Abstract
Citrus fruit production generates substantial by-products, primarily from juice processing, which represent significant environmental and economic challenges. However, these residues, rich in polysaccharides, flavonoids, essential oils, and enzymes, offer an untapped resource for biotechnological applications. This review explores the potential of citrus by-products as substrates for enzyme production, focusing on key industrial enzymes such as cellulases, pectinases, xylanases, ligninases, lipases, and proteases. Various microbial strains have demonstrated the ability to convert citrus residues into high-value enzymes through solid-state and submerged fermentation. The optimization of fermentation conditions—including temperature, pH, moisture content, and the carbon-to-nitrogen ratio—further enhances enzymatic yields. The valorization of citrus waste aligns with circular economy principles, reducing environmental impacts while supporting sustainable bioproduct development for the food, biofuel, pharmaceutical, and textile industries. Future research should focus on scaling up enzyme production using citrus waste to improve economic feasibility and advance industrial biorefineries.
1. Introduction
Citrus fruits, which belong to the Rutaceae family, are one of the most produced fruits worldwide [1]. Among the citrus fruits, oranges, lemons, limes, tangerine, and grapefruits are some of the most widely cultivated citrus varieties and are consumed globally [2]. The most important aspects influencing their prominent production include their outstanding properties such as nutritional eminence and sensory properties (flavor, taste, and smell) [3].
According to the literature, in 2023–2024, the worldwide production of citrus fruits reached more than 1.02 × 1011 kg, with oranges being in the lead, representing almost half of the production [4]. Against this background, Brazil is the largest orange fruit and orange juice producer in the world [4]. However, processing oranges to obtain orange juice results in a large amount of waste, as more than 50% of the fruit becomes a by-product in the process [5]. Amongst these by-products, leftover products from orange juice processing are materials that can contain compounds of industrial and/or commercial interest, such as fermentable sugars (glucose, fructose and sucrose), polysaccharides (cellulose, xylan, and pectin), flavonoids, polyphenols, essential oils (limonene mainly), and enzymes [6].
Enzymes, viz. cellulases, pectinases, lipases and amylases, are fundamental to industries such as the food, textiles, pharmaceuticals and biofuels industries [7]. According to the Market Analysis Report: Enzymes Market Size review recently released by Grand View Research, the global enzyme market was valued at USD 60.48 billion during 2023 and is estimated to increase at a compound annual growth rate of 6.5% from 2024 to 2030 [8]. However, the main propellant of the enzyme market includes advanced technologies presented to improve the cost-effectiveness and efficiency of biocatalysts [9].
Over the years, there has been a growing interest in the valorization of citrus by-products, as reflected in the increasing number of scientific publications on the topic. According to the Scopus database, the number of publications including “citrus by-product” in the title, abstract, or keywords, has grown from 41 documents in 2015 to 155 in 2024. This trend highlights the recognition of citrus waste as a valuable resource with scope for the development of sustainable and high-value applications. Advances in bioprocessing technologies and circular economy approaches have further contributed to this expansion, reinforcing the potential of citrus by-products in various industrial sectors
In this way, due to the wealth of components present in citrus by-products, the use of them to produce biomolecules, such as enzymes, is a noble alternative with the aim of obtaining bioproducts of commercial value and interest. As such, within the framework of large-scale citrus biorefineries in citrus-producing countries such as Brazil, China, South Africa, and the USA, the bioproduction of molecules based on the biorefinery concept is promising [10]. Therefore, this review focuses on the hidden potential of citrus by-products as a valuable source for obtaining enzymes of industrial interest, analyzing valorization strategies for sustainable production.
2. Global Overview of Citrus By-Product
2.1. Citric Fruits
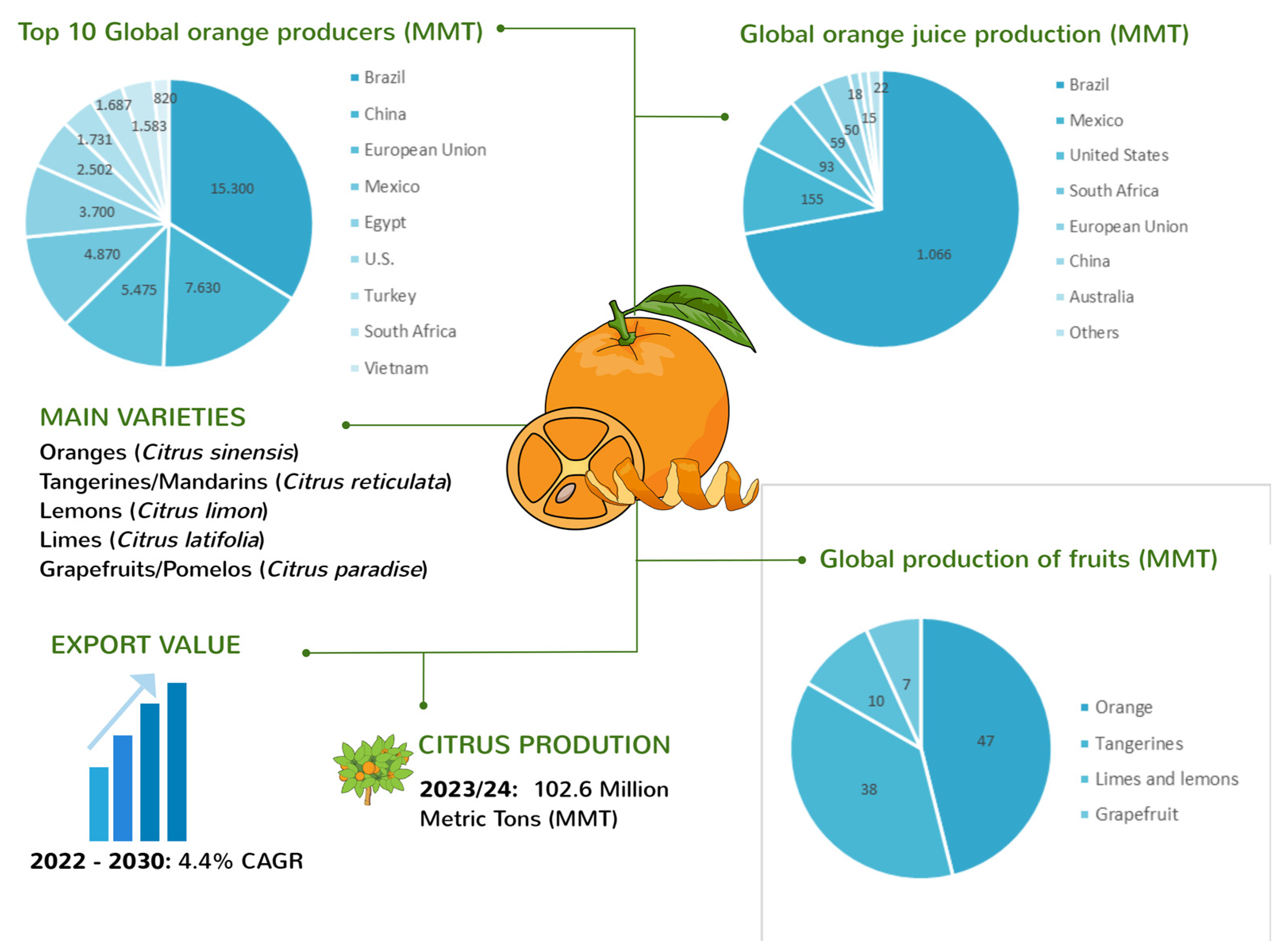
Citrus fruits stand out as one of the most significant fruit crops globally. Oranges (Citrus sinensis and Citrus aurantium), lemons (Citrus limon), limes (Citrus latifolia, Citrus aurantifolia, and Citrus limettioides), tangerines/mandarins (Citrus reticulata, Citrus delicia, Citrus clementina, Citrus unshiu, and Citrus nobilis), and grapefruits/pomelos (Citrus paradise and Citrus maxima) are some of the most widely cultivated citrus varieties, commonly traded and consumed as fresh fruit, juice, or concentrate [2]. According to the Citrus: World Markets and Trade report recently released by the U.S. Department of Agriculture’s Foreign Agricultural Service [4], the global production of citrus fruits reached 102.6 million metric tons in 2023/24 with a 4.4% compound annual growth rate (CAGR) from 2022 to 2030. Fresh orange production dominates the global citrus industry, accounting for 47.4 million tons, which is 46.2% of the total citrus output (Figure 1).
