Migration and Conversion of Al Element in the Hydrometallurgical Preparation of Al2O3 from Secondary Aluminium Dross
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Impact of Water Washing on SAD
3.2. Acid Leaching Mechanism
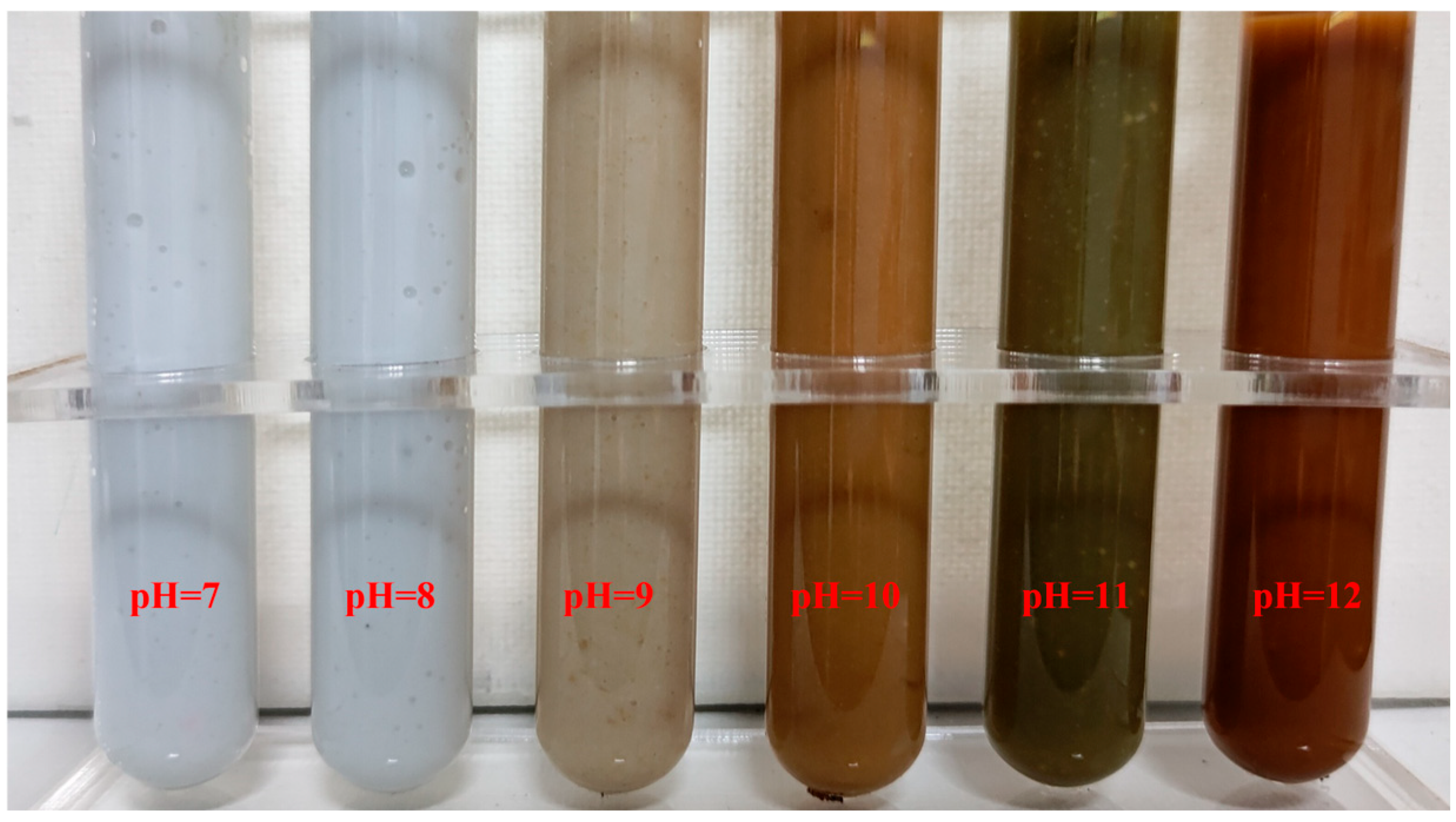
3.3. Hydrolytic Precipitation
3.4. Migration and Transformation of Al Element
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Primary Aluminium Production. Available online: https://international-aluminium.org/statistics/primary-aluminium-production/ (accessed on 12 March 2025).
