Removal of Glyphosate in Agricultural Runoff Using Subsurface Constructed Wetlands in Monocultures and Polycultures of Tropical Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Preparation of the Glyphosate Solution
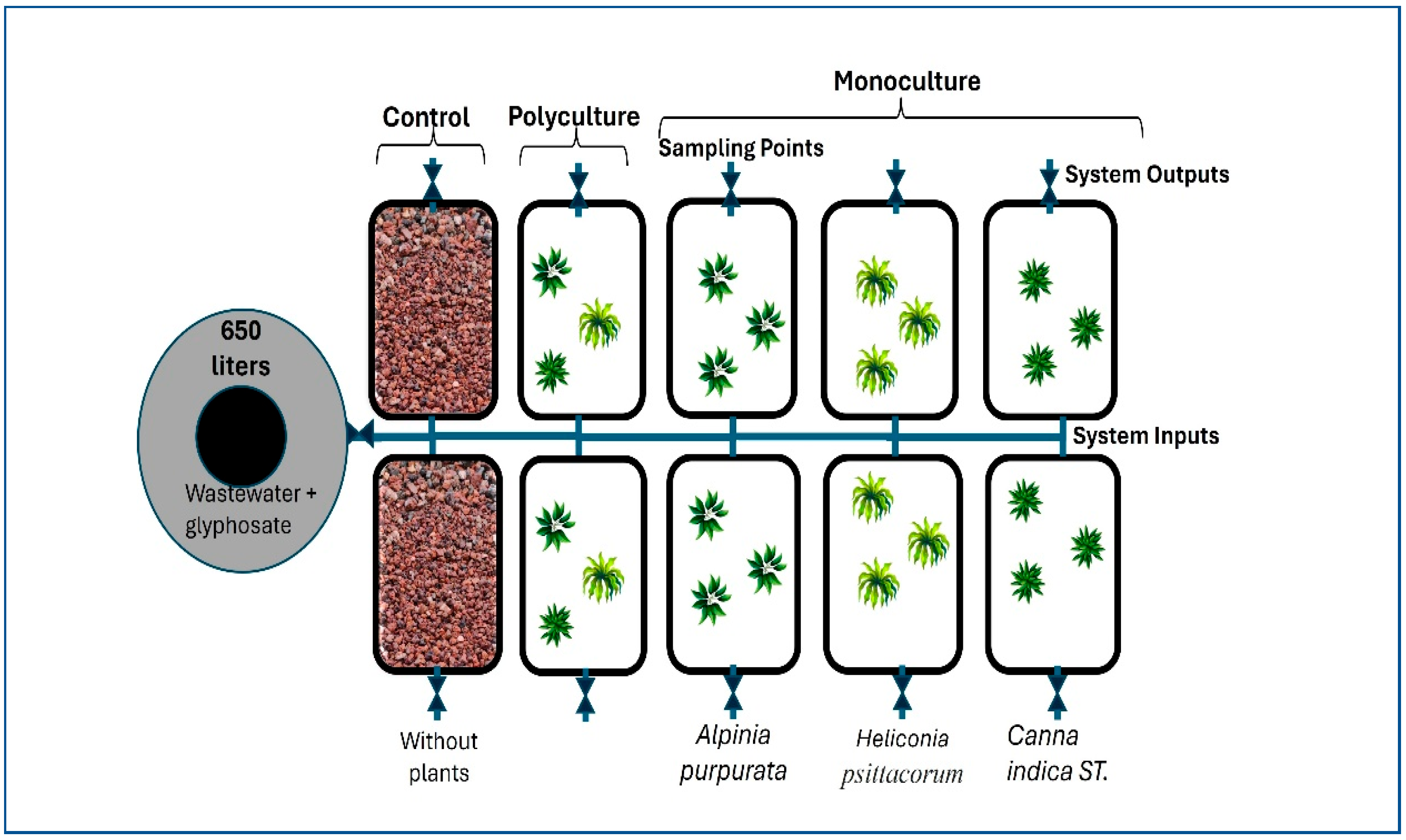
2.3. HSFW Design
2.4. Plant Selection
2.5. Parameters and Measurements of Pollutants
2.5.1. Plant Development in Mesocosms
2.5.2. Biomass in Mesocosms
2.6. Measurement of Physicochemical Parameters in Water
2.6.1. Physicochemical Parameters
2.6.2. Glyphosate Analysis
2.7. Experimental Design and Statistical Analysis
3. Results
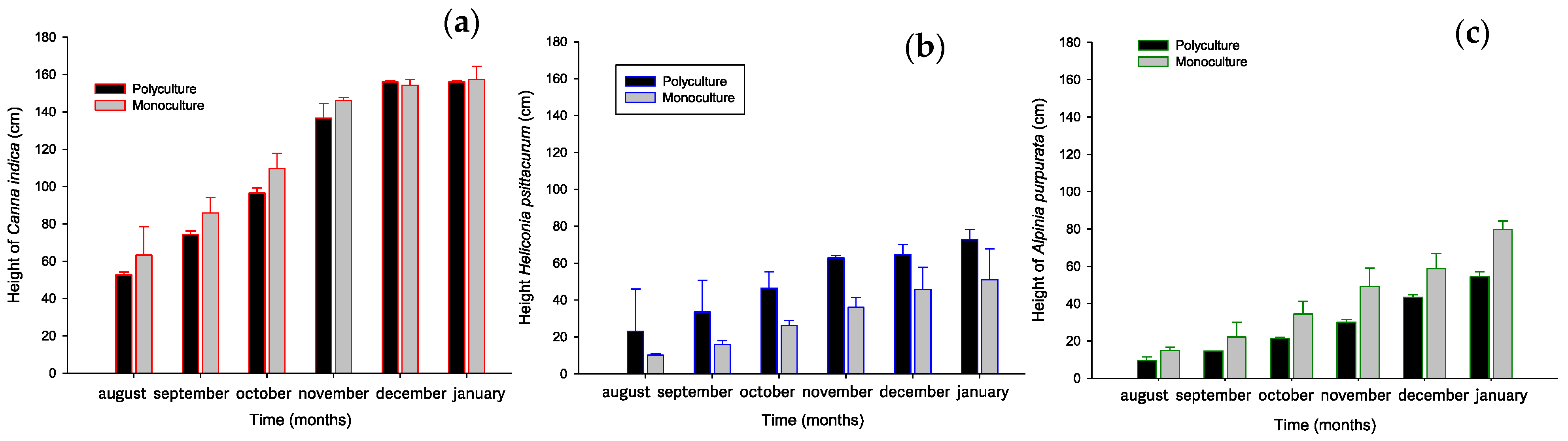
3.1. Plant Development
3.1.1. Plant Growth and Biomass Production
3.1.2. Biomass
3.2. Measurement of Physicochemical Parameters in the Water
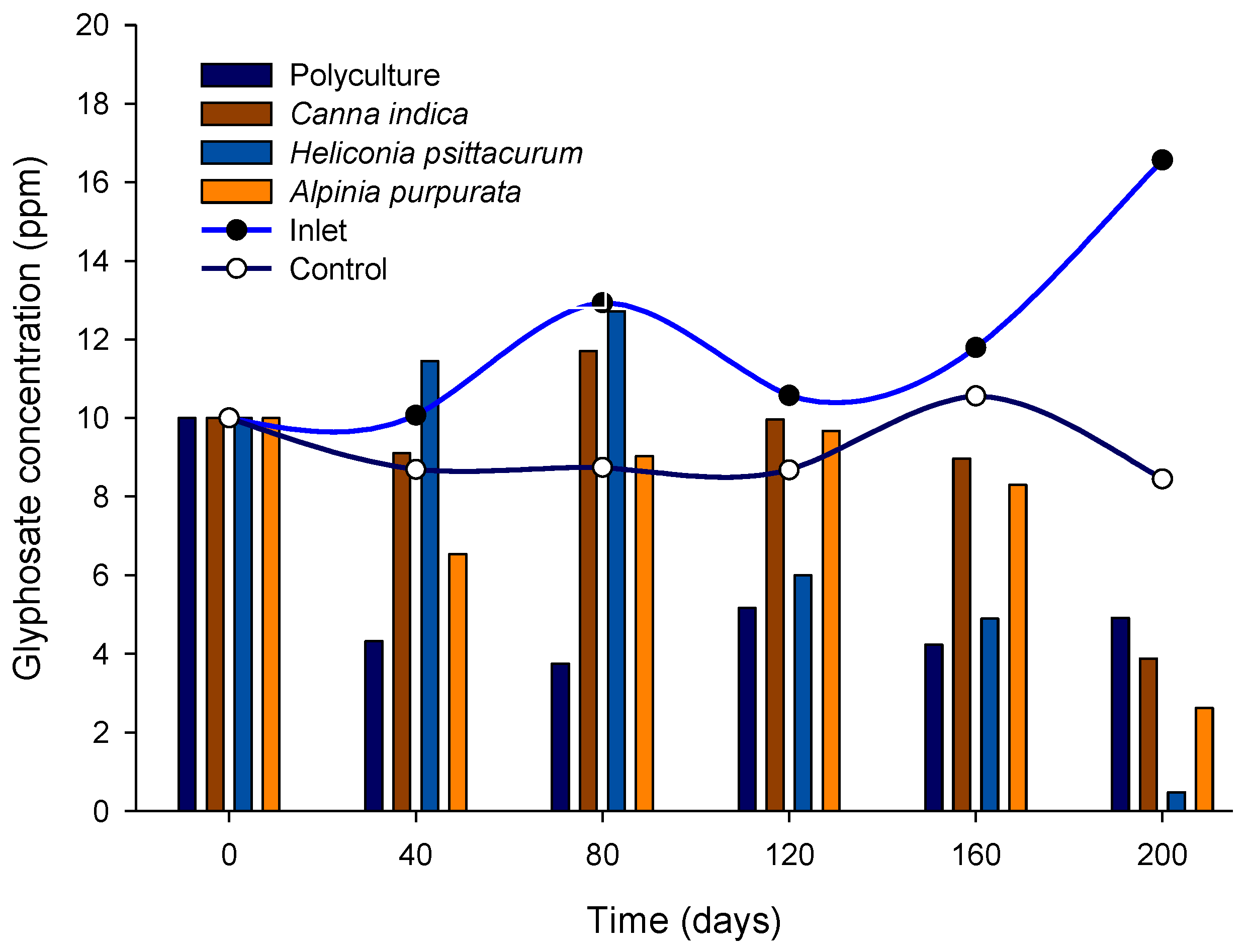
3.3. Glyphosate Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Aclnowledgements
Conflicts of Interest
References
- Lacroix, R.; Kurrasch, D.M. Glyphosate toxicity: In vivo, in vitro, and epidemiological evidence. Toxicol. Sci. 2023, 192, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Rapisarda, P.; Grasso, A.; Favara, C.; Conti, G.O. Glyphosate and environmental toxicity with ‘One Health’ approach, a review. Environ. Res. 2023, 235, 116678. [Google Scholar] [CrossRef] [PubMed]
- Le Du-Carrée, J.; Cabon, L.; Louboutin, T.; Morin; Danion, M. Changes in defense capacity to infectious hematopoietic necrosis virus (IHNv) in rainbow trout intergenerationally exposed to glyphosate. Fish Shellfish Immunol. 2022, 122, 67–70. [Google Scholar] [CrossRef]
- Álvarez Bayona, M.A.; Maturana Córdoba, A.; Gallardo Amaya, R.J.; Muñoz Acevedo, A. Occurrence of glyphosate in surface and drinking water sources in Cúcuta, Norte de Santander, and its removal using membrane technology. Front. Environ. Sci. 2022, 10, 941836. [Google Scholar] [CrossRef]
- Edwards, W.M.; Triplett, G.B.; Kramer, R.M. A Watershed Study of Glyphosate Transport in Runoff. J. Environ. Qual. 1980, 9, 661–665. [Google Scholar] [CrossRef]
- Lima, I.B.; Boëchat, I.G.; Fernandes, M.D.; Monteiro, J.A.F.; Rivaroli, L.; Gücker, B. Glyphosate pollution of surface runoff, stream water, and drinking water resources in Southeast Brazil. Environ. Sci. Pollut. Res. 2022, 30, 27030–27040. [Google Scholar] [CrossRef]
