Structural Characterization of Low-Rank Coals in the Ningdong Coalfield Under the Control of the First Coalification Jump
Abstract
1. Introduction
2. Materials and Methods
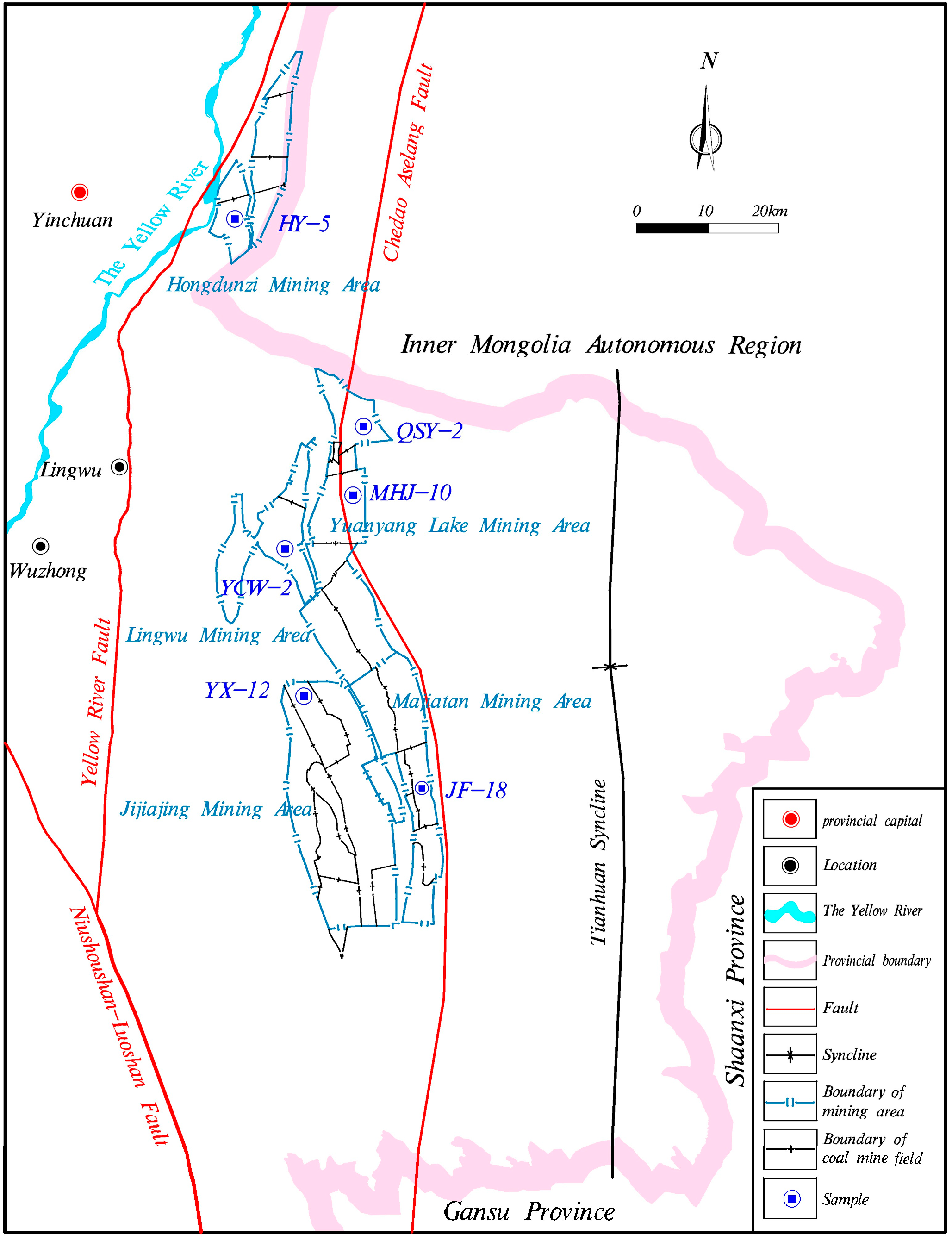
2.1. Coal Sampling and Preparation
2.2. 13C NMR Experiments
2.3. FTIR Experiments