- Yang, J.; Tian, L.; Meng, L.; Wang, F.; Die, Q.; Yu, H.; Yang, Y.; Huang, Q. Thermal Utilization Techniques and Strategies for Secondary Aluminum Dross: A Review. J. Environ. Manag. 2024, 351, 119939. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, B.; Ekberg, C.; Zhang, S. Harmless Disposal and Resource Utilization for Secondary Aluminum Dross: A Review. Sci. Total Environ. 2021, 760, 143968. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, J.; Yang, Y.; Huang, Q.; Liu, T. Pyrometallurgical Process and Multipollutant Co-Conversion for Secondary Aluminum Dross: A Review. J. Mater. Res. Technol. 2022, 21, 1196–1211. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Shen, H.; Lou, B.; Zhang, B. Recycling of Secondary Aluminum Dross to Make Alumina by Hydrometallurgy: A Review. J. Mater. Res. Technol. 2024, 32, 4234–4245. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, K.; Su, Z.; Xu, J.; Jiang, T. Self-Driven Hydrolysis Mechanism of Secondary Aluminum Dross (SAD) in the Hydrometallurgical Process without Any Additives. Chem. Eng. J. 2023, 466, 143141. [Google Scholar] [CrossRef]
- Zuo, Z.; Lv, H.; Li, R.; Liu, F.; Zhao, H. A New Approach to Recover the Valuable Elements in Black Aluminum Dross. Resour. Conserv. Recycl. 2021, 174, 105768. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, Q.; Li, Y.; Ren, B.; Fu, M.; Li, H.; Tian, D.; Ding, M. Innovative Technology for Defluorination of Secondary Aluminum Dross by Alkali Leaching. Miner. Eng. 2021, 172, 107134. [Google Scholar] [CrossRef]