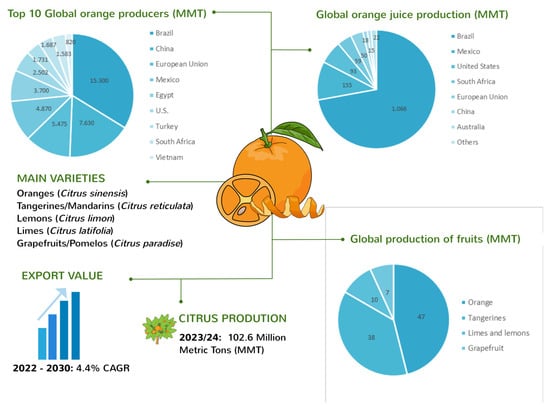
Figure 1.
Overview of global citrus fruit production showing main citrus fruits varieties commercialized, export value growth, global citrus fruits production, top 10 orange producers, and orange juice producers in MMT.
The leading producers of citrus fruits are Brazil and China. Following oranges, tangerines are the next most prominent citrus fruit, with a harvest of 38.2 million tons, representing 37.2% of citrus production. Limes and lemons contribute 10.1 million tons, making up 9.8% of citrus production. Grapefruit yields alone add 6.9 million tons to the global market, representing 6.7% of citrus production. Additionally, the global orange juice market reached 1.5 million tons [4]. Brazil is the leading producer. However, production is down due to a reduced availability of fruit for processing due to drought, extremely high temperatures, and some incidences of citrus greening. Meanwhile, Mexico claims the title of the largest producer of lemons and limes. Other major citrus-producing nations include the European Union, Egypt, the USA, Turkey, and South Africa, rounding out the top 10.
Citrus fruit processing results in a significant volume of by-products, such as peels, seeds, pomace, and wastewater, which can make up 55–60% of the fruit’s total weight [5]. It is estimated that approximately 40% of the world’s citrus fruits are used exclusively by juice processing industries, leading to the generation of substantial amounts of citrus waste [11]. These wastes are linked to substantial environmental harm and financial losses related to waste mismanagement [12,13]. With their high moisture content and abundance of compounds like sugars and oils, they present unique challenges for waste management. These characteristics complicate the drying process, making the waste highly fermentable, because of the presence of a high carbohydrate content which accelerates its degradation [14]. As a result, proper handling of these by-products is often a difficult task for the citrus industry.
It is equally vital to recover by-products from the food supply chain, supporting the principles of a circular economy. There is growing recognition that this problem can be solved through the utilization of waste as a resource and using green and sustainable technologies. Many industries are embracing the circular economy model by transforming food waste, such as citrus peels and seeds, into valuable products like essential oils, animal feed, medicines, cosmetics, and food supplements [15]. These practices not only reduce waste but also create new revenue streams, demonstrating a growing commitment to sustainability and resource efficiency in food processing [16].
2.2. Chemical Composition of Citrus Wastes
The citrus processing industry is typically organized into transporting harvested fruits to the processing facility, followed by washing, grading, and sorting, then juice extraction and heat treatment, and finally packaging the finished product. After juice extraction, substantial solid residues and wastewater remain. The most crucial concern of processing industries is solid waste management which accounts for around 90% of the total waste generated. Typically, this solid waste comprises peel, rags, pulp residues, and seeds that remain after the process of juice extraction [17]. Developing effective technologies for converting citrus by-products requires a deep understanding of their composition, which varies significantly based on fruit variety, climate, and the juice extraction method used [18]. The typical proximate compositions of some citrus by-products have been summarized in Table 1.

Table 1.
Composition (% dry weight) of citrus by-products after extraction of juice.
The chemical composition of citrus by-products differs regarding their constituents and their values. The most commonly reported components in the literature include cellulose, hemicellulose, lignin, ash, protein, fats, sugars, and pectin. Citrus peels and rags are the most important type of waste representing nearly 50–55% of the wet fruit mass [29]. As shown in Table 1 it should be noted that citrus by-products present a wide range of variability in their composition. Orange peels and pulp, for example, include a pectin content ranging from 11.18 to 35.30%. In addition, they contain valuable compounds, including flavonoids (such as naringin, hesperidin, tangeretin, nobiletin, rutin, quercetin, and kaempferol), phenolic acids (like caffeic, p-coumaric, ferulic, and sinapic acids), essential oils, and dietary fibers. Extensive research is underway to explore the potential of citrus peels for extracting biologically active, high-value compounds that can be applied in different industrial fields such as the pharmaceutical, food, and chemical industries [16]. Seeds, which make up around 2–7% of the total weight of citrus fruits, are the unused portion. However, they are recognized as a valuable source of oil, comprising 20 to 40% of their weight [30]. During juice extraction, by-products like seeds are easily separated and, if managed effectively, can significantly reduce the amount of waste produced. However, the concentration of valuable compounds in citrus processing waste can fluctuate based on various factors, including species, variety, climate, cultivation practices, and waste management methods [3,28].
3. Enzyme-Based Biorefinery of Citrus By-Products: Universal Overview
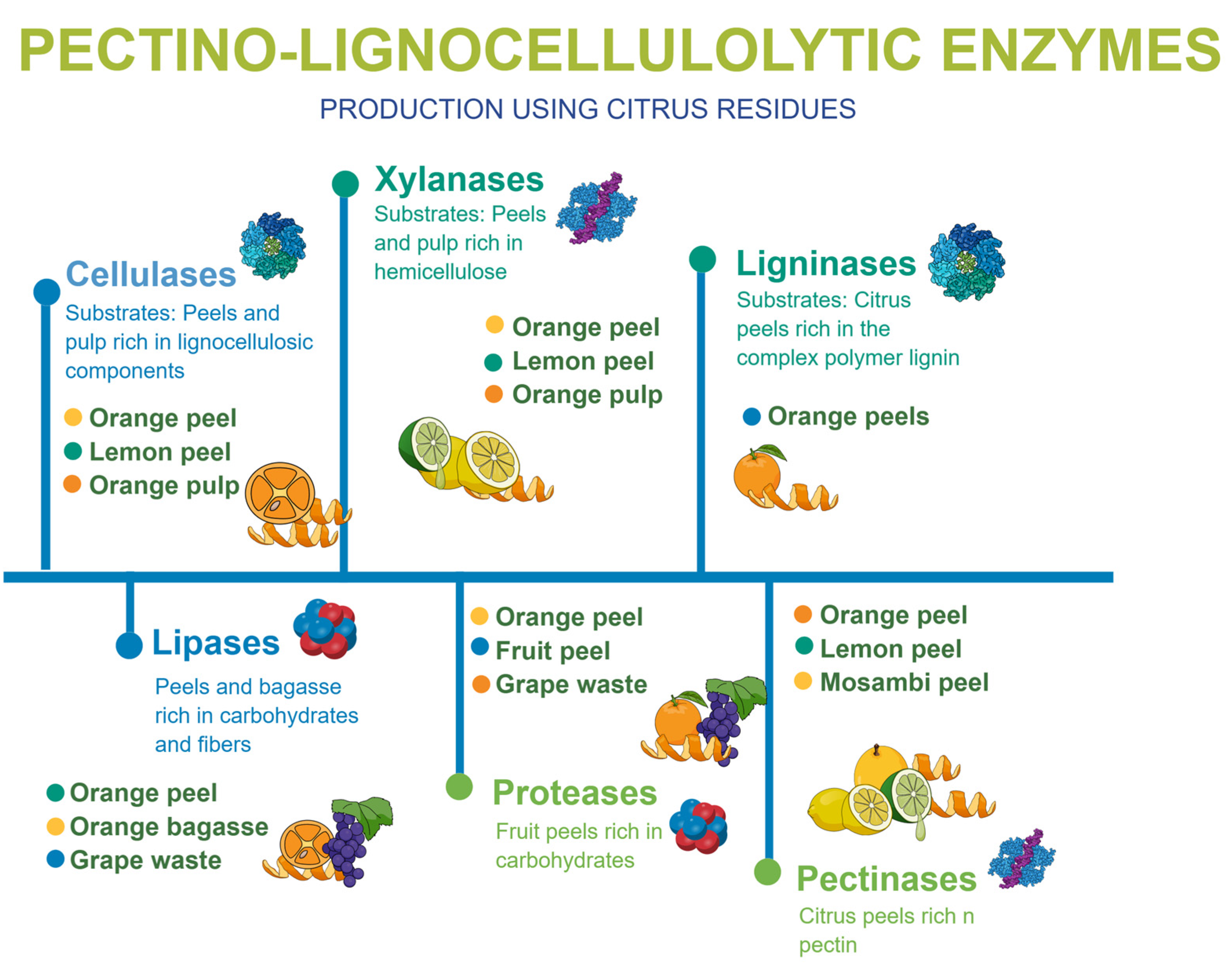
Due to the rich nutritional composition of citrus by-products, they have become a valuable substrate for many microorganisms that are able to biosynthesize products of industrial interest, such as enzymes, which have been highlighted as sources of biocatalysts with applications in food, pharmaceutical and energy industrial processes [31,32]. Figure 2 demonstrates the different enzymes that can be produced using citrus by-products.