2.4. Raman Experiments
2.5. XPS Experiments
2.6. Spectrum Deconvolution
3. Results and Discussion
3.1. Coal Conventional Characteristics
3.2. 13C NMR Characteristics
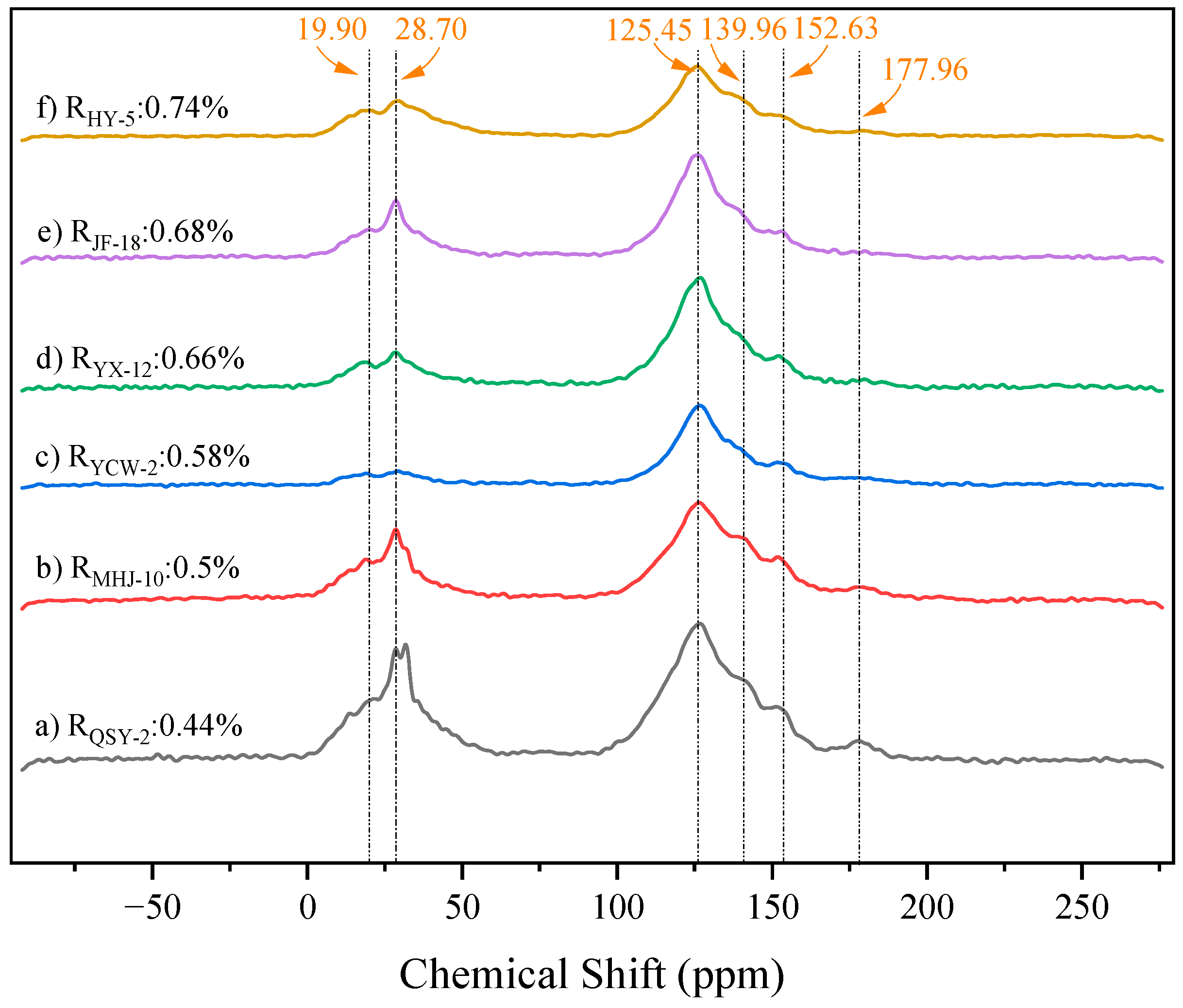
3.2.1. Forms of Carbon in Samples
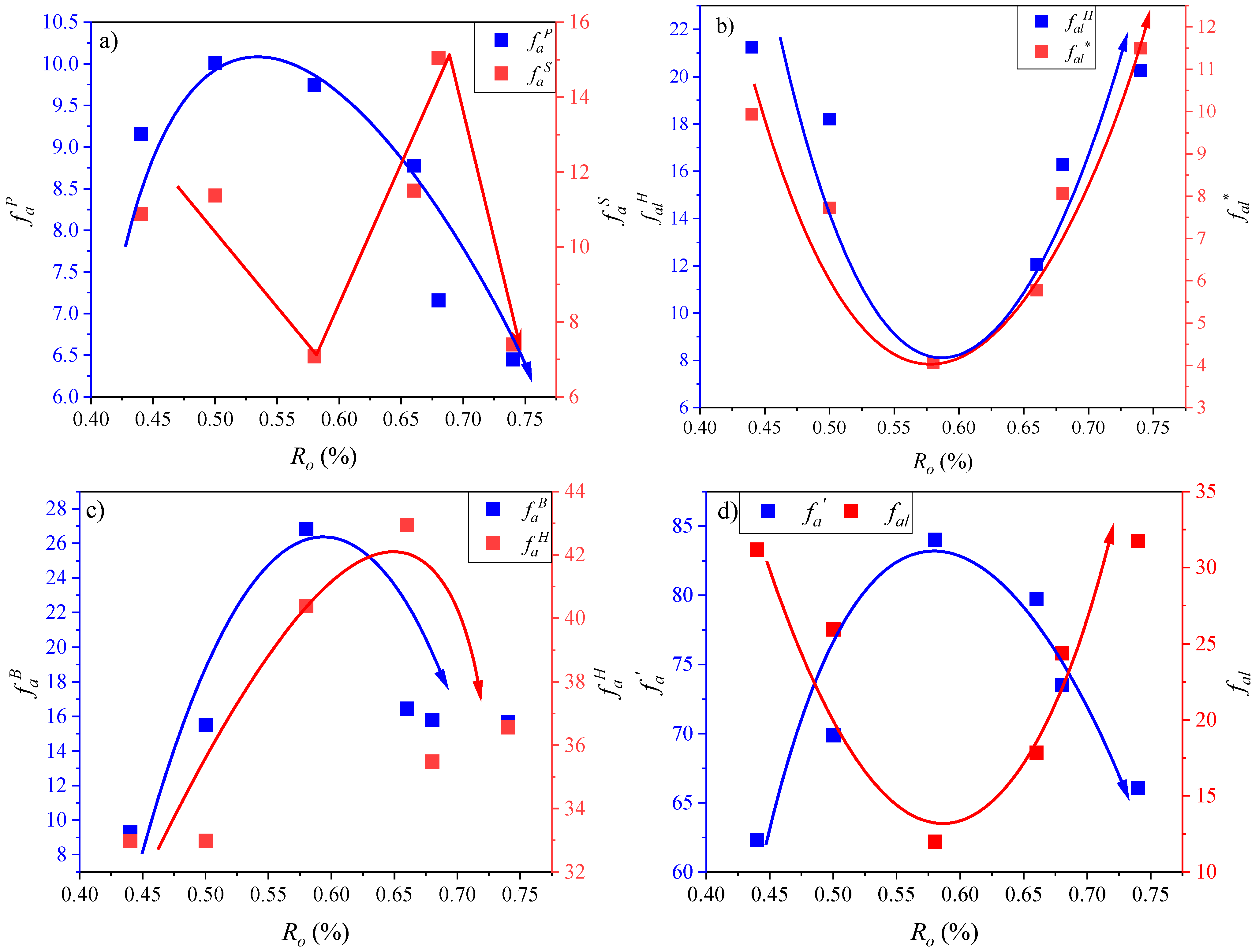