- Lin, K.; Su, Z.; Xu, J.; Jiang, T.; Zhang, Y. Double-Edged Effects of Aluminosilicates Formation on Denitrification and Desalination during the Leaching Process of Secondary Aluminum Dross (SAD). Sep. Purif. Technol. 2025, 353, 128383. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Li, C.; Qu, Y.; Wang, H.; Peng, L.; Wang, Y. Hazardous Characteristics and Transformation Mechanism in Hydrometallurgical Disposing Strategy of Secondary Aluminum Dross. J. Environ. Chem. Eng. 2021, 9, 106470. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Guo, Y.; He, Y.; Liu, J.; Liu, H. Comprehensive Treatments of Aluminum Dross in China: A Critical Review. J. Environ. Manag. 2023, 345, 118575. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Y.; He, Y.; Li, S.; Liu, J.; Liu, H. Mechanism of the Denitrification of Secondary Aluminum Dross (SAD) during Water Leaching with Delayed Addition of a Low Dosage of Sodium Hydroxide. Hydrometallurgy 2024, 226, 106318. [Google Scholar] [CrossRef]
- Shi, M.; Yu, A.; Li, Y. Production of Alumina from Secondary Aluminum Dross by Hydrometallurgical Process. JOM 2023, 75, 291–300. [Google Scholar] [CrossRef]