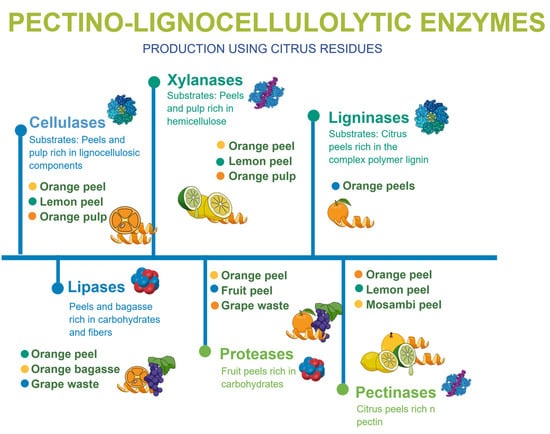
Figure 2.
Variety of enzymes produced using diverse types of citrus by-products.
Studies have highlighted many enzymes produced from citrus by-product, mainly cellulases, xylanases, ligninases, pectinases, lipases and proteases. [31,32,33,34]. Table 2 summarizes the production of enzymes using citrus residues, highlighting key findings and the efficacy of different microbial strains.

Table 2.
Production of pectino-lignocellulolytic enzymes using citrus residues.
3.1. Lignocellulolytic Enzymes
The use of agro-industrial by-products in biotechnology has become a sustainable and economically viable approach towards enzyme production [47]. Among agricultural residues, citrus by-products, such as peels, pulp, and pomace, have emerged as promising sources for this purpose. These by-products are rich in compounds, which can serve as efficient substrates for and induce the production of several enzymes [48].
Industrial enzymes, such as cellulases, pectinases, and amylases, play a pivotal role in industries like food, textiles, pharmaceuticals, and biofuels [48]. The use of citrus residues as a carbon and energy source for enzyme-producing microorganisms, such as fungi and bacteria, not only reduces enzyme production costs but also contributes to sustainable waste management. The conversion of these by-products into high-value biomolecules exemplifies the principles of the circular bioeconomy, transforming environmental liabilities into industrial inputs [49].
In this context, the production of enzymes from citrus by-products relies on the biotransformation promoted by specific microorganisms, which utilize complex components from the waste as a nutrient source and activators of enzymatic synthesis. The main challenges and advances in this approach include optimizing cultivation and fermentation conditions, the selection of efficient microbial strains, and using residues from different citrus varieties. As such, this field of research is expanding, with the potential to revolutionize the enzyme industry and agricultural waste management [49,50,51,52].
3.1.1. Cellulases
Cellulases like endoglucanases, cellobiohydrolases, and β-glucosidases are enzymes that catalyze the breakdown of cellulose into simple sugars, such as glucose, and are widely used in industries like biofuels, textiles, food, and paper [53].
Citrus residues, such as peels and pulp, are rich in lignocellulosic components, making them promising substrates for cellulase production through fermentation using specific microorganisms [54]. Filamentous fungi such as Trichoderma and Aspergillus species have been extensively studied for their ability to produce large quantities of cellulases in submerged (SmF) and solid-state fermentation (SSF) processes. These microorganisms utilize citrus residues as a carbon and energy source, promoting the biotransformation of polysaccharides in the residue [31,55].
For example, studies like Srivastava et al. [54] demonstrated that orange peels could be used as a substrate in solid-state fermentation processes for cellulase production by Emericella variecolor, resulting in a high enzymatic activity. Another example is the study by Al Mousa et al. [56], which explored the submerged fermentation of tangerine peels with Mucor circinelloides and Mucor hiemalis, achieving a high cellulase production compared to other agricultural residues. This research highlighted that the cellulose-rich composition of citrus residues favors the induction of hydrolytic enzyme production, such as cellulases.
Furthermore, the pre-treatment of citrus residues, such as pectin removal or the application of physicochemical processes like acid hydrolysis, can significantly enhance the efficiency of cellulase production. Acid hydrolysis involves using diluted or concentrated acids to break down the lignocellulosic structure, solubilizing hemicellulose and altering lignin composition. These structural modifications reduce cellulose crystallinity and increase the material’s porosity, making the cellulose components more accessible to microbial and enzymatic action. Studies such as those by Mathias et al. [57] and Da Silva et al. [58] have shown that the enzymatic yield significantly increases after pre-treatment due to the improved availability of lignocellulosic components for microbial activity.
Using citrus residues as a substrate for cellulase production not only promotes the valorization of agro-industrial residues but also helps reduce enzyme production costs, as the substrate is low-cost and widely available, particularly in countries with large citrus juice industries, such as Brazil and the United States [11,59].
However, the production of cellulases from citrus residues faces technical challenges, such as the need to optimize fermentation conditions, including factors like pH, temperature, moisture, and substrate proportion. Additionally, the variability in the composition of citrus residues, which can differ between harvests and regions, must be considered in developing consistent processes for enzyme production [55,59].
3.1.2. Xylanases
The production of xylanases and other hemicellulases from citrus residues has garnered increasing interest in biotechnology, mainly due to the essential role of these enzymes in degrading hemicellulose, a significant component of plant cell walls [60]. Xylanases are particularly important for the hydrolysis of xylan, the primary polymer in hemicellulose, releasing simple sugars that can be used in several industrial applications such as biofuel production, food, paper, and animal feed [61].
Citrus residues contain significant amounts of hemicellulose, making them attractive substrates for producing xylanases and other hemicellulases through fermentation with microorganisms. Bacteria like Pseudomonas spp. [62] and fungi such as Trichoderma spp. [63] and Talaromyces spp. [64] have proven to be highly effective in xylanase production when grown on citrus-derived substrates. These microorganisms can utilize the complex polysaccharides present in the residues to induce the production of hemicellulases, including xylanases [62,63,64].
A study by Saha and Ghosh (2014) [65] demonstrated that using orange peel as a substrate in submerged fermentation with Penicillium citrinum resulted in high xylanase production. In this case, the cultivation conditions, such as temperature, pH, and fermentation time, were optimized to maximize enzyme production. Another study by Gooruee et al. [31] also highlighted the use of citrus residues, such as lemon peels, for xylanase production by Trichoderma spp., resulting in significant enzymatic activity, especially in submerged fermentation processes.
In another example, Silva et al. [63] investigated the production of hemicellulases from orange pulp using Trichoderma reesei in submerged fermentation. The study demonstrated that, in addition to xylanases, the presence of other hemicellulases, such as β-xylosidase and arabinofuranosidase, was significantly increased, reinforcing the potential of citrus residues as natural inducers of specific enzymes for hemicellulose degradation.
In addition to fungi, certain bacteria have also shown significant potential for xylanase and hemicellulase production from citrus residues. For instance, Zerva et al. [62] investigated xylanase production using Pseudomonas psychrotolerans and Pseudomonas oryzihabitans, demonstrating that the orange peels served as an efficient carbon source, resulting in high levels of xylanolytic activity. To enhance enzyme production efficiency, various physicochemical processes—such as steam explosion, acid or alkaline hydrolysis, and microwave-assisted treatments—can be applied to the biomass. These methods help disrupt the lignocellulosic matrix, increase substrate porosity, and improve the accessibility of carbohydrates for microbial metabolism, thereby boosting enzymatic yields.
Pre-treatment of citrus residues can also significantly increase hemicellulase production. The work of Mathias et al. [57] showed that the removal of components such as pectin and lignin from citrus residues through physical-chemical processes can increase the availability of hemicellulose, resulting in a higher enzyme production.
However, despite promising advances, challenges remain, such as optimizing fermentation conditions, variability in residue composition, and the need for proper pre-treatment to maximize enzymatic efficiency. Further studies are needed to expand the use of citrus residues as substrates in industrial hemicellulase production processes to meet the growing market demands [66].