3.2.2. Structural Parameter Calculation
3.3. FTIR Characteristics
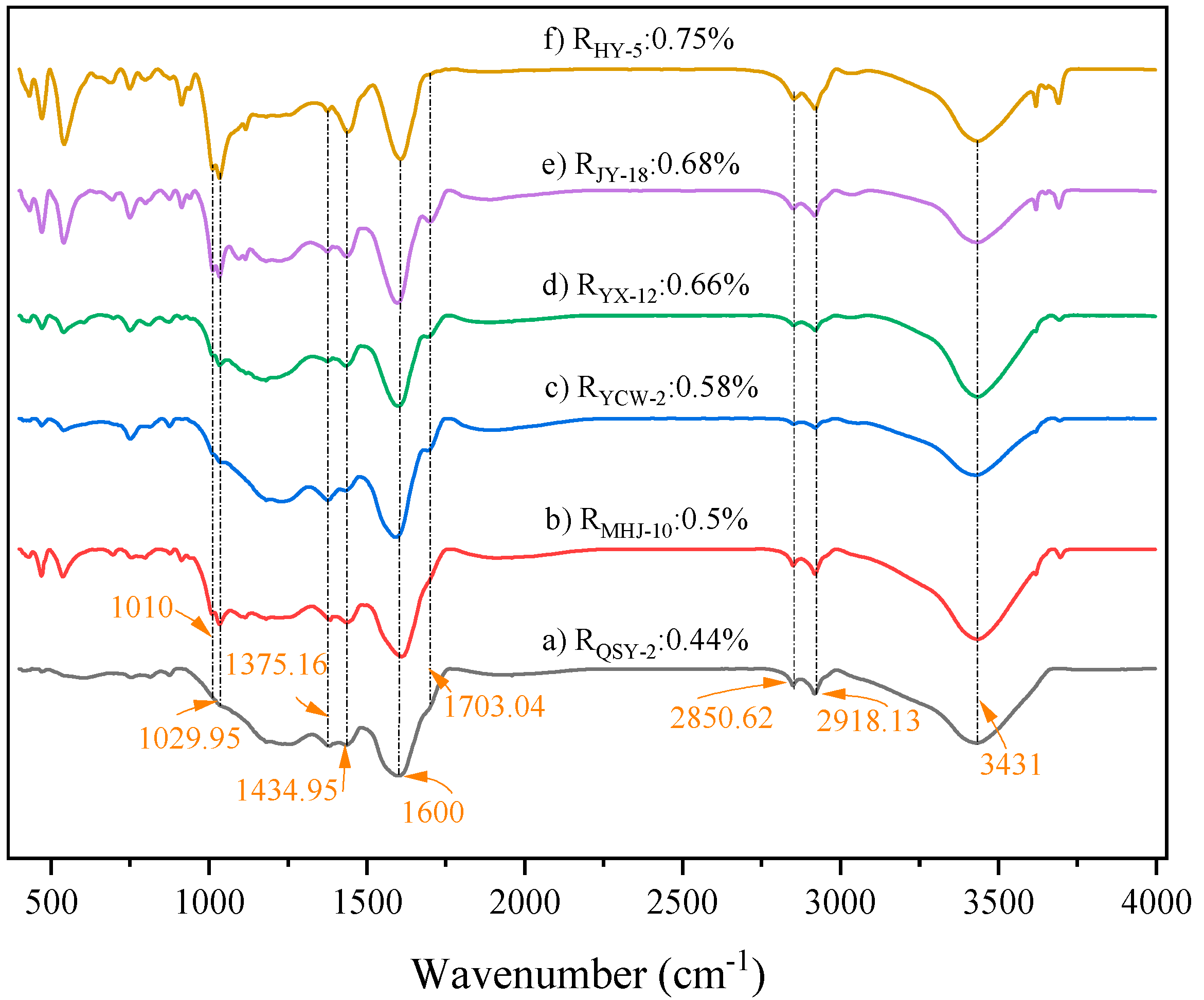
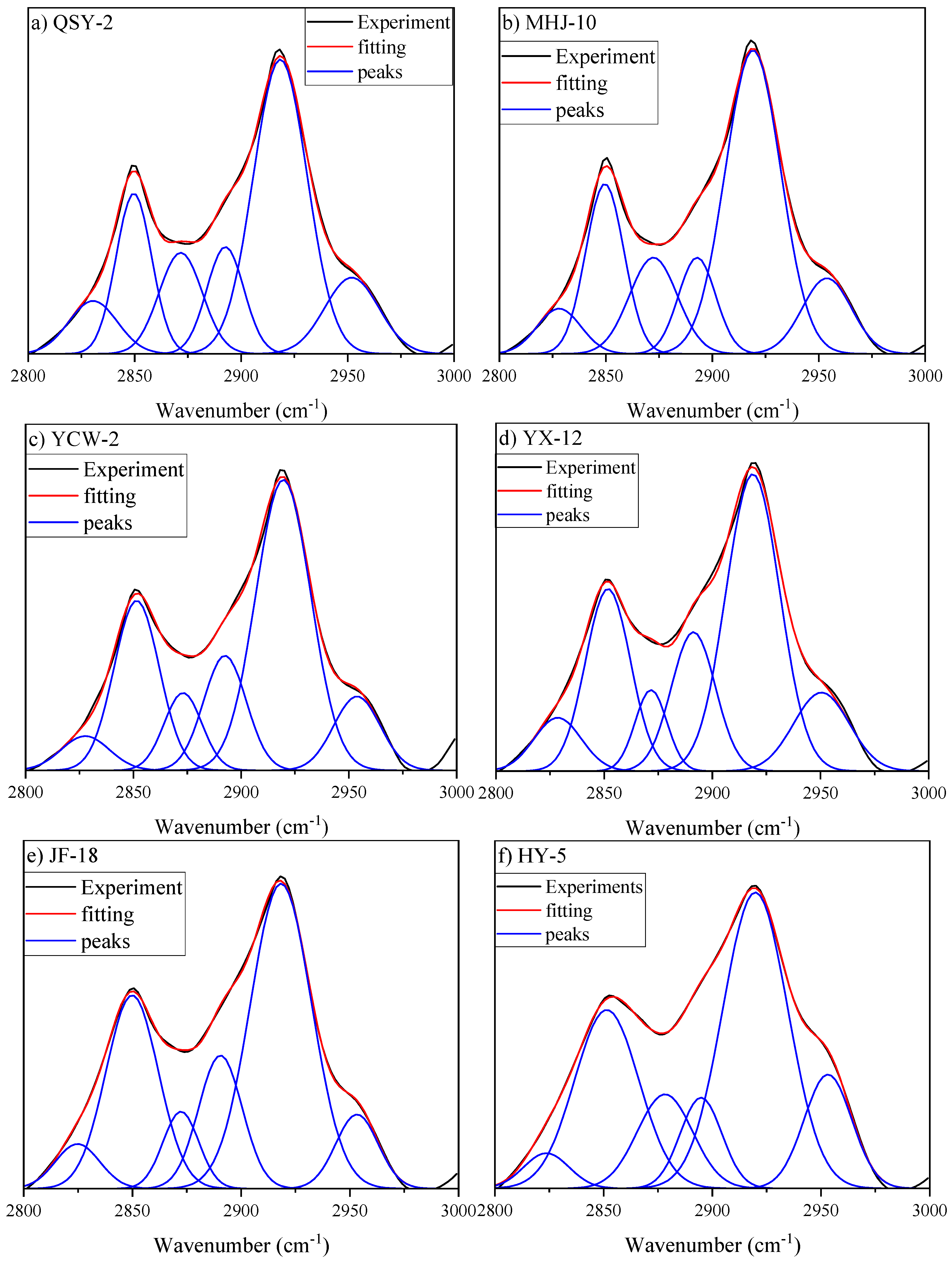
3.3.1. Aliphatic Structure Absorption Region
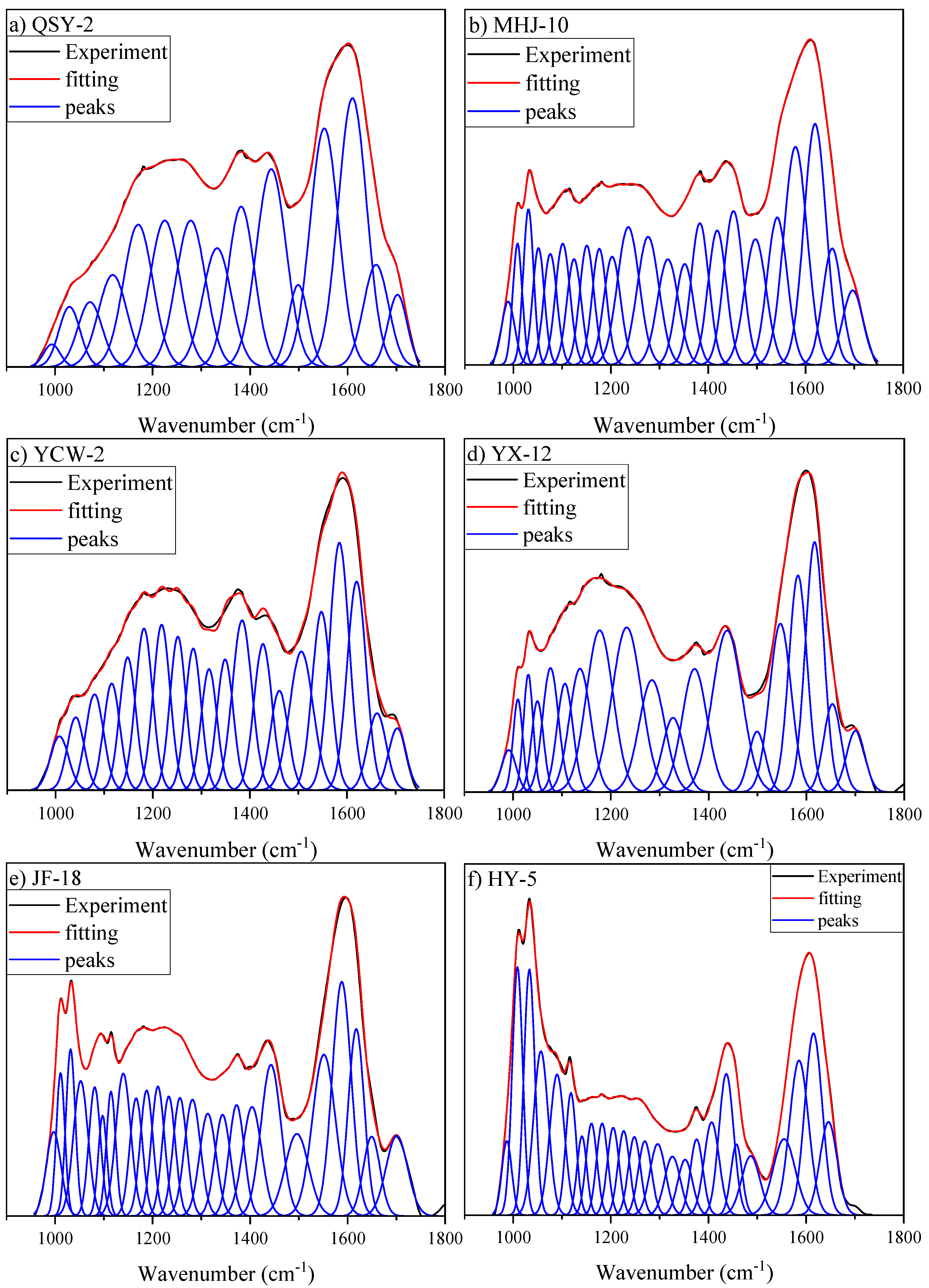
3.3.2. Oxygen-Containing Functional Group Region
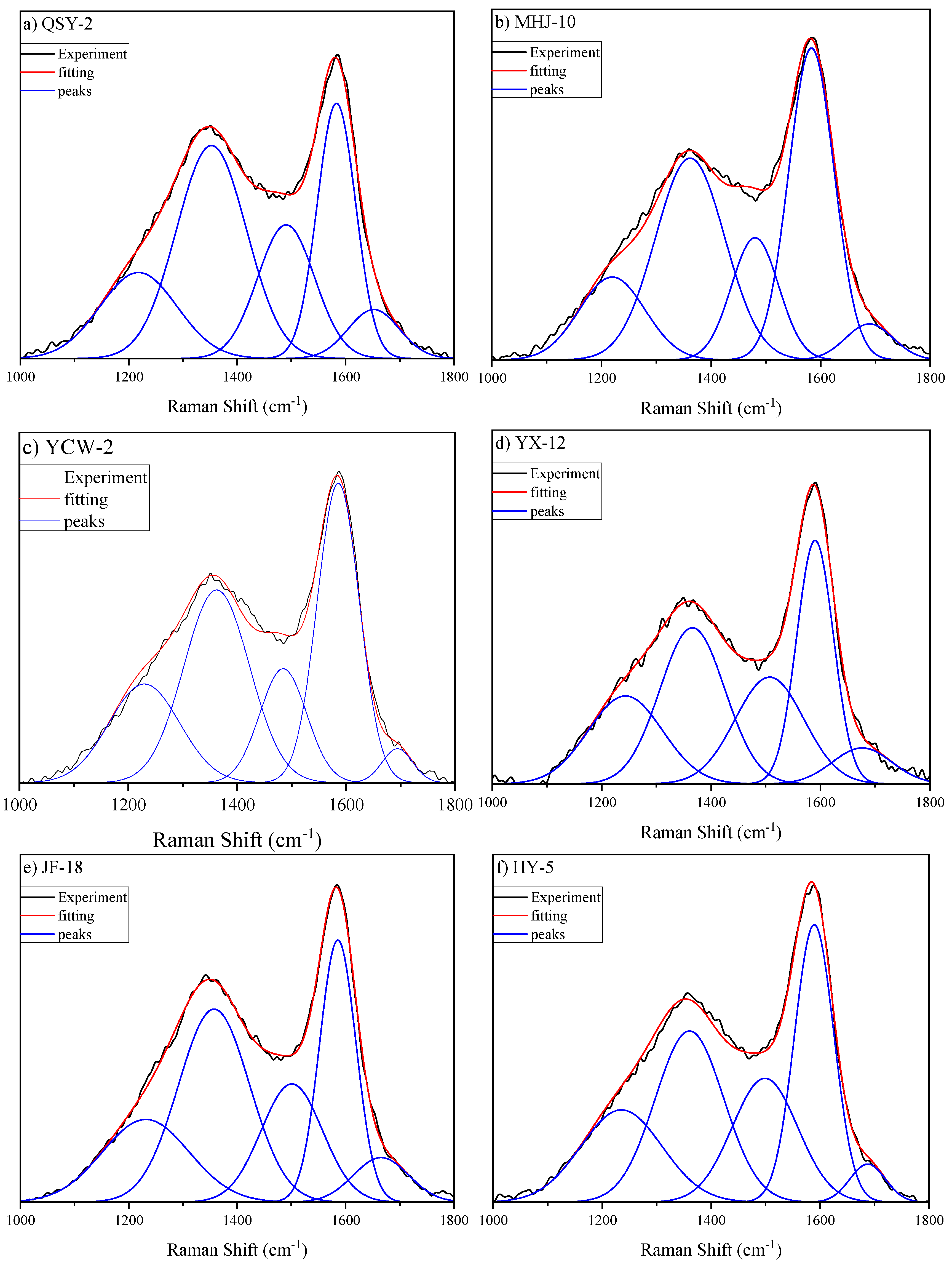