- Chávez-Ortiz, P.; Tapia-Torres, Y.; Larsen, J.; García-Oliva, F. Glyphosate-based herbicides alter soil carbon and phosphorus dynamics and microbial activity. Appl. Soil Ecol. 2022, 169, 104256. [Google Scholar] [CrossRef]
- Cano-García, A.; Cerna Chávez, E.; Cerna Chávez, Y. Plaguicidas detectados en suelo de colonias de Cynomys mexicanus en San Luis Potosí y Zacatecas, México. Abanico Vet. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- López, A.; Ruiz, P.; Fuentes, E.; Yusà, V.; Dualde, P.; Miralles, P.; Coscollà, C. Simultaneous direct determination of Glyphosate and AMPA in the ambient air and inhalation risk assessment in a Mediterranean Region (Spain). Atmos. Environ. 2024, 317, 120204. [Google Scholar] [CrossRef]
- Vasseur, C.; Serra, L.; El Balkhi, S.; Lefort, G.; Ramé, C.; Froment, P.; Dupont, J. Glyphosate presence in human sperm: First report and positive correlation with oxidative stress in an infertile French population. Ecotoxicol. Environ. Saf. 2024, 278, 116410. [Google Scholar] [CrossRef]
- Acquavella, J.F.; Alexander, B.H.; Mandel, J.S.; Gustin, C.; Baker, B.; Chapman, P.; Bleeke, M. Glyphosate biomonitoring for farmers and their families: Results from the Farm Family Exposure Study. Environ. Health Perspect. 2004, 112, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Orduña-Gaytán, F.; Vallejo-Cantú, N.A.; Alvarado-Vallejo, A.; Rosas-Mendoza, E.S.; Sandoval-Herazo, L.C.; Alvarado-Lassman, A. Evaluation of the Removal of Organic Matter and Nutrients in the Co-Treatment of Fruit and Vegetable Waste Using a Bioreactor-Constructed Wetlands System. Processes 2022, 10, 278. [Google Scholar] [CrossRef]
- Rad, S.M.; Ray, A.K.; Barghi, S. Water Pollution and Agriculture Pesticide. Clean Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Ikehata, K.; Gamal El-Din, M. Aqueous Pesticide Degradation by Ozonation and Ozone-Based Advanced Oxidation Processes: A Review (Part I). Ozone Sci. Eng. 2005, 27, 83–114. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wu, L.; Jin, Y.; Gong, Y.; Li, A.; Li, J.; Li, F. Recycled aggregates from construction and demolition waste as wetland substrates for pollutant removal. J. Clean. Prod. 2021, 311, 127766. [Google Scholar] [CrossRef]
- Boucher-Carrier, O.; Brisson, J.; Abas, K.; Duy, S.V.; Sauvé, S.; Kõiv-Vainik, M. Effects of macrophyte species and biochar on the performance of treatment wetlands for the removal of glyphosate from agricultural runoff. Sci. Total Environ. 2022, 838, 156061. [Google Scholar] [CrossRef]