- Shi, M.; Li, Y. Extraction of Aluminum Based on NH4HSO4 Roasting and Water Leaching from Secondary Aluminum Dross. JOM 2022, 74, 3239–3247. [Google Scholar] [CrossRef]
- Deng, W.; Xie, Q.; Zhou, R.; Tuo, H.; Liu, X.; Deng, H.; Lin, Z. Mechanism Study of Synergistic Aluminum Extraction and Purification by Ammonium for High-Value Conversion of Secondary Aluminum Dross. J. Environ. Chem. Eng. 2024, 12, 113298. [Google Scholar] [CrossRef]
- Tang, J.; Liu, G.; Qi, T.; Zhou, Q.; Peng, Z.; Li, X.; Yan, H.; Hao, H. Two-Stage Process for the Safe Utilization of Secondary Aluminum Dross in Combination with the Bayer Process. Hydrometallurgy 2022, 209, 105836. [Google Scholar] [CrossRef]
- Zhu, B.; Feng, H.; Zhu, X.; Jin, Q. Preparation of Adsorbent from Secondary Aluminum Dross by Deep Hydrolysis of Active Aluminum Components. J. Environ. Chem. Eng. 2024, 12, 112501. [Google Scholar] [CrossRef]
- Dos Santos, L.H.D.N.; Pereira, B.D.R.; Rosset, M.; Espinosa, D.C.R.; Botelho Junior, A.B. High Purity Alumina Production by Leaching-Ion Exchange Process: Design and Flowchart Proposal. Miner. Eng. 2024, 217, 108946. [Google Scholar] [CrossRef]
- Shen, H.; Liu, B.; Shi, Z.; Zhao, S.; Zhang, J.; Zhang, S. Reduction for Heavy Metals in Pickling Sludge with Aluminum Nitride in Secondary Aluminum Dross by Pyrometallurgy, Followed by Glass Ceramics Manufacture. J. Hazard. Mater. 2021, 418, 126331. [Google Scholar] [CrossRef]
