Integration of Emerging and Conventional Technologies for Obtaining By-Products from Cocoa Pod Husk and Their Application
Abstract
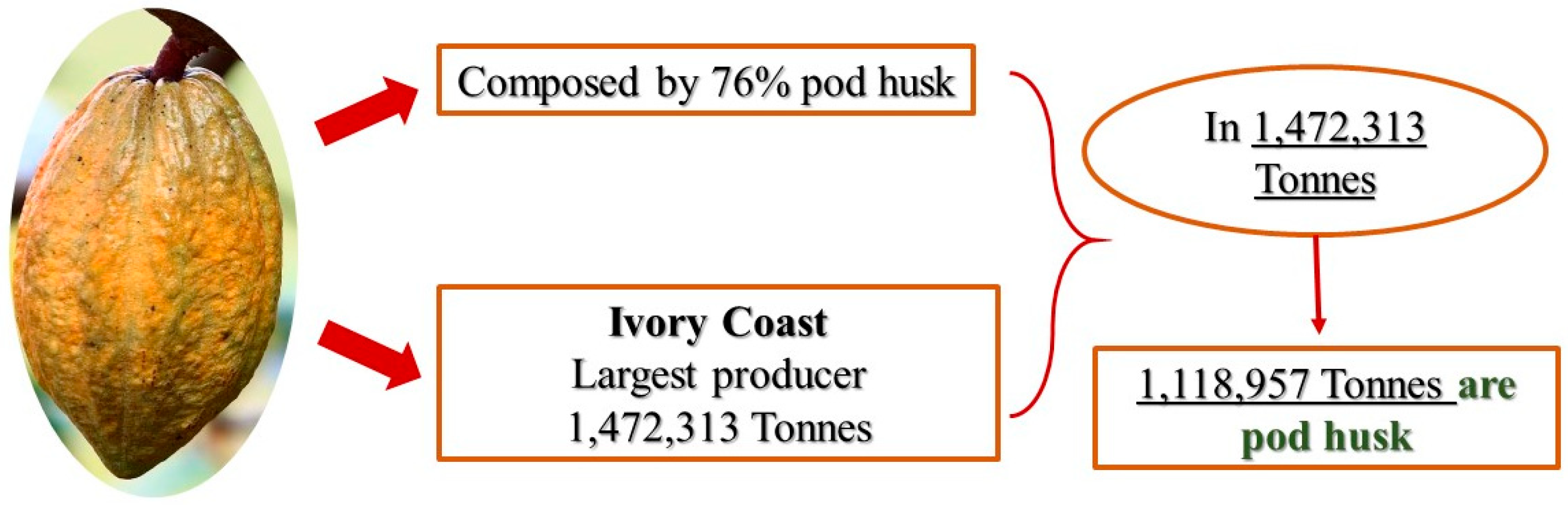
1. Introduction
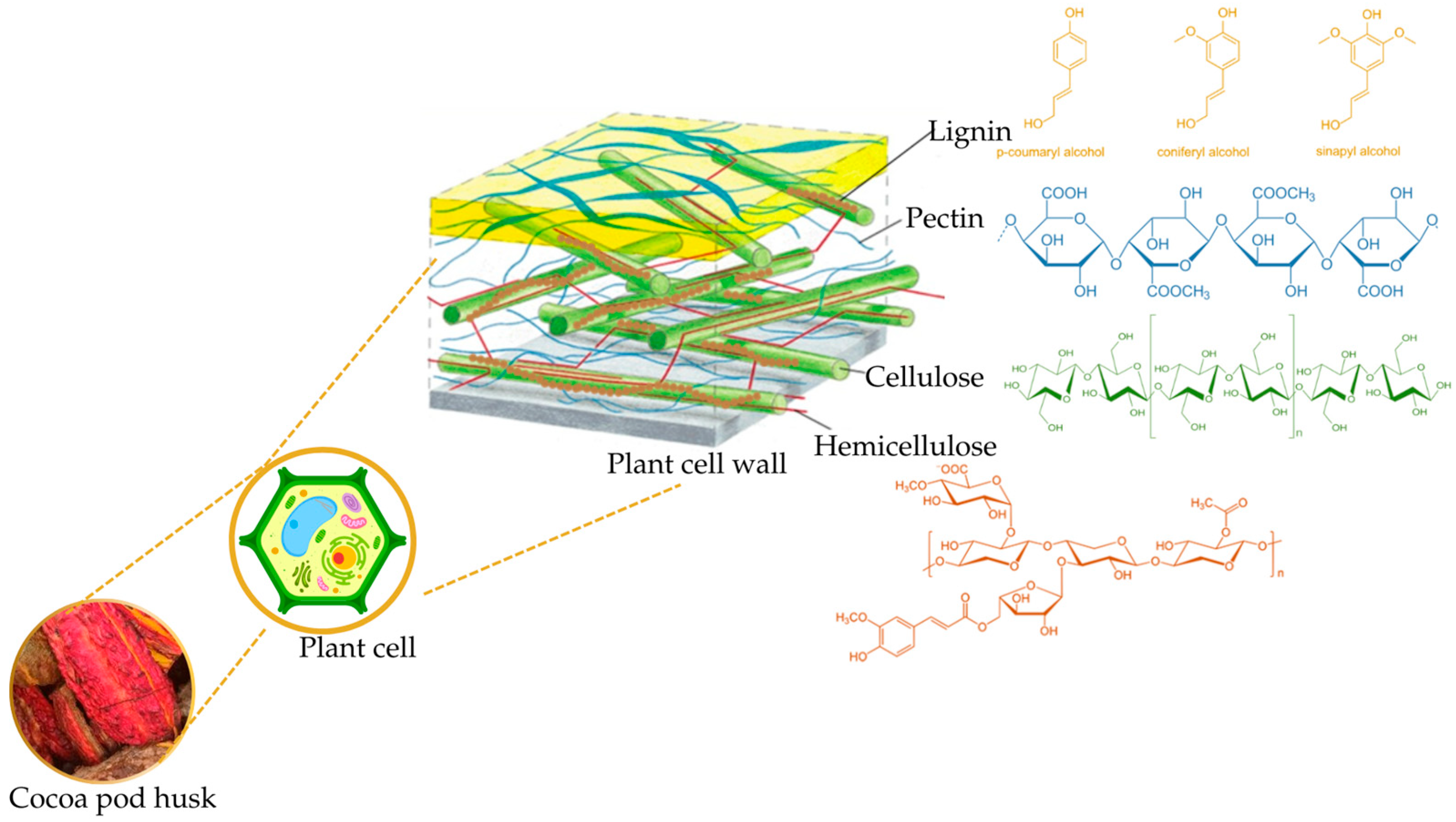
2. Cocoa Pod Husk Compounds
2.1. Cellulose
2.2. Hemicellulose
2.3. Lignin
2.4. Pectin
| Extraction Methods | pH | DE (%) | References |
|---|---|---|---|
| Acid hydrolysis with citric acid | 2.0 | 83.99 | [55] |
| 2.5 | 69.25 | [55] | |
| 3.0 | 48.84 | [55] | |
| 3.71 | 52.20 | [9] | |
| 3.0 | 40.3 | [56] | |
| Acid hydrolysis with acetic acid | 2.0 | 15.26 | [55] |
| 2.5 | 18.43 | [55] | |
| 3.0 | 20.22 | [55] | |
| Alkaline extraction | 12.0 | 40.1 | [57] |
| Enzymatic extraction | 5.0 | 48.5 | [57] |
| Hot aqueous extraction | 6.7 | 26.8 | [58] |
3. Conventional Technology for Obtaining By-Products from the Cocoa Pod Husk
3.1. Acidic Treatment
3.2. Alkaline Treatment
3.3. Fermentation
3.3.1. Aerobic Fermentation
3.3.2. Anaerobic Fermentation
4. Emerging Technology for Obtaining By-Products from the Cocoa Pod Husk
4.1. High-Voltage Electric Discharge Pretreatment
4.2. Pyrolysis
| By-Product from CPH | Characteristics | Parameters | Yield (%) | References |
|---|---|---|---|---|
| Biochar | High nutrient content (calcium and potassium) | Slow pyrolysis T: 450 °C | 31.1% | [87] |
| Increased porosity due to post-acid treatment | Slow pyrolysis T: 400 °C Integrate posttreatment with HCl 0.25 M | 40.70% | [88] | |
| It has polycondensed aromatic structures, which are responsible for the stability of biochar when applied to soils More rigid and porous structure | Fast pyrolysis T: 800 °C Integrate posttreatment with HCl 0.25 M | 30.22% | ||
| Bio-oil | pH: 2.8 Density: 1150 kg m−3 Viscosity: 140 mm2 s−1 High heating value: 8.64 MJ/kg High water content: 30% | Fast pyrolysis Feed rate: 110 g/h T: 600 °C | 58% | [89] |
| High water content: 50% Contain chemicals grouped into ketones, carboxylic acids, aldehydes, furans, heterocyclic aromatics, phenols, benzenediols, and other chemicals. Requires stabilization with catalysts | Slow pyrolysis T: 500 °C | 59.63% | [90] |
4.3. Fluid-Assisted Extraction Techniques
4.3.1. Supercritical Fluids (SCFs)
4.3.2. Subcritical Water Extraction (SWE)
4.3.3. Ionic Fluid
4.3.4. Deep Eutectic Solvents (DESs)
4.4. Separately from Intensification Techniques
4.4.1. Ultrasound (US)
4.4.2. Microwave-Assisted Extraction
4.5. Hydrothermal Treatment
4.6. Enzymatic Hydrolysis
| Enzymatic Hydrolysis | By-Products Obtained from Hydrolysis | By-Products Obtained After Fermentation | References |
|---|---|---|---|
| Cellic HTec 2®/Cellic CTec® from Novozymes—Araucaria, Brazil (1/9 ratio) | Glucose (58.9%) Xylose (16.1%) | Bioethanol | [9] |
| Xylanase-X2753, 1500 U/g (Pentopan Mono BG®) and Cellulase-C2730, 700 U/g (Celluclast®) from Sigma-Aldrich, Taiwan | Glucose Xylose | Not applicable | [109] |
| Celluclast® | Pectin (8.28%) | Not applicable | [117] |
| Cellulase enzyme Viscozyme Cassava CL (Novozymes A/S, Denmark) | Reducing sugars | Butanol, acetone–butanol–ethanol | [3] |
| Cellic Ctec2, Novozyme | Sugars (98.75%) | Ethanol | [68] |
| Emerging Technology | Parameters | By-Products Obtain | Characteristics | Yield | References |
|---|---|---|---|---|---|
| Subcritical water extraction | T:121 °C Pressure: 103.4 bar Time: 30 min | Pectin | Higher pectin yield, higher galacturonic acid content, and higher degree of methyl esterification; fewer interfering compounds derived from other cell wall polysaccharides | 10.9% | [97] |
| Microwave-assisted extraction | Time extraction: 30 min Microwave power: 450 W Solvent concentration: 10% T: 104 °C | Pectin | Galacturonic acid content of 72.86% | 21.1% | [120] |
| Microwave-assisted extraction + deep eutectic solvents | Time extraction: 30 min Microwave irradiation: 200 W DES proportion: 2:1:1 (p-toluenesulfonic acid/choline chloride/glycerol) Relation solid/liquid: 5% | Lignin | Larger particle sizes and structural diversity and higher H/G sub-unit ratio | 95.5% * | [121] |
| T: 121 °C Time: 15 min Microwave irradiation: 450 W DES proportion: 1:1 (choline chloride/citric acid) | Cellulose | Increased cellulose crystallinity due to the removal of amorphous components | 28.12% | [109] | |
| Hemicellulose | Removal of acetyl and uronic groups from hemicellulose | 7.76% | |||
| Lignin | DES treatment allows lignin to be removed; it was not characterized | 16.31% | |||
| Ionic fluids | Ionic fluid: 100 mL ionic liquid, 1-ethyl-3-methylimidazolium methanesulfonate (C7H14N2O3S) T1: room temperature (2 h) T2: 121 °C (15 min) | Cellulose | Minimum decrease in cellulose crystallinity | 47% | [101] |
| Hemicellulose | Was not characterized | 25% | |||
| Lignin | Was not characterized | 9% |
| Emerging Technology | Conventional Technology | By-Products Obtain | Yield (%) | References |
|---|---|---|---|---|
| Ultrasonic-assisted treatment (T, 50 °C; 15 min; frequency, 40 kHz) | Acidic treatment (pH 3.0, using citric acid) | Pectin | 9.31% from epicarp, 6.57% from mesocarp, 8.22% from endocarp | [126] |
| Hydrothermal treatment | Acidic treatment (pH 2.0–4.0, using citric acid) | Cellulose | 14.14% | [9] |
| Hemicellulose | 10.41% | |||
| Lignin | 27.75% | |||
| Pectin | 19.26% | |||