3.1.3. Ligninases
The production of ligninases from citrus residues has attracted interest from the field of biotechnology due to the potential of these enzymes to degrade lignin, a complex and resistant polymer that is part of the plant cell wall [40,41]. Lignin is one of the most significant obstacles to the efficiency of processes aimed at converting lignocellulosic biomass into fermentable sugars and other value-added products, such as biofuels. Therefore, the production of ligninases, such as laccases, manganese peroxidases (MnP), and lignin peroxidases (LiP), is of great importance for industries that deal with biomass processing and plant waste [67].
Citrus residues contain significant amounts of lignocellulosic components. Although lignin is not as predominant in these residues compared to cellulose and hemicellulose, its presence makes these by-products a potential source for inducing ligninase production using microorganisms, mainly ligninolytic fungi. Fungi like Pleurotus eryngii, Pleurotus pulmonarius, and Trametes hirsuta have proven effective in ligninase production using citrus residues as substrates, especially in solid-state fermentation processes [40,41,68]. For example, a study by Chairin et al. [69] demonstrated that orange peels were used as a substrate in solid-state fermentation for laccase production by Trametes polyzona. The use of this residue as a natural inducer of ligninases resulted in considerable enzymatic activity, showing the potential of citrus residues for enzyme production. Another study conducted by Inácio et al. [70] explored the use of citrus residues in the production of ligninase by P. pulmonarius. They highlighted the effectiveness of solid-state fermentation as a promising strategy to improve laccase production.
In addition to fungi, some bacteria can also produce ligninases, though at a smaller scale. For example, Bacillus aquimaris have demonstrated the ability to produce a thermostable laccase using lignocellulosic substrates such as citrus residues [33]. The study by Sharma et al. [29] showed that submerged fermentation with Rheinheimera sp. using the peels of citrus fruits led to considerable laccase production, indicating that citrus residues can also be exploited for this application in bacteria.
Moreover, the pre-treatment of citrus residues can significantly enhance ligninase production. Processes like pectin removal and alkaline or acidic hydrolysis can make the lignocellulosic components more accessible to microorganisms, increasing enzymatic production efficiency. For example, Rosales et al. [71] showed that the pre-treatment of orange residues increased laccase production by T. hirsuta in solid-state fermentation.
The use of citrus residues for ligninase production represents a sustainable solution for both reducing environmental liabilities and obtaining high-value enzymes. Countries that produce large amounts of citrus residues have the potential to harness these by-products in industrial processes for ligninase production, increasing the sustainability of production chains and generating new products with commercial value [40,41].
3.1.4. Pectinases
The production of pectinases from citrus residues is a promising area in the study of biotechnology since these enzymes play a pivotal role in the degradation of pectin, a polysaccharide in the plant cell walls, especially in fruits. Pectinases, including polygalacturonase, pectin lyase, and pectin esterase, are widely used in several industries, such as food and beverage, paper, textiles, and in the extraction of essential oils and fibers. As citrus fruits are rich in pectin, the resulting residues from their production offer an ideal substrate for pectinase production through microbial fermentation [72,73,74].
Microorganisms such as fungi (e.g., Aspergillus spp., Penicillium spp.) and bacteria (e.g., B. subtilis, Serratia marcescens) have shown great potential for pectinase production using citrus residues as a substrate [37,75,76,77]. The use of orange and lemon peels, as well as other by-products from the citrus industry, is advantageous due to the high concentration of pectin and other nutrients in these residues, which promotes microbial growth and enzyme synthesis [31,78].
A study conducted by Esawy et al. [73] demonstrated that pectinase production by A. niger was significantly increased when orange peels were used as a substrate in solid-state fermentation. This study showed that citrus residues provide the nutrients necessary for fungal growth and act as efficient inducers of pectinase production. Another study by Núñez-Serrano et al. [37] reported the production of pectinases by Penicillium crustosum using lemon and orange residues as substrates in submerged fermentation. This study highlighted that cultivation conditions, such as pH, temperature, and agitation, were critical factors for optimizing enzymatic production.
Fungi from the genus Penicillium are also highlighted for use in the production of pectinase from citrus residues. A study by Majumder et al. [79] demonstrated that using orange peels as a substrate for the fermentation of Pleurotus ostreatus resulted in efficient polygalacturonase production. The solid-state and submerged fermentation processes were identified as economically and environmentally efficient strategies for the large-scale production of pectinase from agricultural residues.
In addition to fungi, some bacteria are also good for the production of pectinases from citrus residues. A study by Prajapati et al. [80] showed that using orange peels as substrates for B. subtilis resulted in high concentrations of pectinases, with potential applications in fruit juice clarification.
The production of pectinases is of great industrial interest, as these enzymes are essential for pectin degradation in processes such as juice clarification, fiber extraction, and viscosity reduction in processed foods. Using citrus residues as a substrate for producing these enzymes offers a sustainable and economically viable alternative by transforming by-products from the citrus industry into high-value products, while also reducing the environmental liabilities associated with improper waste disposal [74,81].
3.2. Lipases and Proteases
The use of agro-industrial waste in the production of enzymes has proven to be a sustainable and economical alternative, contributing to the reduction in the environmental impact of the citrus industry and adding value to by-products that would otherwise be discarded. In recent years, several studies have focused on the production of enzymes, such as lipases and proteases, using fruit waste and other agricultural materials, through solid-state fermentation (SSF) or liquid fermentation processes. Table 2 shows the main results of studies on the production of these enzymes, highlighting their methodologies, substrates used and activity values. The articles analyzed highlight the potential of citrus fruit waste as substrates in the production of lipases and proteases, promoting the circular economy. The results of the articles analyzed here affirm that microorganisms have emerged as essential and indispensable candidates that can take advantage of and valorize a variety of citrus fruit wastes into valuable products, thus promoting the biocircular economy [82]. Full use of citrus fruit and agricultural waste can be made through solid-state fermentation, which is an efficient technique for obtaining enzymes with desirable characteristics for various industrial applications, such as the production of biodiesel, pharmaceuticals, detergents and food. These studies highlight the importance of continuing to explore new types of waste from the citrus industry and optimizing processes to increase the efficiency and economic viability of enzyme production.
The use of waste from the citrus industry is not new, as Table 2 shows studies from past decades. However, these studies have gained relevance due to the constant appeal of green chemistry for more sustainable processes and also due to the great demand for cheaper enzyme production processes.
The study by Okino-Delgado and Fleuri (2014) [83] explored the production of lipases using by-products from orange juice processing, such as peels and bagasse. These residues, rich in carbohydrates and fibers, are a promising substrate for the production of lipases by solid-state fermentation (SSF). The article highlighted the efficiency of using citrus residues in the production of lipases, contributing to the development of sustainable biotechnological processes and the valorization of industrial by-products. The lipases obtained showed high enzymatic activity, with potential applications in the food and cosmetics industries.
In the study by Kavitha [45], fruit peel residues, such as orange and banana, were used for the production of amylase and protease by solid-state fermentation using Bacillus subtilis. The research demonstrated that fruit residues are effective substrates for the production of these enzymes, which have great potential for application in the food and detergent industries. Solid-state fermentation proved to be an efficient and low-cost method for obtaining amylases and proteases on a large scale.
The study by Athanázio-Heliodoro et al. [42] improved the lipase production system using orange residues and solid-state fermentation. The article addressed the optimization of the fermentation process and characterization of the lipases obtained, which showed high stability and efficiency in industrial processes, especially in the production of biodiesel. The application of citrus residues for the production of enzymes stood out as a sustainable and effective approach for industrial biotechnology.
4. Major Parameters Affecting the Enzyme Production Utilizing Citrus By-Product
The production of enzymes from citrus by-products is influenced by several critical parameters, including temperature, humidity, pH, pretreatment of the biomass, light exposure, production time, and the addition of carbon and nitrogen sources. These parameters are important for optimizing yields and enhancing enzyme activity, as shown in the literature.
4.1. Temperature
Temperature is a major parameter in enzyme production, as it directly influences the metabolic activity of the microorganisms involved in fermentation. Optimal enzyme production often occurs within a narrow temperature range, which varies depending on the microbial strain. Aspergillus niger is known for pectinase production at temperatures ranging between 30 °C and 45 °C [55,84]. Higher temperatures may lead to protein denaturation, thereby reducing enzyme activity. In contrast, temperatures below the optimal range slow down microbial growth and enzyme synthesis [85].