- Lin, K.; Su, Z.; Xu, J.; Jiang, T.; Zhang, Y. Waste Liquid Recirculation-Driven Hydrolysis Mechanism of Secondary Aluminum Dross (SAD) in the Hydrometallurgical Processes. Process Saf. Environ. Prot. 2025, 194, 593–603. [Google Scholar] [CrossRef]
- Lin, K.; Su, Z.; Xu, J.; Jiang, T.; Zhang, Y. Calcium-Driven Hydrolysis Mechanisms of Secondary Aluminum Dross (SAD) in the Hydrometallurgical Process to Simultaneous Denitrification, Desalination, and Fluoride Fixation. Chem. Eng. J. 2024, 499, 155910. [Google Scholar] [CrossRef]
- Feng, H.; Liu, Y.; Jin, Q. Effect of In-Situ Nano-Alumina on the Properties of Alumina Ceramics from Secondary Aluminum Dross. Ceram. Int. 2024, 50, 46227–46238. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Lou, B.; Shen, H. Formation of Aluminum Nitride in Dross by Contact-Diffusion Reaction during Aluminum Recycling. J. Alloys Compd. 2025, 1010, 177432. [Google Scholar] [CrossRef]
- Tang, J.; Liu, G.; Qi, T.; Zhou, Q.; Peng, Z.; Li, X.; Wang, Y.; Shen, L.; Zhao, J.; Hao, H. Rich Microbubbles Modifying the Leaching Mechanism of Aluminum and Aluminum Nitride of Secondary Aluminum Dross in Sodium Aluminate Solution. Hydrometallurgy 2022, 214, 105962. [Google Scholar] [CrossRef]