| Microwave-assisted | Acidic deep eutectic solvent (ChCl/citric acid) | Xylooligosaccharides | 68.22 mg/g | [109] |
| Enzymatic hydrolysis | Alkaline treatment | Reducing sugars | 98.75% | [68] |
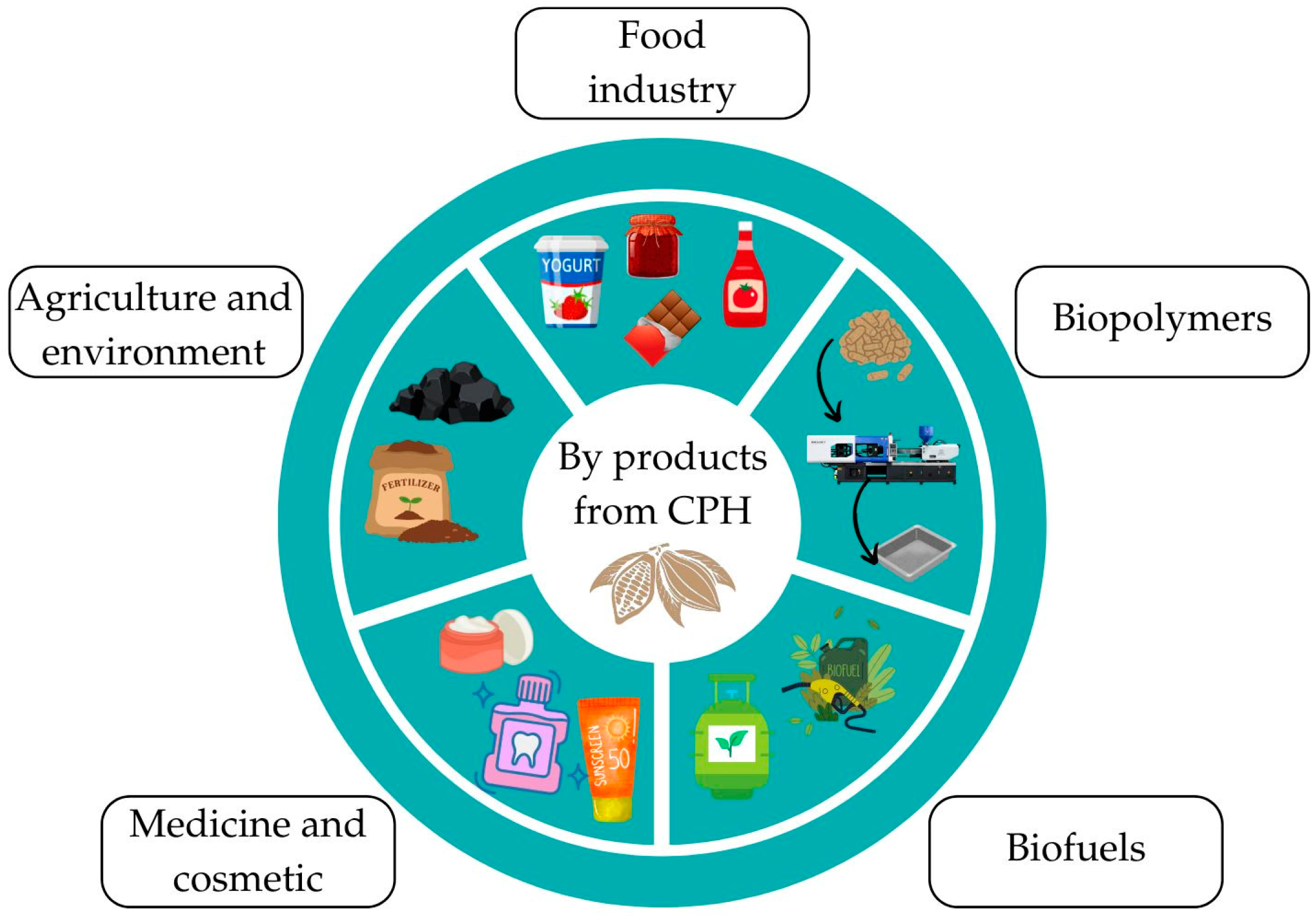
5. Application of By-Products from the Cocoa Pod Husk
5.1. Food Industry
5.2. Biopolymers
5.3. Biofuels
5.4. Medicine and Cosmetic Industry
5.5. Agriculture and Environment Sector
6. Concluding Remarks and Future Trends
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brahma, R.; Ray, S. Optimization of Extraction Conditions for Cellulose from Jackfruit Peel Using RSM, Its Characterization and Comparative Studies to Commercial Cellulose. Meas. Food 2024, 13, 100130. [Google Scholar] [CrossRef]
- Gutiérrez-Macías, P.; Mirón-Mérida, V.A.; Rodríguez-Nava, C.O.; Barragán-Huerta, B.E. Chapter 13-Cocoa: Beyond Chocolate, a Promising Material for Potential Value-Added Products. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 267–288. ISBN 978-0-12-824044-1. [Google Scholar]
- Muharja, M.; Darmayanti, R.F.; Fachri, B.A.; Palupi, B.; Rahmawati, I.; Rizkiana, M.F.; Amini, H.W.; Putri, D.K.Y.; Setiawan, F.A.; Asrofi, M.; et al. Biobutanol Production from Cocoa Pod Husk through a Sequential Green Method: Depectination, Delignification, Enzymatic Hydrolysis, and Extractive Fermentation. Bioresour. Technol. Rep. 2023, 21, 101298. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) Pod Husk: Renewable Source of Bioactive Compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Kley Valladares-Diestra, K.; Porto De Souza Vandenberghe, L.; Ricardo Soccol, C. A Biorefinery Approach for Pectin Extraction and Second-Generation Bioethanol Production from Cocoa Pod Husk. Bioresour. Technol. 2022, 346, 126635. [Google Scholar] [CrossRef]
- Santiago-Gómez, I.; Carrera-Lanestosa, A.; González-Alejo, F.A.; Guerra-Que, Z.; García-Alamilla, R.; Rivera-Armenta, J.L.; García-Alamilla, P. Pectin Extraction Process from Cocoa Pod Husk (Theobroma cacao L.) and Characterization by Fourier Transform Infrared Spectroscopy. ChemEngineering 2025, 9, 25. [Google Scholar] [CrossRef]
- Jarrín-Chacón, J.P.; Núñez-Pérez, J.; Espín-Valladares, R.d.C.; Manosalvas-Quiroz, L.A.; Rodríguez-Cabrera, H.M.; Pais-Chanfrau, J.M. Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study. Foods 2023, 12, 590. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; Porto De Souza Vandenberghe, L.; Zevallos Torres, L.A.; Zandoná Filho, A.; Lorenci Woiciechowski, A.; Ricardo Soccol, C. Citric Acid Assisted Hydrothermal Pretreatment for the Extraction of Pectin and Xylooligosaccharides Production from Cocoa Pod Husks. Bioresour. Technol. 2022, 343, 126074. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Ramola, S.; Thakur, M.; Chemat, F.; Cravotto, G. Bioactive Compounds from Cocoa Husk: Extraction, Analysis and Applications in Food Production Chain. Foods 2022, 11, 798. [Google Scholar] [CrossRef]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, Y.; White, J.C.; Dhankher, O.P. Mitigating Toxic Metals Contamination in Foods: Bridging Knowledge Gaps for Addressing Food Safety. Trends Food Sci. Technol. 2024, 153, 104725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, Z. Recent Advances in Minimizing Cadmium Accumulation in Wheat. Toxics 2022, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Porto De Souza Vandenberghe, L.; Kley Valladares-Diestra, K.; Amaro Bittencourt, G.; Fátima Murawski De Mello, A.; Sarmiento Vásquez, Z.; Zwiercheczewski De Oliveira, P.; Vinícius De Melo Pereira, G.; Ricardo Soccol, C. Added-Value Biomolecules’ Production from Cocoa Pod Husks: A Review. Bioresour. Technol. 2022, 344, 126252. [Google Scholar] [CrossRef]
- Baidoo, M.F.; Asiedu, N.Y.; Darkwah, L.; Arhin-Dodoo, D.; Zhao, J.; Jerome, F.; Amaniampong, P.N. Conventional and Unconventional Transformation of Cocoa Pod Husks into Value-Added Products. In Biomass Biorefineries Bioeconomy; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Meza-Sepúlveda, D.C.; Hernandez-Urrea, C.; Valencia-Sanchez, H. Caracterización Termoquímica de La Celulosa Extraída de La Cáscara de La Mazorca de Cacao de Theobroma cacao L. Ind. Crops Prod. 2025, 226, 120579. [Google Scholar] [CrossRef]
- Hasan, M.H.; Hossain, S.; Rahman, M.L.; Rahman, G.M.S.; Khan, M.A.; Al Mamun, M.A. Effect of Hydrolysis Agitation and Suspension Drying Temperature on the Synthesis of Crystalline Cellulose from Jute Fiber. Carbohydr. Polym. Technol. Appl. 2025, 10, 100769. [Google Scholar] [CrossRef]
- Jain, B.; Jain, R.; Jha, R.R.; Ghosh, A.; Basu, D.; Abourehab, M.A.S.; Bajaj, A.; Chauhan, V.; Kaur, S.; Sharma, S. Cellulose Paper Sorptive Extraction (CPSE): A Simple and Affordable Microextraction Method for Analysis of Basic Drugs in Blood as a Proof of Concept. J. Chromatogr. B 2023, 1214, 123551. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F. Production and Modification of Nanofibrillated Cellulose Composites and Potential Applications. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–141. ISBN 978-0-08-102177-4. [Google Scholar]
