2014, 89, 106–112. [Google Scholar] [CrossRef]
- Shu, H.; Liu, X. Tuning Electronic and Optical Properties of Graphene/h-BN Heterobilayer via Surface Modification. Appl. Surf. Sci. J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2022, 605, 154591. [Google Scholar] [CrossRef]
- Abdullah, N.R.; Rashid, H.O.; Tang, C.S.; Manolescu, A.; Gudmundsson, V. Role of Interlayer Spacing on Electronic, Thermal and Optical Properties of BN-codoped Bilayer Graphene: Break Influence of the Interlayer and the Induced Dipole-dipole Interactions. J. Phys. Chem. Solids 2021, 155, 110095. [Google Scholar] [CrossRef]
- Hussein, T.A.; Shiltagh, N.M.; Alaarage, W.K.; Abbas, R.R.; Jawad, R.A.; Nasria, A.H.A. Electronic and Optical Properties of the BN Bilayer as Gas Sensor for CO2, SO2, and NO2 Molecules: A DFT Study. Results Chem. 2023, 5, 100978. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as Toxic Gas Scavenger: A First-principles Study. Nanoscale Adv. 2019, 1, 772. [Google Scholar] [CrossRef]
- Chen, Y.; Gui, Y.; Ding, Z.; Zu, L.; Chen, X. Adsorption and Gas-sensing Properties of Pdn-GaNNTs to C2H2 and H2 Gases. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 136, 115004. [Google Scholar] [CrossRef]
- Kim, M.-C.; Sim, E.; Burke, K. Understanding and Reducing Errors in Density Functional Calculations. Phys. Rev. Lett. 2013, 111, 073003. [Google Scholar] [CrossRef]
- Li, T.; Hu, S.L.; Ma, R.; Sang, T.; Chen, Q.; Ma, L.; Chen, Y.; Liao, Y.; Yang, G.; Huang, Y.; et al. The Electronic Properties and Adsorption Mechanism of Agn, Aun (n = 1–4) Modified GeSe Monolayer towards Hazardous gases (H2S, NH3, NO2 and SOF2): A First-principles Study. Surf. Interfaces 2022, 32, 102150. [Google Scholar] [CrossRef]
- Bond, A.D.; Solanko, K.A.; van de Streek, J.; Neumann, M.A. Experimental Verification of a Subtle Low-temperature Phase transition Suggested by DFT-D Energy Minimisation. CrystEngComm 2011, 13, 1768–1771. [Google Scholar] [CrossRef]
- Soni, H.; Singh, A.; Mishra, A.K. Biaxial Strain Induced Tunable Electronic Properties Study of ZnO Nanoparticles via First-Principles Density Functional Theory. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2023, 288, 116186. [Google Scholar] [CrossRef]
- Jonuarti, R.; Kurniawan, R.; Darma, Y.; Suprijadi; Hidayat, R. Tiny (ZnO) Clusters Supported on Graphene for Solar Energy Trapping: A Density Functional Theory Study. J. Taiwan Inst. Chem. Eng. 2023, 144, 104769. [Google Scholar] [CrossRef]
- Pentyala, P.; Biswas, P.; Jha, P.K. A Density Functional Theory Study of Ni-x (x = 4–16) Cluster Impregnation Effects in Multi-metal (Ce, Ti) UiO-66 Metal-organic Frameworks. New J. Chem. 2023, 47, 8549–8557. [Google Scholar] [CrossRef]


| Cr-BN(B-Vac) | Cr-BN(N-Vac) | |
|---|---|---|
| Emod | −1.289 | −1.889 |
| QCr | 0.292 | −0.358 |
| Material | Gas | Model | Eads/eV | Distance/Å | Charge Transfer/eV |
|---|---|---|---|---|---|
| Cr-BN(B-Vac) | CO | M1 | −1.029 | 2.018 | 0.124 |
| O3 | M2 | −4.452 | 1.607 | −0.512 | |
| NO2 | M3 | −2.576 | 1.982 | −0.386 | |
| Cr-BN(N-Vac) | CO | N1 | −1.550 | 1.973 | 0.032 |
| O3 | N2 | −8.577 | 1.736 | −1.017 | |
| NO2 | N3 | −3.024 | 1.866 | −0.339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Gao, P.; Wang, Y.; Wang, Z.; Li, J.; Zou, H. Online Monitoring of Faulty Gases (O3, NO2, CO) in Substation Secondary Equipment Based on Cr-Doped BN Sensor: Insights from Density Functional Theory. Processes 2025, 13, 746. https://doi.org/10.3390/pr13030746
Guo Z, Gao P, Wang Y, Wang Z, Li J, Zou H. Online Monitoring of Faulty Gases (O3, NO2, CO) in Substation Secondary Equipment Based on Cr-Doped BN Sensor: Insights from Density Functional Theory. Processes. 2025; 13(3):746. https://doi.org/10.3390/pr13030746
Chicago/Turabian StyleGuo, Zhiqi, Peifeng Gao, Yibo Wang, Zhiqiang Wang, Jinchen Li, and Hongbo Zou. 2025. "Online Monitoring of Faulty Gases (O3, NO2, CO) in Substation Secondary Equipment Based on Cr-Doped BN Sensor: Insights from Density Functional Theory" Processes 13, no. 3: 746. https://doi.org/10.3390/pr13030746
APA StyleGuo, Z., Gao, P., Wang, Y., Wang, Z., Li, J., & Zou, H. (2025). Online Monitoring of Faulty Gases (O3, NO2, CO) in Substation Secondary Equipment Based on Cr-Doped BN Sensor: Insights from Density Functional Theory. Processes, 13(3), 746. https://doi.org/10.3390/pr13030746