- Poiger, T.; Keller, M.; Buerge, I.J.; Balmer, M.E. Behavior of Glyphosate in Wastewater Treatment Plants. Chimia 2020, 74, 156. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Le, P.T.; Le, D.N.; Nguyen, T.H.; Bui, H.T.; Pham, L.A.; Nguyen, L.L.; Nguyen, Q.S.; Nguyen, T.P.; Dang, T.H.; Duong, T.T.; et al. On the Degradation of Glyphosate by Photocatalysis Using TiO2/Biochar Composite Obtained from the Pyrolysis of Rice Husk. Water 2021, 13, 3326. [Google Scholar] [CrossRef]
- Zhang, W.; You, Q.; Shu, J.; Wang, A.; Lin, H.; Yan, X. Photocatalytic degradation of glyphosate using Ce/N co-doped TiO2 with oyster shell powder as carrier under the simulated fluorescent lamp. Front. Environ. Sci. 2023, 11, 1131284. [Google Scholar] [CrossRef]
- Lu, J.; Liu, S.Y.; Xu, N. Treatment of Glyphosate Mother Liquor by Catalytic Wet Oxidation. Asian J. Chem. 2017, 29, 1817–1820. [Google Scholar] [CrossRef]
- Ahmed, N.; Vione, D.; Rivoira, L.; Castiglioni, M.; Beldean-Galea, M.S.; Bruzzoniti, M.C. Feasibility of a Heterogeneous Nanoscale Zero-Valent Iron Fenton-like Process for the Removal of Glyphosate from Water. Molecules 2023, 28, 2214. [Google Scholar] [CrossRef]
- Li, X.; Xiao, B.; Wu, M.; Wang, L.; Chen, R.; Wei, Y.; Liu, H. In-situ generation of multi-homogeneous/heterogeneous Fe-based Fenton catalysts toward rapid degradation of organic pollutants at near neutral pH. Chemosphere 2020, 245, 125663. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, F.; Chen, Y.; Wu, J.; Cheng, S. The Interaction Effects of Aeration and Plant on the Purification Performance of Horizontal Subsurface Flow Constructed Wetland. Int. J. Environ. Res. Public Health 2022, 19, 1583. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leontievsky, A.A. Microbial degradation of glyphosate herbicides (Review). Appl. Biochem. Microbiol. 2015, 51, 188–195. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, D.; Hu, J.; Zhang, J.; Liu, Z.; Li, A.; Li, R. Glyphosate and nutrients removal from simulated agricultural runoff in a pilot pyrrhotite constructed wetland. Water Res. 2020, 168, 115154. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Feng, X.; Pyo, S.H. Current problems and countermeasures of constructed wetland for wastewater treatment: A review. J. Water Process Eng. 2024, 57, 104569. [Google Scholar] [CrossRef]
