Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management
Abstract
1. Introduction
2. Soil Organic Pollutants in Forest Ecosystems
2.1. Hazards of Organic Pollutants in Forest Soil
2.2. Sources of Organic Matter Contamination in Forest Soils
2.3. Microbial Treatment
3. Mechanism of Microbial Degradation of Soil Organic Pollutants
3.1. Complex Processes and Key Species
| Type of Pollutant | Microbial Species | Mechanism of Degradation | Ref. |
|---|---|---|---|
| Pesticides | |||
| DDT | Bacteria: Sphingomonas spp. Fungi: Phanerochaete chrysosporium | Reduction and Hydrolysis: Reduction of DDT to DDE, hydrolysis to DDA (dichlorodiphenyldichloroethane) | [94] |
| Aldrin | Bacteria: Burkholderia cepacia Fungi: Aspergillus niger | Oxidative Degradation: Oxidation of Aldrin to Dieldrin, further degradation to less toxic forms | [95] |
| Endrin | Bacteria: Pseudomonas putida Fungi: Trichoderma harzianum | Dechlorination: Dechlorination and hydrolysis of Endrin to less toxic products | [78] |
| Chlordane | Bacteria: Mycobacterium spp. Fungi: Cunninghamella echinulata | Oxidative and Hydrolytic Degradation: Oxidation of Chlordane to less toxic metabolites | [96] |
| Lindane | Bacteria: Rhodococcus spp. Fungi: White-rot fungi | Ring Cleavage: Ring cleavage and mineralization of Lindane to non-toxic products | [82] |
| Industrial Chemicals | |||
| PCBs | Bacteria: Burkholderia spp. Fungi: Phanerochaete chrysosporium | Dechlorination: Microbial dechlorination of PCBs to less chlorinated and less toxic forms | [97] |
| Dioxins | Bacteria: Dechloromonas spp. Fungi: Cunninghamella elegans | Reductive Dechlorination: Reduction of chlorine atoms from dioxins to less toxic forms | [98] |
| Furans | Bacteria: Pseudomonas spp. Fungi: Lentinus edodes | Oxidative Degradation: Oxidation of furans to less harmful products | [99] |
| Alkanes | |||
| n-Hexane | Bacteria: Pseudomonas putida Fungi: Phanerochaete chrysosporium | Oxidative Biodegradation: Conversion of n-Hexane to less harmful products through hydroxylation | [100] |
| n-Heptane | Bacteria: Mycobacterium spp. Fungi: Aspergillus niger | Hydroxylation: Oxidation of n-Heptane to heptane-1-ol, followed by further oxidation | [101] |
| n-Octane | Bacteria: Rhodococcus spp. Fungi: White-rot fungi | Terminal Oxidation: Terminal oxidation of n-Octane to fatty acids and further degradation | [102] |
| Aromatic Hydrocarbons | |||
| Benzene | Bacteria: Pseudomonas putida Fungi: Phanerochaete chrysosporium | Ring Cleavage: Conversion of benzene to catechol and further breakdown via ring cleavage | [103] |
| Toluene | Bacteria: Pseudomonas putida Fungi: Aspergillus niger | Monooxygenation: Oxidation of toluene to toluene-4-monooxygenase, further oxidized to benzoic acid | [79] |
| Ethylbenzene | Bacteria: Pseudomonas spp. Fungi: White-rot fungi | Oxidative Degradation: Oxidation of ethylbenzene to ethylbenzene-1,2-diol, then to catechol | [80] |
| Xylenes | Bacteria: Pseudomonas putida Fungi: Phanerochaete chrysosporium | Oxidative Degradation: Oxidation of xylenes to methylbenzoic acids and further breakdown | [104] |
| PAHs | |||
| Naphthalene | Bacteria: Pseudomonas putida Fungi: Phanerochaete chrysosporium | Ring Cleavage: Conversion of naphthalene to catechol and further breakdown | [74] |
| Anthracene | Bacteria: Mycobacterium spp. Fungi: Aspergillus niger | Ring Cleavage and Oxidation: Conversion to anthraquinone and further breakdown | [75] |
| Phenanthrene | Bacteria: Sphingomonas spp. Fungi: White-rot fungi | Ring Cleavage and Oxidation: Conversion to phenanthrene-2,3-diol and further breakdown | [76] |
| Benzo[a]pyrene | Bacteria: Mycobacterium spp. Fungi: Phanerochaete chrysosporium | Ring Cleavage and Oxidation: Conversion to less toxic metabolites | [77] |
| Antibiotics | |||
| Ciprofloxacin | Bacteria: Pseudomonas spp. Fungi: Aspergillus niger | Oxidative Degradation: Conversion to less toxic derivatives | [105] |
| Tetracycline | Bacteria: Bacillus spp. Fungi: Trichoderma harzianum | Hydrolysis and Oxidation: Hydrolysis to inactive forms and oxidative cleavage | [106] |
| Hormones | |||
| Estrogens | Bacteria: Comamonas testosteroni Fungi: Phanerochaete chrysosporium | Hydroxylation and Oxidation: Conversion to less active metabolites | [107] |
| Progesterone | Bacteria: Sphingomonas spp. Fungi: White-rot fungi | Oxidative Degradation: Conversion to less active forms through hydroxylation | [108] |
| Sunscreens | |||
| Oxybenzone | Bacteria: Pseudomonas putida Fungi: Aspergillus niger | Oxidative Degradation: Conversion to less toxic metabolites | [109] |
| Octocrylene | Bacteria: Pseudomonas spp. Fungi: Penicillium chrysogenum | Hydrolysis and Oxidation: Conversion to less harmful products through hydrolysis | [110] |
| Synthetic Fragrances | |||
| Phthalates | Bacteria: Burkholderia spp. Fungi: Aspergillus niger | Hydrolysis: Hydrolysis of phthalates to phthalic acid and further degradation | [111] |
| Musk Compounds | Bacteria: Sphingomonas spp. Fungi: White-rot fungi | Oxidative Degradation: Oxidation of musk compounds to less toxic metabolites | [112] |
| Solvents | |||
| Acetone | Bacteria: Pseudomonas spp. Fungi: Aspergillus niger | Oxidative Degradation: Conversion to acetic acid and further oxidation | [81] |
| Ethanol | Bacteria: Zymomonas mobilis Fungi: Saccharomyces cerevisiae | Fermentation: Conversion to acetaldehyde and acetic acid via fermentation | [113] |
| Methanol | Bacteria: Methylobacterium spp. Fungi: Aspergillus niger | Oxidative Degradation: Conversion to formaldehyde and further oxidation | [114] |
| Industrial Emissions | |||
| Formaldehyde | Bacteria: Methylobacterium spp. Fungi: Phanerochaete chrysosporium | Oxidative Degradation: Conversion to formic acid and further breakdown | [115] |
| Styrene | Bacteria: Pseudomonas putida Fungi: Aspergillus niger | Oxidative Degradation: Conversion to styrene oxide and further breakdown | [116] |
| Trichloroethylene | Bacteria: Dehalococcoides spp. Fungi: White-rot fungi | Reductive Dechlorination: Conversion to less toxic forms via dechlorination | [117] |
| BTEX | |||
| Benzene | Bacteria: Pseudomonas putida Fungi: Phanerochaete chrysosporium | Ring Cleavage: Conversion to catechol and further breakdown | [118] |
| Toluene | Bacteria: Pseudomonas putida Fungi: Aspergillus niger | Monooxygenation: Conversion to benzoic acid | [119] |
| Xylenes | Bacteria: Pseudomonas putida Fungi: Phanerochaete chrysosporium | Oxidative Degradation: Conversion to methylbenzoic acids | [119] |
3.2. Microbial Groups and Their Roles in Soil Degradation
3.2.1. SRB and Archaea
3.2.2. SOB and Archaea
3.2.3. IRB and Archaea
3.2.4. IOB and Archaea
4. Factors Influencing Microbial Degradation Efficiency
4.1. Forest Cover Types
4.2. Soil Properties
4.2.1. pH
4.2.2. Temperature
4.2.3. Oxygen Levels
4.2.4. Redox Potential
4.2.5. Humidness
4.2.6. Soil Organic Matter
4.3. Types of Pollutant
5. Technological Advances and Methodological Approaches
5.1. Genetic Engineering of Microorganisms
5.2. Next-Generation Microbial Agents
5.3. Nanotechnologies
5.4. Bio-Electrochemical Technologies
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bharat Raj, S.; Lal, R.; Blum, W.H.; Valentin, C.; Stewart, B.A. Soil Pollution and Contamination. In Methods for Assessment of Soil Degradation; CRC Press eBooks; Taylor & Francis Group: Abington, UK, 2020. [Google Scholar] [CrossRef]
- Zhikun, C.; Muhammad, I.; Guanghua, J.; Weixi, W.; Biao, H.; Yingmei, L.; Yanxia, Z.; Yizhe, Y.; Qingtao, L.; Zhao, Z.; et al. Toxic elements pollution risk as affected by various input sources in soils of greenhouses, kiwifruit orchards, cereal fields, and forest/grassland. Environ. Pollut. 2023, 338, 122639. [Google Scholar] [CrossRef]
- Su, C.T.; Zou, S.Z.; Tang, J.S.; Liang, B. Influence of different ecosystems on the soil moisture and karst effect in Luota, West Hunan Province of China. Carbonates Evaporites 2018, 33, 187–193. [Google Scholar] [CrossRef]
- Vanguelova, E.I.; Benham, S.; Pitman, R.; Moffat, A.J.; Broadmeadow, M.; Nisbet, T.; Durrant, D.; Barsoum, N.; Wilkinson, M.; Bochereau, F.; et al. Chemical fluxes in time through forest ecosystems in the UK—Soil response to pollution recovery. Environ. Pollut. 2010, 158, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.; Rebeca, B. Microbial Remediation of Heavy Metals Contamination in Soil. Zenodo (CERN European Organization for Nuclear Research). 2022. Available online: https://zenodo.org/records/7081192 (accessed on 1 November 2021).
- Janick, F.A.; James, W.; Stanislav, M.; Michael, A.C. Soil and Land Pollution. In Environmental and Pollution Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- María, J.G.-G.; Houshang, A.; Izzie, A.; Vikki, A.; Alexandra, M.; Brett, R.; Sky, H.; Prosser, J.A.; Jacqui, H. Phytoremediation of microbial contamination in soil by New Zealand native plants. Appl. Soil Ecol. 2021, 167, 104040. [Google Scholar] [CrossRef]
- Niego, A.G.T.; Rapior, S.; Thongklang, N.; Raspé, O.; Hyde, K.D.; Mortimer, P. Reviewing the contributions of macrofungi to forest ecosystem processes and services. Fungal Biol. Rev. 2023, 44, 100294. [Google Scholar] [CrossRef]
- Martina, S.G.; Colin, D.C.; Killham, K.; James, I.P.; Lesley Anne, G. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 2005, 7, 301–313. [Google Scholar] [CrossRef]
- Corsolini, S.; Sarà, G. The trophic transfer of persistent pollutants (HCB, DDTs, PCBs) within polar marine food webs. Chemosphere 2017, 177, 189–199. [Google Scholar] [CrossRef]
- Talreja, N.; Hegde, C.; Kumar, E.M.; Chavali, M. Emerging Environmental Contaminants: Sources, Consequences and Future Challenges. In Green Technologies for Industrial Contaminants; John Wiley & Sons: Hoboken, NJ, USA, 2025; pp. 119–149. [Google Scholar]
- Sonibare, O.; Alimi, H.; Jarvie, D.; Ehinola, O. Origin and occurrence of crude oil in the Niger delta, Nigeria. J. Pet. Sci. Eng. 2008, 61, 99–107. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Yin, C.; She, L.; Ren, J.; Xu, Q.; Wang, S.; Peng, Y. Occurrence of contaminants of emerging concern in surface and waste water from the Yangtze River chemical contiguous zone, China: Distribution, sources and ecological risk assessment. Sci. Total Environ. 2024, 949, 175151. [Google Scholar] [CrossRef]
- Gu, S.; Guenther, A.; Faiola, C. Effects of anthropogenic and biogenic volatile organic compounds on Los Angeles air quality. Environ. Sci. Technol. 2021, 55, 12191–12201. [Google Scholar] [CrossRef]
- Okweye, P.; Garner, K.; McCullers, K.; Hutchinson, M.; Sheeley, N. The Presence of Contaminants of Emerging Concern (CECs) and Volatile Organic Compounds (VOCs) in Northern Alabama Aquatic Ecosystems. J. Environ. Sci. Eng. B 2021, 10, 77–89. [Google Scholar]
