Multistep Kinetics Study on Hydrogen Reduction of 0.25–0.5 mm Iron Oxide Particles
Abstract
1. Introduction
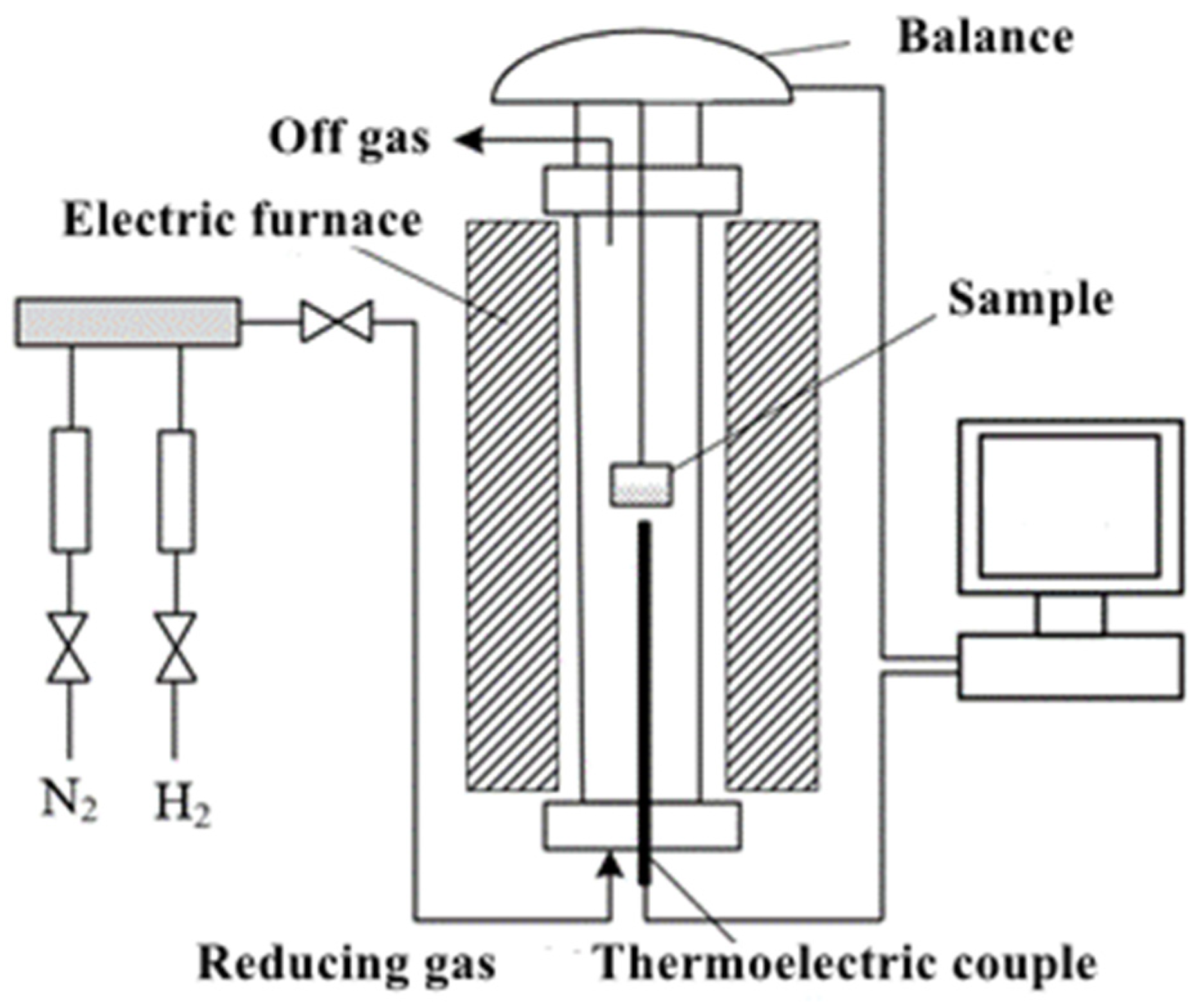
2. Experimental Scheme
3. Experimental Process
3.1. Experimental Sample
3.2. Experimental Method
4. Experimental Results and Analysis
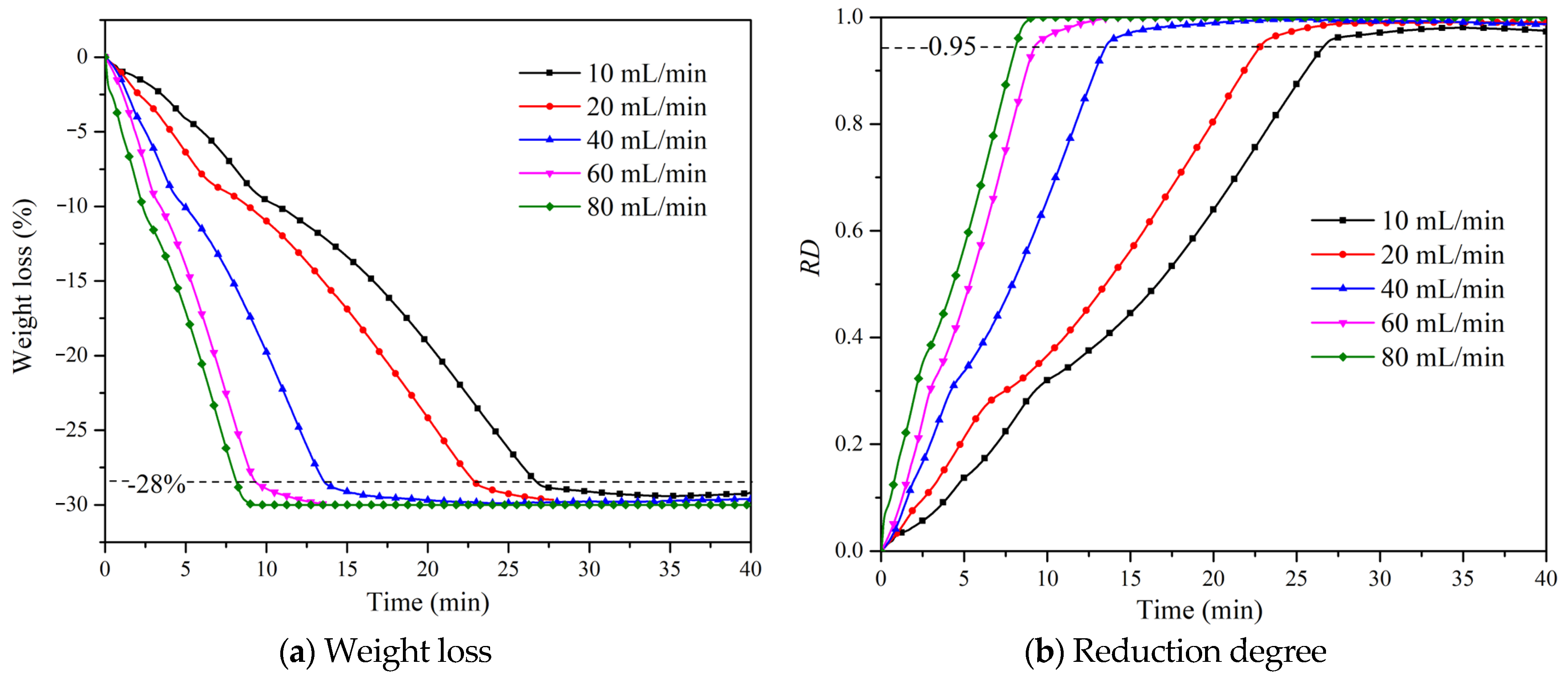
4.1. Effect of Gas Flow on Reduction Degree
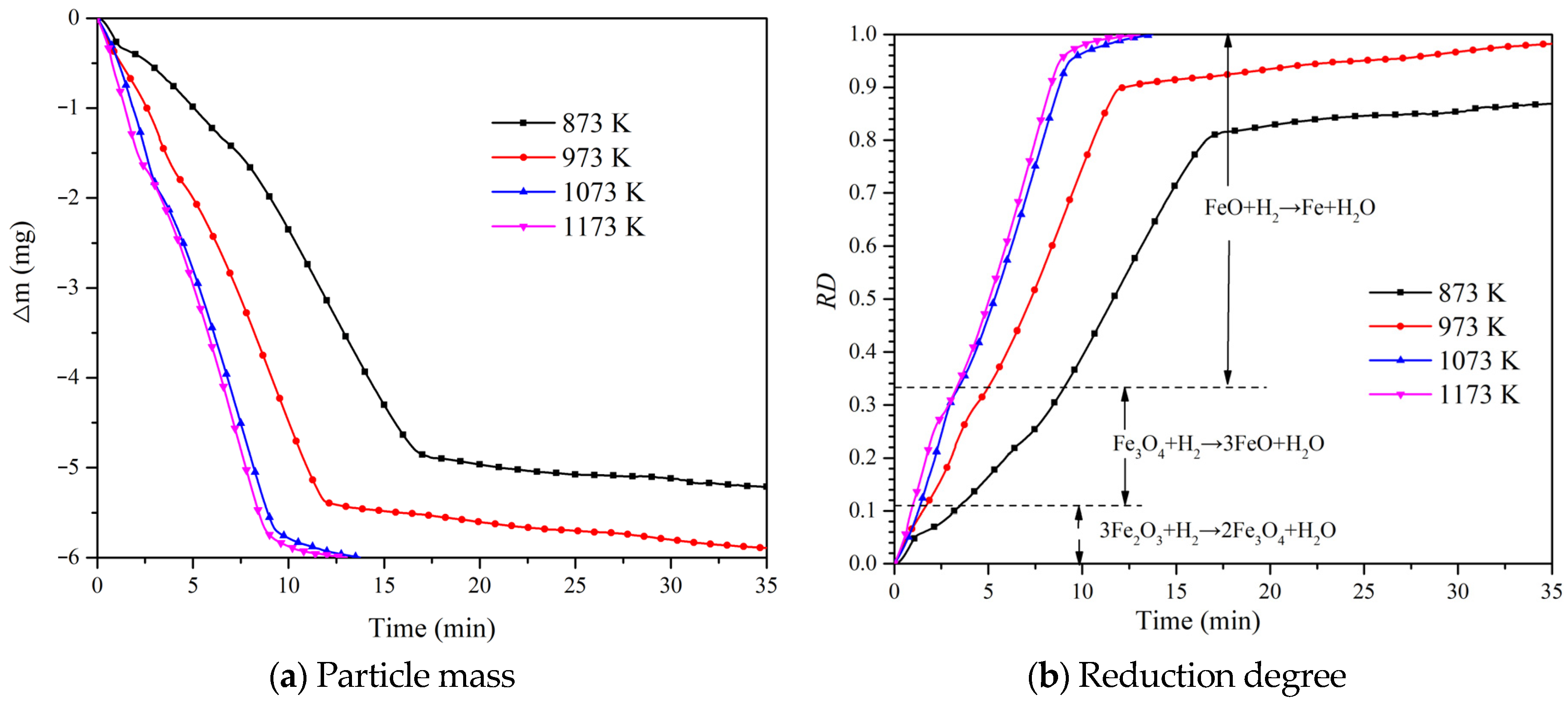
4.2. Effect of Temperature on Reduction Degree
4.3. Calculation and Evaluation of Apparent Activation Energy
5. Conclusions
- (1)
- The improvement of the degree of reduction is promoted by both the inlet flow rate and temperature. Moreover, the time required for iron oxide to reach the maximum degree of reduction will be shortened as the inlet flow rate and temperature become higher.
- (2)
- The whole process of iron oxide reduction can be divided into three stages, namely the Fe2O3 → Fe3O4 stage, the Fe3O4 → FeO stage and the FeO → Fe stage. The control mechanisms of each stage are, respectively, based on interfacial chemical reactions, second-order chemical reactions and interfacial chemical reactions. All R2 values are greater than 0.94, indicating a good linearity.