3.4. Raman Characteristics
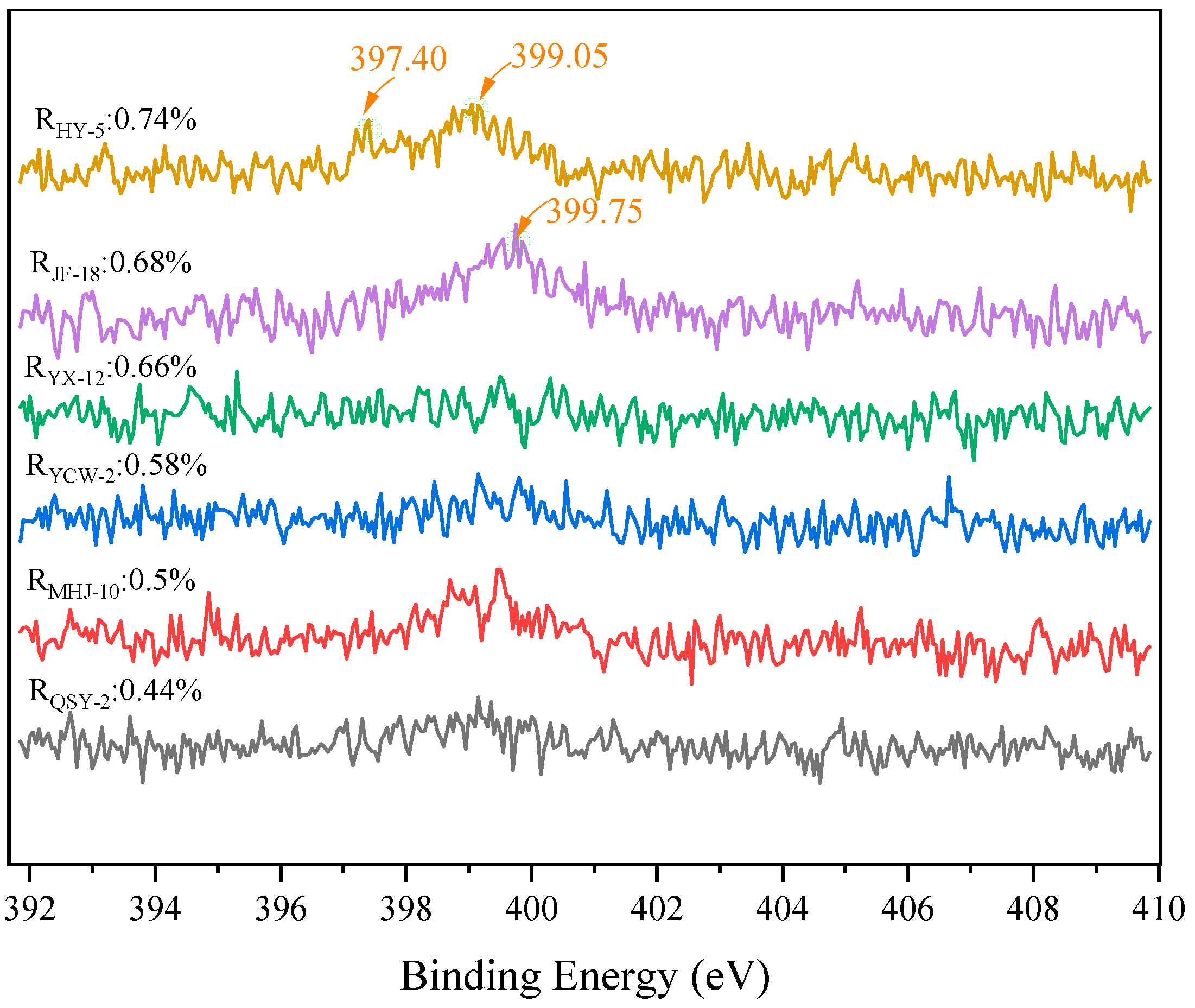
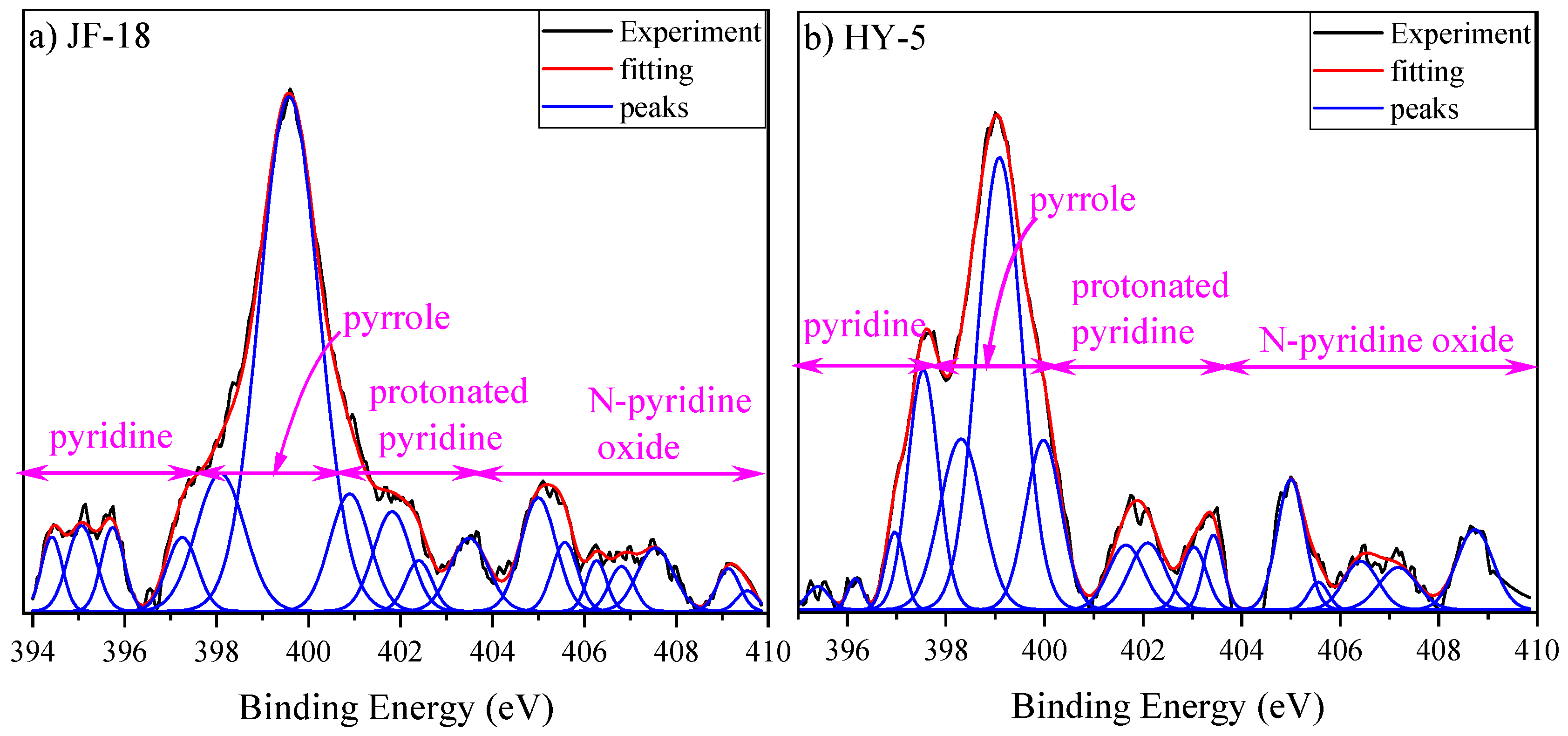
3.5. XPS Characteristics
Nitrogen Atomic Structure
3.6. Implications of the FCJ for the Molecular Structure
4. Conclusions
- (1)
- Low-rank coals have a complex and unstable chemical structure, and the high-temperature environment provided by the FCJ facilitates abrupt changes in their microstructure. The long-chain aliphatic structure and oxygen-containing functional groups gradually detach from the aromatic ring, providing the possibility for the formation of CH4 and CO2.