- Clarke, N.; Fuksová, K.; Gryndler, M.; Lachmanová, Z.; Liste, H.-H.; Rohlenová, J.; Schroll, R.; Schröder, P.; Matucha, M. The formation and fate of chlorinated organic substances in temperate and boreal forest soils. Environ. Sci. Pollut. Res. 2009, 16, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cao, Y.; Yu, T.; Yang, J.; Fan, S.; Feng, C.; Liu, Z.; Huang, C. A Scientometric Analysis and Visualization of Forest Soil Contamination Research from Global Perspectives. Forests 2024, 15, 1068. [Google Scholar] [CrossRef]
- Aichner, B.; Bussian, B.; Lehnik-Habrink, P.; Hein, S. Levels and spatial distribution of persistent organic pollutants in the environment: A case study of German forest soils. Environ. Sci. Technol. 2013, 47, 12703–12714. [Google Scholar] [CrossRef] [PubMed]
- Štrbac, S.; Kašanin-Grubin, M.; Stojić, N.; Pezo, L.; Lončar, B.; Tognetti, R.; Pucarević, M. Persistent organic pollutants in soil samples from mountain beech forests across Europe. Plant Soil 2024, 495, 313–339. [Google Scholar] [CrossRef]
- Belis, C.; Offenthaler, I.; Uhl, M.; Nurmi-Legat, J.; Bassan, R.; Jakobi, G.; Kirchner, M.; Knoth, W.; Kräuchi, N.; Levy, W. A comparison of Alpine emissions to forest soil and spruce needle loads for persistent organic pollutants (POPs). Environ. Pollut. 2009, 157, 3185–3191. [Google Scholar] [CrossRef]
- Gong, P.; Xu, H.; Wang, C.; Chen, Y.; Guo, L.; Wang, X. Persistent organic pollutant cycling in forests. Nat. Rev. Earth Environ. 2021, 2, 182–197. [Google Scholar] [CrossRef]
- Nam, J.J.; Gustafsson, O.; Kurt-Karakus, P.; Breivik, K.; Steinnes, E.; Jones, K.C. Relationships between organic matter, black carbon and persistent organic pollutants in European background soils: Implications for sources and environmental fate. Environ. Pollut. 2008, 156, 809–817. [Google Scholar] [CrossRef]
- Valentin, L.; Nousiainen, A.; Mikkonen, A. Introduction to organic contaminants in soil: Concepts and risks. In Emerging Organic Contaminants in Sludges: Analysis, Fate and Biological Treatment; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–29. [Google Scholar]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Jones, K.C.; De Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K.; Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Ecological impacts of total petroleum hydrocarbons. In Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 95–138. [Google Scholar]
- Pinedo, J.; Ibáñez, R.; Lijzen, J.; Irabien, A. Assessment of soil pollution based on total petroleum hydrocarbons and individual oil substances. J. Environ. Manag. 2013, 130, 72–79. [Google Scholar] [CrossRef]
- Sharma, I.; Khare, N.; Singh, K.; Dahiya, V.S. Environmental Applications and Emerging Pollutants: Monitoring and Remediation Techniques. In Biotechnology for Environmental Sustainability; Springer: Berlin/Heidelberg, Germany, 2025; pp. 637–660. [Google Scholar]
- Zhu, X.; Liu, S.; Gao, X.; Gu, Y.; Yu, Y.; Li, M.; Chen, X.; Fan, M.; Jia, Y.; Tian, L. Typical emerging contaminants in sewage treatment plant effluent, and related watersheds in the Pearl River Basin: Ecological risks and source identification. J. Hazard. Mater. 2024, 476, 135046. [Google Scholar] [CrossRef] [PubMed]
- Havugimana, E.; Bhople, B.S.; Kumar, A.; Byiringiro, E.; Mugabo, J.P.; Kumar, A. Soil pollution–major sources and types of soil pollutants. Environ. Sci. Eng. 2017, 11, 53–86. [Google Scholar]
- Duarte, R.M.; Matos, J.T.; Senesi, N. Organic pollutants in soils. In Soil Pollution; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–126. [Google Scholar]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Alloway, B. Soil pollution and land contamination. In Pollution, Causes, Effects and Control; Royal Society of Chemistry: London, UK, 1996; Volume 3, pp. 352–377. [Google Scholar]
- Fischer, J.R.; Zapata, F.; Dubelman, S.; Mueller, G.M.; Jensen, P.D.; Levine, S.L. Characterizing a novel and sensitive method to measure dsRNA in soil. Chemosphere 2016, 161, 319–324. [Google Scholar] [CrossRef]
- Knox, A.; Gamerdinger, A.; Adriano, D.; Kolka, R.; Kaplan, D. Sources and practices contributing to soil contamination. Bioremediation Contam. Soils 1999, 37, 53–87. [Google Scholar]
- Cachada, A.; Pato, P.; Rocha-Santos, T.; da Silva, E.F.; Duarte, A. Levels, sources and potential human health risks of organic pollutants in urban soils. Sci. Total Environ. 2012, 430, 184–192. [Google Scholar] [CrossRef]
- Cui, X.; Cao, X.; Xue, W.; Xu, L.; Cui, Z.; Zhao, R.; Ni, S.-Q. Integrative effects of microbial inoculation and amendments on improved crop safety in industrial soils co-contaminated with organic and inorganic pollutants. Sci. Total Environ. 2023, 873, 162202. [Google Scholar] [CrossRef]
- Reid, A.S.; Jerald, L.S. Phytoremediation, Bioaugmentation, and the Plant Microbiome. Environ. Sci. Technol. 2022, 56, 16602–16610. [Google Scholar] [CrossRef]
- Sanjana, M.; Prajna, R.; Katti, U.S.; Kavitha, R.V. Bioremediation—The recent drift towards a sustainable environment. Environ. Sci. Adv. 2024, 3, 1097–1110. [Google Scholar] [CrossRef]
- Muhammad, M.; Batool, S.; Hivare, V.; Li, W.-J.; Waheed, A.; Sinha, D. Chapter 1—Bioremediation techniques—Classification, principles, advantages, limitations, and prospects. In Microbiome-Assisted Bioremediation; Parray, J.A., Li, W.-J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 1–23. [Google Scholar] [CrossRef]
- Sun, Y.; Shaheen, S.M.; Ali, E.F.; Abdelrahman, H.; Sarkar, B.; Song, H.; Rinklebe, J.; Ren, X.; Zhang, Z.; Wang, Q. Enhancing microplastics biodegradation during composting using livestock manure biochar. Environ. Pollut. 2022, 306, 119339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yin, X.; Wang, N.; Chen, N.; Jiang, Y.; Deng, L.; Xiao, W.; Zhou, K.; He, Y.; Zhao, X.; et al. Optimizing the management of aerobic composting for antibiotic resistance genes elimination: A review of future strategy for livestock manure resource utilization. J. Environ. Manag. 2024, 370, 122766. [Google Scholar] [CrossRef]
- Lahori, A.H.; Tunio, M.; Ahmed, S.R.; Mierzwa-Hersztek, M.; Vambol, V.; Afzal, A.; Kausar, A.; Vambol, S.; Umar, A.; Muhammad, A. Role of pressmud compost for reducing toxic metals availability and improving plant growth in polluted soil: Challenges and recommendations. Sci. Total Environ. 2024, 951, 175493. [Google Scholar] [CrossRef]
- Lin, C.; Cheruiyot, N.K.; Bui, X.T.; Ngo, H.H. Composting and its application in bioremediation of organic contaminants. Bioengineered 2022, 13, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- López, A.M.Q.; dos Santos Silva, A.L. Biostimulation and Bioaugmentation. In Genomics Approach to Bioremediation; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 53–68. [Google Scholar] [CrossRef]
- Kumar, R.; Kaushal, S.; Verma, N.; Kumar, P.; Thakur, N.; Kumar, A.; Kumar, S.; Umar, A.; Almas, T.; Pal, K.; et al. Nano bioaugmentation for textile dye remediation: A sustainable approach for health and environment management. J. Mol. Liq. 2024, 415, 126254. [Google Scholar] [CrossRef]
- Kansour, M.K.; Al-Mailem, D.M. Bioremediation of two oil-contaminated Kuwaiti hyper-saline soils by cross bioaugmentation and the role of indigenous halophilic/halotolerant hydrocarbonoclastic bacteria. Environ. Technol. Innov. 2023, 32, 103259. [Google Scholar] [CrossRef]
- Shan, X.; Guo, H.; Ma, F.; Shan, Z. Enhanced treatment of synthetic wastewater by bioaugmentation with a constructed consortium. Chemosphere 2023, 338, 139520. [Google Scholar] [CrossRef]
- Nivetha, N.; Srivarshine, B.; Sowmya, B.; Rajendiran, M.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Pham, T.H.T.; Shanmugam, V.; Dragoi, E.-N. A comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration. Chemosphere 2023, 312, 137099. [Google Scholar] [CrossRef]
- Milano, G.; Colosio, A.; Minotta Quebradas, M.J.; Pratobevera, A.; Daffara, V.; Saccomanno, M.F. Biologic augmentation of rotator cuff repair with microfragmented autologous subacromial bursal tissue enveloped in a patch of compressed autologous long head of biceps tendon tissue: The Bio-Ravioli technique. JSES Int. 2024, 8, 1010–1015. [Google Scholar] [CrossRef]
- Eyupoglu, S. 8—Characterization techniques for bio-fillers/bio-plasticizers. In Sustainable Fillers/Plasticizers for Polymer Composites; Suyambulingam, I., Divakaran, D., Rangappa, S.M., Siengchin, S., Eds.; Elsevier Science Ltd: Amsterdam, The Netherlands, 2025; pp. 185–210. [Google Scholar] [CrossRef]
- Samaei, M.R.; Mortazavi, S.B.; Bakhshi, B.; Jafari, A.J.; Shamsedini, N.; Mehrazmay, H.; Ansarizadeh, M. Investigating the effects of combined bio-enhancement and bio-stimulation on the cleaning of hexadecane-contaminated soils. J. Environ. Chem. Eng. 2022, 10, 106914. [Google Scholar] [CrossRef]
- Vijay Pradhap Singh, M.; Ravi Shankar, K. Next-generation hybrid technologies for the treatment of pharmaceutical industry effluents. J. Environ. Manag. 2024, 353, 120197. [Google Scholar] [CrossRef]
- Min, T.; Luo, T.; He, H.; Qin, J.; Wang, Y.; Cheng, L.; Ru, S.; Li, J. Dissolved organic matter–assisted phytoremediation potential of cotton for Cd-contaminated soil: A relationship between dosage and phytoremediation efficiency. Environ. Sci. Pollut. Res. 2022, 29, 84640–84650. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, J.; Du, J.; Hou, C.; Zhou, X.; Chen, J.; Zhang, Y. Non-phytoremediation and phytoremediation technologies of integrated remediation for water and soil heavy metal pollution: A comprehensive review. Sci. Total Environ. 2024, 948, 174237. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, A.; Bindra, S.; Sharma, A. Phytoremediation: An emerging green technology for dissipation of PAHs from soil. J. Geochem. Explor. 2024, 259, 107426. [Google Scholar] [CrossRef]
- Yin, F.; Li, J.; Wang, Y.; Yang, Z. Biodegradable chelating agents for enhancing phytoremediation: Mechanisms, market feasibility, and future studies. Ecotoxicol. Environ. Saf. 2024, 272, 116113. [Google Scholar] [CrossRef]
- Montreemuk, J.; Stewart, T.N.; Prapagdee, B. Bacterial-assisted phytoremediation of heavy metals: Concepts, current knowledge, and future directions. Environ. Technol. Innov. 2024, 33, 103488. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.-D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Xiaodan, S.; Yuqian, Y.; Jiahui, L.; Andrey, S.; Smirnov, P.V.; Yakov, K. Organic Mulching Increases Microbial Activity in Urban Forest Soil. Forests 2022, 13, 1352. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Dai-Viet, N.V.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Kanwar, P.; Yadav, N.; Srivastava, S. Microbial Biodiversity and Bioremediation: A Systematic, Biological and Metabolic Engineering Tool. In Microbiology-2.0 Update for a Sustainable Future; Gupta, J., Verma, A., Eds.; Springer Nature: Singapore, 2024; pp. 77–93. [Google Scholar]