- Zhu, J.; Shao, C.; Hao, S.; Zhang, J.; Ren, W.; Wang, B.; Xiao, L.; Wang, C.; Shao, L. Recent Progress on the Dissolution of Cellulose in Deep Eutectic Solvents. Ind. Crops Prod. 2025, 228, 120844. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Hozman-Manrique, A.S.; Garcia-Brand, A.J.; Hernández-Carrión, M.; Porras, A. Isolation and Characterization of Cellulose Microfibers from Colombian Cocoa Pod Husk via Chemical Treatment with Pressure Effects. Polymers 2023, 15, 664. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alkhalidi, H.M.; Alharbi, W.S.; Md, S.; Sindi, A.M.; Ali, S.A.; Bakhaidar, R.B.; Almehmady, A.M.; Alfayez, E.; Kurakula, M. Recent Trends in Assessment of Cellulose Derivatives in Designing Novel and Nanoparticulate-Based Drug Delivery Systems for Improvement of Oral Health. Polymers 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Evangelista-Osorio, A.; Muchaypiña-Flores, K.G.; Marzano-Barreda, L.A.; Paredes-Concepción, P.; Palacin-Baldeón, H.; Dos Santos, M.S.N.; Tres, M.V.; Zabot, G.L.; Olivera-Montenegro, L. Polymeric Materials Obtained by Extrusion and Injection Molding from Lignocellulosic Agroindustrial Biomass. Polymers 2023, 15, 4046. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef] [PubMed]
- Roldi-Oliveira, M.; Diniz, L.M.; Elias, A.L.; Luz, S.M. Hemicellulose Films from Curaua Fibers (Ananas Erectifolius): Extraction and Thermal and Mechanical Characterization. Polymers 2022, 14, 2999. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The Production of Furfural Directly from Hemicellulose in Lignocellulosic Biomass: A Review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Shaheen, H.; Wu, A.-M. Cell Wall Hemicellulose for Sustainable Industrial Utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, L.; Zhou, Z.; Chen, S.; Chen, L.; Li, Y.; Wu, A.; Li, H. Understanding the Dynamic Evolution of Hemicellulose during Pinus taeda L. Growth. Int. J. Biol. Macromol. 2024, 273, 132914. [Google Scholar] [CrossRef]
- Nechita, P.; Mirela, R.; Ciolacu, F. Xylan Hemicellulose: A Renewable Material with Potential Properties for Food Packaging Applications. Sustainability 2021, 13, 13504. [Google Scholar] [CrossRef]
- Bajpai, P. Chapter 2-Xylan Occurrence and Structure. In Microbial Xylanolytic Enzymes; Bajpai, P., Ed.; Progress in Biochemistry and Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 13–28. ISBN 978-0-323-99636-5. [Google Scholar]
- Scapini, T.; Dos Santos, M.S.N.; Bonatto, C.; Wancura, J.H.C.; Mulinari, J.; Camargo, A.F.; Klanovicz, N.; Zabot, G.L.; Tres, M.V.; Fongaro, G.; et al. Hydrothermal Pretreatment of Lignocellulosic Biomass for Hemicellulose Recovery. Bioresour. Technol. 2021, 342, 126033. [Google Scholar] [CrossRef]
- Jacob, S.; Dilshani, A.; Rishivanthi, S.; Khaitan, P.; Vamsidhar, A.; Rajeswari, G.; Kumar, V.; Rajak, R.C.; Din, M.F.M.; Zambare, V. Lignocellulose-Derived Arabinose for Energy and Chemicals Synthesis through Microbial Cell Factories: A Review. Processes 2023, 11, 1516. [Google Scholar] [CrossRef]
- Fehér, C. Novel Approaches for Biotechnological Production and Application of L-Arabinose. J. Carbohydr. Chem. 2018, 37, 251–284. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Drăgoi, E.N.; Danu, M.; Ailiesei, G.-L.; Balan, C.D.; Chicet, D.-L.; Nechita, M.T. Optimization of Alkaline Extraction of Xylan-Based Hemicelluloses from Wheat Straws: Effects of Microwave, Ultrasound, and Freeze–Thaw Cycles. Polymers 2023, 15, 1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Cheng, H.; Zhou, H. Hemicellulose Degradation: An Overlooked Issue in Acidic Deep Eutectic Solvents Pretreatment of Lignocellulosic Biomass. Ind. Crops Prod. 2022, 187, 115335. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; De Souza Vandenberghe, L.P.; Vieira, S.; Goyzueta-Mamani, L.D.; De Mattos, P.B.G.; Manzoki, M.C.; Soccol, V.T.; Soccol, C.R. The Potential of Xylooligosaccharides as Prebiotics and Their Sustainable Production from Agro-Industrial by-Products. Foods 2023, 12, 2681. [Google Scholar] [CrossRef]
- Manicardi, T.; Baioni E Silva, G.; Longati, A.A.; Paiva, T.D.; Souza, J.P.M.; Pádua, T.F.; Furlan, F.F.; Giordano, R.L.C.; Giordano, R.C.; Milessi, T.S. Xylooligosaccharides: A Bibliometric Analysis and Current Advances of This Bioactive Food Chemical as a Potential Product in Biorefineries’ Portfolios. Foods 2023, 12, 3007. [Google Scholar] [CrossRef]
- Obrzut, N.; Hickmott, R.; Shure, L.; Gray, K.A. The Effects of Lignin Source and Extraction on the Composition and Properties of Biorefined Depolymerization Products. RSC Sustain. 2023, 1, 2328–2340. [Google Scholar] [CrossRef]
- Huang, J.; Fu, S.; Gan, L. (Eds.) Chapter 2-Structure and Characteristics of Lignin. In Lignin Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–50. ISBN 978-0-12-813941-7. [Google Scholar]
- Erfani Jazi, M.; Narayanan, G.; Aghabozorgi, F.; Farajidizaji, B.; Aghaei, A.; Kamyabi, M.A.; Navarathna, C.M.; Mlsna, T.E. Structure, Chemistry and Physicochemistry of Lignin for Material Functionalization. SN Appl. Sci. 2019, 1, 1094. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Vijayalakshmi, S.; Barathikannan, K.; Sultan, G.; Liu, S.; Oh, D.-H. A Comprehensive Mini-Review on Lignin-Based Nanomaterials for Food Applications: Systemic Advancement and Future Trends. Molecules 2023, 28, 6470. [Google Scholar] [CrossRef]
- Suota, M.J.; da Silva, T.A.; Zawadzki, S.F.; Sassaki, G.L.; Hansel, F.A.; Paleologou, M.; Ramos, L.P. Chemical and Structural Characterization of Hardwood and Softwood LignoForceTM Lignins. Ind. Crops Prod. 2021, 173, 114138. [Google Scholar] [CrossRef]