Controlling the temperature in solid-state fermentation (SSF) bioreactors, such as rotating drum or packed bed types, is challenging due to heat accumulation. Effective temperature management strategies include controlling the air temperature and ensuring proper ventilation to remove excess heat, as seen in [86]. Additionally, temperature gradients within the fermentation medium may impact enzyme yields, with some regions experiencing higher enzyme activity than others.
4.2. Moisture
Moisture content, or water activity, is a critical factor in solid-state fermentation (SSF), as it directly influences substrate availability and microbial growth. Excessive moisture can limit oxygen transfer, while low moisture content may restrict enzyme activity due to substrate dehydration. The optimal water activity (aw) for enzyme production using citrus by-products typically ranges from 0.6 to 0.8 [85,87]. The addition of secondary biomasses, such as sugarcane bagasse, to orange peel substrates helps maintain these optimal moisture levels by acting as a water-retaining agent. Due to its high water absorption capacity and fibrous structure, sugarcane bagasse prevents rapid dehydration of the substrate, ensuring consistent water availability throughout the fermentation process. This moisture retention improves the overall fermentation environment, enhancing pectinase production by maintaining favorable conditions for microbial metabolism and enzyme secretion [85].
In systems using rotating drum bioreactors, continuous agitation can maintain an even moisture distribution, thereby improving enzyme production yields [88]. The interplay between moisture content and temperature is particularly critical, as the evaporation of water can lead to substrate drying, reducing microbial activity [85].
4.3. pH
The pH of the fermentation medium plays a significant role in enzyme production. For most enzymes produced by Aspergillus sp., an acidic pH is favorable. Enzymes such as pectinase show optimal production at pH levels between 4.5 and 6.0 [89,90]. Variations in pH can alter the ionization state of substrates and enzymes, affecting enzyme binding and catalysis.
Adjusting the pH can also influence the availability of nutrients and cofactors, which are necessary for microbial metabolism. In some cases, buffering agents such as calcium carbonate are added to the fermentation medium to stabilize pH fluctuations [84].
4.4. Pretreatment of Biomass
Pretreatment of citrus by-products allows the enzyme’s access to the substrate to be enhanced. Physical, chemical, or enzymatic pretreatments are employed to break down the complex lignocellulosic structure of citrus residues, increasing the surface area available for microbial attack [55].
Common pretreatment methods include steam explosion, dilute acid treatment, and enzymatic hydrolysis. These processes disrupt the lignin and hemicellulose matrix, releasing fermentable sugars that serve as substrates for microbial growth [89]. The choice of pretreatment method depends on the desired enzyme production process. Li et al. [90] used ultrasound-assisted extraction to enhance enzyme activity in SSF by increasing the mass transfer and reducing the viscosity of the fermentation medium.
4.5. Light
Light exposure, although not commonly considered a major factor in enzyme production, may influence certain microbial activities. Studies on Aspergillus sp. have shown that some strains are sensitive to light, which can affect spore formation and secondary metabolite production [89]. However, the specific role of light in citrus by-product fermentation requires further investigation.
4.6. Production Time
The duration of the fermentation process impacts enzyme yields and microbial growth. Enzyme production typically follows a sigmoidal growth curve, with a lag phase, exponential phase, and stationary phase [91]. For pectinases and cellulases, maximum activity is often observed between 72 and 96 h of fermentation, depending on the strain and substrate used [90,91]. Extending the fermentation time beyond the optimal period may lead to nutrient depletion and enzyme degradation due to proteolytic activities [86].
4.7. Carbon-to-Nitrogen (C/N) Ratio
The carbon-to-nitrogen (C/N) ratio affects enzyme synthesis, influencing metabolic regulation mechanisms and the efficiency of enzyme production. Citrus by-products are rich in pectin and sugars, making them excellent carbon sources [85,92]. However, to optimize enzyme yields, additional nitrogen sources such as ammonium sulfate, yeast extract, or peptone are often supplemented [89].
The optimal C/N ratio varies depending on the microbial strain but generally falls between 10:1 and 30:1 [90]. Imbalances in the C/N ratio can lead to metabolic shifts, affecting enzyme production. High nitrogen concentrations may lead to catabolic repression, reducing enzyme synthesis [88].
4.8. Multiproduction of Enzymes from Citrus By-Products
An additional advantage of utilizing citrus by-products as fermentation substrates lies in the potential to simultaneously produce multiple enzymes from a single waste source. Due to their complex lignocellulosic structure, citrus residues contain cellulose, hemicellulose, lignin, and pectin, which can serve as inducers for various enzymatic pathways [57,63]. For example, microbial strains such as Trichoderma reesei and Pleurotus pulmonarius have demonstrated the capacity to produce cellulase and laccase concurrently when cultivated on orange peel substrates [40,70]. The simultaneous induction of cellulases, which hydrolyze cellulose into glucose, and laccases, which degrade lignin compounds, can be particularly advantageous for integrated bioprocessing applications [41,71]. Additionally, bacteria such as Pseudomonas spp. have been reported to produce xylanases and hemicellulases from citrus-derived substrates, expanding the range of potential enzymes obtained from a single fermentation process [64]. However, the feasibility of multiproduction depends on factors such as the microbial strain, fermentation conditions, and substrate composition. If the structural composition of the citrus residue favors the selective production of specific enzymes, the process conditions can be adjusted accordingly to optimize the desired enzymatic activity without compromising overall yield [57,63].
The efficiency of enzyme production from citrus waste can be evaluated based on the enzymatic yield per unit of raw material, which varies depending on the microbial strain, fermentation method, and pre-treatment applied to the substrate. Studies have reported cellulase yields ranging from 10.96 ± 0.51 U/mL using Trichoderma afroharzianum on lemon peel under submerged fermentation [31] to 497.3 ± 2.06 U/mL with Trichoderma asperellum under solid-state fermentation using mosambi peel [35]. Similarly, xylanase production from Penicillium crustosum using lemon peel substrates in submerged fermentation reached 47.26 ± 1.36 U/mL [37], whereas Bacillus altitudinis achieved up to 99.2 ± 11.7 U/g in submerged fermentation using citrus peel [39]. These results highlight the potential for high enzymatic yields from citrus residues, especially when optimized fermentation conditions and appropriate pre-treatment methods are applied to increase substrate accessibility and enhance microbial metabolism.
In addition to enzyme production, the fate of residual biomass post-fermentation is an important aspect of the process’s sustainability. After enzyme extraction, the remaining solid biomass, primarily consisting of unconverted lignin, residual cellulose, and other insoluble compounds, can be further valorized. This residual material can be processed into bioenergy products, such as biogas through anaerobic digestion, or used as a soil amendment due to its organic content and nutrient value [85]. Moreover, the liquid fraction of the fermentation broth, rich in soluble sugars and organic acids, can be recycled into subsequent fermentation processes or utilized for the production of secondary metabolites. By integrating enzyme production with other valorization strategies, the overall efficiency of citrus waste biorefineries can be improved, aligning with the principles of a circular economy and reducing environmental impacts.
5. Conclusions
Recent trends in enzyme production show that citrus by-products can be applied in the production of a wide range of enzymes. The articles in this review presented the value-added products that can be obtained from citrus by-products, demonstrating the technical, sustainable, and economic feasibility of the process. Despite the possibilities, it is important to highlight that some pretreatments and techniques may be required to improve enzyme yields. Additionally, the variability in the composition of citrus waste requires alterations in preliminary treatment methods to optimize enzyme production efficiency. Therefore, although the potential and feasibility at the laboratory scale are well-documented, it is essential that the scientific community intensify efforts to develop viable biorefinery arrangements on an industrial scale. Moreover, enzymes produced using citrus by-products as a carbon source can be extracted and applied not only in the biotransformation of agro-industrial residues but can also have a universal effect, broadening their industrial relevance to several sectors. In conclusion, the production of enzymes from citrus residues presents an effective strategy for valorizing agricultural by-products while contributing to the biotechnological landscape. The utilization of these residues not only addresses environmental concerns associated with waste disposal but also provides a valuable resource for enzyme production, benefiting various industries, including food, biofuels, and bioremediation. This approach enhances the economic viability of enzyme production and fosters a Circular Economy within the agricultural sector.