- Harirchi, S.; Rafieyan, S.; Nojoumi, S.A.; Etemadifar, Z. Microbial Biodegradation and Metagenomics in Remediation of Environmental Pollutants: Enzymes and Mechanisms. In Omics Insights in Environmental Bioremediation; Kumar, V., Thakur, I.S., Eds.; Springer Nature: Singapore, 2022; pp. 487–514. [Google Scholar]
- Karigar, C.S.; Rao, S.S. Role of microbial enzymes in the bioremediation of pollutants: A review. Enzym. Res. 2011, 2011, 805187. [Google Scholar] [CrossRef]
- Aysha, A.; Muhammad, R.; Ashraf, S.S. Oxidoreductases for the remediation of organic pollutants in water—A critical review. Crit. Rev. Biotechnol. 2018, 38, 971–988. [Google Scholar] [CrossRef]
- Dave, S.; Das, J. Chapter 13—Role of microbial enzymes for biodegradation and bioremediation of environmental pollutants: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 325–346. [Google Scholar] [CrossRef]
- Pankaj Kumar, G.; Manvi, G. Bioremediation of Organic Pollutants in Soil–Water System: A Review. Biotech 2023, 12, 36. [Google Scholar] [CrossRef]
- Salishcheva, O.; Burlachenko, A.; Tarasova, Y.; Moldagulova, N.; Yustratov, V. Biodegradation of organic compounds in wastewater. BIO Web Conf. 2023, 64, 01003. [Google Scholar] [CrossRef]
- Marcela Alejandra, S.; Ana Silvia, T.; Mónica Lucrecia, B.; Mariana, G.; César Nicolás, P.; María Isabel, F.; Laura, L.; Laura Lidia, V. Evaluation of bioremediation strategies for treating recalcitrant halo-organic pollutants in soil environments. Ecotoxicol. Environ. Saf. 2020, 202, 110929. [Google Scholar] [CrossRef]
- Chakraborty, J.; Das, S. Molecular perspectives and recent advances in microbial remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2016, 23, 16883–16903. [Google Scholar] [CrossRef]
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of organic pollutants by white rot fungal cytochrome P450: The role and mechanism of CYP450 in biodegradation. Chemosphere 2022, 301, 134776. [Google Scholar] [CrossRef]
- Pieper, D.H.; Martins dos Santos, V.t.A.P.; Golyshin, P.N. Genomic and mechanistic insights into the biodegradation of organic pollutants. Curr. Opin. Biotechnol. 2004, 15, 215–224. [Google Scholar] [CrossRef]
- Lü, H.; Wei, J.-L.; Tang, G.-X.; Chen, Y.-S.; Huang, Y.-H.; Hu, R.; Mo, C.-H.; Zhao, H.-M.; Xiang, L.; Li, Y.-W.; et al. Microbial consortium degrading of organic pollutants: Source, degradation efficiency, pathway, mechanism and application. J. Clean. Prod. 2024, 451, 141913. [Google Scholar] [CrossRef]
- Zehnle, H.; Otersen, C.; Benito Merino, D.; Wegener, G. Potential for the anaerobic oxidation of benzene and naphthalene in thermophilic microorganisms from the Guaymas Basin. Front. Microbiol. 2023, 14, 1279865. [Google Scholar] [CrossRef]
- Garcés Mejía, A.C.; Pino, N.J.; Peñuela, G.A. Effect of Secondary Metabolites Present in Brassica nigra Root Exudates on Anthracene and Phenanthrene Degradation by Rhizosphere Microorganism. Environ. Eng. Sci. 2017, 35, 203–209. [Google Scholar] [CrossRef]
- Dong, J.; Kang, Y.; Kuang, S.; Ma, H.; Li, M.; Xiao, J.; Wang, Y.; Guo, Z.; Wu, H. Combined biological effects of polystyrene microplastics and phenanthrene on Tubifex tubifex and microorganisms in wetland sediment. Chem. Eng. J. 2023, 462, 142260. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Gong, X.; Wang, C.; Wang, H. Anaerobic biodegradation of pyrene and benzo[a]pyrene by a new sulfate-reducing Desulforamulus aquiferis strain DSA. J. Hazard. Mater. 2023, 459, 132053. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.; Perczyk, P.; Wydro, P.; Broniatowski, M. Incorporation of cyclodiene pesticides and their polar metabolites to model membranes of soil bacteria. J. Mol. Liq. 2020, 298, 112019. [Google Scholar] [CrossRef]
- Su, F.; Wang, F.; Zhang, C.; Lu, T.; Zhang, S.; Zhang, R.; Qi, X.; Liu, P. Ameliorating substance accessibility for microorganisms to amplify toluene degradation and power generation of microbial fuel cell by using activated carbon anode. J. Clean. Prod. 2022, 377, 134481. [Google Scholar] [CrossRef]
- Toabaita, M.; Vangnai, A.S.; Thiravetyan, P. Removal of Ethylbenzene from Contaminated Air by Zamioculcas Zamiifolia and Microorganisms Associated on Z. Zamiifolia Leaves. Water Air Soil Pollut. 2016, 227, 115. [Google Scholar] [CrossRef]
- Kita, Y.; Amao, Y. Acetoacetate Production from CO2 and Acetone with Acetone Carboxylase from Photosynthetic Bacteria Rhodobacter Capsulatus. Catal. Surv. Asia 2023, 27, 67–74. [Google Scholar] [CrossRef]
- Shanthi, T.R.; Vasanthy, M.; Mohamed Hatha, A.A. Bioremediation of Lindane-Contaminated Soil and Water Ecosystems: A Review. In Organic Pollutants: Toxicity and Solutions; Vasanthy, M., Sivasankar, V., Sunitha, T.G., Eds.; Springer International Publishing: Cham, Switzlerand, 2022; pp. 199–227. [Google Scholar]
- Kadri, T.; Magdouli, S.; Rouissi, T.; Brar, S.K. Ex-situ biodegradation of petroleum hydrocarbons using Alcanivorax borkumensis enzymes. Biochem. Eng. J. 2018, 132, 279–287. [Google Scholar] [CrossRef]
- Deng, S.; Wang, B.; Zhang, H.; Qu, R.; Sun, S.; You, Q.; She, Y.; Zhang, F. Degradation and enhanced oil recovery potential of Alcanivorax borkumensis through production of bio-enzyme and bio-surfactant. Bioresour. Technol. 2024, 400, 130690. [Google Scholar] [CrossRef]
- Cheng, J.; Yuan, J.; Li, S.; Yang, X.; Lu, Z.; Xu, J.; He, Y. Promoted reductive removal of chlorinated organic pollutants co-occurring with facilitated methanogenesis in anaerobic environment: A systematic review and meta-analysis. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2582–2609. [Google Scholar] [CrossRef]
- Khomyakova, M.; Slobodkin, A. Transformation of methoxylated aromatic compounds by anaerobic microorganisms. Microbiology 2023, 92, 97–118. [Google Scholar] [CrossRef]
- Jaiswal, S.; Singh, D.K.; Shukla, P. Degradation effectiveness of hexachlorohexane (γ-HCH) by bacterial isolate Bacillus cereus SJPS-2, its gene annotation for bioremediation and comparison with Pseudomonas putida KT2440. Environ. Pollut. 2023, 318, 120867. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xie, M.; Han, Z.; Xiao, Y.; Wang, R.; Shen, C.; Hashmi, M.Z.; Sun, F. Resuscitation-promoting factor accelerates enrichment of highly active tetrachloroethene/polychlorinated biphenyl-dechlorinating cultures. Appl. Environ. Microbiol. 2023, 89, e0195122. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zhou, L.; Yu, D.; Chen, Y.; Luo, Y.; Lin, T. Effects of polystyrene microplastics on the metabolic level of Pseudomonas aeruginosa. Sci. Total Environ. 2024, 922, 171335. [Google Scholar] [CrossRef]
- Safdar, A.; Ismail, F.; Iftikhar, H.; Majid Khokhar, A.; Javed, A.; Imran, M.; Safdar, B. Determination of Biodegradation Potential of Aspergillus niger, Candida albicans, and Acremonium sclerotigenum on Polyethylene, Polyethylene Terephthalate, and Polystyrene Microplastics. Int. J. Microbiol. 2024, 2024, 7682762. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, S.; Pandey, R.; Yu, Z.G.; Kumar, M.; Khoo, K.S.; Thakur, T.K.; Show, P.L. Microplastics in terrestrial ecosystems: Un-ignorable impacts on soil characterises, nutrient storage and its cycling. TrAC Trends Anal. Chem. 2023, 158, 116869. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H. Systematical review of interactions between microplastics and microorganisms in the soil environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Q. Bioelectrochemical systems—A potentially effective technology for mitigating microplastic contamination in wastewater. J. Clean. Prod. 2024, 450, 141931. [Google Scholar] [CrossRef]
- Lew, S.; Lew, M.; Biedunkiewicz, A.; Szarek, J. Impact of Pesticide Contamination on Aquatic Microorganism Populations in the Littoral Zone. Arch. Environ. Contam. Toxicol. 2013, 64, 399–409. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.-V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Zhu, G.; Du, R.; Du, D.; Qian, J.; Ye, M. Keystone taxa shared between earthworm gut and soil indigenous microbial communities collaboratively resist chlordane stress. Environ. Pollut. 2021, 283, 117095. [Google Scholar] [CrossRef]
- Zeldes, B.M.; Keller, M.W.; Loder, A.J.; Straub, C.T.; Adams, M.W.W.; Kelly, R.M. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front. Microbiol. 2015, 6, 1209. [Google Scholar] [CrossRef]
- Schlüter, M.; Hentzel, T.; Suarez, C.; Koch, M.; Lorenz, W.G.; Böhm, L.; Düring, R.-A.; Koinig, K.A.; Bunge, M. Synthesis of novel palladium(0) nanocatalysts by microorganisms from heavy-metal-influenced high-alpine sites for dehalogenation of polychlorinated dioxins. Chemosphere 2014, 117, 462–470. [Google Scholar] [CrossRef]
- Zanellati, A.; Spina, F.; Bonaterra, M.; Dinuccio, E.; Varese, G.C.; Scarpeci, T.E. Screening and evaluation of phenols and furans degrading fungi for the biological pretreatment of lignocellulosic biomass. Int. Biodeterior. Biodegrad. 2021, 161, 105246. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, L.; He, J.; Zhou, G.; Chen, Z.; Wang, Z.; Chen, J.; Hayat, K.; Hrynsphan, D.; Tatsiana, S. Mechanisms of N, N-dimethylacetamide-facilitated n-hexane removal in a rotating drum biofilter packed with bamboo charcoal-polyurethane composite. Bioresour. Technol. 2023, 372, 128600. [Google Scholar] [CrossRef]
- Joshi, N.; Keshav, A.; Poonia, A.K. Reactive separation of gallic acid using tributyl phosphate dissolved in 2-octanone, laurylalcohol and heptane. Chem. Data Collect. 2020, 25, 100325. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, C.-J.; Liu, P.-F.; Fu, L.; Laso-Pérez, R.; Yang, L.; Bai, L.-P.; Li, J.; Yang, M.; Lin, J.-Z.; et al. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature 2022, 601, 257–262. [Google Scholar] [CrossRef]
- Panwar, R.; Mathur, J. Remediation of polycyclic aromatic hydrocarbon-contaminated soils using microbes and nanoparticles: A review. Pedosphere 2023, 33, 93–104. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, D.; Sun, L.; Xu, S.; Wan, S. Conductive and hydrophobically modified sodium alginate hydrogels for enhanced gaseous para-xylene removal by functional bacteria. Chem. Eng. J. 2023, 461, 142005. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, Y.; Ke, L.; Qian, L.; Zhou, X.; Shao, X. Ciprofloxacin enhances cadmium toxicity to earthworm Eisenia fetida by altering the gut microorganism composition. Environ. Pollut. 2023, 333, 122106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, X.; Yang, Q.; Chen, Y.; Du, B. Evolution of microbial community and drug resistance during enrichment of tetracycline-degrading bacteria. Ecotoxicol. Environ. Saf. 2019, 171, 746–752. [Google Scholar] [CrossRef]
- Mantegazza, G.; Dalla Via, A.; Licata, A.; Duncan, R.; Gardana, C.; Gargari, G.; Alamprese, C.; Arioli, S.; Taverniti, V.; Karp, M.; et al. Use of kefir-derived lactic acid bacteria for the preparation of a fermented soy drink with increased estrogenic activity. Food Res. Int. 2023, 164, 112322. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-S.; Chen, J.; Zhang, J.-N.; Liu, Y.-S.; Hu, L.-X.; Chen, X.-W.; Liu, S.; Xu, X.-R.; Ying, G.-G. Microbial transformation of progesterone and dydrogesterone by bacteria from swine wastewater: Degradation kinetics and products identification. Sci. Total Environ. 2020, 701, 134930. [Google Scholar] [CrossRef]