- Flores, E.M.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-Assisted Biomass Valorization to Industrial Interesting Products: State-of-the-Art, Perspectives and Challenges. Ultrason. Sonochem. 2021, 72, 105455. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A Review on Trends in Lignin Extraction and Valorization of Lignocellulosic Biomass for Energy Applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Liang, W.; Liao, J.; Qi, J.-R.; Jiang, W.; Yang, X. Physicochemical Characteristics and Functional Properties of High Methoxyl Pectin with Different Degree of Esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef] [PubMed]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The Primary, Secondary, and Structures of Higher Levels of Pectin Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Forouhar, A.; Hamdami, N.; Djelveh, G.; Gardarin, C.; Pierre, G.; Ursu, A.V.; Michaud, P. The Effect of Ultrasound Pretreatment on Pectin Extraction from Watermelon Rind Using Microwave-Assisted Extraction. Appl. Sci. 2023, 13, 5558. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, S. The Effect of Degree of Esterification of Pectin on the Interaction between Pectin and Wheat Gluten Protein. Food Hydrocoll. 2023, 136, 108272. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Ramírez-Herrera, C.A.; Araújo, R.G.; Flores-Contreras, E.A.; Iqbal, H.M.N.; Parra-Saldívar, R.; Melchor-Martínez, E.M. Enhancing Pectin Extraction from Orange Peel through Citric Acid-Assisted Optimization Based on a Dual Response. Int. J. Biol. Macromol. 2024, 263, 130230. [Google Scholar] [CrossRef]
- Valdivia-Rivera, S.; Herrera-Pool, I.E.; Ayora-Talavera, T.; Lizardi-Jiménez, M.A.; García-Cruz, U.; Cuevas-Bernardino, J.C.; Cervantes-Uc, J.M.; Pacheco, N. Kinetic, Thermodynamic, Physicochemical, and Economical Characterization of Pectin from Mangifera indica L. cv. Haden Residues. Foods 2021, 10, 2093. [Google Scholar] [CrossRef]
- Jong, S.H.; Abdullah, N.; Muhammad, N. Effect of Acid Type and Concentration on the Yield, Purity, and Esterification Degree of Pectin Extracted from Durian Rinds. Results Eng. 2023, 17, 100974. [Google Scholar] [CrossRef]
- Marsiglia, D.E.; Ojeda, K.A.; Ramírez, M.C.; Sánchez, E. Pectin Extraction from Cocoa Pod Husk (Theobroma cacao L.) by hydrolysis with citric and acetic acid. Int. J. ChemTech Res. 2016, 9, 97–507. [Google Scholar]
- Vriesmann, L.C.; Teófilo, R.F.; Lúcia de Oliveira Petkowicz, C. Extracción y Caracterización de Pectina de Cáscaras de Mazorca de Cacao (Theobroma cacao L.) Con Ácido Cítrico. LWT 2012, 49, 108–116. [Google Scholar] [CrossRef]
- Girón-Hernández, J.; Tombe, A.; Chemban Koyilot, M.; Salas-Calderón, K.T.; Charlton, A.; Wills, C.; Gentile, P. From Cocoa Waste to Sustainable Bioink: Valorising Pectin for Circular Economy-Driven Tissue Engineering. Eur. Polym. J. 2024, 210, 112967. [Google Scholar] [CrossRef]
- Adi-Dako, O.; Ofori-Kwakye, K.; Frimpong Manso, S.; Boakye-Gyasi, M.E.; Sasu, C.; Pobee, M. Physicochemical and Antimicrobial Properties of Cocoa Pod Husk Pectin Intended as a Versatile Pharmaceutical Excipient and Nutraceutical. J. Pharm. 2016, 2016, 7608693. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, A.; Wan Daud, W.M.A.; Patah, M.F.A.; Zuki, F.M.; Ai, S.P.; Azman, D.Q.; Chen, W.-H. Breaking Barriers for a Green Future: A Comprehensive Study on Pre-Treatment Techniques for Empty Fruit Bunches in the Bio-Based Economy. Process Saf. Environ. Prot. 2024, 182, 535–558. [Google Scholar] [CrossRef]
- Basak, B.; Kumar, R.; Bharadwaj, A.V.S.L.S.; Kim, T.H.; Kim, J.R.; Jang, M.; Oh, S.-E.; Roh, H.-S.; Jeon, B.-H. Advances in Physicochemical Pretreatment Strategies for Lignocellulose Biomass and Their Effectiveness in Bioconversion for Biofuel Production. Bioresour. Technol. 2023, 369, 128413. [Google Scholar] [CrossRef]
- Ong, V.Z.; Wu, T.Y.; Chu, K.K.L.; Sun, W.Y.; Shak, K.P.Y. A Combined Pretreatment with Ultrasound-Assisted Alkaline Solution and Aqueous Deep Eutectic Solvent for Enhancing Delignification and Enzymatic Hydrolysis from Oil Palm Fronds. Ind. Crops Prod. 2021, 160, 112974. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Ivančić Šantek, M. The Production of Bioethanol from Lignocellulosic Biomass: Pretreatment Methods, Fermentation, and Downstream Processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Díaz-González, A.; Luna, M.Y.P.; Morales, E.R.; Saldaña-Trinidad, S.; Blanco, L.R.; de la Cruz-Arreola, S.; Pérez-Sariñana, B.Y.; Robles-Ocampo, J.B. Assessment of the Pretreatments and Bioconversion of Lignocellulosic Biomass Recovered from the Husk of the Cocoa Pod. Energies 2022, 15, 3544. [Google Scholar] [CrossRef]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance–Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Sarmiento-Vásquez, Z.; Vandenberghe, L.; Rodrigues, C.; Tanobe, V.O.A.; Marín, O.; de Melo Pereira, G.V.; Ghislain Rogez, H.L.; Góes-Neto, A.; Soccol, C.R. Cocoa Pod Husk Valorization: Alkaline-Enzymatic Pre-Treatment for Propionic Acid Production. Cellulose 2021, 28, 4009–4024. [Google Scholar] [CrossRef]