Author Contributions
Conceptualization, C.A.L., A.K.F.C. and H.B.S.B.; writing—original draft preparation, C.A.L., A.G.C., F.d.O., S.S.d.S., V.B.H., M.I., B.C.G., A.K.F.C. and H.B.S.B.; writing—review and editing, F.d.O., S.S.d.S., V.B.H., M.I., B.C.G., A.K.F.C. and H.B.S.B.; supervision, A.K.F.C. and H.B.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Process numbers 2021/09175-4 and 2023/09789-8, CNPq (National Council for Scientific and Technological Development, Brazil) grant #406564/2022-1 and CAPES (Coordination of Superior Level Staff Improvement, Brazil) finance code 001.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dubey, P.; Tripathi, G.; Mir, S.S.; Yousuf, O. Current scenario and global perspectives of citrus fruit waste as a valuable resource for the development of food packaging film. Trends Food Sci. Technaol. 2023, 141, 104190. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Markets and Trade. 2024. Available online: https://www.fao.org/markets-and-trade/commodities-overview/food-and-agriculture-market-analysis-(FAMA)/citrus/en (accessed on 23 September 2024).
- Suri, S.; Singh, A.; Nema, P.K. Recent advances in valorization of citrus fruits processing waste: A way forward towards environmental sustainability. Food Sci. Biotechnol. 2021, 30, 1601–1626. [Google Scholar] [CrossRef] [PubMed]
- USDA FAS. United States Department of Agriculture Foreign Agricultural Service. 2024. Available online: https://fas.usda.gov/data/citrus-world-markets-and-trade-07252024 (accessed on 23 September 2024).
- FAO. Citrus Fruit Fresh and Processed Statistical Bulletin 2020; Food and Agriculture Organizations of the United Nations: Rome, Italy, 2021; Available online: http://www.fao.org/3/cb6492en/cb6492en.pdf (accessed on 23 September 2024).
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Biswas, S.; Goyal, A. Enzymes of Industrial Significance and Their Applications. In Industrial Microbiology and Biotechnology; Verma, P., Ed.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Industrial Enzymes Market Size, Share & Trends Analysis Report by Product (Carbohydrase, Proteases), by Source (Plants, Animals, Microorganisms), by Application (Food & Beverages, Detergents, Animal Feed), by Region, and Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/industrial-enzymes-market (accessed on 9 October 2024).
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Danzì, C.; Timpanaro, G.; Di Stefano, V.; Avellone, G.; Pagliaro, M. Fragrant bioethanol: A valued bioproduct from orange juice and essential oil extraction. Sustain. Chem. Pharm. 2018, 9, 42–45. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The second life of citrus fruit waste: A valuable source of bioactive compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Mourad, M. Recycling, recovering and preventing “food waste”: Competing solutions for food systems sustainability in the United States and France. J. Clean. Prod. 2016, 126, 461–477. [Google Scholar] [CrossRef]
- Iftikhar, M.; Wahab, S.; Haq, N.U.; Malik, S.N.; Amber, S.; Taran, N.U.; Rehman, S.U. Utilization of citrus plant waste (peel) for the development of food product. Pure Appl. Biol. 2019, 8, 1991–1998. [Google Scholar] [CrossRef]
- Satari, B.; Palhed, J.; Karimi, K.; Lundin, M.; Taherzadeh, M.J.; Zamani, A. Process Optimization for Citrus Waste Biorefinery via Simultaneous Pectin Extraction and Pretreatment. BioResources 2017, 12, 1706–1722. [Google Scholar] [CrossRef]
- Kovačević, D.B.; Kljusurić, J.G.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Omarini, A.B.; González-Aguirre, J.A.; Baglioni, M.; Zygadlo, J.A.; Breccia, J.; D’Souza, R.; Lemesoff, L.; Bodeain, M.; Cardona-Alzate, C.A.; et al. Valorization Routes of Citrus Waste in the Orange Value Chain through the Biorefinery Concept: The Argentina Case Study. Chem. Eng. Process.-Process Intensif. 2023, 189, 109407. [Google Scholar] [CrossRef]
- Dubey, P.; Yousuf, O. An overview of fruit by-products valorization: A step towards sustainable utilization. Ind. J. Pure Appl. Biosci. 2021, 9, 46–55. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Forrest, B.; Oh, J.; Boussert, S.M.; Hamann, M.T. Lemon yellow #15 a new highly stable, water soluble food colorant from the peel of Citrus limon. Food Chem. 2019, 270, 251–256. [Google Scholar]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. Byproducts from Different Citrus Processes as a Source of Customized Functional Fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Pourbafrani, M.; Forgács, G.; Horváth, I.S.; Niklasson, C.; Taherzadeh, M.J. Production of Biofuels, Limonene and Pectin from Citrus Wastes. Bioresour. Technol. 2010, 101, 4246–4250. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Nanjundaswamy, A.; Bansal, S.; Singh, S.; Kaur, S.; Babbar, N. Enhanced Ethanol Production from Kinnow Mandarin (Citrus reticulata) Waste via a Statistically Optimized Simultaneous Saccharification and Fermentation Process. Bioresour. Technol. 2011, 102, 1593–1601. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, Y.M.; Jae, J.; Watanabe, C.; Kim, S.; Jung, S.C.; Kim, S.C.; Park, Y.K. Pyrolysis and Catalytic Upgrading of Citrus unshiu Peel. Bioresour. Technol. 2015, 194, 312–319. [Google Scholar] [CrossRef]
- Orozco, R.S.; Hernández, P.B.; Morales, G.R.; Núñez, F.U.; Villafuerte, J.O.; Lugo, V.L.; Ramírez, N.F.; Díaz, C.E.B.; Vázquez, P.C. Characterization of Lignocellulosic Fruit Waste as an Alternative Feedstock for Bioethanol Production. BioResources 2014, 9, 1873–1885. [Google Scholar]
- Alvarez, J.; Hooshdaran, B.; Cortazar, M.; Amutio, M.; Lopez, G.; Freire, F.B.; Haghshenasfard, M.; Hossein, S.; Olazar, M. Valorization of Citrus Wastes by Fast Pyrolysis in a Conical Spouted Bed Reactor. Fuel 2018, 224, 111–120. [Google Scholar] [CrossRef]
- Mohsin, A.; Zhang, K.; Hu, J.; Salim-Ur-Rehman; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized Biosynthesis of Xanthan via Effective Valorization of Orange Peels Using Response Surface Methodology: A Kinetic Model Approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of Citrus Waste Peel for Induced Pectinase Production by Aspergillus niger; Its Purification and Characterization. J. Radiat. Res. Appl. Sci. 2016, 9, 148–154. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral Use of Orange Peel Waste through the Biorefinery Concept: An Experimental, Technical, Energy, and Economic Assessment. Biomass Convers. Biorefin. 2020, 11, 645–659. [Google Scholar] [CrossRef]
- Lima, C.A.; Bento, H.B.; Picheli, F.P.; Paz-Cedeno, F.R.; Mussagy, C.U.; Masarin, F.; Acosta, M.A.T.; Santos-Ebinuma, V.C. Process development and techno-economic analysis of co-production of colorants and enzymes valuing agro-industrial citrus waste. Sustain. Chem. Phar. 2023, 35, 101204. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Rosa, A.; Era, B.; Masala, C.; Nieddu, M.; Scano, P.; Fais, A.; Porcedda, S.; Piras, A. Supercritical CO extraction of waste citrus seeds: Chemical composition, nutritional and biological properties of edible fixed oils. Europ. J. Lip. Sci. Technol. 2019, 121, 1800502. [Google Scholar] [CrossRef]
- Gooruee, R.; Hojjati, M.; Behbahani, B.A.; Shahbazi, S.; Askari, H. Extracellular enzyme production by different species of Trichoderma fungus for lemon peel waste bioconversion. Biomass Convers. Bioref. 2024, 14, 2777–2786. [Google Scholar] [CrossRef]