- Chen, S.; Xie, J.; Wen, Z. Removal of pharmaceutical and personal care products (PPCPs) from waterbody using a revolving algal biofilm (RAB) reactor. J. Hazard. Mater. 2021, 406, 124284. [Google Scholar] [CrossRef]
- Suleiman, M.; Schröder, C.; Kuhn, M.; Simon, A.; Stahl, A.; Frerichs, H.; Antranikian, G. Microbial biofilm formation and degradation of octocrylene, a UV absorber found in sunscreen. Commun. Biol. 2019, 2, 430. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Xu, W.; Tian, R.; Zeng, J. Dibutyl phthalate contamination accelerates the uptake and metabolism of sugars by microbes in black soil. Environ. Pollut. 2020, 262, 114332. [Google Scholar] [CrossRef]
- Kruis, A.J.; Bohnenkamp, A.C.; Patinios, C.; van Nuland, Y.M.; Levisson, M.; Mars, A.E.; van den Berg, C.; Kengen, S.W.M.; Weusthuis, R.A. Microbial production of short and medium chain esters: Enzymes, pathways, and applications. Biotechnol. Adv. 2019, 37, 107407. [Google Scholar] [CrossRef]
- Senne de Oliveira Lino, F.; Bajic, D.; Vila, J.C.C.; Sánchez, A.; Sommer, M.O.A. Complex yeast–bacteria interactions affect the yield of industrial ethanol fermentation. Nat. Commun. 2021, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.C.; Maher, D.T.; Chiri, E.; Leung, P.M.; Nauer, P.A.; Arndt, S.K.; Tait, D.R.; Greening, C.; Johnston, S.G. Bark-dwelling methanotrophic bacteria decrease methane emissions from trees. Nat. Commun. 2021, 12, 2127. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, F.; Zhang, J.; Shi, Q.; Wu, Y.; Kong, Q. Treatment of formaldehyde-containing wastewater and power generation by constructed wetland–microbial fuel cells enhanced by formaldehyde-degrading bacteria. J. Water Process Eng. 2024, 59, 104984. [Google Scholar] [CrossRef]
- Oveisi, F.; Fallah, N.; Nasernejad, B. Biodegradation of synthetic wastewater containing styrene in microbial fuel cell: Effect of adaptation of microbial community. Fuel 2021, 305, 121382. [Google Scholar] [CrossRef]
- Ma, J.; Xie, M.; Zhao, N.; Wang, Y.; Lin, Q.; Zhu, Y.; Chao, Y.; Ni, Z.; Qiu, R. Enhanced trichloroethylene biodegradation: The mechanism and influencing factors of combining microorganism and carbon-iron materials. Sci. Total Environ. 2023, 878, 162720. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.G.; Pedraz, M.A.; Sánchez, J.M. Application of immobilized microorganisms in the biodegradation of alkyl sulphate and linear alkyl benzene sulphonate surfactants. Int. Biodeterior. Biodegrad. 1996, 37, 258–259. [Google Scholar] [CrossRef]
- Verkholiak, N.S.; Peretyatko, T.B. Destruction of toluene and xylene by sulfate-reducing bacteria. Ecol. Noospherol. 2019, 30, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Stams, A.J. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef]
- Ranadev, P.; Revanna, A.; Bagyaraj, D.J.; Shinde, A.H. Sulfur oxidizing bacteria in agro ecosystem and its role in plant productivity—A review. J. Appl. Microbiol. 2023, 134, lxad161. [Google Scholar] [CrossRef]
- Wang, H.; Xing, L.; Zhang, H.; Gui, C.; Jin, S.; Lin, H.; Li, Q.; Cheng, C. Key factors to enhance soil remediation by bioelectrochemical systems (BESs): A review. Chem. Eng. J. 2021, 419, 129600. [Google Scholar] [CrossRef]
- Emerson, D.; Moyer, C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl. Environ. Microbiol. 1997, 63, 4784–4792. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Timmis, K.N. Ferroplasma and relatives, recently discovered cell wall-lacking archaea making a living in extremely acid, heavy metal-rich environments. Environ. Microbiol. 2005, 7, 1277–1288. [Google Scholar] [CrossRef]
- Huber, H.; Stetter, K.O. Archaeoglobus. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–5. [Google Scholar]
- Liying, J.; Timothy, P.; Buenfeld, N.R.; Stephen, R.S. A critical review of the physiological, ecological, physical and chemical factors influencing the microbial degradation of concrete by fungi. Build. Environ. 2022, 214, 108925. [Google Scholar] [CrossRef]
- Ruyan, Z.; Xiaolu, Y.; Qifeng, T.; Xinggang, W.; Chang, L.; Chengliang, L.; Feng, L. Soil microbial community structure, metabolic potentials and influencing factors in a subtropical mountain forest ecosystem of China. Environ. Pollut. Bioavailab. 2020, 32, 69–78. [Google Scholar] [CrossRef]
- Yao, Z.; Lan, C.; Jie-Ying, W.; Yi, L.; Jun, W.; Yaoxin, G.; Chengjie, R.; Halbert, B.; Huihui, S.; Fazhu, Z. Differences and influencing factors of microbial carbon use efficiency in forest rhizosphere soils at different altitudes in Taibai Mountain, China. Chin. J. Plant Ecol. 2023, 47, 275–288. [Google Scholar] [CrossRef]
- Jadhav, S.S.; Sharma, S.B.; Sibi, G. Microbial Degradation of Petroleum Hydrocarbons and Factors Influencing the Degradation Process. Bioprocess Eng. 2019, 3, 6–11. [Google Scholar] [CrossRef]
- Hugo, S.; Martin, L.; Farrukh, J. An Analysis of Fast Learning Methods for Classifying Forest Cover Types. Appl. Artif. Intell. 2020, 34, 691–709. [Google Scholar] [CrossRef]
- Boyka, M. Microbial diversity and enzymatic activity of soils in coniferous forest ecosystems. Bulg. J. Soil Sci. Agro-Chem. Ecol. 2020, 54, 43–54. [Google Scholar]
- Teresa, F.; Ana Cristina, G. Editorial: Forest species and stands regeneration. Front. For. Glob. Change 2023, 6, 1208267. [Google Scholar] [CrossRef]
- Su, F.; Xu, S.; Sayer, E.J.; Chen, W.; Du, Y.; Lu, X. Distinct storage mechanisms of soil organic carbon in coniferous forest and evergreen broadleaf forest in tropical China. J. Environ. Manag. 2021, 295, 113142. [Google Scholar] [CrossRef]
- Waring, R.H.; Franklin, J.F.J.S. Evergreen Coniferous Forests of the Pacific Northwest: Massive long-lived conifers dominating these forests are adapted to a winter-wet, summer-dry environment. Science 1979, 204, 1380–1386. [Google Scholar] [CrossRef]
- Yi-Zhen, S.; Senlin, W.; Yushan, L.; Yun, C.; Zuyuan, H.; Jing, W.; Fengqin, L.; Zuoqiang, Y. Importance of Bark Physicochemical Properties in an Epiphytic Bryophyte Community within a Temperate Deciduous Broadleaf Forest. Diversity 2023, 15, 688. [Google Scholar] [CrossRef]
- Johansson, M.; Gyllin, M.; Witzell, J.; Küller, M.J.U.F.; Greening, U. Does biological quality matter? Direct and reflected appraisal of biodiversity in temperate deciduous broad-leaf forest. Urban For. Urban Green. 2014, 13, 28–37. [Google Scholar] [CrossRef]
- Renhui, M.; Jun, M.; Yinzhan, L.; Yanchun, L.; Zhongling, Y.; Ming, G. Variability of Aboveground Litter Inputs Alters Soil Carbon and Nitrogen in a Coniferous–Broadleaf Mixed Forest of Central China. Forests 2019, 10, 188. [Google Scholar] [CrossRef]
- Knoke, T.; Ammer, C.; Stimm, B.; Mosandl, R. Admixing broadleaved to coniferous tree species: A review on yield, ecological stability and economics. Eur. J. For. Res. 2008, 127, 89–101. [Google Scholar] [CrossRef]
- Andressa Monteiro, V.; Júlia Brandão, G.; Jéssica, A.M.; Erika, B.; Kabir, G.P.; Siu Mui, T.; Brendan, J.M.B. Soil microbes under threat in the Amazon Rainforest. Trends Ecol. Evol. 2023, 38, 693–696. [Google Scholar] [CrossRef]
- Francielle Roberta Dias de, L.; Polyana, P.; Ediu, C.S.; Isabela, C.F.V.; Jakeline Rosa de, O.; Cláudia Carvalhinho, W.; Alberto Vasconcellos, I.; David, C.W.; Nilton, C.; Bruno Teixeira, R.; et al. Geochemistry signatures of mercury in soils of the Amazon rainforest biome. Environ. Res. 2022, 215, 114147. [Google Scholar] [CrossRef]
- Koné, A.W.; Kassi, S.-P.A.Y.; Koffi, B.Y.; Masse, D.; Maïga, A.A.; Tondoh, J.E.; Kisaka, O.M.; Touré, G.-P.T. Chromolaena odorata (L.) K&R (Asteraceae) invasion effects on soil microbial biomass and activities in a forest-savanna mosaic. CATENA 2021, 207, 105619. [Google Scholar] [CrossRef]
- Eduarda Martiniano de Oliveira, S.; Marcela de Castro Nunes Santos, T.; Hanster, S.; Eduardo Eiji, M.; Fausto Weimar Acerbi, J.; José Roberto Soares, S. Carbon-diversity hotspots and their owners in Brazilian southeastern Savanna, Atlantic Forest and Semi-Arid Woodland domains. For. Ecol. Manag. 2019, 452, 117575. [Google Scholar] [CrossRef]
- Liao, Y.-C.-Z.; Pu, H.-X.; Jiao, Z.-W.; Palviainen, M.; Zhou, X.; Heinonsalo, J.; Berninger, F.; Pumpanen, J.; Köster, K.; Sun, H. Enhancing boreal forest resilience: A four-year impact of biochar on soil quality and fungal communities. Microbiol. Res. 2024, 283, 127696. [Google Scholar] [CrossRef]
- Roman, D.; Colin, M.; Rebecca, E.H.; Amy, M.W.; Wong, R.K.; David Livingston, C.; Madeline, G.Z.; Patrick, F.S. Arctic sea ice retreat fuels boreal forest advance. Science 2024, 383, 877–884. [Google Scholar] [CrossRef]
- Naji, M.B.; Nicholas, B.; Jonathan, R.L. Microbial degradation of isosaccharinic acid at high pH. ISME J. 2014, 9, 310–320. [Google Scholar] [CrossRef]
- Brulé, C.; Frey-Klett, P.; Pierrat, J.C.; Courrier, S.; Gérard, F.; Lemoine, M.C.; Rousselet, J.L.; Sommer, G.; Garbaye, J. Survival in the soil of the ectomycorrhizal fungus Laccaria bicolor and the effects of a mycorrhiza helper Pseudomonas fluorescens. Soil Biol. Biochem. 2001, 33, 1683–1694. [Google Scholar] [CrossRef]
- Duo, Y.; Xiaolong, C.; Jingbo, H.; Zhao, F.J.; Peng, L. Soil properties and microbial functional attributes drive the response of soil multifunctionality to long-term fertilization management. Appl. Soil Ecol. 2023, 192, 105095. [Google Scholar] [CrossRef]
- Vaithyanathan, V.K.; Cabana, H.; Vaidyanathan, V.K. Remediation of trace organic contaminants from biosolids: Influence of various pre-treatment strategies prior to Bacillus subtilis aerobic digestion. Chem. Eng. J. 2021, 419, 129966. [Google Scholar] [CrossRef]
- Deni, J.; Penninckx, M.J. Influence of long-term diesel fuel pollution on nitrite-oxidising activity and population size of nitrobacter spp. in soil. Microbiol. Res. 2004, 159, 323–329. [Google Scholar] [CrossRef]
- List, C.; Hosseini, Z.; Lederballe Meibom, K.; Hatzimanikatis, V.; Bernier-Latmani, R. Impact of iron reduction on the metabolism of Clostridium acetobutylicum. Environ. Microbiol. 2019, 21, 3548–3563. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.; Acharya, D.; Sakariya, R.; Sharma, D.; Patel, P.; Shah, M.; Prajapati, M. A comprehensive study of bioremediation for pharmaceutical wastewater treatment. Clean. Chem. Eng. 2022, 4, 100073. [Google Scholar] [CrossRef]
- Aparicio, J.D.; Raimondo, E.E.; Saez, J.M.; Costa-Gutierrez, S.B.; Álvarez, A.; Benimeli, C.S.; Polti, M.A. The current approach to soil remediation: A review of physicochemical and biological technologies, and the potential of their strategic combination. J. Environ. Chem. Eng. 2022, 10, 107141. [Google Scholar] [CrossRef]