- Hernández-Mendoza, A.G.; Saldaña-Trinidad, S.; Martínez-Hernández, S.; Pérez-Sariñana, B.Y.; Láinez, M. Optimization of Alkaline Pretreatment and Enzymatic Hydrolysis of Cocoa Pod Husk (Theobroma cacao L.) for Ethanol Production. Biomass Bioenergy 2021, 154, 106268. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Osueke, C.O.; Olayanju, T.M.A.; Lawal, A.I. Co-Digestion of Theobroma cacao (Cocoa) Pod Husk and Poultry Manure for Energy Generation: Effects of Pretreatment Methods. Bioresour. Technol. 2019, 283, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Igbinadolor, R.O.; Onilude, A.A. Bioprocess Systems Applied for the Production of Bio-Ethanol from Lignocellulosic Biomass of Cocoa Pod Husk (Theobroma cacao L.) and Other Agricultural Residues: A Review. Afr. J. Biotechnol. 2013, 12, 5375–5388. [Google Scholar] [CrossRef]
- de Oliveira, P.Z.; de Souza Vandenberghe, L.P.; Rodrigues, C.; de Melo Pereira, G.V.; Soccol, C.R. Exploring Cocoa Pod Husks as a Potential Substrate for Citric Acid Production by Solid-State Fermentation Using Aspergillus Niger Mutant Strain. Process Biochem. 2022, 113, 107–112. [Google Scholar] [CrossRef]
- Velásquez-Jiménez, D.; Luzardo-Ocampo, I.; Gaytán-Martínez, M.; Campos-Vega, R. Design and Characterization of a Solid-State Fermented Cacao Pods and Husk-Based Functional Ingredient to Potentially Modulate Circadian Rhythm-Associated Proteins. Food Biosci. 2023, 56, 103199. [Google Scholar] [CrossRef]
- Acosta, N.; De Vrieze, J.; Sandoval, V.; Sinche, D.; Wierinck, I.; Rabaey, K. Cocoa Residues as Viable Biomass for Renewable Energy Production through Anaerobic Digestion. Bioresour. Technol. 2018, 265, 568–572. [Google Scholar] [CrossRef]
- Alvarez-Barreto, J.F.; Larrea, F.; Pinos, M.C.; Benalcázar, J.; Oña, D.; Andino, C.; Viteri, D.A.; Leon, M.; Almeida-Streitwieser, D. Chemical Pretreatments on Residual Cocoa Pod Shell Biomass for Bioethanol Production. Bionatura 2021, 6, 1490–1500. [Google Scholar] [CrossRef]
- Perwitasari, U.; Agustina, N.T.; Pangestu, R.; Amanah, S.; Saputra, H.; Andriani, A.; Fahrurrozi; Juanssilfero, A.B.; Thontowi, A.; Widyaningsih, T.D.; et al. Cacao Pod Husk for Citric Acid Production under Solid State Fermentation Using Response Surface Method. Biomass Convers. Biorefinery 2023, 13, 7165–7173. [Google Scholar] [CrossRef]
- Santana, N.B.; Teixeira Dias, J.C.; Rezende, R.P.; Franco, M.; Silva Oliveira, L.K.; Souza, L.O. Production of Xylitol and Bio-Detoxification of Cocoa Pod Husk Hemicellulose Hydrolysate by Candida Boidinii XM02G. PLoS ONE 2018, 13, e0195206. [Google Scholar] [CrossRef]
- Santos, R.X.; Oliveira, D.A.; Sodré, G.A.; Gosmann, G.; Brendel, M.; Pungartnik, C. Antimicrobial Activity of Fermented Theobroma Cacao Pod Husk Extract. Genet. Mol. Res. 2014, 13, 7725–7735. [Google Scholar] [CrossRef] [PubMed]
- Antwi, E.; Engler, N.; Nelles, M.; Schüch, A. Anaerobic Digestion and the Effect of Hydrothermal Pretreatment on the Biogas Yield of Cocoa Pods Residues. Waste Manag. 2019, 88, 131–140. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Ferraz Dutra, J.; Passos, M.F.; García, G.J.Y.; Gomes, R.F.; Magalhães, T.A.; dos Santos Freitas, A.; Laguna, J.G.; da Costa, F.M.R.; da Silva, T.F.; Rodrigues, L.S.; et al. Anaerobic Digestion Using Cocoa Residues as Substrate: Systematic Review and Meta-Analysis. Energy Sustain. Dev. 2023, 72, 265–277. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of Valuable Biocompounds Assisted by High Voltage Electrical Discharges: A Review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Sanito, R.C.; You, S.-J.; Wang, Y.-F. Degradation of Contaminants in Plasma Technology: An Overview. J. Hazard. Mater. 2022, 424, 127390. [Google Scholar] [CrossRef]
- Marček, T.; Kovač, T.; Jukić, K.; Lončarić, A.; Ižaković, M. Application of High Voltage Electrical Discharge Treatment to Improve Wheat Germination and Early Growth under Drought and Salinity Conditions. Plants 2021, 10, 2137. [Google Scholar] [CrossRef]
- Barišić, V.; Kerovec, D.; Flanjak, I.; Jozinović, A.; Babić, J.; Lončarić, Z.; Šubarić, D.; Miličević, B.; Ačkar, Đ. Effect of High-Voltage Electrical Discharge Treatment on Multi-Element Content in Cocoa Shell and Chocolates with Cocoa Shell. LWT 2022, 155, 112944. [Google Scholar] [CrossRef]
- Pinzon-Nuñez, D.A.; Adarme-Duran, C.A.; Vargas-Fiallo, L.; Rodriguez-Lopez, N.; Rios-Reyes, C.A. Biochar as a Waste Management Strategy for Cadmium Contaminated Cocoa Pod Husk Residues. Int. J. Recycl. Org. Waste Agric. 2022, 11, 101–115. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Li, Q.; Zhang, Y.; Zhou, H. Sustainable Carbon Materials from the Pyrolysis of Lignocellulosic Biomass. Mater. Today Sustain. 2022, 19, 100209. [Google Scholar] [CrossRef]
- Pecha, M.B.; Garcia-Perez, M. Pyrolysis of Lignocellulosic Biomass: Oil, Char, and Gas. In Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 581–619. ISBN 978-0-12-815497-7. [Google Scholar]
- Luperón, L.M.; Rodríguez, M.H.; Hernández, J.F.; Otero-Calvis, A. Obtaining Bioproducts by Slow Pyrolysis of Coffee and Cocoa Husks as Suitable Candidates for Being Used as Soil Amendment and Source of Energy. Rev. Colomb. Quím. 2020, 49, 23–29. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Hsu, C.-H.; Lin, Y.-Q.; Tsai, C.-H.; Chen, W.-S.; Chang, Y.-T. Enhancing the Pore Properties and Adsorption Performance of Cocoa Pod Husk (CPH)-Derived Biochars via Post-Acid Treatment. Processes 2020, 8, 144. [Google Scholar] [CrossRef]