- Amadi, O.C.; Awodiran, I.P.; Moneke, A.N.; Nwagu, T.N.; Egong, J.E.; Chukwu, G.C. Concurrent production of cellulase, xylanase, pectinase and immobilization by combined Cross-linked enzyme aggregate strategy—Advancing tri-enzyme biocatalysis. Bioresour. Technol. Rep. 2022, 18, 101019. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Bilal, M.; Chandra, R. Sustainable production of thermostable laccase from agro-residues waste by Bacillus aquimaris AKRC02. Catal. Lett. 2022, 152, 1784–1800. [Google Scholar] [CrossRef]
- Camargo, D.A.; Pereira, M.S.; dos Santos, A.G.; Fleuri, L.F. Isolated and fermented orange and grape wastes: Bromatological characterization and phytase, lipase and protease source. Innov. Food Sci. Emerg. Technol. 2022, 77, 102978. [Google Scholar] [CrossRef]
- Singh, B.; Garg, N.; Mathur, P.; Soni, S.K.; Vaish, S.; Kumar, S. Microbial production of multienzyme preparation from mosambi peel using Trichoderma asperellum. Arch. Microbiol. 2022, 204, 313. [Google Scholar] [CrossRef]
- Ahmed, T.; Rana, M.R.; Zzaman, W.; Ara, R.; Aziz, M.G. Optimization of substrate composition for pectinase production from Satkara (Citrus macroptera) peel using Aspergillus niger—ATCC 1640 in solid—State fermentation. Heliyon 2021, 7, e08133. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Serrano, A.; García-Reyes, R.B.; García-González, A. Optimization of hydrolases production by Penicillium crustosum in submerged fermentation using agro-waste residues as cosubstrate. Biocatal. Agric. Biotechnol. 2024, 57, 103116. [Google Scholar] [CrossRef]
- Belorkar, S.A.; Kausar, H. Valorization parameters to determine the fermentative applicability of selected fruit peels for xylanase production. Waste Biomass Valorization 2023, 14, 185–196. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S.; Baxi, N.N. Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through Response Surface Method. Sci. Rep. 2020, 10, 3824. [Google Scholar] [CrossRef] [PubMed]
- Contato, A.G.; Inácio, F.D.; Brugnari, T.; de Araújo, C.A.V.; Maciel, G.M.; Haminiuk, C.W.I.; Peralta, R.M.; de Souza, C.G.M. Solid-state fermentation with orange waste: Optimization of laccase production from Pleurotus pulmonarius CCB-20 and decolorization of synthetic dyes. Acta Sci. Biol. Sci. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Ortolan, G.G.; Contato, A.G.; Aranha, G.M.; Salgado, J.C.S.; Alnoch, R.C.; Polizeli, M.L.T.M. Enhancing Laccase Production by Trametes hirsuta GMA-01 Using Response Surface Methodology and Orange Waste: A Novel Breakthrough in Sugarcane Bagasse Saccharification and Synthetic Dye Decolorization. Reactions 2024, 5, 635–650. [Google Scholar] [CrossRef]
- Athanázio-Heliodoro, J.C.; Okino-Delgado, C.H.; Fernandes, C.J.D.C.; Zanutto, M.R.; Prado, D.Z.D.; da Silva, R.A.; Facanali, R.; Zambuzzi, W.F.; Marques, M.O.M.; Fleuri, L.F. Improvement of lipase obtaining system by orange waste-based solid-state fermentation: Production, characterization and application. Prep. Biochem. Biotechnol. 2018, 48, 565–573. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Pereira, M.S.; da Silva, J.V.I.; Kharfan, D.; do Prado, D.Z.; Fleuri, L.F. Lipases obtained from orange wastes: Commercialization potential and biochemical properties of different varieties and fractions. Biotechnol. Prog. 2019, 35, e2734. [Google Scholar] [CrossRef]
- Johnvesly, B.; Manjunath, B.R.; Naik, G.R. Pigeon pea waste as a novel, inexpensive, substrate for production of a thermostable alkaline protease from thermoalkalophilic Bacillus sp. JB-99. Bioresour. Technol. 2002, 82, 61–64. [Google Scholar] [CrossRef]
- Kavitha, R. Production of amylase and protease from fruit peels using Bacillus subtilis by solid-state fermentation. Int. J. Sci. Res. Rev. 2018, 7, 652–663. [Google Scholar]
- Chimbekujwo, K.I.; Ja’afaru, M.I.; Adeyemo, O.M. Purification, characterization and optimization conditions of protease produced by Aspergillus brasiliensis strain BCW2. Sci. Afr. 2020, 8, e00398. [Google Scholar] [CrossRef]
- Contato, A.G.; Borelli, T.C.; de Carvalho, A.K.F.; Bento, H.B.S.; Buckeridge, M.S.; Rogers, J.; Hartson, S.; Prade, R.A.; Polizeli, M.L.T.M. Comparative Analysis of CAZymes from Trichoderma longibrachiatum LMBC 172 Cultured with Three Different Carbon Sources: Sugarcane Bagasse, Tamarind Seeds, and Hemicellulose Simulation. Clean Technol. 2024, 6, 994–1010. [Google Scholar] [CrossRef]
- Benny, N.; Shams, R.; Dash, K.K.; Pandey, V.K.; Bashir, O. Recent trends in utilization of citrus fruits in production of eco-enzyme. J. Agric. Food Res. 2023, 13, 100657. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Girardi, E.A.; Sola, J.G.P.; Scapin, M.D.S.; Moreira, A.S.; Bassanezi, R.B.; Ayres, A.J.; Peña, L. The perfect match: Adjusting high tree density to rootstock vigor for improving cropping and land use efficiency of sweet orange. Agronomy 2021, 11, 2569. [Google Scholar] [CrossRef]
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent trends on the valorization strategies for the management of citrus by-products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Scarcella, A.S.A.; Pasin, T.M.; de Lucas, R.C.; Ferreira-Nozawa, M.S.; de Oliveira, T.B.; Contato, A.G.; Grandis, A.; Buckeridge, M.S.; Polizeli, M.L.T.M. Holocellulase production by filamentous fungi: Potential in the hydrolysis of energy cane and other sugarcane varieties. Biomass Convers. Bioref. 2023, 13, 1163–1174. [Google Scholar] [CrossRef]
- Srivastava, N.; Mohammad, A.; Pal, D.B.; Srivastava, M.; Alshahrani, M.Y.; Ahmad, I.; Singh, R.; Mishra, P.K.; Yoon, T.; Gupta, V.K. Enhancement of fungal cellulase production using pretreated orange peel waste and its application in improved bioconversion of rice husk under the influence of nickel cobaltite nanoparticles. Biomass Convers. Bioref. 2024, 14, 6687–6696. [Google Scholar] [CrossRef]
- Areeshi, M.Y. Microbial cellulase production using fruit wastes and its applications in biofuels production. Int. J. Food Microbiol. 2022, 378, 109814. [Google Scholar] [CrossRef]
- Al Mousa, A.A.; Hassane, A.M.; Gomaa, A.E.R.F.; Aljuriss, J.A.; Dahmash, N.D.; Abo-Dahab, N.F. Response-surface statistical optimization of submerged fermentation for pectinase and cellulase production by Mucor circinelloides and M. hiemalis. Fermentation 2022, 8, 205. [Google Scholar] [CrossRef]
- Mathias, D.J.; Kumar, S.; Rangarajan, V. An investigation on citrus peel as the lignocellulosic feedstock for optimal reducing sugar synthesis with an additional scope for the production of hydrolytic enzymes from the aqueous extract waste. Biocatal. Agric. Biotechnol. 2019, 20, 101259. [Google Scholar] [CrossRef]
- da Silva, A.F.V.; Santos, L.A.D.; de Melo, A.H.F.; Jucá, J.F.T.; Santos, A.F.M.S.; Porto, T.S. Use of Cellulase Obtained from Solid-State Fermentation of Orange and Passion Fruit Peels as an Enzymatic Pre-treatment Step for Anaerobic Digestion. BioEnergy Res. 2024, 17, 1288–1301. [Google Scholar] [CrossRef]
- Bhati, N.; Shreya; Sharma, A.K. Cost-effective cellulase production, improvement strategies, and future challenges. J. Food Process Eng. 2021, 44, e13623. [Google Scholar]
- Taghizadeh-Alisaraei, A.; Hosseini, S.H.; Ghobadian, B.; Motevali, A. Biofuel production from citrus wastes: A feasibility study in Iran. Renew. Sustain. Energy Rev. 2017, 69, 1100–1112. [Google Scholar] [CrossRef]
- Chaudhary, R.; Kuthiala, T.; Singh, G.; Rarotra, S.; Kaur, A.; Arya, S.K.; Kumar, P. Current status of xylanase for biofuel production: A review on classification and characterization. Biomass Convers. Bioref. 2021, 13, 8773–8791. [Google Scholar] [CrossRef]
- Zerva, I.; Remmas, N.; Ntougias, S. Diversity and biotechnological potential of xylan-degrading microorganisms from orange juice processing waste. Water 2019, 11, 274. [Google Scholar] [CrossRef]