- Kumar, V.; Agrawal, S.; Bhat, S.A.; Américo-Pinheiro, J.H.P.; Shahi, S.K.; Kumar, S. Environmental impact, health hazards, and plant-microbes synergism in remediation of emerging contaminants. Clean. Chem. Eng. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Zhang, P.; Wu, Y.; Gou, X.; Song, Y.; Tian, Z.; Zeng, G. Two-stage anoxic/oxic combined membrane bioreactor system for landfill leachate treatment: Pollutant removal performances and microbial community. Bioresour. Technol. 2017, 243, 738–746. [Google Scholar] [CrossRef]
- Jasu, A.; Lahiri, D.; Nag, M.; Ray, R.R. Chapter 17—Fungi in bioremediation of soil organic pollutants. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Sharma, V.K., Shah, M.P., Parmar, S., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 381–405. [Google Scholar] [CrossRef]
- Baldrian, P. Wood-inhabiting ligninolytic basidiomycetes in soils: Ecology and constraints for applicability in bioremediation. Fungal Ecol. 2008, 1, 4–12. [Google Scholar] [CrossRef]
- Lladó, S.; Žifčáková, L.; Větrovský, T.; Eichlerová, I.; Baldrian, P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol. Fertil. Soils 2016, 52, 251–260. [Google Scholar] [CrossRef]
- Berger, T.; Poyntner, C.; Margesin, R. Culturable bacteria from an Alpine coniferous forest site: Biodegradation potential of organic polymers and pollutants. Folia Microbiol. 2021, 66, 87–98. [Google Scholar] [CrossRef]
- Ling, L.; Fu, Y.; Jeewani, P.H.; Tang, C.; Pan, S.; Reid, B.J.; Gunina, A.; Li, Y.; Li, Y.; Cai, Y.; et al. Organic matter chemistry and bacterial community structure regulate decomposition processes in post-fire forest soils. Soil Biol. Biochem. 2021, 160, 108311. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, D.; Song, M.; Guan, G.; Sun, Y.; Li, J.; Cheng, X.; Luo, C.; Zhang, G. The positive role of root decomposition on the bioremediation of organic pollutants contaminated soil: A case study using PCB-9 as a model compound. Soil Biol. Biochem. 2022, 171, 108726. [Google Scholar] [CrossRef]
- Krohn, C.; Jin, J.; Wood, J.L.; Hayden, H.L.; Kitching, M.; Ryan, J.; Fabijański, P.; Franks, A.E.; Tang, C. Highly decomposed organic carbon mediates the assembly of soil communities with traits for the biodegradation of chlorinated pollutants. J. Hazard. Mater. 2021, 404, 124077. [Google Scholar] [CrossRef] [PubMed]
- Uttarotai, T.; Sutheeworapong, S.; Crombie, A.T.; Murrell, J.C.; Mhuantong, W.; Noirungsee, N.; Wangkarn, S.; Bovonsombut, S.; McGenity, T.J.; Chitov, T. Genome characterisation of an isoprene-degrading Alcaligenes sp. isolated from a tropical restored forest. Biology 2022, 11, 519. [Google Scholar] [CrossRef]
- Laffite, A.; Florio, A.; Andrianarisoa, K.S.; Creuze des Chatelliers, C.; Schloter-Hai, B.; Ndaw, S.M.; Periot, C.; Schloter, M.; Zeller, B.; Poly, F.; et al. Biological inhibition of soil nitrification by forest tree species affects populations. Environ. Microbiol. 2020, 22, 1141–1153. [Google Scholar] [CrossRef]
- Zenteno-Rojas, A.; Martínez-Romero, E.; Castañeda-Valbuena, D.; Rincón-Molina, C.I.; Ruíz-Valdiviezo, V.M.; Meza-Gordillo, R.; Villalobos-Maldonado, J.J.; Vences-Guzmán, M.Á.; Rincón-Rosales, R. Structure and diversity of native bacterial communities in soils contaminated with polychlorinated biphenyls. AMB Express 2020, 10, 124. [Google Scholar] [CrossRef]
- Sarwan, J. Role of isolates of Bacillus species for biodegradation of multiple contaminants. J. Sustain. Environ. Manag. 2022, 1, 292–298. [Google Scholar] [CrossRef]
- Sato, Y.; Tsukamoto, T.; Sato, M. Rapid degradation of carbaryl by two novel strains of Arthrobacter spp. isolated from forest soil. J. For. Res. 1999, 4, 275–280. [Google Scholar] [CrossRef]
- Young, P.C. Data-based mechanistic modelling and forecasting globally averaged surface temperature. Int. J. Forecast. 2018, 34, 314–335. [Google Scholar] [CrossRef]
- Malhi, Y.; Aragão, L.E.O.C.; Galbraith, D.; Huntingford, C.; Fisher, R.; Zelazowski, P.; Sitch, S.; McSweeney, C.; Meir, P. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl. Acad. Sci. USA 2009, 106, 20610–20615. [Google Scholar] [CrossRef]
- Silva de Miranda, P.L.; Dexter, K.G.; Swaine, M.D.; de Oliveira-Filho, A.T.; Hardy, O.J.; Fayolle, A. Dissecting the difference in tree species richness between Africa and South America. Proc. Natl. Acad. Sci. USA 2022, 119, e2112336119. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, R.A.; Dash, J.; Rodriguez-Galiano, V.F.; Janous, D.; Pavelka, M.; Marek, M.V. Extreme warm temperatures alter forest phenology and productivity in Europe. Sci. Total Environ. 2016, 563–564, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, G.J.; Abrams, M.D. Is climate an important driver of post-European vegetation change in the Eastern United States? Glob. Change Biol. 2015, 21, 314–334. [Google Scholar] [CrossRef] [PubMed]
- Naveendrakumar, G.; Vithanage, M.; Kwon, H.-H.; Chandrasekara, S.S.K.; Iqbal, M.C.M.; Pathmarajah, S.; Fernando, W.C.D.K.; Obeysekera, J. South Asian perspective on temperature and rainfall extremes: A review. Atmos. Res. 2019, 225, 110–120. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, Y.; Zhang, R. An Asian–Pacific teleconnection in summer tropospheric temperature and associated Asian climate variability. Clim. Dyn. 2007, 29, 293–303. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Li, D.; Wu, J. Effects of soil organism interactions and temperature on carbon use efficiency in three different forest soils. Soil Ecol. Lett. 2021, 3, 156–166. [Google Scholar] [CrossRef]
- Tang, Z.; Sun, X.; Luo, Z.; He, N.; Sun, O.J. Effects of temperature, soil substrate, and microbial community on carbon mineralization across three climatically contrasting forest sites. Ecol. Evol. 2018, 8, 879–891. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.G.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Khan, M.I.U.; Khattak, M.N.K.; Zhang, J.; Li, K.; Hassan, I.U. Diversity and distribution of thermophilic microorganisms and their applications in biotechnology. J. Basic Microbiol. 2022, 62, 95–108. [Google Scholar] [CrossRef]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Temperature affects microbial decomposition of cadavers (Rattus rattus) in contrasting soils. Appl. Soil Ecol. 2008, 40, 129–137. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Ai, Z.; Zhang, J.; Zhang, C.; Xue, S.; Liu, G. Effects of the interaction between temperature and revegetation on the microbial degradation of soil dissolved organic matter (DOM)—A DOM incubation experiment. Geoderma 2019, 337, 812–824. [Google Scholar] [CrossRef]
- Mallick, S.; Chakraborty, J.; Dutta, T.K. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: A review. Crit. Rev. Microbiol. 2011, 37, 64–90. [Google Scholar] [CrossRef]
- Mirza, B.S.; Sorensen, D.L.; Dupont, R.R.; McLean, J.E. Dehalococcoides abundance and alternate electron acceptor effects on large, flow-through trichloroethene dechlorinating columns. Appl. Microbiol. Biotechnol. 2016, 100, 2367–2379. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Wick, L.Y.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef]
- Friis, A.K.; Heimann, A.C.; Jakobsen, R.; Albrechtsen, H.-J.; Cox, E.; Bjerg, P.L. Temperature dependence of anaerobic TCE-dechlorination in a highly enriched Dehalococcoides-containing culture. Water Res. 2007, 41, 355–364. [Google Scholar] [CrossRef]
- Hong, X.; Qin, J.; Chen, R.; Yuan, L.; Zha, J.; Huang, C.; Li, N.; Ji, X.; Wang, Z. Phenanthrene-induced apoptosis and its underlying mechanism. Environ. Sci. Technol. 2017, 51, 14397–14405. [Google Scholar] [CrossRef] [PubMed]
- Ayumi, K.; Naoki, M.; Akira, O. Response of microbial respiration from fine root litter decomposition to root water content in a temperate broad-leaved forest. Plant Root 2013, 7, 77–82. [Google Scholar] [CrossRef]
- Zhu, M.; Zheng, J.; Xie, J.; Zhao, D.; Qiao, Z.-W.; Huang, D.; Luo, H.-B. Effects of environmental factors on the microbial community changes during medium-high temperature Daqu manufacturing. Food Res. Int. 2022, 153, 110955. [Google Scholar] [CrossRef]
- Aytac, Z.; Xu, J.; Raman Pillai, S.K.; Eitzer, B.D.; Xu, T.; Vaze, N.; Ng, K.W.; White, J.C.; Chan-Park, M.B.; Luo, Y. Enzyme-and relative humidity-responsive antimicrobial fibers for active food packaging. ACS Appl. Mater. Interfaces 2021, 13, 50298–50308. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons-Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Das, S. Potential and prospects of Actinobacteria in the bioremediation of environmental pollutants: Cellular mechanisms and genetic regulations. Microbiol. Res. 2023, 273, 127399. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Sun, L.; Yuan, C.; Han, Y.; Huang, Z. The combined enhancement of RL, nZVI and AQDS on the microbial anaerobic-aerobic degradation of PAHs in soil. Chemosphere 2022, 307, 135609. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Bull, I.D.; Journeaux, T.; Chadwick, D.R.; Jones, D.L. Volatile organic compounds (VOCs) allow sensitive differentiation of biological soil quality. Soil Biol. Biochem. 2021, 156, 108187. [Google Scholar] [CrossRef]
- Sengupta, K.; Pal, S. A review on microbial diversity and genetic markers involved in methanogenic degradation of hydrocarbons: Futuristic prospects of biofuel recovery from contaminated regions. Environ. Sci. Pollut. Res. 2021, 28, 40288–40307. [Google Scholar] [CrossRef]
- Wang, J.; Bao, H.; Pan, G.; Zhang, H.; Li, J.; Li, J.; Cai, J.; Wu, F. Combined application of rhamnolipid and agricultural wastes enhances PAHs degradation via increasing their bioavailability and changing microbial community in contaminated soil. J. Environ. Manag. 2021, 294, 112998. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Chen, D.; Parales, R.E.; Jiang, J. Oxygenases as powerful weapons in the microbial degradation of pesticides. Annu. Rev. Microbiol. 2022, 76, 325–348. [Google Scholar] [CrossRef]
- Tiessen, H.; Cuevas, E.; Chacon, P. The role of soil organic matter in sustaining soil fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Filonov, A.E.; Puntus, I.F.; Karpov, A.V.; Kosheleva, I.A.; Kashparov, K.I.; Slepenkin, A.V.; Boronin, A.M. Efficiency of naphthalene biodegradation by Pseudomonas putida G7 in soil. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2004, 79, 562–569. [Google Scholar] [CrossRef]
- Quero, G.M.; Cassin, D.; Botter, M.; Perini, L.; Luna, G.M. Patterns of benthic bacterial diversity in coastal areas contaminated by heavy metals, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Front. Microbiol. 2015, 6, 1053. [Google Scholar] [CrossRef] [PubMed]
- Calogero, R.; Arcadi, E.; Fabiano, F.; Rizzo, C.; Romeo, T.; Greco, S. PCB bioremediation potential of thermophilic strains from shallow hydrothermal vent (Vulcano Island). J. Water Process Eng. 2024, 61, 105330. [Google Scholar] [CrossRef]