- Adjin-Tetteh, M.; Asiedu, N.; Dodoo-Arhin, D.; Karam, A.; Amaniampong, P.N. Thermochemical Conversion and Characterization of Cocoa Pod Husks a Potential Agricultural Waste from Ghana. Ind. Crops Prod. 2018, 119, 304–312. [Google Scholar] [CrossRef]
- Mansur, D.; Tago, T.; Masuda, T.; Abimanyu, H. Conversion of Cacao Pod Husks by Pyrolysis and Catalytic Reaction to Produce Useful Chemicals. Biomass Bioenergy 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Kumar, P.; Kermanshahi-pour, A.; Brar, S.K.; Brooks, M.S. Conversion of Lignocellulosic Biomass to Reducing Sugars in High Pressure and Supercritical Fluids: Greener Alternative for Biorefining of Renewables. Adv. Sustain. Syst. 2021, 5, 2000275. [Google Scholar] [CrossRef]
- Escobar, E.L.N.; Da Silva, T.A.; Pirich, C.L.; Corazza, M.L.; Pereira Ramos, L. Supercritical Fluids: A Promising Technique for Biomass Pretreatment and Fractionation. Front. Bioeng. Biotechnol. 2020, 8, 252. [Google Scholar] [CrossRef]
- Lee, S.E.; Lim, J.S.; Park, Y.-K.; Shong, B.; Lee, H. Utilizing Cocoa Bean Husk Residues from Supercritical Extraction for Biofuel Production through Hydrothermal Liquefaction. J. Supercrit. Fluids 2025, 215, 106416. [Google Scholar] [CrossRef]
- Ferreira, C.; Moreira, M.M.; Delerue-Matos, C.; Sarraguça, M. Subcritical Water Extraction to Valorize Grape Biomass—A Step Closer to Circular Economy. Molecules 2023, 28, 7538. [Google Scholar] [CrossRef]
- Park, J.-S.; Han, J.-M.; Park, S.-W.; Kim, J.-W.; Choi, M.-S.; Lee, S.-M.; Haq, M.; Zhang, W.; Chun, B.-S. Subcritical Water Extraction of Undaria Pinnatifida: Comparative Study of the Chemical Properties and Biological Activities across Different Parts. Mar. Drugs 2024, 22, 344. [Google Scholar] [CrossRef]
- Žagar, T.; Frlan, R.; Kočevar Glavač, N. Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties. Antibiotics 2024, 13, 334. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural Characterisation of Pectin Obtained from Cacao Pod Husk. Comparison of Conventional and Subcritical Water Extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Lu, X.; Zheng, X.; Yan, D.; Xin, J.; El-Tantawy El-Sayed, I.; Kang, Y.; Yang, J. Highly Efficient Enzymolysis and Fermentation of Corn Stalk into L-Lactic Acid by Enzyme-Bacteria Friendly Ionic Liquid Pretreatment. Green Chem. Eng. 2022, 3, 321–327. [Google Scholar] [CrossRef]
- Amini, E.; Valls, C.; Roncero, M.B. Ionic Liquid-Assisted Bioconversion of Lignocellulosic Biomass for the Development of Value-Added Products. J. Clean. Prod. 2021, 326, 129275. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef]
- Idi, A.; Salleh, M.M.; Ibrahim, Z.; Mohamad, S.E. Pretreatment of Cocoa Waste for Bioethanol Production Using Ionic Liquid. J. Teknol. Sci. Eng. 2012, 59, 49–56. [Google Scholar] [CrossRef][Green Version]
- Sathitsuksanoh, N.; George, A.; Zhang, Y.H.P. New Lignocellulose Pretreatments Using Cellulose Solvents: A Review. J. Chem. Technol. Biotechnol. 2013, 88, 169–180. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Mohedano, A.F.; de la Rubia, A. Pretreatment of Lignocellulosic Biomass with 1-Ethyl-3-Methylimidazolium Acetate for Its Eventual Valorization by Anaerobic Digestion. Resources 2021, 10, 118. [Google Scholar] [CrossRef]
- Quesada-Salas, M.C.; Vuillemin, M.E.; Dillies, J.; Dauwe, R.; Firdaous, L.; Bigan, M.; Lambertyn, V.; Cailleu, D.; Jamali, A.; Froidevaux, R.; et al. 1-Ethyl-3-Methyl Imidazolium Acetate, Hemicellulolytic Enzymes and Laccase-Mediator System: Toward an Integrated Co-Valorization of Polysaccharides and Lignin from Miscanthus. Ind. Crops Prod. 2023, 197, 116627. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Ganorkar, S.B.; Hadole, P.M.; Patil, M.R.; Pardeshi, C.V.; Bobade, P.S.; Shirkhedkar, A.A.; Heyden, Y.V. Deep Eutectic Solvents in Analysis, Delivery and Chemistry of Pharmaceuticals. Int. J. Pharm. 2025, 672, 125278. [Google Scholar] [CrossRef]
- Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Z.; Zhang, X.; Li, K.; Jin, Y.; Wu, W. DES: Their Effect on Lignin and Recycling Performance. RSC Adv. 2023, 13, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Sharma, V.; Tsai, M.-L.; Sharma, D.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Dong, C.-D. Synergistic Microwave and Acidic Deep Eutectic Solvent-Based Pretreatment of Theobroma Cacao Pod Husk Biomass for Xylooligosaccharides Production. Bioresour. Technol. 2024, 400, 130702. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, V.; Tsai, M.-L.; Nargotra, P.; Yadav, A.; Sun, P.-P.; Chen, C.-W.; Dong, C.-D. Recent Advances in Ultrasound-Assisted Technologies for Improved Bioproduct Recovery in Lignocellulosic and Microalgal Biorefineries. J. Ind. Eng. Chem. 2025, in press. [Google Scholar] [CrossRef]