- (2)
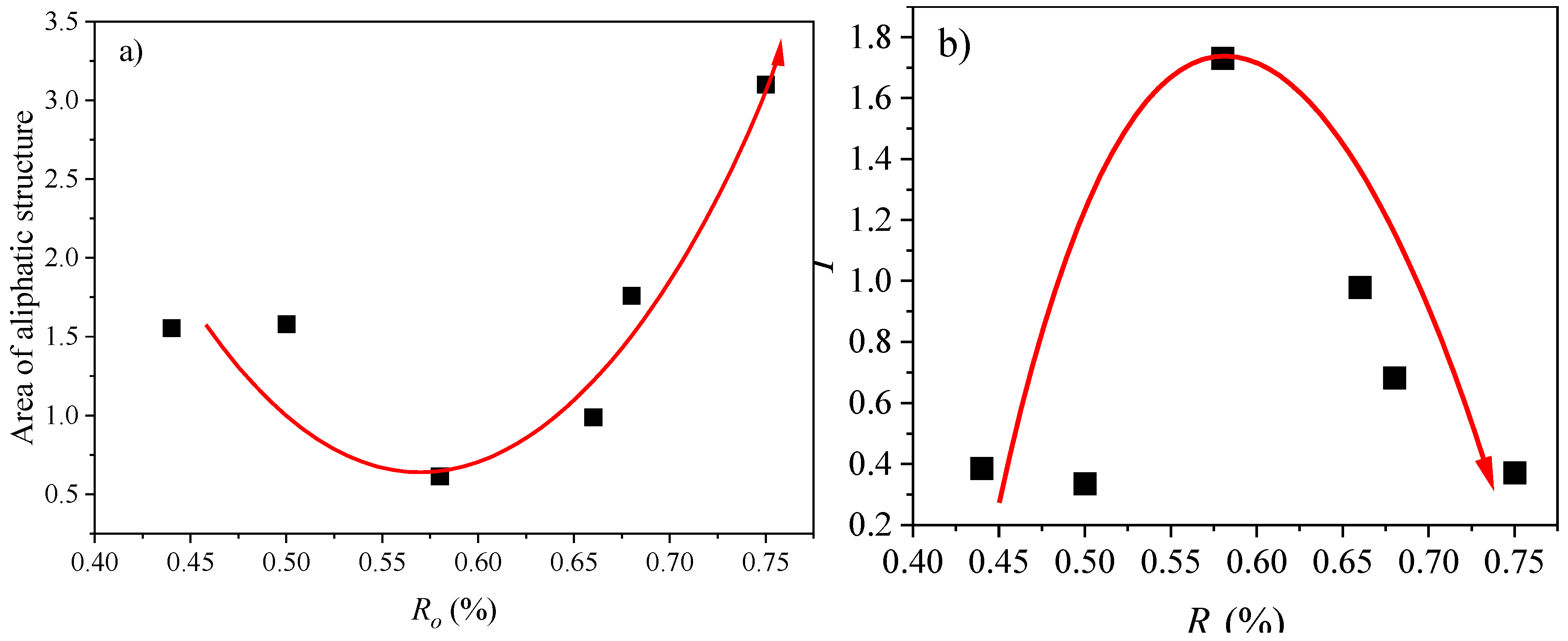
- Demethylation produces benzene substances that gradually form dense aromatic rings through condensation polymerization, significantly enhancing the aromaticity of YCW-2. The aliphatic structural evolution follows a V-shaped trend, with an inflection point at approximately 0.6% as the degree of coal metamorphism increases.
- (3)
- The impact of the FCJ on heteroatomic functional groups is almost negligible. Additionally, the peak position difference in the Raman spectra shows a discrete distribution related to the degree of metamorphism. The feasibility of applying Raman spectroscopy to the study of low-rank coal still needs to be verified.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shang, F.; Tang, X.; Hu, Q.; Wang, Y.; Meng, X.; Zhu, Y. Chemical structure transformations in kerogen from longmaxi shales in response to tectonic stress as investigated by HRTEM, FTIR, and 13C NMR. Energy Fuels 2021, 35, 19496–19506. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Liu, T.; Lin, B.; Sang, S.; Yang, W.; Liu, T.; Liu, S.; Zheng, S.; Wang, T. Control Mechanisms of Macromolecular Compositions and Structures of Coals on the Evolution of Nanopores during Coalification. Energy Fuels 2024, 38, 13002–13018. [Google Scholar] [CrossRef]
- Adsul, T.; Ghosh, S.; Ojha, A.; Bhattacharyya, S.; Varma, A.K. Spectral narratives of microstructural restyling and their controls on hydrocarbon generation potential from coal. Int. J. Coal Sci. Technol. 2023, 10, 33. [Google Scholar]
- Jiang, J.; Yang, W.; Cheng, Y.; Liu, Z.; Zhang, Q.; Zhao, K. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification. Fuel 2019, 239, 559–572. [Google Scholar] [CrossRef]
- Yao, P.; Zhang, J.; Lv, D.; Vandeginste, V.; Chang, X.; Zhang, X.; Wang, D.; Han, S.; Liu, Y. Effect of water occurrence in coal reservoirs on the production capacity of coalbed methane by using NMR simulation technology and production capacity simulation. Geoenergy Sci. Eng. 2024, 243, 213353. [Google Scholar] [CrossRef]
- Yao, N.; Cao, J.; Pang, X.; Zhao, F.; Cai, S.; Xu, M.; An, J.; Xu, X.; Yin, J. Research progress in catalytic reforming of low rank coal pyrolysis volatiles. Chem. Ind. Eng. Prog. 2024, 43, 2279–2293. (In Chinese) [Google Scholar]
- Li, M.; Zeng, F.; Zhao, Y.; Liang, H.; Xiang, J. Structural evolution around first coalification jump revealed by TG/MS and FTIR. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 562–569. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Stańczyk, K. An experimental ex-situ study of the suitability of a high moisture ortho-lignite for underground coal gasification (UCG) process. Fuel 2016, 179, 150–155. [Google Scholar] [CrossRef]
- Ding, J.; Wu, C.; Gao, B.; Zhang, S.; Zhang, J.; Tan, K. Research on the underground gasification of lignite through an oxygen enrichment process: Insights from experimental study and Aspen Plus process model. RSC Adv. 2024, 14, 36398–36409. [Google Scholar] [CrossRef] [PubMed]
- Bustin, R.M.; Guo, Y. Abrupt changes (jumps) in reflectance values and chemical compositions of artificial charcoals and inertinite in coals. Int. J. Coal Geol. 1999, 38, 237–260. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Wang, G.; Jiang, B. Characterization of coalification jumps during high rank coal chemical structure evolution. Fuel 2016, 185, 298–304. [Google Scholar] [CrossRef]
- Xin, F.; Xu, H.; Tang, D.; Cao, C. Differences in accumulation patterns of low-rank coalbed methane in China under the control of the first coalification jump. Fuel 2022, 324, 124657. [Google Scholar] [CrossRef]
- Liu, Y. Effect of Coal Vitrinite Macromolecular Structure Evolution on Methane Adsorption Capacity at Molecular Level; China University of Mining and Technology: Xuzhou, China, 2019. (In Chinese) [Google Scholar]
- Rodrigues, S.; Suárez-Ruiz, I.; Marques, M.; Camean, I.; Flores, D. Microstructural evolution of high temperature treated anthracites of different rank. Int. J. Coal Geol. 2011, 87, 204–211. [Google Scholar] [CrossRef]
- Hower, J.C.; Taulbee, D.N.; Rimmer, S.M.; Morrell, L.G. Petrographic and Geochemical Anatomy of Lithotypes from the Blue Gem Coal Bed, Southeastern Kentucky. Energy Fuels 1994, 8, 719–728. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Gao, B.; Wu, C.; Song, Y.; Zhou, D.; Niu, Q.; Zhou, H.; Jiang, X. Structural characterization of high fidelity for bituminous and semi-anthracite: Insights from spectral analysis and modeling. Fuel 2022, 315, 123183. [Google Scholar] [CrossRef]
- Solum, M.S.; Sarofim, A.F.; Pugmire, R.J.; Fletcher, T.H.; Zhang, H. 13C NMR Analysis of Soot Produced from Model Compounds and a Coal. Energy Fuels 2001, 15, 961–971. [Google Scholar] [CrossRef]
- Tao, S.; Chen, S.; Tang, D.; Zhao, X.; Xu, H.; Li, S. Material composition, pore structure and adsorption capacity of low-rank coals around the first coalification jump: A case of eastern Junggar Basin, China. Fuel 2018, 211, 804–815. [Google Scholar] [CrossRef]
- Lloyd, M.K.; Trembath-Reichert, E.; Dawson, K.S.; Feakins, S.J.; Mastalerz, M.; Orphan, V.J.; Sessions, A.L.; Eiler, J.M. Methoxyl stable isotopic constraints on the origins and limits of coal-bed methane. Science 2021, 374, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, D.; Mochimaru, H.; Tamaki, H.; Yamamoto, K.; Yoshioka, H.; Suzuki, Y.; Kamagata, Y.; Sakata, S. Methane production from coal by a single methanogen. Science 2016, 354, 222–225. [Google Scholar] [CrossRef]
- Hoffmann, K.; Huculak-Mączka, M. The utilization possibility of waste lignite as a raw material in the process of obtaining humic acids preparations. Pol. J. Chem. Technol. 2012, 14, 1–6. [Google Scholar] [CrossRef]
- Singh, A.L.; Singh, P.K.; Singh, M.P. Biomethanization of coal to obtain clean coal energy: A review. Energy Explor. Exploit. 2012, 30, 837–852. [Google Scholar] [CrossRef]
- GB/T 19222-2003; Sampling of Coal Petrology. China Coal Industry Association: Xi’an, China, 2003.