- Silva, D.F.; Hergesel, L.M.; Campioni, T.S.; Carvalho, A.F.A.; Oliva-Neto, P. Evaluation of different biological and chemical treatments in agroindustrial residues for the production of fungal glucanases and xylanases. Process Biochem. 2018, 67, 29–37. [Google Scholar] [CrossRef]
- Barbieri, G.S.; Bento, H.B.; de Oliveira, F.; Picheli, F.P.; Dias, L.M.; Masarin, F.; Santos-Ebinuma, V.C. Xylanase production by Talaromyces amestolkiae valuing agroindustrial byproducts. BioTech 2022, 11, 15. [Google Scholar] [CrossRef]
- Saha, S.P.; Ghosh, S. Optimization of xylanase production by Penicillium citrinum xym2 and application in saccharification of agro-residues. Biocatal. Agric. Biotechnol. 2014, 3, 188–196. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Sybuia, P.A.; Contato, A.G.; de Araújo, C.A.V.; Zanzarin, D.M.; Maciel, G.M.; Pilau, E.J.; Peralta, R.M.; de Souza, C.G.M. Application of the white-rot fungus Trametes sp.(C3) laccase in the removal of acetaminophen from aqueous solutions. J. Water Process Eng. 2024, 57, 104677. [Google Scholar] [CrossRef]
- Akpinar, M.; Urek, R.O. Induction of fungal laccase production under solid state bioprocessing of new agroindustrial waste and its application on dye decolorization. 3 Biotech 2017, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Chairin, T.; Nitheranont, T.; Watanabe, A.; Asada, Y.; Khanongnuch, C.; Lumyong, S. Purification and characterization of the extracellular laccase produced by Trametes polyzona WR710–1 under solid-state fermentation. J. Basic Microbiol. 2014, 54, 35–43. [Google Scholar] [CrossRef]
- Inácio, F.D.; Ferreira, R.O.; De Araujo, C.A.V.; Peralta, R.M.; de Souza, C.G.M. Production of Enzymes and Biotransformation of Orange Waste by Oyster Mushroom, Pleurotus pulmonarius (Fr.) Quél. Adv. Microbiol. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Rosales, E.; Couto, S.R.; Sanromán, M.A. Increased laccase production by Trametes hirsuta grown on ground orange peelings. Enzym. Microb. Technol. 2007, 40, 1286–1290. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, D.; Lv, C.; Zhang, Y.; Gelbic, I.; Ye, X. Archives of microbiology: Screening of pectinase-producing bacteria from citrus peel and characterization of a recombinant pectate lyase with applied potential. Arch. Microbiol. 2020, 202, 1005–1013. [Google Scholar] [CrossRef]
- Esawy, M.A.; Gamal, A.A.; Kamel, Z. Optimization of Aspergillus niger NRC1ami pectinase using citrus peel pectin, purification, and thermodynamic characterization of the free and modified enzyme. Waste Biomass Valorization 2022, 13, 4823–4837. [Google Scholar] [CrossRef]
- Guimarães, N.C.A.; Glienke, N.N.; Contato, A.G.; Galeano, R.M.S.; Marchetti, C.R.; Rosa, M.P.G.; Teles, J.S.S.; Simas, A.L.O.; Zanoelo, F.F.; Masui, D.C.; et al. Production and Biochemical Characterization of Aspergillus japonicus Pectinase Using a Low-Cost Alternative Carbon Source for Application in the Clarification of Fruit Juices. Waste Biomass Valorization 2024, 15, 177–186. [Google Scholar] [CrossRef]
- Haile, S.; Masi, C.; Tafesse, M. Isolation and characterization of pectinase-producing bacteria (Serratia marcescens) from avocado peel waste for juice clarification. BMC Microbiol. 2022, 22, 145. [Google Scholar] [CrossRef]
- Mahto, R.B.; Yadav, M.; Muthuraj, M.; Sharma, A.K.; Bhunia, B. Biochemical properties and application of a novel pectinase from a mutant strain of Bacillus subtilis. Biomass Convers. Bioref. 2023, 13, 10463–10474. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Bhattacharyya, R.; Bhattacharya, S.; Alnafisi, B.K. Valorisation of Citrus limetta peel for Aspergillus terreus FP6 mediated pectinase fermentation and application in grape juice clarification. J. King Saud. Univ. -Sci. 2024, 36, e103454. [Google Scholar] [CrossRef]
- Qadir, F.; Ejaz, U.; Sohail, M. Co-culturing corncob-immobilized yeasts on orange peels for the production of pectinase. Biotechnol. Lett. 2020, 42, 1743–1753. [Google Scholar] [CrossRef]
- Majumder, K.; Paul, B.; Sundas, R. An analysis of exo-polygalacturonase bioprocess in submerged and solid-state fermentation by Pleurotus ostreatus using pomelo peel powder as carbon source. J. Genet. Eng. Biotechnol. 2020, 18, 47. [Google Scholar] [CrossRef]
- Prajapati, J.; Dudhagara, P.; Patel, K. Production of thermal and acid-stable pectinase from Bacillus subtilis strain BK-3: Optimization, characterization, and application for fruit juice clarification. Biocatal. Agric. Biotechnol. 2021, 35, e102063. [Google Scholar] [CrossRef]
- John, J.; Kaimal, K.S.; Smith, M.L.; Rahman, P.K.; Chellam, P.V. Advances in upstream and downstream strategies of pectinase bioprocessing: A review. Int. J. Biol. Macromol. 2020, 162, 1086–1099. [Google Scholar] [CrossRef]
- Najar, I.N.; Sharma, P.; Das, R.; Tamang, S.; Mondal, K.; Thakur, N.; Kumar, V. From waste management to circular economy: Leveraging thermophiles for sustainable growth and global resource optimization. J. Environ. Manag. 2024, 360, e121136. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Fleuri, L.F. Obtaining lipases from byproducts of orange juice processing. Food Chem. 2014, 163, 103–107. [Google Scholar] [CrossRef]
- Maciel, M.; Ottoni, C.; Santos, C.; Lima, N.; Moreira, K.; Souza-Motta, C. Production of Polygalacturonases by Aspergillus section Nigri Strains in a Fixed Bed Reactor. Molecules 2013, 18, 1660–1671. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Najafpour, G.D.; Mohammadi, M. Bioconversion of Agroindustrial Wastes to Pectinases Enzyme via Solid State Fermentation in Trays and Rotating Drum Bioreactors. Biocatal. Agric. Biotechnol. 2019, 21, 101280. [Google Scholar] [CrossRef]
- Poletto, P.; Polidoro, T.A.; Zeni, M.; da Silveira, M.M. Evaluation of the Operating Conditions for the Solid-State Production of Pectinases by Aspergillus niger in a Bench-Scale, Intermittently Agitated Rotating Drum Bioreactor. LWT—Food Sci. Technol. 2017, 79, 92–101. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, J.; Li, X.; Lu, X.; Liu, G.; Qu, Y.; Zhao, J. Review of research progress on the production of cellulase from filamentous fungi. Int. J. Biol. Macromol. 2024, 277, 134539. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, T.; Mandalari, G. Valorization of Agro-Industrial Orange Peel By-Products through Fermentation Strategies. Fermentation 2024, 10, 224. [Google Scholar] [CrossRef]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Optimization of the fermentation parameters to maximize the production of cellulases and xylanases using DDGS as the main feedstock in stirred tank bioreactors. Biocatal. Agric. Biotechnol. 2022, 45, e102514. [Google Scholar] [CrossRef]
- Li, P.; Xia, J.; Shan, Y.; Nie, Z. Comparative Study of Multi-Enzyme Production from Typical Agro-Industrial Residues and Ultrasound-Assisted Extraction of Crude Enzyme in Fermentation with Aspergillus japonicus PJ01. Bioprocess Biosyst. Eng. 2015, 38, 2013–2022. [Google Scholar] [CrossRef]
- Demir, H.; Göğüs, N.; Tari, C.; Heerd, D.; Lahore, M.F. Optimization of the Process Parameters for the Utilization of Orange Peel to Produce Polygalacturonase by Solid-State Fermentation from an Aspergillus sojae Mutant Strain. Turk. J. Biol. 2012, 36, 394–404. [Google Scholar] [CrossRef]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of Pectinases by Solid-State Fermentation of a Mixture of Citrus Waste and Sugarcane Bagasse in a Pilot-Scale Packed-Bed Bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).