- Ramirez Cabrera, P.A.; Lozano Pérez, A.S.; Guerrero Fajardo, C.A. Innovative Design of a Continuous Ultrasound Bath for Effective Lignocellulosic Biomass Pretreatment Based on a Theorical Method. Inventions 2024, 9, 105. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the Recent Advances of Microwave Pretreatment Technologies for the Conversion of Lignocellulosic Biomass into Sustainable Biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A Review on the Environment-Friendly Emerging Techniques for Pretreatment of Lignocellulosic Biomass: Mechanistic Insight and Advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Puligundla, P.; Oh, S.-E.; Mok, C. Microwave-Assisted Pretreatment Technologies for the Conversion of Lignocellulosic Biomass to Sugars and Ethanol: A Review. Carbon Lett. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Susanti, R.F.; Wiratmadja, R.G.R.; Kristianto, H.; Arie, A.A.; Nugroho, A. Synthesis of High Surface Area Activated Carbon Derived from Cocoa Pods Husk by Hydrothermal Carbonization and Chemical Activation Using Zinc Chloride as Activating Agent. Mater. Today Proc. 2022, 63, S55–S60. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Vasco-Correa, J.; Paz Astudillo, I.C. Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L. Molecules 2021, 26, 1473. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Himanshu; Hemansi; Kaur, A.; Mathur, A. Strategies to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass for Biorefinery Applications: A Review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Wijffels, R.H.; Marzocchella, A.; Russo, M.E. Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass. Catalysts 2021, 11, 680. [Google Scholar] [CrossRef]
- Sarah, M.; Hasibuan, I.M.; Misran, E.; Maulina, S. Optimization of Microwave-Assisted Pectin Extraction from Cocoa Pod Husk. Molecules 2022, 27, 6544. [Google Scholar] [CrossRef]
- Mao, Y.; Gerrow, A.; Ray, E.; Perez, N.D.; Edler, K.; Wolf, B.; Binner, E. Lignin Recovery from Cocoa Bean Shell Using Microwave-Assisted Extraction and Deep Eutectic Solvents. Bioresour. Technol. 2023, 372, 128680. [Google Scholar] [CrossRef]
- Anoraga, S.B.; Shamsudin, R.; Hamzah, M.H.; Sharif, S.; Saputro, A.D. Cocoa by-Products: A Comprehensive Review on Potential Uses, Waste Management, and Emerging Green Technologies for Cocoa Pod Husk Utilization. Heliyon 2024, 10, e35537. [Google Scholar] [CrossRef]
- Anoraga, S.B.; Shamsudin, R.; Hamzah, M.H.; Sharif, S.; Saputro, A.D.; Basri, M.S.M. Optimization of Subcritical Water Extraction for Pectin Extraction from Cocoa Pod Husks Using the Response Surface Methodology. Food Chem. 2024, 459, 140355. [Google Scholar] [CrossRef]
- Muharja, M.; Darmayanti, R.F.; Palupi, B.; Rahmawati, I.; Fachri, B.A.; Setiawan, F.A.; Amini, H.W.; Rizkiana, M.F.; Rahmawati, A.; Susanti, A.; et al. Optimization of Microwave-Assisted Alkali Pretreatment for Enhancement of Delignification Process of Cocoa Pod Husk. Bull. Chem. React. Eng. Catal. 2021, 16, 31–43. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from Fruits: Relationships between Extraction Methods, Structural Characteristics, and Functional Properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Yauri, S.; Fissore, E.N.; Chavez, S.G.; Rojas, A.M. Advanced Valorization of Cocoa (Theobroma cacao L.) Pod Husks through 40 kHz-Ultrasonic Bath Assisted Extraction of Pectins. Sustain. Chem. Pharm. 2024, 42, 101869. [Google Scholar] [CrossRef]
- Yi, L.; Cheng, L.; Yang, Q.; Shi, K.; Han, F.; Luo, W.; Duan, S. Source, Extraction, Properties, and Multifunctional Applications of Pectin: A Short Review. Polymers 2024, 16, 2883. [Google Scholar] [CrossRef] [PubMed]
- Barišić, V.; Stokanović, M.C.; Flanjak, I.; Doko, K.; Jozinović, A.; Babić, J.; Šubarić, D.; Miličević, B.; Cindrić, I.; Ačkar, Đ. Cocoa Shell as a Step Forward to Functional Chocolates—Bioactive Components in Chocolates with Different Composition. Molecules 2020, 25, 5470. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahab, A.; Chuppava, B.; Siebert, D.-C.; Visscher, C.; Kamphues, J. Digestibility of a Lignocellulose Supplemented Diet and Fecal Quality in Beagle Dogs. Animals 2022, 12, 1965. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Martuscelli, M.; Grande-Tovar, C.D.; Lucas-González, R.; Molina-Hernandez, J.B.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Chaves-López, C. Cacao Pod Husk Flour as an Ingredient for Reformulating Frankfurters: Effects on Quality Properties. Foods 2021, 10, 1243. [Google Scholar] [CrossRef]
- Barrios-Rodríguez, Y.F.; Salas-Calderón, K.T.; Orozco-Blanco, D.A.; Gentile, P.; Girón-Hernández, J. Cocoa Pod Husk: A High-Pectin Source with Applications in the Food and Biomedical Fields. ChemBioEng Rev. 2022, 9, 462–474. [Google Scholar] [CrossRef]
- Adi-Dako, O.; Ofori-Kwakye, K.; Kukuia, K.K.E.; Asiedu-Larbi, J.; Nyarko, A.; Osei-Asare, C. Subchronic Toxicity Studies of Cocoa Pod Husk Pectin. J. Pharm. Pharmacogn. Res. 2018, 6, 271–284. [Google Scholar] [CrossRef]
- Pinkaew, T.; Inthachat, W.; Khemthong, C.; Kemsawasd, V.; On-Nom, N.; Temviriyanukul, P. High Pectin Recovery from Cocoa Husks Using an Autoclave Approach: An Analysis of Its Physicochemical, Structural, and Genotoxicity Properties. Foods 2024, 13, 669. [Google Scholar] [CrossRef]
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Babić, J.; Pajin, B.; Miličević, B.; Guberac, S.; Vrdoljak, A.; Šubarić, D. Cocoa Husk Application in the Enrichment of Extruded Snack Products. J. Food Process. Preserv. 2019, 43, e13866. [Google Scholar] [CrossRef]
- Cruz-Santos, M.M.; Antunes, F.A.F.; Arruda, G.L.; Shibukawa, V.P.; Prado, C.A.; Ortiz-Silos, N.; Castro-Alonso, M.J.; Marcelino, P.R.F.; Santos, J.C. Production and Applications of Pullulan from Lignocellulosic Biomass: Challenges and Perspectives. Bioresour. Technol. 2023, 385, 129460. [Google Scholar] [CrossRef]