- GB/T 6948-2008; Method of Determining Microscopically the Reflectance of Vitrinite in Coal. China Coal Industry Association: Xi’an, China, 2009.
- GB/T 8899-2013; Determination of Maceral Group Composition and Minerals in Coal. China Coal Industry Association: Xi’an, China, 2014.
- Solomon, P.R.; Carangelo, R.M. FTIR analaysis of coal. 1. Techniques and determination of hydroxyl concentrations. Fuel 1981, 61, 663–669. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.; Cai, Y.; Li, Q.; Zhou, Y. Spontaneous imbibition in coal with in-situ dynamic micro-CT imaging. J. Pet. Sci. Eng. 2022, 208, 109296. [Google Scholar] [CrossRef]
- Ping, A.; Xia, W.; Peng, Y.; Xie, G. Construction of bituminous coal vitrinite and inertinite molecular assisted by 13C NMR, FTIR and XPS. J. Mol. Struct. 2020, 1222, 128959. [Google Scholar] [CrossRef]
- Cui, X.; Yan, H.; Zhao, P.; Yang, Y.; Xie, Y. Modeling of molecular and properties of anthracite base on structural accuracy identification methods. J. Mol. Struct. 2019, 1183, 313–323. [Google Scholar] [CrossRef]
- Liu, J.; Luo, L.; Ma, J.; Zhang, H.; Jiang, X. Chemical Properties of Superfine Pulverized Coal Particles. 3. Nuclear Magnetic Resonance Analysis of Carbon Structural Features. Energy Fuels 2016, 30, 6321–6329. [Google Scholar] [CrossRef]
- Li, Z.; Wei, X.; Yan, H.; Zong, Z. Insight into the structural features of Zhaotong lignite using multiple techniques. Fuel 2015, 153, 176–182. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, D.; Karpyn, Z.T.; Cai, Y.; Yao, Y. Effect of coalification jumps on petrophysical properties of various metamorphic coals from different coalfields in China. J. Nat. Gas Sci. Eng. 2018, 60, 63–76. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.H.; Guo, Y.N.; Su, Y. FTIR and 13C NMR Investigation of Coal Component of Late Permian Coals from Southern China. Energy Fuels 2011, 25, 5672–5677. [Google Scholar] [CrossRef]
- Wang, S.; Griffiths, P.R. Resolution enhancement of diffuse reflectance i.r. spectra of coals by Fourier self-deconvolution. Fuel 1985, 64, 229–236. [Google Scholar] [CrossRef]
- Liu, X.; Song, D.; He, X.; Nie, B.; Wang, L. Insight into the macromolecular structural differences between hard coal and deformed soft coal. Fuel 2019, 245, 188–197. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Clifford, D.J. The organic geochemistry of coal: From plant materials to coal. Org. Geochem. 1997, 27, 251–274. [Google Scholar] [CrossRef]
- Solum, M.S.; Pugmire, R.J.; Grant, D.M. 13C solid-state NMR of argonne premium coals. Energy Fuels 1989, 3, 187–193. [Google Scholar] [CrossRef]
- Erdenetsogt, B.; Lee, I.; Lee, S.K.; Ko, Y.; Bat-Erdene, D. Solid-state C-13 CP/MAS NMR study of Baganuur coal, Mongolia: Oxygen-loss during coalification from lignite to subbituminous rank. Int. J. Coal Geol. 2010, 82, 37–44. [Google Scholar] [CrossRef]
- Olcay Mert, Asgar Kayan, Synthesis of silyliminophenolate zirconium compounds and their catalytic activity over lactide/epoxide. Appl. Catal. A Gen. 2013, 464, 322–331.
- Hao, P.; Meng, Y.; Zeng, F.; Yan, T.; Xu, G. Quantitative study of chemical structures of different rank coals based on infrared spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 787–792. (In Chinese) [Google Scholar]
- Liang, H.; Wang, G.; Zeng, F.; Li, M.; Xiang, J. Effect of demineralization on lignite structure from Yinmin coalfield by FTIR investigation. J. Fuel Chem. Technol. 2014, 42, 129–137. (In Chinese) [Google Scholar]
- Okolo, G.N.; Neomagus, H.W.J.P.; Everson, R.C.; Roberts, M.J.; Bunt, J.R.; Sakurovs, R.; Mathews, J.P. Chemical-structural properties of South African bituminous coals: Insights from wide angle XRD-carbon fraction analysis, ATR-FTIR, solid state 13C NMR, and HRTEM techniques. Fuel 2015, 158, 779–792. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Pan, J.; Wang, Z.; Niu, Q.; Du, M. Research on Molecular Structure Characteristics of Vitrinite and Inertinite from Bituminous Coal with FTIR, Micro-Raman, and XRD Spectroscopy. Energy Fuels 2021, 35, 1322–1335. [Google Scholar] [CrossRef]
- Liang, C.; Liang, W.; Li, W. Functional groups of different coal ranks based on infrared spectroscopy. Coal Sci. Technol. 2020, 48, 182–186. (In Chinese) [Google Scholar]
- Wang, C.; Zeng, F.; Li, C.; Xu, Q.; Chen, P. Insight into the molecular structural evolution of a series of medium-rank coals from China by XRD, Raman and FTIR. J. Mol. Struct. 2024, 1303, 137616. [Google Scholar] [CrossRef]
- Morga, R. Micro-Raman spectroscopy of carbonized semifusinite and fusinite. Int. J. Coal Geol. 2011, 87, 253–267. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Chen, S.; Wang, Y.; Song, Y. Evaluation of Spatial Alignment of Kerogen in Shale Using High-Resolution Transmission Electron Microscopy, Raman Spectroscopy, and Fourier Transform Infrared. Energy Fuels 2018, 32, 10616–10627. [Google Scholar] [CrossRef]
- Ulyanova, E.V.; Molchanov, A.N.; Prokhorov, I.Y.; Grinyov, V.G. Fine structure of Raman spectra in coals of different rank. Int. J. Coal Geol. 2014, 121, 37–43. [Google Scholar] [CrossRef]


