Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies
Abstract
:1. Introduction
2. Major Mechanisms
2.1. The Driving Force




2.2. Factors Opposing the Driving Force
2.2.1. Concentration Polarization (CP)
- (a)
- the concentration of rejected solute near the membrane surface,
- (b)
- the precipitation of sparingly soluble macromolecular polymeric and inorganic (gel layer formation and scaling, respectively) at the membrane surface and
- (c)
- the accumulation of retained solids on the membrane (cake layer formation).
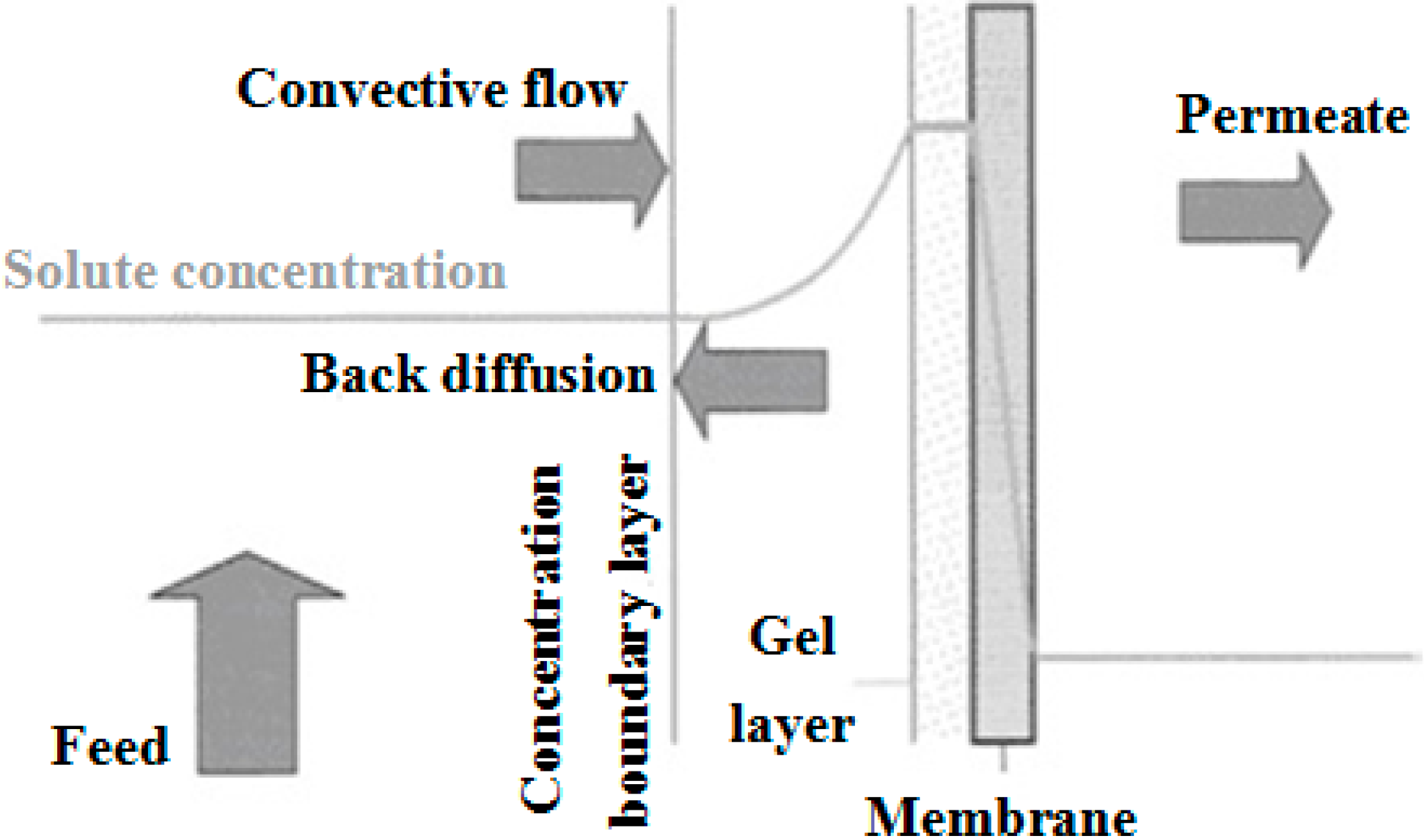


2.2.2. Fouling
- -
- inorganic fouling which refers to the deposit of inorganic material like salts, clay and metal oxides,
- -
- organic fouling which includes all kind of deposit of organic material like grease, oil, surfactants, proteins, polysaccharides, humic substances and other organic biopolymers and
- -
- biofouling which designates the formation of biofilms by compounds and microorganisms attached and growing at the membrane surface [3].
2.2.3. Clogging
- The solids agglomeration rate in the channels relates to the rate at which water is drained from the sludge. This in turn is dependent on both the flux and the residence time of the sludge in the membrane channels, since the extent of dewatering increases at longer residence times.
- The residence time in the membrane channel itself is directly related to membrane aeration, with respect to both the distribution of the air bubbles throughout the channels and the overall aeration rate.
- Agglomeration must also depend both on the concentration and the characteristics of the particles, since particles which, for whatever reason, more readily adhere to the membrane and/or each other can be expected to agglomerate faster. These may be presumed to be partly related to feedwater physicochemical parameters, since these are known to impact on sludge quality and the physical nature of the inert solids specifically.