- Xie, H.; Guo, Z.; Xu, R.; Zhang, Y. Particle Sorting to Improve the Removal of Fluoride and Aluminum Nitride from Secondary Aluminum Dross by Roasting. Env. Sci. Pollut. Res. 2023, 30, 54536–54546. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Liu, W.; Liu, S.; Huang, Y.; Qin, W. Optimization of AlN Hydrolysis in Aluminum Dross Based on Response Surface Methodology and Reaction Kinetics. J. Cent. South Univ. 2023, 30, 2993–3005. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, Q.; Ye, Z. Life Cycle Environmental and Economic Assessment of Alumina Recovery from Secondary Aluminum Dross in China. J. Clean. Prod. 2020, 277, 123291. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Aluminum Recovery as a Product with High Added Value Using Aluminum Hazardous Waste. J. Hazard. Mater. 2013, 261, 316–324. [Google Scholar] [CrossRef]
- Dash, B.; Das, B.R.; Tripathy, B.C.; Bhattacharya, I.N.; Das, S.C. Acid Dissolution of Alumina from Waste Aluminium Dross. Hydrometallurgy 2008, 92, 48–53. [Google Scholar] [CrossRef]
- Huang, K.; Wang, L.; Li, M.; Mi, T.; Zhang, J.; Liu, J.; Yi, X. Mechanism of Porous Ceramic Fabrication Using Second Aluminum Dross Assisted by Corn Stalk as Pore-Forming Agent. Environ. Technol. Innov. 2023, 31, 103195. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, X.; Jin, Q. Exergoeconomic and Exergoenvironmental Analyses of a Promising Alumina Extraction Process from Secondary Aluminum Dross in China. J. Environ. Chem. Eng. 2023, 11, 109658. [Google Scholar] [CrossRef]