| Sample | Ro (%) | Demineralized Base (%) | Containing Mineral Base (%) | Standard Deviation (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Exinite | TOC | Clay | Sulfide | Carbonate | Silicon Oxide | Other | |||
| QSY-2 | 0.44 | 81.62 | 16.81 | 1.57 | 96.98 | 0.76 | 2.26 | - | - | - | 0.029 |
| MHJ-10 | 0.5 | 78.85 | 18.51 | 2.64 | 94.59 | 4.58 | - | 0.83 | - | - | 0.038 |
| YCW-2 | 0.58 | 2.87 | 95.90 | 1.23 | 93.49 | 1.53 | 0.38 | 4.60 | - | - | 0.039 |
| YX-12 | 0.66 | 26.13 | 72.73 | 1.14 | 95.66 | 0.36 | 3.98 | - | - | - | 0.034 |
| JF-18 | 0.68 | 21.69 | 73.89 | 4.42 | 95.04 | 3.82 | 0.38 | 0.38 | 0.38 | - | 0.041 |
| HY-5 | 0.74 | 77.82 | 13.58 | 8.60 | 90.95 | 8.23 | - | 0.82 | - | - | 0.032 |
| Sample | fac | faP | faS | faB | faH | falO | falH | fal* | faN | fa’ | fa | fal | XBP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QSY-2 | 4.01 | 9.16 | 10.89 | 9.30 | 32.97 | 2.47 | 21.25 | 9.94 | 29.35 | 62.32 | 66.34 | 31.19 | 0.18 |
| MHJ-10 | 4.16 | 10.01 | 11.38 | 15.52 | 32.99 | 0.00 | 18.21 | 7.73 | 36.91 | 69.90 | 74.06 | 25.94 | 0.29 |
| YCW-2 | 3.97 | 9.75 | 7.08 | 26.82 | 40.39 | 0.00 | 7.93 | 4.07 | 43.64 | 84.03 | 88.00 | 12.00 | 0.47 |
| YX-12 | 2.45 | 8.78 | 11.51 | 16.46 | 42.94 | 0.00 | 12.07 | 5.78 | 36.75 | 79.70 | 82.15 | 17.85 | 0.26 |
| JF-18 | 2.11 | 7.16 | 15.04 | 15.82 | 35.49 | 0.00 | 16.30 | 8.07 | 38.02 | 73.51 | 75.62 | 24.38 | 0.27 |
| HY-5 | 2.15 | 6.45 | 7.40 | 15.67 | 36.57 | 0.00 | 20.26 | 11.50 | 29.51 | 66.08 | 68.24 | 31.76 | 0.31 |
| Assignment | QSY-2 | MHJ-10 | YCW-2 | YX-12 | JF-18 | HY-5 |
| Sym. R2CH2 | 22.64 | 22.77 | 25.55 | 26.93 | 28.52 | 28.01 |
| Sym. RCH3 | 11.96 | 12.41 | 8.00 | 6.50 | 6.49 | 11.11 |
| R3CH | 10.85 | 9.64 | 13.39 | 15.42 | 13.67 | 8.07 |
| Asym. R2CH2 | 43.28 | 45.20 | 43.69 | 40.14 | 43.95 | 41.21 |
| Asym. RCH3 | 11.27 | 9.98 | 9.37 | 11.00 | 7.38 | 11.60 |
| Attribution | QSY-2 | MHJ-10 | YCW-2 | YX-12 | JF-18 | HY-5 |
|---|---|---|---|---|---|---|
| Ash | 0.71 | 3.74 | 2.08 | 3.18 | 5.13 | 8.98 |
| Alkyl ether C-O | 2.43 | 5.66 | 2.58 | 4.68 | 7.39 | 13.67 |
| Aryl ether C-O | 8.06 | 9.10 | 11.77 | 7.82 | 11.91 | 9.02 |
| Phenol OH | 31.21 | 28.77 | 25.51 | 34.29 | 27.55 | 25.94 |
| Sym. CH3 | 8.10 | 4.81 | 11.44 | 6.48 | 3.54 | 2.56 |
| α-CH2 angular vibration | 11.27 | 4.67 | 5.55 | 10.09 | 4.35 | 9.34 |
| CH3CH2 asymmetric deformation vibration | 3.08 | 5.61 | 3.67 | 2.13 | 6.36 | 5.08 |
| Aromatic C=C | 12.95 | 20.42 | 23.86 | 16.06 | 21.14 | 12.50 |
| Conjugated C=O vibration | 19.54 | 14.34 | 11.27 | 12.90 | 9.01 | 12.90 |
| C=O stretching vibration | 2.65 | 2.87 | 2.27 | 2.37 | 3.62 | 0.00 |
| Sample | Subpeak Center Position | Difference (G-D1) | ||||
|---|---|---|---|---|---|---|
| D4 | D1 | D3 | G | D2 | ||
| QSY-2 | 1218.09 | 1352.68 | 1490.47 | 1583.26 | 1652.00 | 230.58 |
| MHJ-10 | 1219.76 | 1361.84 | 1480.36 | 1583.16 | 1688.97 | 221.32 |
| YCW-2 | 1229.50 | 1362.43 | 1484.53 | 1585.67 | 1694.28 | 223.24 |
| YX-12 | 1243.51 | 1365.90 | 1507.04 | 1590.32 | 1676.24 | 224.42 |
| JF-18 | 1231.56 | 1357.49 | 1500.95 | 1585.78 | 1665.46 | 228.29 |
| HY-5 | 1235.50 | 1360.50 | 1499.00 | 1589.50 | 1687.52 | 229.00 |
| Sample | Elemental Proportion (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C1s | O1s | N1s | Na1s | Si2p | Al2p | Cl2p | S2p | |
| QSY-2 | 75.84 | 21.8 | 0.46 | / | 1.77 | / | / | 0.14 |
| MHJ-10 | 73.97 | 23.34 | 0.39 | / | 2.3 | / | / | / |
| YCW-2 | 76.83 | 21.65 | / | / | 1.52 | / | / | / |
| YX-12 | 75.52 | 22.71 | 0.28 | / | 1.48 | / | / | / |
| JF-18 | 74.67 | 22.11 | 1.33 | / | 1.89 | / | / | / |
| HY-5 | 74.63 | 21.45 | 1.4 | 0.25 | 2.23 | / | / | 0.05 |
| Attribution | Relative Area Ratio (%) | |||
|---|---|---|---|---|
| Pyridine | Pyrrole | Protonated Pyridine | N-Pyridine Oxide | |
| JF-18 | 11.74 | 53.65 | 17.99 | 16.62 |
| HY-5 | 15.98 | 54.46 | 12.55 | 17.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Wu, C.; Gao, B.; Lu, X.; Wang, B.; Liang, Y.; Zhang, X.; Zhang, Z. Structural Characterization of Low-Rank Coals in the Ningdong Coalfield Under the Control of the First Coalification Jump. Processes 2025, 13, 1996. https://doi.org/10.3390/pr13071996
Ji X, Wu C, Gao B, Lu X, Wang B, Liang Y, Zhang X, Zhang Z. Structural Characterization of Low-Rank Coals in the Ningdong Coalfield Under the Control of the First Coalification Jump. Processes. 2025; 13(7):1996. https://doi.org/10.3390/pr13071996
Chicago/Turabian StyleJi, Xiaoyan, Caifang Wu, Bin Gao, Xuezhong Lu, Bei Wang, Yongping Liang, Xiaowu Zhang, and Zhifeng Zhang. 2025. "Structural Characterization of Low-Rank Coals in the Ningdong Coalfield Under the Control of the First Coalification Jump" Processes 13, no. 7: 1996. https://doi.org/10.3390/pr13071996
APA StyleJi, X., Wu, C., Gao, B., Lu, X., Wang, B., Liang, Y., Zhang, X., & Zhang, Z. (2025). Structural Characterization of Low-Rank Coals in the Ningdong Coalfield Under the Control of the First Coalification Jump. Processes, 13(7), 1996. https://doi.org/10.3390/pr13071996