- Sun, Y.; Xiong, X.; He, M.; Xu, Z.; Hou, D.; Zhang, W.; Ok, Y.S.; Rinklebe, J.; Wang, L.; Tsang, D.C. Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review. Chem. Eng. J. 2021, 424, 130387. [Google Scholar] [CrossRef]
- Reijonen, I.; Metzler, M.; Hartikainen, H. Impact of soil pH and organic matter on the chemical bioavailability of vanadium species: The underlying basis for risk assessment. Environ. Pollut. 2016, 210, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, S.; Zhang, Q.; Zou, M.; Yin, Q.; Qiu, Y.; Qin, W. Effect of organic material addition on active soil organic carbon and microbial diversity: A meta-analysis. Soil Tillage Res. 2024, 241, 106128. [Google Scholar] [CrossRef]
- Esmaeilzadeh-Salestani, K.; Bahram, M.; Seraj, R.G.M.; Gohar, D.; Tohidfar, M.; Eremeev, V.; Talgre, L.; Khaleghdoust, B.; Mirmajlessi, S.M.; Luik, A. Cropping systems with higher organic carbon promote soil microbial diversity. Agric. Ecosyst. Environ. 2021, 319, 107521. [Google Scholar] [CrossRef]
- Maron, P.-A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S. High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef] [PubMed]
- Bollag, J.-M.; Myers, C.J.; Minard, R.D. Biological and chemical interactions of pesticides with soil organic matter. Sci. Total Environ. 1992, 123, 205–217. [Google Scholar] [CrossRef]
- Spark, K.; Swift, R. Effect of soil composition and dissolved organic matter on pesticide sorption. Sci. Total Environ. 2002, 298, 147–161. [Google Scholar] [CrossRef]
- Farenhorst, A. Importance of soil organic matter fractions in soil-landscape and regional assessments of pesticide sorption and leaching in soil. Soil Sci. Soc. Am. J. 2006, 70, 1005–1012. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Mead, R.; Xu, Y.; Chong, J.; Jaffé, R. Sediment and soil organic matter source assessment as revealed by the molecular distribution and carbon isotopic composition of n-alkanes. Org. Geochem. 2005, 36, 363–370. [Google Scholar] [CrossRef]
- Wiesenberg, G.L.; Schwarzbauer, J.; Schmidt, M.W.; Schwark, L. Source and turnover of organic matter in agricultural soils derived from n-alkane/n-carboxylic acid compositions and C-isotope signatures. Org. Geochem. 2004, 35, 1371–1393. [Google Scholar] [CrossRef]
- Endo, S.; Grathwohl, P.; Haderlein, S.B.; Schmidt, T.C. Characterization of sorbent properties of soil organic matter and carbonaceous geosorbents using n-alkanes and cycloalkanes as molecular probes. Environ. Sci. Technol. 2009, 43, 393–400. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Smreczak, B.; Klimkowicz-Pawlas, A. Soil organic matter composition as a factor affecting the accumulation of polycyclic aromatic hydrocarbons. J. Soils Sediments 2019, 19, 1890–1900. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, N.; Xue, M.; Tao, S. Impact of soil organic matter on the distribution of polycyclic aromatic hydrocarbons (PAHs) in soils. Environ. Pollut. 2010, 158, 2170–2174. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, N.; Xue, M.; Lu, S.; Tao, S. Effects of soil organic matter on the development of the microbial polycyclic aromatic hydrocarbons (PAHs) degradation potentials. Environ. Pollut. 2011, 159, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tao, S.; Zhang, N.; Zhang, D.; Li, X. The effect of soil organic matter on fate of polycyclic aromatic hydrocarbons in soil: A microcosm study. Environ. Pollut. 2010, 158, 1768–1774. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Lin, S.-S.; Dai, C.-M.; Shi, L.; Zhou, X.-F. Sorption–desorption and transport of trimethoprim and sulfonamide antibiotics in agricultural soil: Effect of soil type, dissolved organic matter, and pH. Environ. Sci. Pollut. Res. 2014, 21, 5827–5835. [Google Scholar] [CrossRef]
- Chen, K.-L.; Liu, L.-C.; Chen, W.-R. Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ. Pollut. 2017, 231, 1163–1171. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Ertani, A. Hormone-like activity of the soil organic matter. Appl. Soil Ecol. 2018, 123, 517–520. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. Evaluation of hormone-like activity of the dissolved organic matter fraction (DOM) of compost and digestate. Sci. Total Environ. 2015, 514, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Misic, C.; Harriague, A.C.; Trielli, F. Organic matter recycling in a beach environment influenced by sunscreen products and increased inorganic nutrient supply (Sturla, Ligurian Sea, NW Mediterranean). Sci. Total Environ. 2011, 409, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ji, Y.; Zeng, C.; Zhang, Y.; Wang, Z.; Yang, X. Aquatic photodegradation of sunscreen agent p-aminobenzoic acid in the presence of dissolved organic matter. Water Res. 2013, 47, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef]
- Rocha, F.; Ratola, N.; Homem, V. Fragrances in the Environment: Properties, Applications, and Emissions; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Tfaily, M.M.; Chu, R.K.; Tolić, N.; Roscioli, K.M.; Anderton, C.R.; Paša-Tolić, L.; Robinson, E.W.; Hess, N.J. Advanced Solvent Based Methods for Molecular Characterization of Soil Organic Matter by High-Resolution Mass Spectrometry. Anal. Chem. 2015, 87, 5206–5215. [Google Scholar] [CrossRef]
- Gentile, L.; Floudas, D.; Olsson, U.; Persson, P.; Tunlid, A. Fungal decomposition and transformation of molecular and colloidal fractions of dissolved organic matter extracted from boreal forest soil. Soil Biol. Biochem. 2024, 195, 109473. [Google Scholar] [CrossRef]
- Ahangar, A.G.; Smernik, R.J.; Kookana, R.S.; Chittleborough, D.J. The effect of solvent-conditioning on soil organic matter sorption affinity for diuron and phenanthrene. Chemosphere 2009, 76, 1062–1066. [Google Scholar] [CrossRef]
- Ran, Y.; Sun, K.; Ma, X.; Wang, G.; Grathwohl, P.; Zeng, E.Y. Effect of condensed organic matter on solvent extraction and aqueous leaching of polycyclic aromatic hydrocarbons in soils and sediments. Environ. Pollut. 2007, 148, 529–538. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Li, H.; Gu, X.; Song, J.; Hui, K.; Chen, G.; Tan, W.; Wang, H.; Jiang, Y.; Yuan, Y. Effects of Soil-groundwater Environmental Factors on BTEX Transport and Transformation: A Review. J. Environ. Chem. Eng. 2024, 12, 113697. [Google Scholar] [CrossRef]
- Ali, M.; Song, X.; Wang, Q.; Zhang, Z.; Che, J.; Chen, X.; Tang, Z.; Liu, X. Mechanisms of biostimulant-enhanced biodegradation of PAHs and BTEX mixed contaminants in soil by native microbial consortium. Environ. Pollut. 2023, 318, 120831. [Google Scholar] [CrossRef]
- Ahmed, N.; Ok, Y.S.; Jeon, B.-H.; Kim, J.R.; Chae, K.-J.; Oh, S.-E. Assessment of benzene, toluene, ethyl-benzene, and xylene (BTEX) toxicity in soil using sulfur-oxidizing bacterial (SOB) bioassay. Chemosphere 2019, 220, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Aydin, S.; Ince, B.; Ince, O. Evaluation of microbial population and functional genes during the bioremediation of petroleum-contaminated soil as an effective monitoring approach. Ecotoxicol. Environ. Saf. 2016, 125, 153–160. [Google Scholar] [CrossRef]
- Qiang, F.; Yi-Zhen, S.; Senlin, W.; Fengqin, L.; Guohang, T.; Yun, C.; Zuoqiang, Y.; Ye, Y. Soil Microbial Distribution Depends on Different Types of Landscape Vegetation in Temperate Urban Forest Ecosystems. Front. Ecol. Evol. 2022, 10, 858254. [Google Scholar] [CrossRef]
- Prasenjit, G.; Soumyo, M. Environmental contamination by heterocyclic Polynuclear aromatic hydrocarbons and their microbial degradation. Bioresour. Technol. 2021, 341, 125860. [Google Scholar] [CrossRef]
- Aboul-Kassim, T.A.; Simoneit, B.R. Interaction mechanisms between organic pollutants and solid phase systems. In Pollutant-Solid Phase Interactions Mechanisms, Chemistry and Modeling; Springer: Berlin/Heidelberg, Germany, 2001; pp. 107–167. [Google Scholar]
- Hayat, M.T.; Xu, J.; Ding, N.; Mahmood, T. Dynamic behavior of persistent organic pollutants in soil and their interaction with organic matter. In Molecular Environmental Soil Science at the Interfaces in the Earth’s Critical Zone; Springer: Berlin/Heidelberg, Germany, 2010; pp. 217–222. [Google Scholar]
- Chen, Y.; Li, H.; Yin, Y.; Shan, S.; Huang, T.; Tang, H. Effect of microplastics on the adherence of coexisting background organic contaminants to natural organic matter in water. Sci. Total Environ. 2023, 905, 167175. [Google Scholar] [CrossRef]
- Thiloka, K.; Paul, D.P.; Julia, H.; Gregory, D. Greener extraction of polycyclic aromatic hydrocarbons from soil and sediment using eucalyptus oil. Environ. Chem. Lett. 2022, 20, 2757–2764. [Google Scholar] [CrossRef]
- Chaudhry, G.R.; Chapalamadugu, S. Biodegradation of halogenated organic compounds. Microbiol. Rev. 1991, 55, 59–79. [Google Scholar] [CrossRef]
- Tratnyek, P.G.; Edwards, E.; Carpenter, L.; Blossom, S. Environmental occurrence, fate, effects, and remediation of halogenated (semi)volatile organic compounds. Environ. Sci. Process. Impacts 2020, 22, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Bai, J.; Chang, P.; Liu, Z.; Wang, Y.; Liu, G.; Cui, B.; Peijnenburg, W.; Vijver, M.G. Microplastics in terrestrial ecosystem: Exploring the menace to the soil-plant-microbe interactions. TrAC Trends Anal. Chem. 2024, 174, 117667. [Google Scholar] [CrossRef]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Ren, X.; Yin, S.; Wang, L.; Tang, J. Microplastics in plant-microbes-soil system: A review on recent studies. Sci. Total Environ. 2022, 816, 151523. [Google Scholar] [CrossRef]
- Ioannis, A.; Ioannis, P.; Berkant, K.; Dimitrios, K. Microplastics as carriers of hydrophilic pollutants in an aqueous environment. J. Mol. Liq. 2022, 350, 118182. [Google Scholar] [CrossRef]
- Dziurzynski, M.; Gorecki, A.; Pawlowska, J.; Istel, L.; Decewicz, P.; Golec, P.; Styczynski, M.; Poszytek, K.; Rokowska, A.; Gorniak, D.; et al. Revealing the diversity of bacteria and fungi in the active layer of permafrost at Spitsbergen island (Arctic)—Combining classical microbiology and metabarcoding for ecological and bioprospecting exploration. Sci. Total Environ. 2023, 856, 159072. [Google Scholar] [CrossRef]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, 990. [Google Scholar] [CrossRef]
- Gavin, J.K.; Jennifer, A.D. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866–869. [Google Scholar] [CrossRef]
- Gaurav, P.; Deviram, G.; Urvashi, A.; Prasuna, R.G.; Thangavel, M.; Arivalagan, P. Biological approaches practised using genetically engineered microbes for a sustainable environment: A review. J. Hazard. Mater. 2021, 405, 124631. [Google Scholar] [CrossRef]
- Koichi, F. Microbial Degradation of Polychlorinated Biphenyls (PCBs). In Biodegradation and Detoxification of Environmental Pollutants; CRC Press eBooks; Taylor & Francis Group: Abington, UK, 2018. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Rajagopal, M.; Krishnan, A.; Sreerama, S.K. A review on recent trends in nanomaterials and nanocomposites for environmental applications. Curr. Anal. Chem. 2021, 17, 202–243. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Biodegradation of organic pollutants at low temperatures. In Biotechnological Applications of Cold-Adapted Organisms; Margesin, R., Schinner, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 271–289. [Google Scholar] [CrossRef]