- Pandey, D.; Singh, S.V.; Savio, N.; Bhutto, J.K.; Srivastava, R.K.; Yadav, K.K.; Sharma, R.; Nandipamu, T.M.K.; Sarkar, B. Biochar application in constructed wetlands for wastewater treatment: A critical review. J. Water Process Eng. 2025, 69, 106713. [Google Scholar] [CrossRef]
- Schierano, M.C.; Panigatti, M.C.; Maine, M.A.; Griffa, C.A.; Boglione, R. Horizontal subsurface flow constructed wetland for tertiary treatment of dairy wastewater: Removal efficiencies and plant uptake. J. Environ. Manag. 2020, 272, 111094. [Google Scholar] [CrossRef] [PubMed]
- Brisson, J.; Carvalho, P.; Stein, O.; Weber, K.; Brix, H.; Zhao, Y.; Zurita, F. Small-scale experiments: Using mesocosms and microcosms for testing hypotheses in treatment wetland research. Ecol. Eng. 2024, 208, 107378. [Google Scholar] [CrossRef]
- Liang, M.Q.; Zhang, C.F.; Peng, C.L.; Lai, Z.L.; Chen, D.F.; Chen, Z.H. Plant growth, community structure, and nutrient removal in monoculture and mixed constructed wetlands. Ecol. Eng. 2011, 37, 309–316. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Zitácuaro-Contreras, I.; Ortega-Pineda, G.; López-Roldán, A.; Vidal-Álvarez, M.; Martínez-Aguilar, K.E.; Álvarez-Hernández, L.M.; Zamora-Castro, S. Phytoremediation Performance with Ornamental Plants in Monocultures and Polycultures Conditions Using Constructed Wetlands Technology. Plants 2024, 13, 1051. [Google Scholar] [CrossRef]
- Calderon, R.; García-Hernández, J.; Palma, P.; Leyva-Morales, J.B.; Zambrano-Soria, M.; Bastidas-Bastidas, P.J.; Godoy, M. Assessment of pesticide residues in vegetables commonly consumed in Chile and Mexico: Potential impacts for public health. J. Food Compos. Anal. 2022, 108, 104420. [Google Scholar] [CrossRef]
- Fernández-Echeverría, E.; Sandoval Herazo, L.C.; Zurita, F.; Betanzo-Torres, E.; Sandoval-Herazo, M. Development of Heliconia latispatha in constructed wetlands, for the treatment of swine/domestic wastewater in tropical climates, with PET as a substitute for the filter medium. Rev. Mex. Ing. Química 2022, 2, IA2811. [Google Scholar] [CrossRef]