- Machineni, L.; Rao Anupoju, G. Review on Valorization of Lignocellulosic Biomass for Green Plastics Production: Sustainable and Cleaner Approaches. Sustain. Energy Technol. Assess. 2022, 53, 102698. [Google Scholar] [CrossRef]
- Holguín Posso, A.M.; Macías Silva, J.C.; Castañeda Niño, J.P.; Mina Hernandez, J.H.; Fajardo Cabrera De Lima, L.D.P. Characterization and Implementation of Cocoa Pod Husk as a Reinforcing Agent to Obtain Thermoplastic Starches and Bio-Based Composite Materials. Polymers 2024, 16, 1608. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, L.; Henríquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from Lignocellulosic Biomass: A Comprehensive Review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Niaz, N.; Waseem, M.; Ashraf, W.; Hussain, M.; Khalid, M.U.; Tahir, A.B.; Raza, A.; Khan, I.M. Xylooligosaccharides: A Comprehensive Review of Production, Purification, Characterization, and Quantification. Food Res. Int. 2025, 201, 115631. [Google Scholar] [CrossRef] [PubMed]
- Ruan, R.; Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Ding, K.; Peng, P.; Addy, M.; Cheng, Y.; et al. Biofuels: Introduction. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–43. ISBN 978-0-12-816856-1. [Google Scholar]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a Sustainable Future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Datta, A.; Hossain, A.; Roy, S. An Overview on Biofuels and Their Advantages and Disadvantages. Asian J. Chem. 2019, 31, 1851–1858. [Google Scholar] [CrossRef]
- Sakhakarmy, M.; Kemp, A.; Biswas, B.; Kafle, S.; Adhikari, S. A Comparative Analysis of Bio-Oil Collected Using an Electrostatic Precipitator from the Pyrolysis of Douglas Fir, Eucalyptus, and Poplar Biomass. Energies 2024, 17, 2800. [Google Scholar] [CrossRef]
- Corvianindya, Y.; Maduratna, E.; Pudji, R.; Elsayed, D. Analysis of antioxidant and antibacterial activity of cocoa podhusk extract (Theobroma cacao L.). Dental Journal 2023, 4, 220–225. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; De Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; De Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Tantapakul, C.; Khat-udomkiri, N.; Sitthichai, P.; Chomsak, A.; Thananusak, N.; Phukhatmuen, P.; Vinardell, M.P.; Sripisut, T. Exploring the Physicochemical Properties of Polysaccharides Extracted from Cocoa Pod Husk Waste and Their Efficacy in Skin Hydration. Ind. Crops Prod. 2024, 222, 119940. [Google Scholar] [CrossRef]
- Liang, T.; Ma, Y.; Jiang, Z.; Remón, J.; Zhou, Y.; Shi, B. New Insights into Greener Skin Healthcare Protection: Lignin Nanoparticles as Additives to Develop Natural-Based Sunscreens with High UV Protection. Carbon Resour. Convers. 2024, 7, 100227. [Google Scholar] [CrossRef]
- Ayilara, M.; Olanrewaju, O.; Babalola, O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
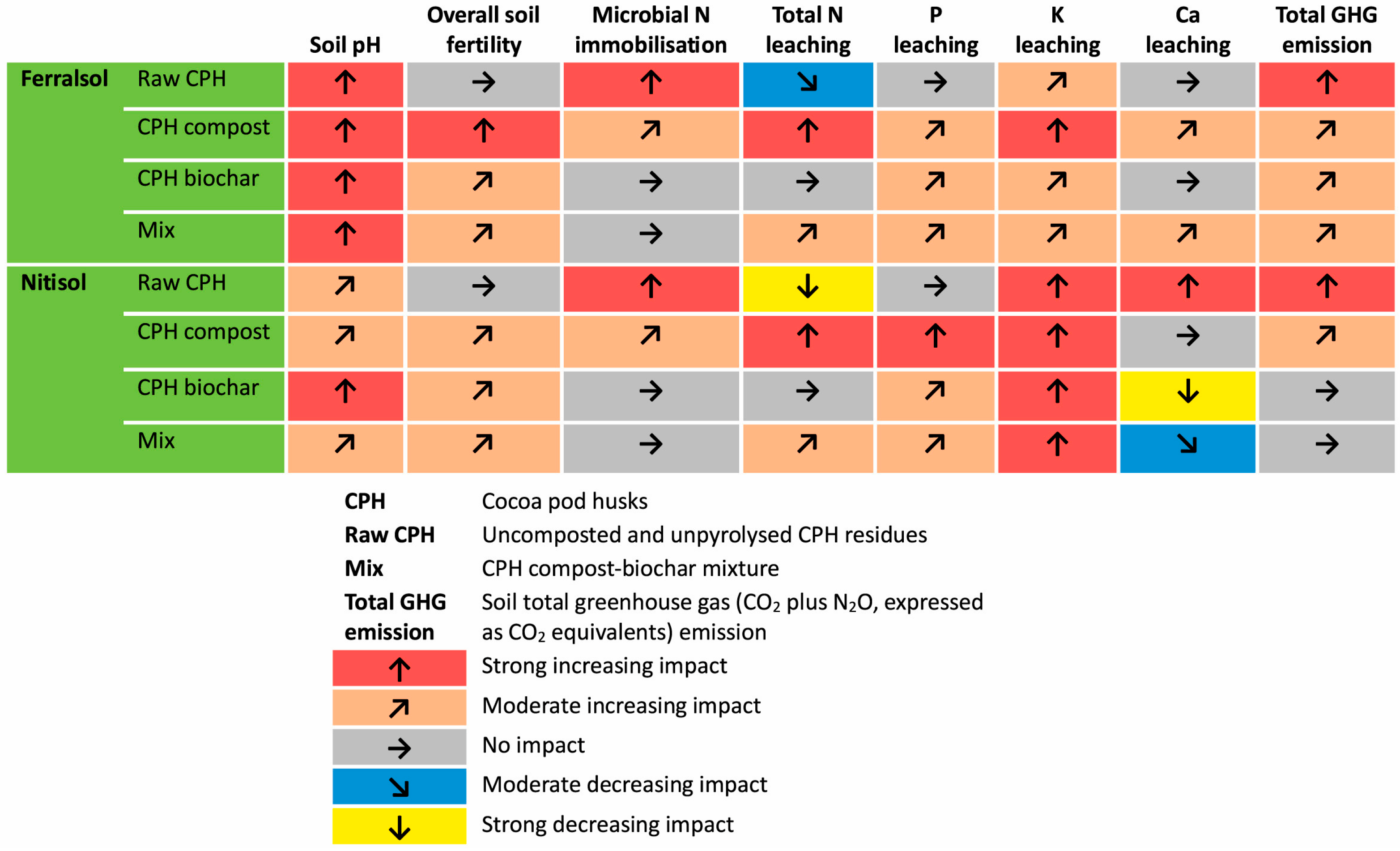
- Mwafulirwa, L.; Sizmur, T.; Daymond, A.; Atuah, L.; Quaye, A.K.; Coole, S.; Robinson, S.; Hammond, J.; Awudzi, G.; Awunyo-Vitor, D.; et al. Cocoa Pod Husk-Derived Organic Soil Amendments Differentially Affect Soil Fertility, Nutrient Leaching, and Greenhouse Gas Emissions in Cocoa Soils. J. Clean. Prod. 2024, 479, 144065. [Google Scholar] [CrossRef]
- Tusar, H.M.; Uddin, M.K.; Mia, S.; Suhi, A.A.; Wahid, S.B.A.; Kasim, S.; Sairi, N.A.; Alam, Z.; Anwar, F. Biochar-Acid Soil Interactions—A Review. Sustainability 2023, 15, 13366. [Google Scholar] [CrossRef]
- Abbey, C.Y.B.; Duwiejuah, A.B.; Quianoo, A.K. Removal of Toxic Metals from Aqueous Phase Using Cacao Pod Husk Biochar in the Era of Green Chemistry. Appl. Water Sci. 2022, 13, 57. [Google Scholar] [CrossRef]
| Product | Fermentation | Reference |
|---|---|---|
| Citric acid | Solid-state fermentation | [70] |
| Phenolic compounds | Solid-state fermentation | [71] |
| Biogas | Anaerobic digestion | [72] |
| Bioethanol | Alcoholic fermentation | [73] |
| Methane | Anaerobic digestion | [72] |
| Propionic acid | Submerged fermentation | [66] |
| Biobutanol | Extractive fermentation | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugarin, A.; Iquise, A.; Motta Dolianitis, B.; Vinícius Tres, M.; Zabot, G.L.; Olivera-Montenegro, L. Integration of Emerging and Conventional Technologies for Obtaining By-Products from Cocoa Pod Husk and Their Application. Processes 2025, 13, 1264. https://doi.org/10.3390/pr13051264
Bugarin A, Iquise A, Motta Dolianitis B, Vinícius Tres M, Zabot GL, Olivera-Montenegro L. Integration of Emerging and Conventional Technologies for Obtaining By-Products from Cocoa Pod Husk and Their Application. Processes. 2025; 13(5):1264. https://doi.org/10.3390/pr13051264
Chicago/Turabian StyleBugarin, Alejandra, Angela Iquise, Bianca Motta Dolianitis, Marcus Vinícius Tres, Giovani Leone Zabot, and Luis Olivera-Montenegro. 2025. "Integration of Emerging and Conventional Technologies for Obtaining By-Products from Cocoa Pod Husk and Their Application" Processes 13, no. 5: 1264. https://doi.org/10.3390/pr13051264
APA StyleBugarin, A., Iquise, A., Motta Dolianitis, B., Vinícius Tres, M., Zabot, G. L., & Olivera-Montenegro, L. (2025). Integration of Emerging and Conventional Technologies for Obtaining By-Products from Cocoa Pod Husk and Their Application. Processes, 13(5), 1264. https://doi.org/10.3390/pr13051264