- Zambrano-Pinto, M.V.; Tinizaray-Castillo, R.; Riera, M.A.; Maddela, N.R.; Luque, R.; Díaz, J.M.R. Microplastics as vectors of other contaminants: Analytical determination techniques and remediation methods. Sci. Total Environ. 2024, 908, 168244. [Google Scholar] [CrossRef]
- Qutob, M.; Rafatullah, M.; Muhammad, S.A.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review of Pyrene Bioremediation Using Mycobacterium Strains in a Different Matrix. Fermentation 2022, 8, 260. [Google Scholar] [CrossRef]
- Manzano, M.; Morán, A.C.; Tesser, B.; González, B. Role of eukaryotic microbiota in soil survival and catabolic performance of the 2,4-D herbicide degrading bacteria Cupriavidus necator JMP134. Antonie Van Leeuwenhoek 2007, 91, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Upasani, V.N. Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016, 222, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Hoodaji, M.; Tahmourespour, A.; Abdollahi, A.; Baghi, T.; Eslamian, S.; Ostad-Ali-Askari, K. Bioremediation of polycyclic aromatic hydrocarbons by Bacillus Licheniformis ATHE9 and Bacillus Mojavensis ATHE13 as newly strains isolated from oil-contaminated soil. J. Geogr. Environ. Earth Sci. Int. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Pi, Y.; Chen, B.; Bao, M.; Fan, F.; Cai, Q.; Ze, L.; Zhang, B. Microbial degradation of four crude oil by biosurfactant producing strain Rhodococcus sp. Bioresour. Technol. 2017, 232, 263–269. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, E.Y.; Kim, Y.J.; Cho, K.-S.; Ryu, H.W. Degradation of polyaromatic hydrocarbons by Burkholderia cepacia 2A-12. World J. Microbiol. Biotechnol. 2003, 19, 411–417. [Google Scholar] [CrossRef]
- Kagle, J.; Porter, A.W.; Murdoch, R.W.; Rivera-Cancel, G.; Hay, A.G. Chapter 3 Biodegradation of Pharmaceutical and Personal Care Products. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2009; Volume 67, pp. 65–108. [Google Scholar]
- Zheng, Z.; He, J.; Dong, C.; Lo, I.M.C. Photoelectrochemical sewage treatment by sulfite activation over an optimized BiVO4 photoanode to simultaneously promote PPCPs degradation, H2 evolution and E. coli disinfection. Chem. Eng. J. 2021, 419, 129418. [Google Scholar] [CrossRef]
- Górny, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Naproxen ecotoxicity and biodegradation by Bacillus thuringiensis B1(2015b) strain. Ecotoxicol. Environ. Saf. 2019, 167, 505–512. [Google Scholar] [CrossRef]
- Holt, L.M.; Laursen, A.E.; McCarthy, L.H.; Bostan, I.V.; Spongberg, A.L. Effects of land application of municipal biosolids on nitrogen-fixing bacteria in agricultural soil. Biol. Fertil. Soils 2010, 46, 407–413. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Villaverde, S.; Fernández-Polanco, F. Spatial distribution of respiratory activity in Pseudomonas putida 54G biofilms degrading volatile organic compounds (VOC). Appl. Microbiol. Biotechnol. 1999, 51, 382–387. [Google Scholar] [CrossRef]
- Andreolli, M.; Lampis, S.; Poli, M.; Gullner, G.; Biró, B.; Vallini, G. Endophytic Burkholderia fungorum DBT1 can improve phytoremediation efficiency of polycyclic aromatic hydrocarbons. Chemosphere 2013, 92, 688–694. [Google Scholar] [CrossRef]
- Jeong, E.; Hirai, M.; Shoda, M. Removal of o-xylene using biofilter inoculated with Rhodococcus sp. BTO62. J. Hazard. Mater. 2008, 152, 140–147. [Google Scholar] [CrossRef]
- Duc, H.D. Degradation of chlorotoluenes by Comamonas testosterone KT5. Appl. Biol. Chem. 2017, 60, 457–465. [Google Scholar] [CrossRef]
- McNerney, R.; Mallard, K.; Okolo, P.I.; Turner, C. Production of volatile organic compounds by mycobacteria. FEMS Microbiol. Lett. 2012, 328, 150–156. [Google Scholar] [CrossRef]
- Monika, J.; Shilpi, S.; Satyawati, S. Development of next-generation formulation against Fusarium oxysporum and unraveling bioactive antifungal metabolites of biocontrol agents. Sci. Rep. 2021, 11, 22895. [Google Scholar] [CrossRef]
- David, T.J. Setting the standards for machine learning in biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 659–660. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef]
- Mishra, C.S.K.; Samal, S.; Samal, R.R. Evaluating earthworms as candidates for remediating pesticide contaminated agricultural soil: A review. Front. Environ. Sci. 2022, 10, 924480. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H. Microbial Consortia Are Needed to Degrade Soil Pollutants. Microorganisms 2022, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Zhang, L.; Zhang, Z.; Tang, S.; Sun, Y.; Huang, H.; Yu, Y. Degradation of organic pollutants by intimately coupling photocatalytic materials with microbes: A review. Crit. Rev. Biotechnol. 2021, 41, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Top, E.M.; Springael, D.; Boon, N. Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol. Ecol. 2002, 42, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Q.; Blom-Zandstra, M.; Gupta, S.K.; Joner, E. Utilising the Synergy between Plants and Rhizosphere Microorganisms to Enhance Breakdown of Organic Pollutants in the Environment (15 pp). Environ. Sci. Pollut. Res. 2005, 12, 34–48. [Google Scholar] [CrossRef]
- Antonio, V. Nanotechnology, bionanotechnology and microbial cell factories. Microb. Cell Factories 2010, 9, 53. [Google Scholar] [CrossRef]
- Günther, J.; Patrick van, R.; Barbara Santos de, M.; Alexander, B. Ferritin: A Versatile Building Block for Bionanotechnology. Chem. Rev. 2015, 115, 1653–1701. [Google Scholar] [CrossRef]
- Karpenko, A.A.; Odintsov, V.S.; Aleksandra, I. Micro-nano-sized polytetrafluoroethylene (teflon) particles as a model of plastic pollution detection in living organisms. Environ. Sci. Pollut. Res. 2021, 29, 11281–11290. [Google Scholar] [CrossRef]
- Dhanapal, A.R.; Thiruvengadam, M.; Vairavanathan, J.; Venkidasamy, B.; Easwaran, M.; Ghorbanpour, M. Nanotechnology Approaches for the Remediation of Agricultural Polluted Soils. ACS Omega 2024, 9, 13522–13533. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Copty, N.K.; Amr, S.S.A.; Abushammala, M.F.M.; Al Maskari, T. Recent Advances of Nanoremediation Technologies for Soil and Groundwater Remediation: A Review. Water 2021, 13, 2186. [Google Scholar] [CrossRef]
- Yadav, N.; Garg, V.K.; Chhillar, A.K.; Rana, J.S. Detection and remediation of pollutants to maintain ecosustainability employing nanotechnology: A review. Chemosphere 2021, 280, 130792. [Google Scholar] [CrossRef]
- Arshad, I.; Noor, A.; Rashid, H.; Hussan, M.U.; Waqas, M.; Fatima, N.; Anwer, A.; Ijaz, V.; Qayyum, A.; Arshad, F.; et al. A comprehensive review on role of nanotechnology in the soil pollutants remediation. Agric. Sci. J. 2022, 4, 17–38. [Google Scholar] [CrossRef]
- Nakum, J.; Bhattacharya, D. Various Green Nanomaterials Used for Wastewater and Soil Treatment: A Mini-Review. Front. Environ. Sci. 2022, 9, 724814. [Google Scholar] [CrossRef]
- Hemalatha, I.; Harika, D.; Karnena, M.K. Sustainable Nano-Bioremediation Approaches for the Treatment of Polluted Soils. Nat. Environ. Pollut. Technol. 2022, 21, 1818–1826. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Q.; Mo, F.; Li, T.; Liu, J. Bioelectrochemical degradation of petroleum hydrocarbons: A critical review and future perspectives. Environ. Pollut. 2022, 306, 119344. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.; Zhou, Q.; Zhang, Z.; Chen, C. Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol. Bioeng. 2012, 109, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Bio-electrochemical treatment of distillery wastewater in microbial fuel cell facilitating decolorization and desalination along with power generation. J. Hazard. Mater. 2010, 177, 487–494. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, Q.; Fan, M.; Xu, Y.; Chen, Y. Highly efficient anaerobic co-degradation of complex persistent polycyclic aromatic hydrocarbons by a bioelectrochemical system. J. Hazard. Mater. 2020, 381, 120945. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liang, B.; Li, Z.-L.; Yang, J.-Q.; Huang, C.; Lyu, M.; Yuan, Y.; Nan, J.; Wang, A.-J. Bioelectrochemical assisted dechlorination of tetrachloroethylene and 1,2-dichloroethane by acclimation of anaerobic sludge. Chemosphere 2019, 227, 514–521. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Liu, Q.; He, W.; Zhang, W.; Wang, L.; Tang, J. Metagenomic analysis reveals the microbial response to petroleum contamination in oilfield soils. Sci. Total Environ. 2024, 912, 168972. [Google Scholar] [CrossRef]
- Sharma, P.; Sirohi, R.; Tong, Y.W.; Kim, S.H.; Pandey, A. Metal and metal(loids) removal efficiency using genetically engineered microbes: Applications and challenges. J. Hazard. Mater. 2021, 416, 125855. [Google Scholar] [CrossRef] [PubMed]
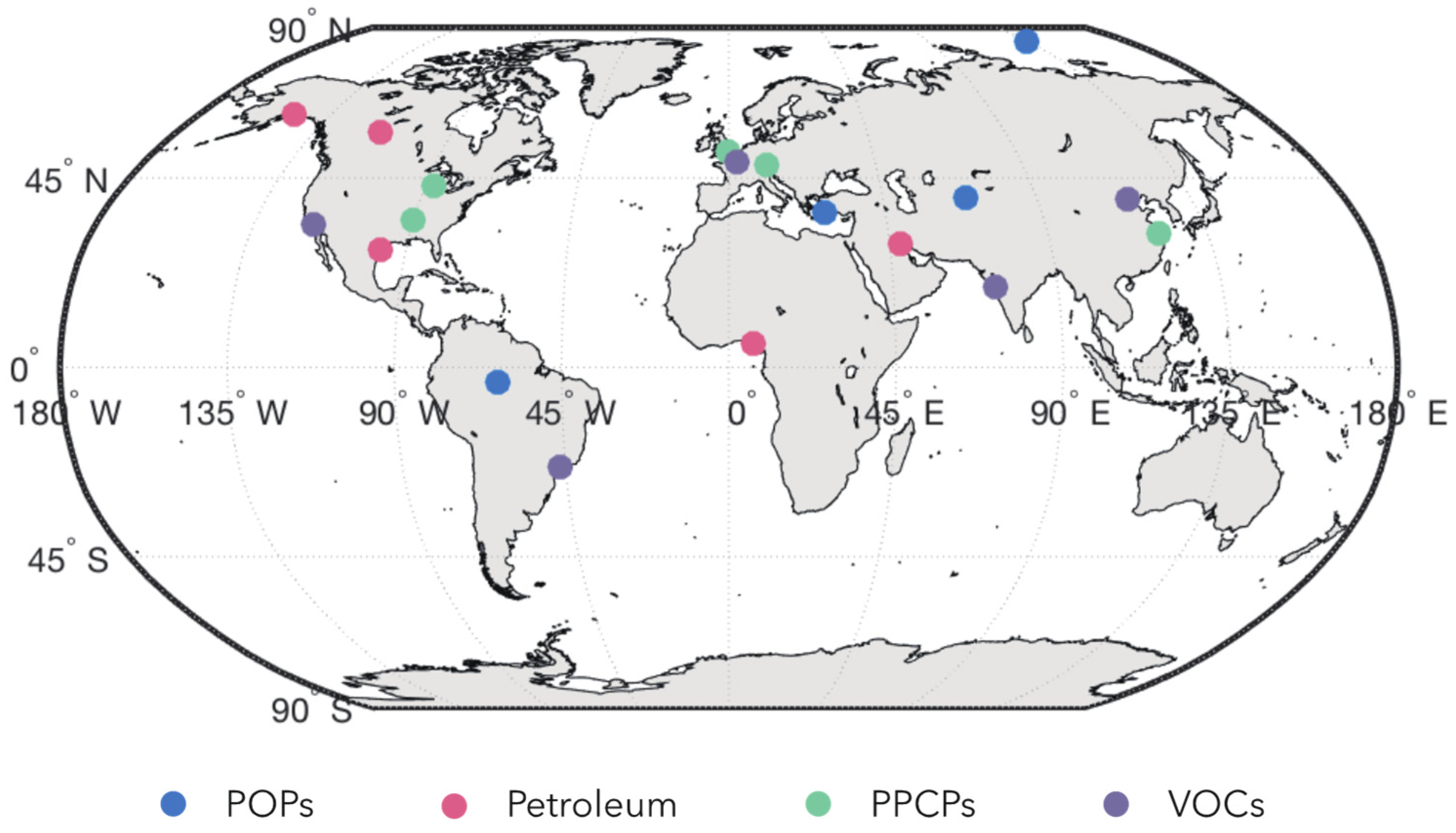
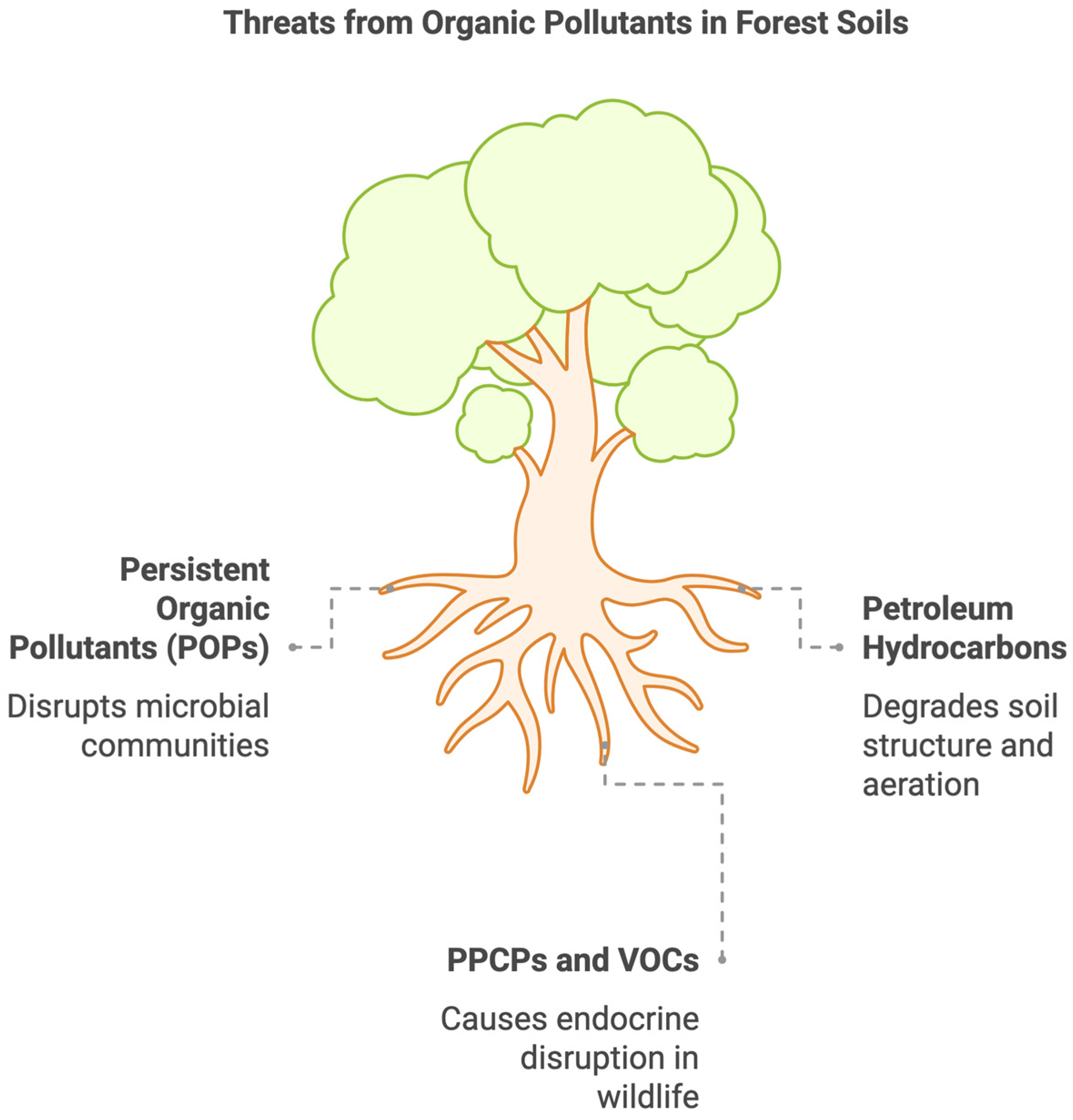
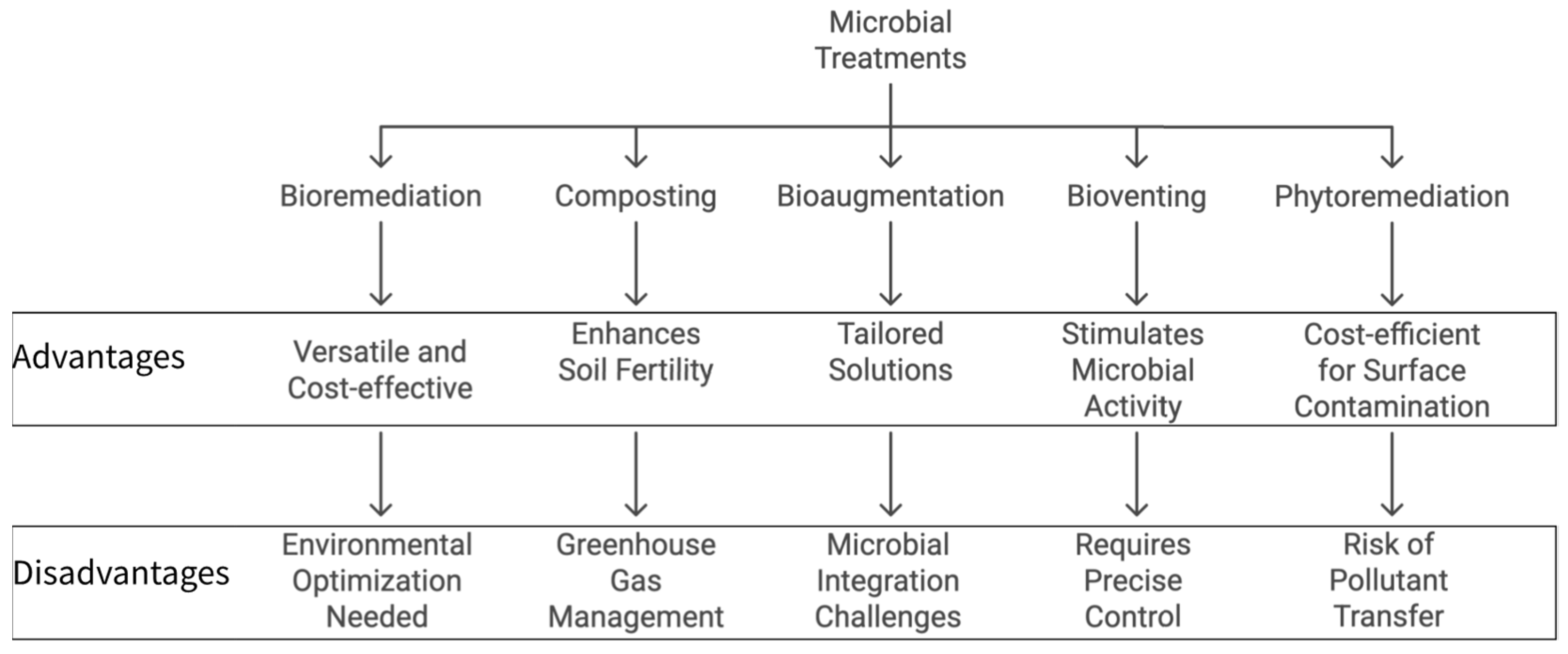
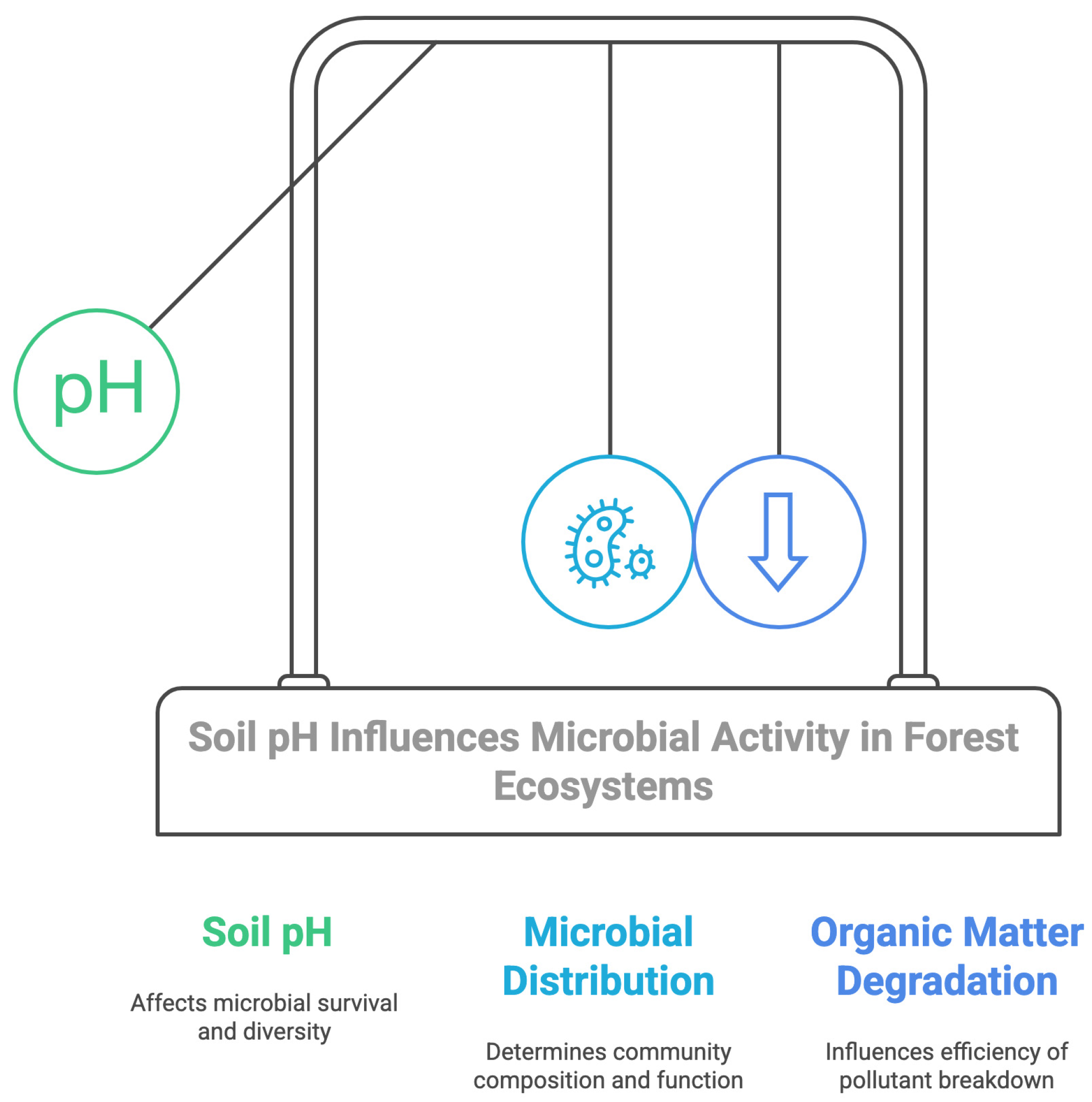
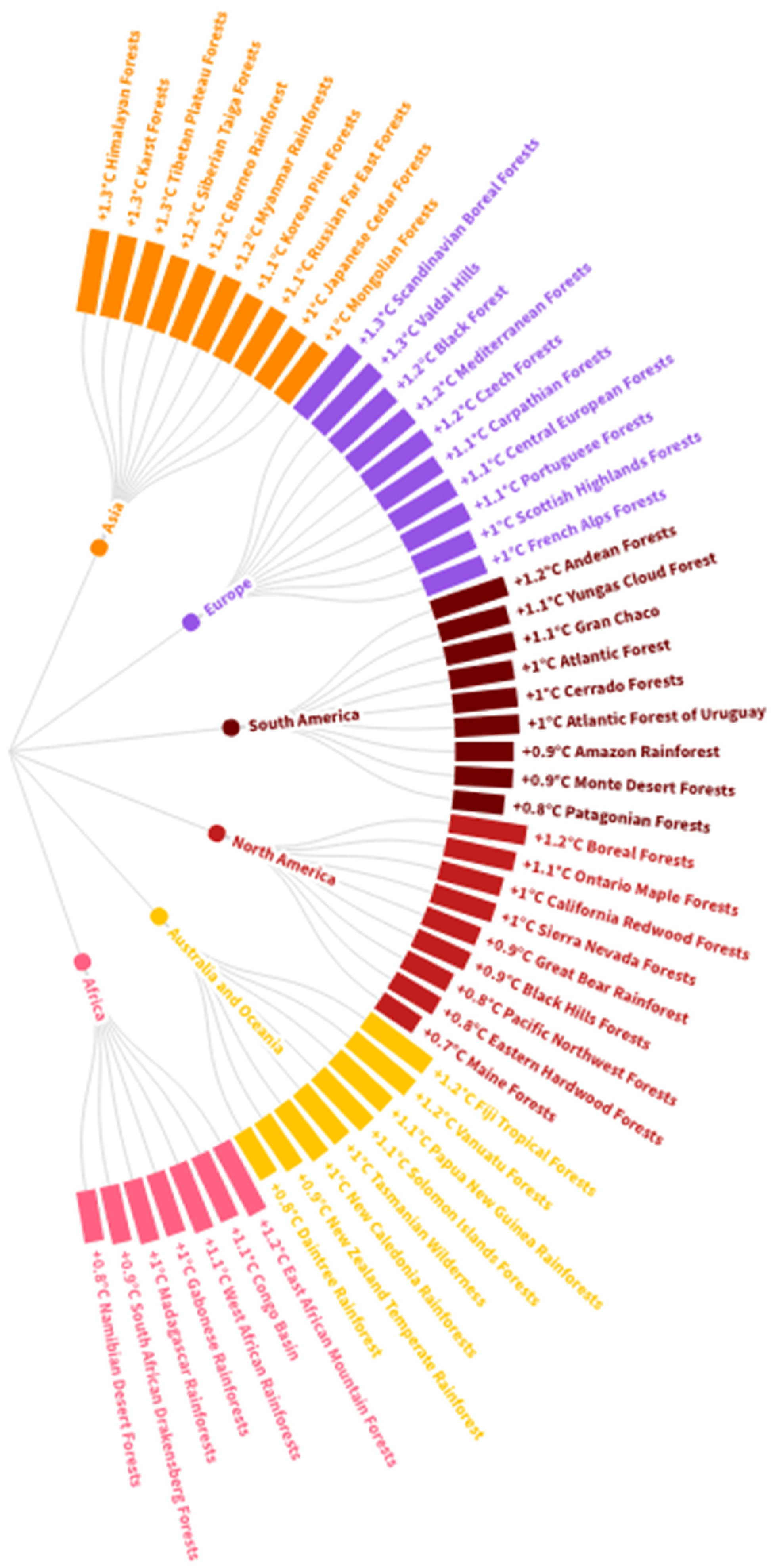

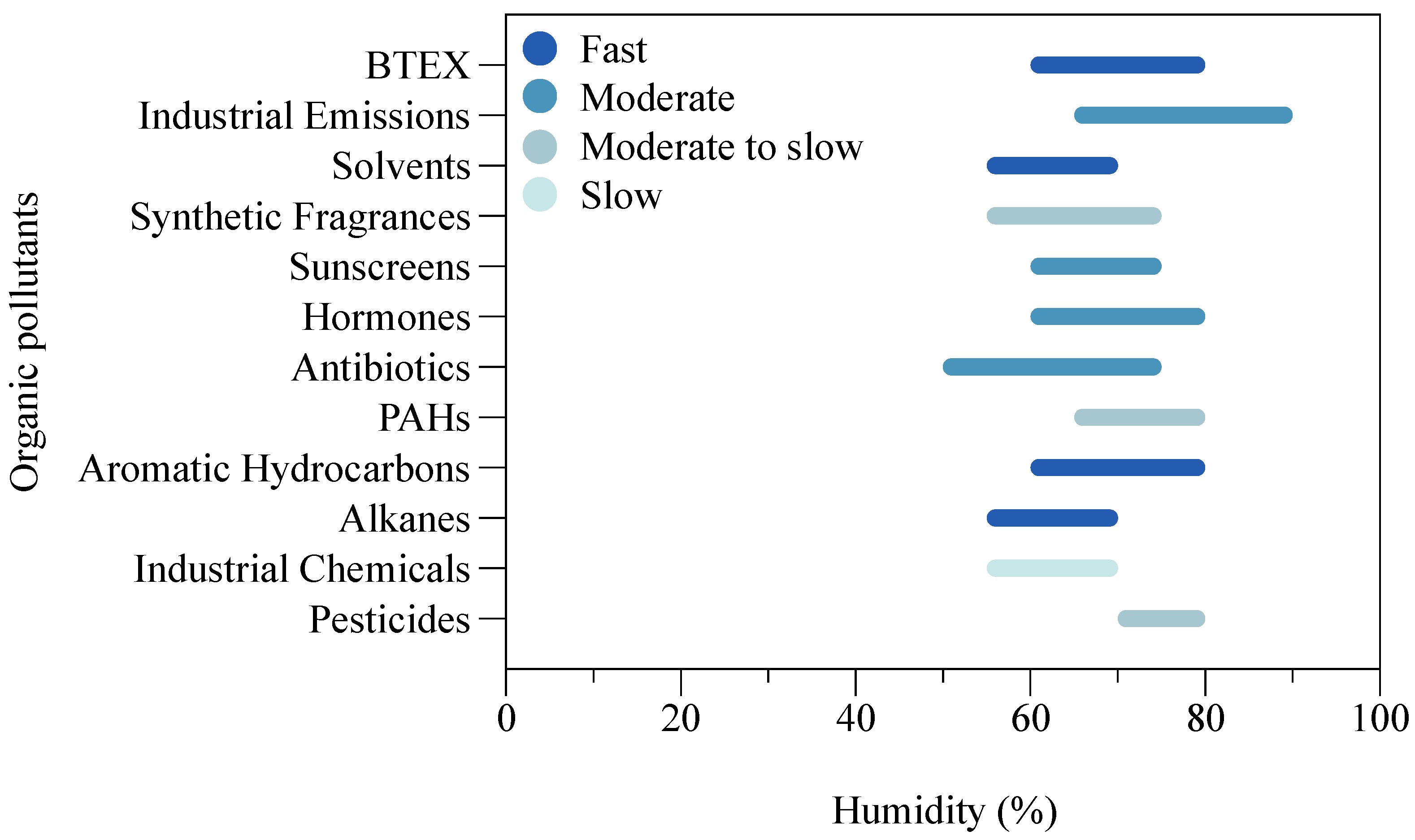
| Types of Enzymatic Reactions | Enzyme | Mechanism | References |
|---|---|---|---|
| Hydrolysis | Hydrolases | Hydrolysis involves the addition of water to break chemical bonds in complex organic molecules, resulting in simpler, often more polar and less toxic products. | [62,63] |
| Oxidation-Reduction (Redox) Reactions | Oxidases, Oxygenases, Dehydrogenases Reductases | Oxidation: Enzymes introduce oxygen into organic molecules or remove electrons, leading to the breakdown of the pollutants. Reduction: Enzymes add electrons to pollutants, often removing halogens or nitro groups, resulting in less toxic or more biodegradable compounds. | [61,64,65,66] |
| Dehalogenases | Dehalogenases | Dehalogenation involves the removal of halogen atoms (e.g., chlorine, bromine) from organic compounds. This process is crucial for detoxifying halogenated pollutants, which are often persistent and toxic. | [67,68] |
| Ring Cleavage | Dioxygenases Peroxidases | Ring cleavage involves the breaking of aromatic rings in complex pollutants, such as PAHs and phenolic compounds. This step is critical for the complete mineralization of these compounds. | [69,70] |
| Hydroxylation | Hydroxylases Monooxygenases | Hydroxylation introduces hydroxyl groups (-OH) into organic molecules, increasing their solubility and making them more susceptible to further degradation. | [71,72,73] |
| Forest Type | Characteristics | Microbial Differences | Ref. |
|---|---|---|---|
| Evergreen Coniferous Forest | pH: Slightly acidic to neutral Temperature: Cool to temperate Humidity: High year-round | High fungal diversity, especially ectomycorrhizal fungi Lower bacterial diversity compared to broadleaf forests | [133,134] |