- Sandoval-Herazo, L.C.; Alvarado-Lassman, A.; López-Méndez, M.C.; Martínez-Sibaja, A.; Aguilar-Lasserre, A.A.; Zamora-Castro, S.; Marín-Muñiz, J.L. Effects of Ornamental Plant Density and Mineral/Plastic Media on the Removal of Domestic Wastewater Pollutants by Home Wetlands Technology. Molecules 2020, 25, 5273. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Ramírez-Martínez, M.; Gómez-Merino, F.C.; García-Albarado, J.C.; Baca-Castillo, G.A.; Tejeda-Sartorius, O. Evaluación física y química de tezontle y su uso en la producción de tulipán. XRev. Mex. Cienc. Agrícolas 2013, 4, 863–876. [Google Scholar]
- Castrejón-Godínez, M.L.; Tovar-Sánchez, E.; Valencia-Cuevas, L.; Rosas-Ramírez, M.E.; Rodríguez, A.; Mussali-Galante, P. Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review. Microorganisms 2021, 9, 2322. [Google Scholar] [CrossRef]
- Lara-Acosta, M.; Lango-Reynoso, F.; Castañeda-Chávez, M.d.R. Use of tropical macrophytes in wastewater treatment. Agro Product. 2022. [Google Scholar] [CrossRef]
- Sandoval, L.; Zurita, F.; Del Ángel-Coronel, O.A.; Adame-García, J.; Marín-Muñíz, J.L. Influence of a new ornamental species (Spathiphyllum blandum) on the removal of COD, nitrogen, phosphorus and fecal coliforms: A mesocosm wetland study with PET and tezontle substrates. Water Sci. Technol. 2020, 81, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Konnerup, D.; Koottatep, T.; Brix, H. Treatment of domestic wastewater in tropical, subsurface flow constructed wetlands planted with Canna and Heliconia. Ecol. Eng. 2009, 35, 248–257. [Google Scholar] [CrossRef]
- Sandoval-Herazo, M.; Martínez-Reséndiz, G.; Fernández Echeverria, E.; Fernández-Lambert, G.; Sandoval Herazo, L.C. Plant Biomass Production in Constructed Wetlands Treating Swine Wastewater in Tropical Climates. Fermentation 2021, 7, 296. [Google Scholar] [CrossRef]
- Norma Mexicana NMX-AA-008-SCFI-2000: Análisis de Agua—Medición del pH en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba; CANCELA A LA NMX-AA-008-SCFI-2011; Secretaría de Economía: Mexico City, Mexico, 2016; Available online: https://www.gob.mx/cms/uploads/attachment/file/166767/NMX-AA-008-SCFI-2016.pdf (accessed on 27 January 2025).
- Norma Mexicana NMX-AA-007-SCFI-2000: Análisis de Agua—Medición de la Temperatura en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba; CANCELA A LA NMX-AA-007-SCFI-2000; Secretaría de Economía: Mexico City, Mexico, 2014; Available online: https://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/agenda/DOFsr/NMX-AA-007-SCFI-2000.pdf (accessed on 27 January 2025).