- (3)
- Eventually, the activation energy values for the three stages are obtained as 39.696 kJ/mol, 28.129 kJ/mol and 19.110 kJ/mol, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patisson, F.; Mirgaux, O. Hydrogen Ironmaking: How It Works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Bararzadeh Ledari, M.; Khajehpour, H.; Akbarnavasi, H.; Edalati, S. Greening steel industry by hydrogen: Lessons learned for the developing world. Int. J. Hydrogen Energy 2023, 48, 36623–36649. [Google Scholar] [CrossRef]
- Wang, R.R.; Zhao, Y.Q.; Babich, A.; Senk, D.; Fan, X.Y. Hydrogen direct reduction (H-DR) in steel industry—An overview of challenges and opportunities. J. Clean. Prod. 2021, 329, 129797. [Google Scholar] [CrossRef]
- Guo, L.; Tu, G.; Feng, N.; Di, Y. Non-isothermal Kinetic Analysis of Magnesium Hydroxide Powder Prepared by Electrolysis Method. J. Mater. Metall. 2023, 22, 166–171. [Google Scholar]
- Qu, Y.; Luo, Y.; Shi, S.; Zhang, B. Flash Reduction Behavior of Hematite Fine Ore in H2 Atmosphere. J. Mater. Metall. 2019, 18, 79–86. [Google Scholar]
- He, J.; Li, K.; Zhang, J.; Conejo, A.N. Reduction Kinetics of Compact Hematite with Hydrogen from 600 to 1050 °C. Metals 2023, 13, 464. [Google Scholar] [CrossRef]
- Si, X.; Zhao, E.; Shao, J.; Pang, Z.; Zhang, J.; Huang, Y. Experimental Study on the Hydrogen Reduction Characteristics of Titanium Concentrate Powder by Thermogravimetric Method. Hebei Metall. 2013, 6, 1–4. [Google Scholar]
- Li, Y.; Hu, X.; Garg, P.; Mao, X.; Li, K.; Zhang, J. Kinetic Study on Hydrogen Reduction of Iron Ore Powder by Thermogravimetric-Mass Spectrometry. Jiangxi Metall. 2020, 40, 1–5. [Google Scholar]
- Lin, C. Mechanism Research on Preparation of Ultra-fine Iron Powder by Low-Temperature Reduction. Ph.D. Thesis, University of Science and Technology Liaoning, Anshan, China, 2017. [Google Scholar]
- Wagner, D.; Devisme, O.; Patisson, F.; Ablitzer, D. A Laboratory Study of the Reduction of Iron Oxides by Hydrogen. arXiv 2008, arXiv:0803.2831. [Google Scholar]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Yin, C.; Minakshi, M.; Ralph, D.E.; Jiang, Z. Hydrothermal synthesis of cubic α-Fe2O3 microparticles using glycine: Surface characterization, reaction mechanism and electrochemical activity. J. Alloys Compd. 2011, 509, 9821–9825. [Google Scholar] [CrossRef]
- Piotrowski, K.; Mondal, K.; Wiltowski, T.; Dydo, P.; Rizeg, G. Topochemical Approach of Kinetics of the Reduction of Hematite to Wüstite. Chem. Eng. J. 2007, 131, 73–82. [Google Scholar] [CrossRef]
- Barde, A.A.; Klausner, J.F.; Mei, R. Solid state reaction kinetics of iron oxide reduction using hydrogen as a reducing agent. Int. J. Hydrogen Energy 2016, 41, 10103–10119. [Google Scholar] [CrossRef]



| Model and Control Mechanism | f(α) | G(α) |
|---|---|---|
| 3D interface control chemical reaction control | 3(1 − α)2/3 | 1 − (1 − α)1/3 |
| First-order chemical reaction model | 1 − α | −ln(1 − α) |
| Second-order chemical reaction model | (1 − α)2 | (1 − α)−1 − 1 |
| Third-order chemical reaction model | (1 − α)3 | 0.5[(1 − α)−2 − 1] |
| Second-order nucleus formation and growth model | 2(1 − α)[−ln(1 − α)]1/2 | [−ln(1 − α)]1/2 |
| Third-order nuclear formation and growth model | 3(1 − α)[−ln(1 − α)]2/3 | [−ln(1 − α)]1/3 |
| Reaction Stage | E (kJ/mol) | A (min−1) | f(α) |
|---|---|---|---|
| Fe2O3 → Fe3O4 | 39.696 | 2.804 | 3(1 − α)2/3 |
| Fe3O4 → FeO | 28.129 | 3.880 | (1 − α)2 |
| FeO → Fe | 19.110 | 0.898 | 3(1 − α)[−ln(1 − α)]2/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, J.; Su, F. Multistep Kinetics Study on Hydrogen Reduction of 0.25–0.5 mm Iron Oxide Particles. Processes 2025, 13, 893. https://doi.org/10.3390/pr13030893
Si J, Su F. Multistep Kinetics Study on Hydrogen Reduction of 0.25–0.5 mm Iron Oxide Particles. Processes. 2025; 13(3):893. https://doi.org/10.3390/pr13030893
Chicago/Turabian StyleSi, Junlong, and Fuyong Su. 2025. "Multistep Kinetics Study on Hydrogen Reduction of 0.25–0.5 mm Iron Oxide Particles" Processes 13, no. 3: 893. https://doi.org/10.3390/pr13030893
APA StyleSi, J., & Su, F. (2025). Multistep Kinetics Study on Hydrogen Reduction of 0.25–0.5 mm Iron Oxide Particles. Processes, 13(3), 893. https://doi.org/10.3390/pr13030893