| Deciduous Broadleaf Forest | pH: Slightly acidic to neutral Temperature: Warm summers, cold winters Humidity: Seasonal variation, high in summer, low in winter | Diverse microbial community, especially during leaf litter decomposition Abundance of decomposer fungi and bacteria during fall | [135,136] |
| Mixed Forest (Coniferous and Broadleaf) | pH: Varies with soil type and tree species Temperature: Variable, depending on location Humidity: Variable, depending on location and season | Microbial diversity reflects a mix of coniferous and broadleaf forests High fungal diversity, including mycorrhizal fungi | [137,138] |
| Tropical Rainforest | pH: Slightly acidic Temperature: Hot and humid year-round Humidity: Very high | Extremely diverse microbial community High abundance of fungi and bacteria due to rich organic matter | [139,140] |
| Dry Forest/Savanna | pH: Slightly acidic to neutral Temperature: Hot during the day, cooler at night Humidity: Low to moderate, seasonal rains | Adapted to drought conditions, lower microbial diversity Fungi and bacteria tolerant to dry conditions | [141,142] |
| Boreal Forest | pH: Slightly acidic Temperature: Cold year-round Humidity: High, with significant snowfall | Low microbial diversity due to cold temperatures Adapted to cold conditions, including psychrophilic microorganisms | [143,144] |
| Microorganism | Types of Organic Matter | Specific Organic Matter | Degradation Principle | Ref. |
|---|---|---|---|---|
| Acidic Soils (pH < 5.5) | ||||
| Ascomycetes | PAHs Phenols | Naphthalene Phenol | Oxidative degradation via enzymes such as laccases and peroxidases. | [155] |
| Basidiomycetes | PAHs Pesticides | Pyrene DDT | Ligninolytic enzymes (laccases, peroxidases) oxidize aromatic compounds. | [156] |
| Acidobacteria | PAHs Pesticides | Fluoranthene Aldrin | Hydrolysis and oxidation of aromatic rings and side chains. | [157] |
| Neutral Soils (pH 6.0–7.5) | ||||
| Actinobacteria | Petroleum Hydrocarbons PPCPs | Toluene Caffeine | Oxidative cleavage of aromatic rings, hydroxylation, and mineralization. | [158] |
| Firmicutes | Petroleum Hydrocarbons PPCPs | Benzene Diclofenac | Anaerobic degradation, hydrolysis, and fermentation of complex compounds. | [159] |
| Proteobacteria | Petroleum Hydrocarbons PPCPs VOCs | Xylene Estrone | Oxidative degradation, cometabolism, and mineralization of organic pollutants. | [160] |
| Bacteroidetes | Pharmaceuticals PPCPs | Erythromycin Bisphenol A | Hydrolytic and oxidative processes, breaking down complex organic molecules. | [161] |
| Alkaline Soils (pH > 7.5) | ||||
| Alcaligenes | Petroleum Hydrocarbons VOCs | Octane Trichloroethylene | Oxygenase-mediated oxidation and degradation of hydrocarbons. | [162] |
| Nitrobacter | PPCPs Organic Nitrogen Compounds | Ammonium Nitrobenzene | Nitrification and oxidation of nitro-containing compounds. | [163] |
| Clostridia | Petroleum Hydrocarbons PPCPs | Butyrate, Hexachlorobenzene | Anaerobic fermentation and degradation of complex organic compounds. | [164] |
| Bacillus | Petroleum Hydrocarbons PPCPs VOCs | Dodecane, Paracetamol | Hydrocarbon oxidation, enzyme-mediated degradation of organic pollutants. | [165] |
| Arthrobacter | PAHs Petroleum Hydrocarbons | Anthracene Cyclohexane | Oxidative degradation of aromatic hydrocarbons and heterocyclic compounds. | [166] |
| Pseudomonas | PAHs Petroleum Hydrocarbons, PPCPs VOCs | Phenanthrene Naphthalene Atrazine Toluene | Enzyme-mediated oxidation, cometabolic degradation, and mineralization. | [158] |
| Pollutant Group | Impact of SOM | Optimal SOM Content (%) | Degradation Rate Impact | Ref. |
|---|---|---|---|---|
| Pesticides | SOM increases bioavailability by adsorbing and releasing pollutants. | 3–5% | Enhanced (70–85%) | [205,206,207] |
| Industrial Chemicals | High SOM can limit degradation by sequestering pollutants. | 2–4% | Moderate (60–75%) | [205,208,209] |
| Alkanes | SOM aids in emulsification, enhancing microbial uptake. | 1–3% | Fast (75–90%) | [210,211,212] |
| Aromatic Hydrocarbons | SOM enhances solubility and bioavailability. | 4–6% | High (80–95%) | [213,214] |
| PAHs | SOM adsorbs PAHs, but microbial enzymes can release them. | 3–5% | Moderate (65–85%) | [214,215,216] |
| Antibiotics | SOM can reduce toxicity and improve microbial tolerance. | 4–6% | Moderate (60–80%) | [217,218] |
| Hormones | SOM enhances stability, promoting slow release. | 2–4% | Moderate (65–75%) | [219,220] |
| Sunscreens | SOM helps in dispersing hydrophobic molecules. | 3–5% | Moderate (60–75%) | [221,222,223] |
| Synthetic Fragrances | SOM reduces volatilization, aiding microbial degradation. | 3–5% | Moderate (70–80%) | [224] |
| Solvents | Low SOM minimizes competition with naturally occurring compounds. | 1–2% | High (80–90%) | [225,226,227,228] |
| Industrial Emissions | SOM can trap volatile compounds but also facilitate microbial uptake. | 3–5% | Moderate (65–80%) | [229,230] |
| BTEX | SOM improves solubility and bioavailability. | 4–6% | High (85–95%) | [231,232,233] |
| Pollutant Category | Microbial Species | Genetic Engineering Technology | Pollutant Name | Degradation Time | Degradation End Product | Ref. |
|---|---|---|---|---|---|---|
| Persistent Organic Pollutants (POPs) | Bacillus subtilis | Expression of dehalogenase genes | DDT | 7 days | Non-toxic products | [252] |
| Pseudomonas putida | Introduction of linA gene | Lindane | 10 days | Degradation products (less toxic) | [253] | |
| Rhodococcus erythropolis | Engineering of dioxygenase genes | PCBs | 14 days | Non-chlorinated compounds | [254] | |
| Mycobacterium vanbaalenii | Genetic modification for biphenyl dioxygenase | PCBs | 12 days | Degradation intermediates | [255] | |
| Cupriavidus necator | Introduction of organophosphorus hydrolase gene | Parathion | 9 days | Non-toxic products | [256] | |
| Petroleum Hydrocarbons | Alcanivorax borkumensis | Alkane hydroxylase genes | Alkanes (crude oil) | 15 days | Carbon dioxide and water | [83] |
| Pseudomonas aeruginosa | Modification of n-alkane-degrading enzymes | Long-chain alkanes | 8 days | Alkanes and fatty acids | [257] | |
| Bacillus licheniformis | PAH dioxygenase genes | PAHs | 11 days | Less toxic degradation products | [258] | |
| Rhodococcus sp. | Enhanced catabolic pathways | Petroleum hydrocarbons | 10 days | Less complex hydrocarbons | [259] | |
| Burkholderia cepacia | PAH-degrading plasmids | High molecular weight PAHs | 12 days | Degradation intermediates | [260] | |
| Pharmaceuticals and Personal Care Products (PPCPs) | Pseudomonas putida | Tetracycline-degrading genes | Tetracycline | 6 days | Degradation products | [261] |
| Escherichia coli | Esterase genes for steroid degradation | Estrone, Estradiol | 7 days | Non-toxic products | [262] | |
| Bacillus thuringiensis | Sulfonamide-degrading enzymes | Sulfonamide antibiotics | 8 days | Degradation products | [263] | |
| Rhizobium leguminosarum | Advanced biotransformation pathways | Various PPCPs | 9 days | Less harmful products | [264] | |
| Acinetobacter baumannii | Synthetic estrogen-degrading pathways | Synthetic estrogens | 10 days | Degradation products | [265] | |
| Volatile Organic Compounds (VOCs) | Pseudomonas putida | Toluene-degrading operon | Toluene | 5 days | Benzene and other derivatives | [266] |
| Burkholderia fungorum | Xylenes-degrading pathways | Xylenes | 7 days | Degradation products | [267] | |
| Rhodococcus sp. | VOC-degrading plasmids | Benzene, Ethylbenzene | 6 days | Less harmful products | [268] | |
| Comamonas testosteroni | Halogenated VOC-degrading pathways | Trichloroethylene | 8 days | Degradation products | [269] | |
| Mycobacterium smegmatis | Specialized VOC degradation pathways | Various VOCs | 9 days | Degradation products | [270] |
| Biological Agent | Target Pollutants | Mechanism | Ref. |
|---|---|---|---|
| Mixotrophic Cyanobacteria and Microalgae | Organic pollutants, hydrocarbons | Mixotrophic pathways enhance degradation efficiency and carbon sequestration | [273] |
| Earthworms | Pesticides and organic pollutants | Gut enzymes and microbial stimulation in processed soil | [274] |
| Microbial Consortia | PAHs, petroleum hydrocarbons, pharmaceuticals | Synergistic microbial interactions for efficient degradation | [275] |
| Photocatalytic Materials with Microbes (ICPB) | Refractory organic pollutants (e.g., VOCs) | Combines photocatalysis and biodegradation | [276] |
| Genetically Modified Microorganisms | PCBs, PAHs, xenobiotic organic compounds | Enhanced gene transfer for pollutant degradation pathways | [277] |
| Rhizosphere Microbial Synergies | Hydrophobic and persistent organic pollutants | Enhanced degradation through plant-microbe interactions in the rhizosphere | [278] |
| Nanotechnology | Target Pollutants | Mechanism | Ref. |
|---|---|---|---|
| Nanobiosurfactants | Organic pollutants, pesticides, and herbicides | Enhances pollutant bioavailability for microbial degradation | [282] |
| Carbon Nanotubes (CNTs) | Persistent organic pollutants (POPs), heavy metals | Facilitates adsorption and transport of pollutants, improving microbial accessibility | [283] |
| Zero-Valent Iron Nanoparticles (nZVI) | Petroleum hydrocarbons, VOCs | Catalyzes degradation and immobilization of contaminants | [284] |
| Nanophytoremediation | Organic pollutants, heavy metals | Assists plant-microbe interactions to detoxify pollutants | [285] |
| Nanobiosorbents | Agrochemicals and organic pollutants | Enhances microbial bioremediation by adsorbing and concentrating pollutants | [282] |
| Green Nanomaterials (e.g., nano-chitosan) | Pesticides and industrial pollutants | Improves pollutant adsorption while minimizing toxicity to microbes | [286] |
| Metallic Nanoparticles | Organic dyes, antibiotics, and pesticides | Facilitates enzymatic degradation and pollutant breakdown | [287] |
| Modified nZVI Nanoparticles | Hydrocarbons, heavy metals | Reduced toxicity to microbes, promoting enhanced microbial activity | [283] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Wen, S.; Zhu, S.; Hu, X.; Wang, Y. Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management. Processes 2025, 13, 916. https://doi.org/10.3390/pr13030916
Liu P, Wen S, Zhu S, Hu X, Wang Y. Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management. Processes. 2025; 13(3):916. https://doi.org/10.3390/pr13030916
Chicago/Turabian StyleLiu, Pengfei, Shizhi Wen, Shanshan Zhu, Xi Hu, and Yamin Wang. 2025. "Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management" Processes 13, no. 3: 916. https://doi.org/10.3390/pr13030916
APA StyleLiu, P., Wen, S., Zhu, S., Hu, X., & Wang, Y. (2025). Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management. Processes, 13(3), 916. https://doi.org/10.3390/pr13030916