- Lv, H.; Xie, M.; Shi, L.; Zhao, H.; Wu, Z.; Li, L.; Li, R.; Liu, F. A Novel Green Process for the Synthesis of High-Whiteness and Ultrafine Aluminum Hydroxide Powder from Secondary Aluminum Dross. Ceram. Int. 2022, 48, 953–962. [Google Scholar] [CrossRef]
- Zuo, Z.; Wu, W.; Zhang, J.; Li, Z.; Liu, F.; Li, S. Mechanism of Mineral Phase Transformation in Preparing Aluminum Alloy by Electrolysis of Molten Salt from Aluminum Alloy Dross. Alex. Eng. J. 2025, 112, 711–722. [Google Scholar] [CrossRef]
- Huang, K.; Yi, X. Resource Utilization and High-Value Targeted Conversion for Secondary Aluminum Dross: A Review. JOM 2023, 75, 279–290. [Google Scholar] [CrossRef]
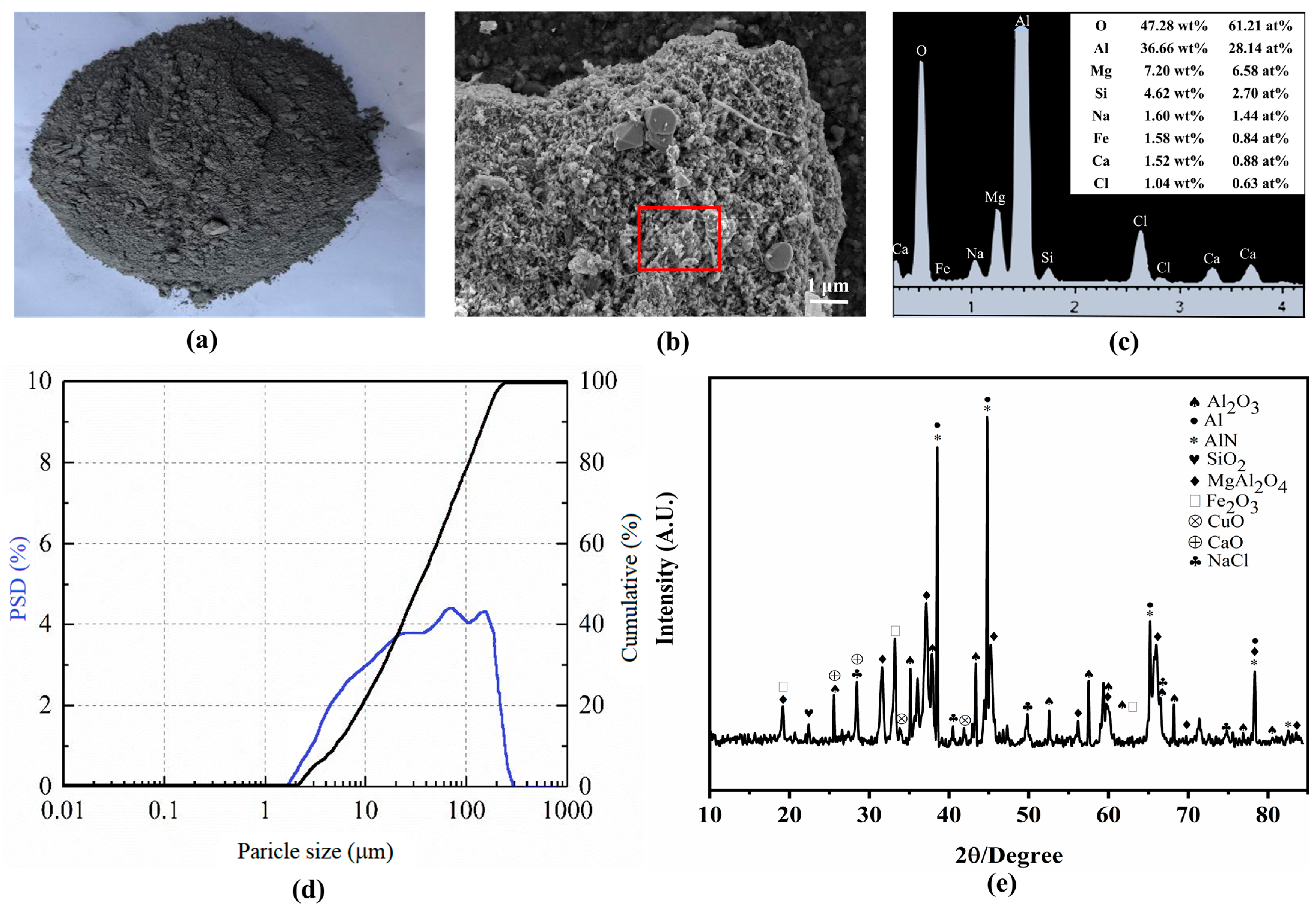
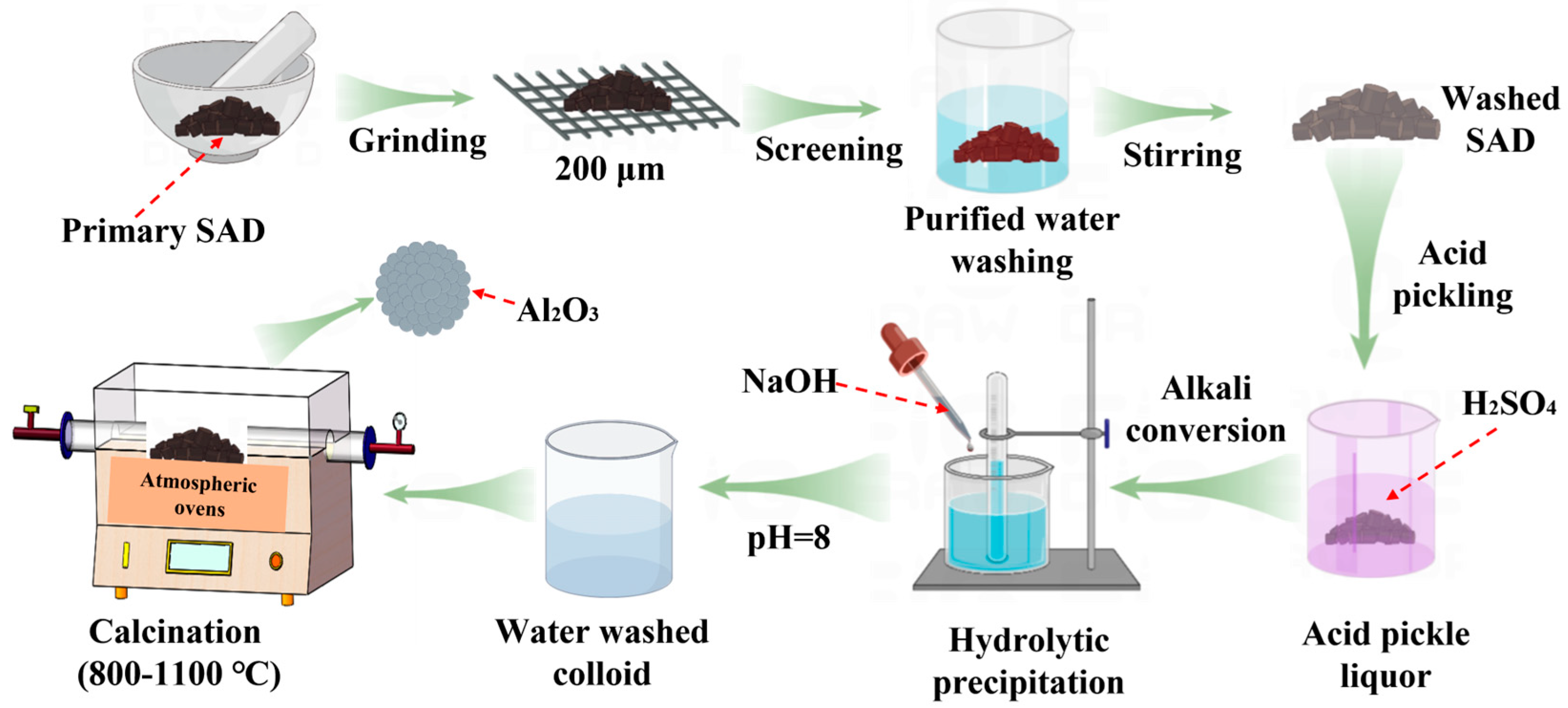
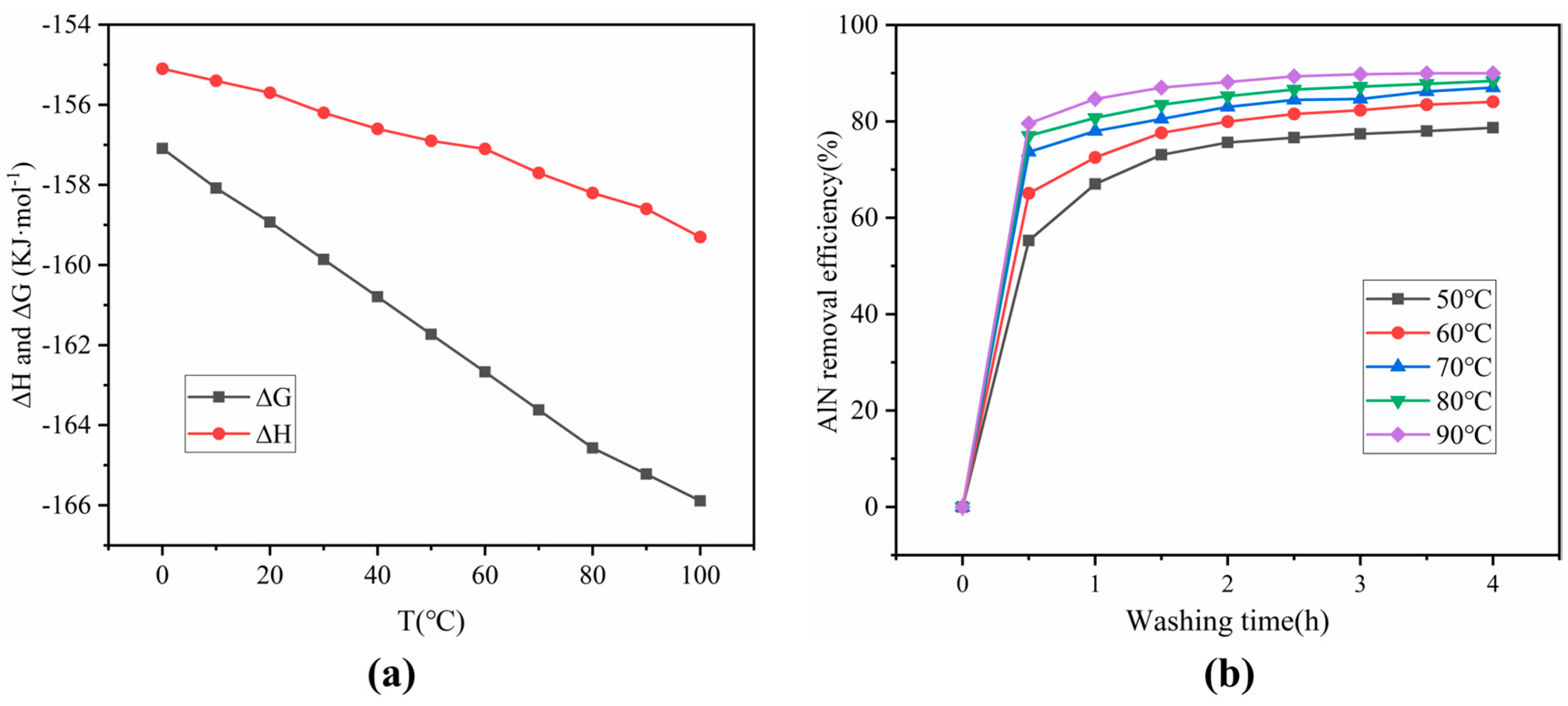
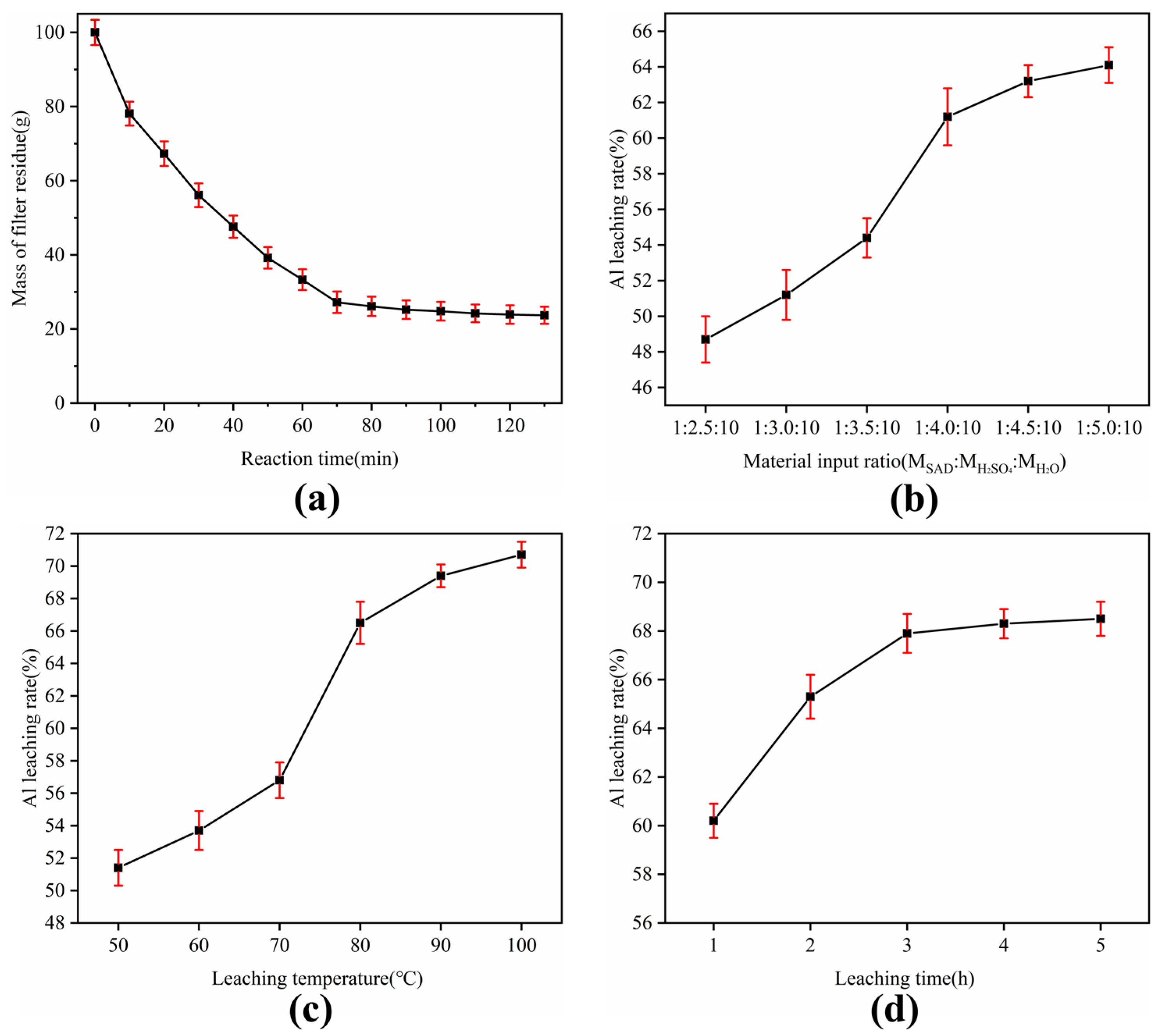


| Component/% | Al2O3 | MgO | SiO2 | Fe2O3 | Cl | CuO | CaO | Other |
|---|---|---|---|---|---|---|---|---|
| Before washed | 72.94 | 7.36 | 4.96 | 3.74 | 3.45 | 1.95 | 1.67 | 2.93 |
| After washed | 74.02 | 6.98 | 5.63 | 5.22 | 0.04 | 2.67 | 1.66 | 3.78 |
| 1 Leaching percentage/% | −1.48 | 5.16 | −13.51 | −39.57 | 98.84 | −36.92 | 0.60 | −29.01 |
| Element | pH Value | ||||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | Washed * | |
| O | 48.20 | 47.50 | 47.30 | 47.20 | 47.50 | 47.50 | 58.2 |
| Na | 19.80 | 21.30 | 23.40 | 23.70 | 24.50 | 24.90 | 0.09 |