- Norma Mexicana NMX-AA-093-SCFI-2018: Análisis de Agua—Medición de la Conductividad Eléctrica en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba; CANCELA A LA NMX-AA-093-SCFI-2000; Secretaría de Economía: Mexico City, Mexico; Available online: https://www.gob.mx/cms/uploads/attachment/file/166800/NMX-AA-093-SCFI-2000.pdf (accessed on 27 January 2025).
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 5220 D Chemical Oxygen Demand (COD). In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA; Available online: https://edgeanalytical.com/wp-content/uploads/Waste_SM5220.pdf (accessed on 27 January 2025).
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 4500-B NO2− nitrogen (nitrite). In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA; Available online: https://www.standardmethods.org/doi/10.2105/SMWW.2882.088 (accessed on 27 January 2025).
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 4500-NO3−E nitrogen (nitrate). In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA; Available online: https://www.standardmethods.org/doi/10.2105/SMWW.2882.089 (accessed on 27 January 2025).
- Jeong, H.; Park, J.; Kim, H. Determination of NH 4 + in Environmental Water with Interfering Substances Using the Modified Nessler Method. J. Chem. 2013, 359217. [Google Scholar] [CrossRef]
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 4500-norg-B: Nitrogen (organic). In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA; Available online: https://www.edgeanalytical.com/wp-content/uploads/SoilsBiosolids_SM4500-Norg.pdf (accessed on 27 January 2025).
- Norma Mexicana NMX-AA-026-SCFI-2010: Análisis de Agua—Medición de Nitrógeno Total Kjeldahl en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba; CANCELA A LA NMX-AA-026-SCFI-2001; Secretaría de Economía: Mexico City, Mexico; Available online: https://www.gob.mx/cms/uploads/attachment/file/166772/NMX-AA-026-SCFI-2010.pdf (accessed on 27 January 2025).
- Standard Methods Committee of the American Public Health Association; American Water Works Association; Water Environment Federation. 4500-P phosphorus. In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA; Available online: https://edgeanalytical.com/wp-content/uploads/SoilsBiosolids_SM4500-P.pdf (accessed on 27 January 2025).
- López-Chávez, M.Y.; Alvarez-Legorreta, T.; Infante-Mata, D.; Dunn, M.F.; Guillén-Navarro, K. Glyphosate-remediation potential of selected plant species in artificial wetlands. Sci. Total Environ. 2021, 781, 146812. [Google Scholar] [CrossRef]
- Vallée, R.; Dousset, S.; Schott, F.-X.; Pallez, C.; Ortar, A.; Cherrier, R.; Munoz, J.-F.; Benoît, M. Do constructed wetlands in grass strips reduce water contamination from drained fields? Environ. Pollut. 2015, 207, 365–373. [Google Scholar] [CrossRef]
- Karungamye, P.N. Potential of Canna indica in Constructed Wetlands for Wastewater Treatment: A Review. Conservation 2022, 2, 499–513. [Google Scholar] [CrossRef]
- Phewnil, O.; Chunkao, K.; Prabhuddham, P.; Pattamapitoon, T. Application of different aquatic plants in an alternated fill and drain wetland system of Phetchaburi municipal wastewater treatment in Thailand. Environ. Sci. Pollut. Res. 2023, 31, 1304–1313. [Google Scholar] [CrossRef]
- Gorme, J.B.; Maniquiz, M.C.; Lee, S.; Kim, L.-H. Seasonal changes of plant biomass at a constructed wetland in a livestock watershed area. Desalination Water Treat. 2012, 45, 136–143. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. Unlocking the potential of wetland biomass: Treatment approaches and sustainable resource management for enhanced utilization. Bioresour. Technol. Rep. 2023, 23, 101553. [Google Scholar] [CrossRef]
- Monteagudo-Hernández, G.; Hernández-Castelán, D.A.; Zamora-Lobato, T.; Sandoval-Herazo, M.; Hernández-Orduña, M.G.; Sandoval Herazo, L.C. Evaluation of the pollutant removal efficiency of swine wastewater through two configurations of hybrid wetlands with tropical ornamental plants. Results Eng. 2024, 24, 102864. [Google Scholar] [CrossRef]