| Al | 13.20 | 12.60 | 11.50 | 10.20 | 9.60 | 4.30 | 37.77 |
| S | 18.40 | 18.20 | 16.70 | 16.80 | 17.30 | 17.10 | 0.26 |
| Mg | 0.26 | 0.37 | 0.38 | 0.78 | 1.02 | 1.98 | 2.55 |
| Ca | 0.14 | 0.26 | 0.33 | 0.65 | 0.66 | 0.71 | 1.13 |
| Cu | 0.03 | 0.02 | 0.04 | 0.11 | 0.14 | 0.19 | —— |
| Fe | 0.22 | 0.47 | 0.69 | 0.75 | 0.75 | 0.86 | —— |
| Other | 0.23 | 0.51 | 1.44 | 1.11 | 1.77 | 0.23 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Zheng, C.; Li, Q.; Qiu, X.; Yi, X. Migration and Conversion of Al Element in the Hydrometallurgical Preparation of Al2O3 from Secondary Aluminium Dross. Processes 2025, 13, 1281. https://doi.org/10.3390/pr13051281
Huang K, Zheng C, Li Q, Qiu X, Yi X. Migration and Conversion of Al Element in the Hydrometallurgical Preparation of Al2O3 from Secondary Aluminium Dross. Processes. 2025; 13(5):1281. https://doi.org/10.3390/pr13051281
Chicago/Turabian StyleHuang, Kepeng, Changjiang Zheng, Qingda Li, Xinyang Qiu, and Xuemei Yi. 2025. "Migration and Conversion of Al Element in the Hydrometallurgical Preparation of Al2O3 from Secondary Aluminium Dross" Processes 13, no. 5: 1281. https://doi.org/10.3390/pr13051281
APA StyleHuang, K., Zheng, C., Li, Q., Qiu, X., & Yi, X. (2025). Migration and Conversion of Al Element in the Hydrometallurgical Preparation of Al2O3 from Secondary Aluminium Dross. Processes, 13(5), 1281. https://doi.org/10.3390/pr13051281