- Hernández-Castelán, D.A.; Zurita, F.; Marín-Peña, O.; Betanzo-Torres, E.A.; Sandoval-Herazo, M.; Castellanos-Rivera, J.; Sandoval Herazo, L.C. Effect of monocultures and polycultures of Typha latifolia and Heliconia psittacorum on the treatment of river waters contaminated with landfill leachate/domestic wastewater in partially saturated vertical constructed wetlands. Int. J. Phytoremediation 2024, 26, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Berego, Y.S.; Sota, S.S.; Ulsido, M.D.; Beyene, E.M. Treatment Performance Assessment of Natural and Constructed Wetlands on Wastewater From Kege Wet Coffee Processing Plant in Dale Woreda, Sidama Regional State, Ethiopia. Environ. Health Insights 2022, 16, 117863022211427. [Google Scholar] [CrossRef] [PubMed]
- Gitau, J.K.; Kitur, E. Efficiency of Tibia wetland in treatment of wastewater. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 59–64. [Google Scholar]
- Ghezali, K.; Bentahar, N.; Barsan, N.; Nedeff, V.; Moșneguțu, E. Potential of Canna indica in Vertical Flow Constructed Wetlands for Heavy Metals and Nitrogen Removal from Algiers Refinery Wastewater. Sustainability 2022, 14, 4394. [Google Scholar] [CrossRef]
- Decezaro, S.T.; Wolff, D.B.; Araújo, R.K.; Faccenda, H.B.; Perondi, T.; Sezerino, P.H. Vertical flow constructed wetland planted with Heliconia psittacorum used as decentralized post-treatment of anaerobic effluent in Southern Brazil. J. Environ. Sci. Health 2018, 53 Pt A, 1131–1138. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; García-González, M.C.; Ruelas-Monjardín, L.C.; Moreno-Casasola, P. Influence of Different Porous Media and Ornamental Vegetation on Wastewater Pollutant Removal in Vertical Subsurface Flow Wetland Microcosms. Environ. Eng. Sci. 2018, 35, 88–94. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Hernández, M.E.; Gallegos-Pérez, M.P.; Amaya-Tejeda, S.I. Plant growth and pollutant removal from wastewater in domiciliary constructed wetland microcosms with monoculture and polyculture of tropical ornamental plants. Ecol. Eng. 2020, 147, 105658. [Google Scholar] [CrossRef]
- Barya, M.P.; Gupta, D.; Thakur, T.K.; Shukla, R.; Singh, G.; Mishra, V.K. Phytoremediation performance of Acorus calamus and Canna indica for the treatment of primary treated domestic sewage through vertical subsurface flow constructed wetlands: A field-scale study. Water Pract. Technol. 2020, 15, 528–539. [Google Scholar] [CrossRef]
- Pinninti, R.; Kasi, V.; Sallangi LK SV, P.; Landa, S.R.; Rathinasamy, M.; Sangamreddi, C.; Dandu Radha, P.R. Performance of Canna Indica based microscale vertical flow constructed wetland under tropical conditions for domestic wastewater treatment. Int. J. Phytoremediat. 2022, 24, 684–694. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Meza-Figueroa, D.; Martínez-Villegas, N.V.; Pedroza-Montero, M.R. Glyphosate impact on human health and the environment: Sustainable alternatives to replace it in Mexico. Chemosphere 2023, 340, 139810. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Tang, Z.; Aryal, N. The removal of glyphosate in agricultural runoff by hybrid constructed wetland mesocosms. In Proceedings of the National Conference on Next-Generation Sustainable Technologies for Small-Scale Producers; Atlantis Press: Dordrecht, the Netherlands, 2023; pp. 186–206. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, Z.; Wang, X.; Guan, W.; Liu, M.; Yu, N.; Tian, H.; Li, J.; Zhang, S.; Gu, Y.; et al. Cladophora can mitigate the shock of glyphosate-containing wastewater on constructed wetlands coupled with microbial fuel cells. Chemosphere 2022, 308, 136273. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Krauss, M.; Zschieschang, S.; Miltner, A.; Butkovskyi, A.; Eggen, T.; Kästner, M.; Nowak, M.K. Superabsorbent polymer as a supplement substrate of constructed wetland to retain pesticides from agricultural runoff. Water Res. 2021, 207, 117776. [Google Scholar] [CrossRef]




| Parameter | Method | Reference |
|---|---|---|
| pH | Electrode | [44] |
| Temperature (°C) | Thermometer | [45] |
| EC (µs cm−1) | Electrode | [46] |
| COD (mg L−1) | Closed Reflux | [47] |
| NO2 | Spectrophotometric | [48] |
| NO3 | Cadmium Reduction technique | [49] |
| NH4 | Nessler | Adapted from [50] |
| TN (mg L−1) | Kjeldahl Digestion Method | [51,52] |
| TP (mg L−1) | Ascorbic Acid Method | [53] |
| Variable | Monoculture Mesocosm | Polyculture Mesocosm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. indica + H. latispatha + A. purpurata | ||||||||||||
| C. indica | H. latispatha | A. purpurata | C. indica | H. latispatha | A. purpurata | |||||||
| i | f | i | f | i | f | i | f | i | f | i | f | |
| Number of Leaves | 25 | 65 a | 15 | 39 a | 125 | 308 a b | 18 | 58 a | 8 | 21 a | 99 | 230 a |
| Number of flowers | 0 | 18 a | 0 | 0 | 0 | 6 a b | 0 | 24 a | 0 | 0 | 0 | 0 |
| Cultivation Method | Plant Specie | Fresh Biomass (g) | Dry Biomass (g) |
|---|---|---|---|
| Monoculture | Canna indica | 8215.1 | 292.8 |
| Heliconia psittacorum | 3115.0 | 786.0 | |
| Alpinia Purpurata | 7320.8 | 3053.0 | |
| Polyculture | Canna indica | 4960.5 | 481.0 |
| Heliconia psittacorum | 7395.9 | 1723.0 | |
| Alpinia Purpurata | 1685.8 | 498.0 |
| Treatments | COD | Ammonium | N-NO3 | N-NO2 | TN | TP | PO4−3 |
|---|---|---|---|---|---|---|---|
| Inlet | 567 ± 65 | 51 ± 15 | 27 ± 2 | 45 ± 3 | 138 ± 26 | 28 ± 2 | 14 ± 1 |
| Control | 377 ± 80 | 26 ± 6 | 16 ± 2 | 38 ± 6 | 77 ± 9 | 16 ± 1 | 10 ± 2 |
| % efficiency | 41 | 49 | 41 | 16 | 44 | 43 | 29 |
| Policulture | 139 ± 28 | 3 ± 1 | 6 ± 1 | 7 ± 2 | 16 ± 4 | 3 ± 0 | 1 ± 0.6 |
| % efficiency | 75 | 94 | 78 | 84 | 88 | 89 | 93 |
| Alpinia Purpurata | 128 ± 18 | 5 ± 3 | 7 ± 2 | 4 ± 3 | 18 ± 5 | 3 ± 1 | 0.31 ± 0.15 |
| % efficiency | 77 | 90 | 74 | 91 | 87 | 89 | 98 |
| Heliconia psittacorum | 130 ± 12 | 6 ± 2 | 5 ± 1 | 4 ± 1 | 17 ± 3 | 3 ± 1 | 1.2 ± 0.2 |
| % efficiency | 77 | 88 | 81 | 91 | 88 | 89 | 91 |
| Canna indica | 141 ± 29 | 3 ± 1 | 6 ± 2 | 5 ± 1 | 13 ± 4 | 2 ± 0 | 0.34 ± 0.09 |
| % efficiency | 75 | 94 | 78 | 89 | 91 | 93 | 98 |
| Treatments | % Removal Efficiency | ||||
|---|---|---|---|---|---|
| 40 Days | 80 Days | 120 Days | 160 Days | 200 Days | |
| Inlet (ppm) | 10.07 ± 0.2 | 12.93 ± 0.5 | 10.57 ± 0.5 | 11.79 ± 0.3 | 16.56 ± 0.5 |
| Control | 13.77 | 32.42 | 17.92 | 10.46 | 49.00 |
| Policulture | 57.11 | 71.03 | 51.11 | 64.13 | 70.31 |
| A. purpurata | 35.05 | 30.21 | 8.54 | 29.58 | 84.19 |
| H. psittacorum | No removal | 1.75 | 43.25 | 58.44 | 97.10 |
| Canna indica | 9.65 | 9.44 | 10.51 | 23.98 | 76.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Cortés, G.; Martínez-Castellanos, G.; Martínez-Reséndiz, G.; Zamora-Castro, S.A.; Monroy-Pineda, M.C.; Sandoval Herazo, L.C. Removal of Glyphosate in Agricultural Runoff Using Subsurface Constructed Wetlands in Monocultures and Polycultures of Tropical Plants. Processes 2025, 13, 860. https://doi.org/10.3390/pr13030860
Aguilar-Cortés G, Martínez-Castellanos G, Martínez-Reséndiz G, Zamora-Castro SA, Monroy-Pineda MC, Sandoval Herazo LC. Removal of Glyphosate in Agricultural Runoff Using Subsurface Constructed Wetlands in Monocultures and Polycultures of Tropical Plants. Processes. 2025; 13(3):860. https://doi.org/10.3390/pr13030860
Chicago/Turabian StyleAguilar-Cortés, Graciano, Gustavo Martínez-Castellanos, Georgina Martínez-Reséndiz, Sergio Aurelio Zamora-Castro, María Cecilia Monroy-Pineda, and Luis Carlos Sandoval Herazo. 2025. "Removal of Glyphosate in Agricultural Runoff Using Subsurface Constructed Wetlands in Monocultures and Polycultures of Tropical Plants" Processes 13, no. 3: 860. https://doi.org/10.3390/pr13030860
APA StyleAguilar-Cortés, G., Martínez-Castellanos, G., Martínez-Reséndiz, G., Zamora-Castro, S. A., Monroy-Pineda, M. C., & Sandoval Herazo, L. C. (2025). Removal of Glyphosate in Agricultural Runoff Using Subsurface Constructed Wetlands in Monocultures and Polycultures of Tropical Plants. Processes, 13(3), 860. https://doi.org/10.3390/pr13030860