2.3. Membrane Fouling in Membrane Bioreactors (MBRs)
2.3.1. Fouling Mechanisms in MBRs
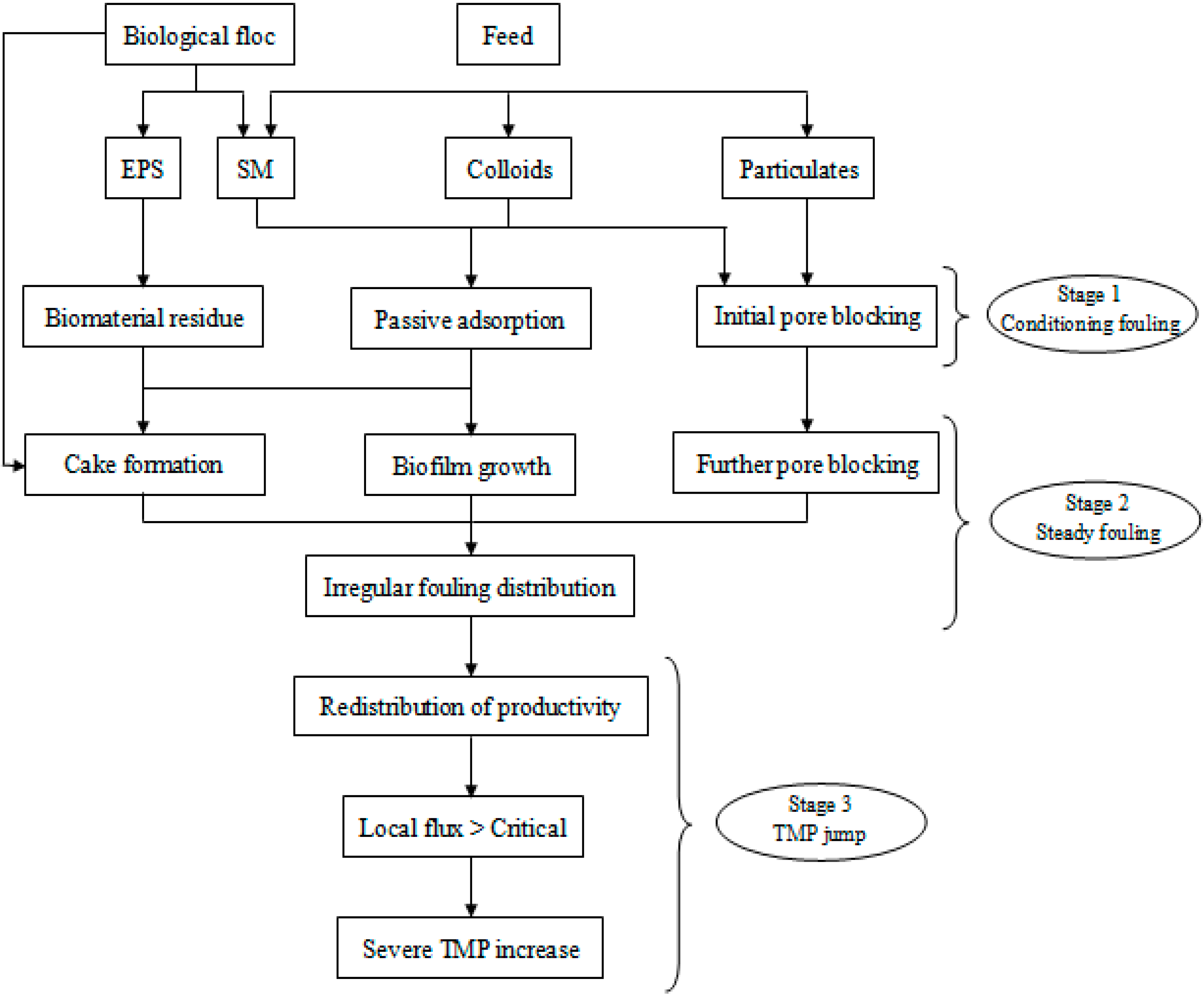
2.3.1.1. Stage 1: Conditioning Fouling
2.3.1.2. Stage 2: Slow/Steady Fouling
2.3.1.3. Stage 3: TMP Jump
- (i)
- Inhomogeneous fouling (area loss) model: This model was proposed to explain the observed TMP profiles in nominally sub-critical filtration of upflow anaerobic sludge [111]. The TMP jump appeared to coincide with a measured loss of local permeability at different positions along the membrane, due to slow fouling by EPS. It was argued that the flux redistribution (to maintain the constant average flux) resulted in regions of sub-critical flux and consequently in rapid fouling and TMP rise.
- (ii)
- Inhomogeneous fouling (pore loss) model: Similar TMP transients have been observed for the crossflow MF of a model biopolymer (alginate) [112]. These trends revealed the TMP transient to occur with relatively simple feeds. The data obtained have been explained by a model that involves flux redistribution among open pores. Local pore velocities eventually exceed the critical flux of alginate aggregates that rapidly block the pores. This idea was also the base of the model proposed by Ognier et al. [113]. While the “area loss” model considers macroscopic redistribution of flux, the “pore loss” model focuses on microscopic scale. In MBR systems, it is expected that both mechanisms occur simultaneously.
- (iii)
- Critical suction pressure model: The two-stage pattern of a gradual TMP rise followed by a more rapid increase has been observed from studies conducted based on dead-end filtration of a fine colloid by an immersed HF. At a critical suction pressure it is suggested coagulation or collapse occurs at the base of the cake, based on membrane autopsy evaluations supplemented with modeling [114]. A very thin dense layer close to the membrane surface, as observed in the study, would account for the rapid increase in resistance leading to the TMP jump. Although this work was based on dead-end rather than crossflow operation, the mechanism could apply to any membrane system where fouling continues until the critical suction pressure is reached, where-upon the depositing compound(s) coalesce or collapse to produce a more impermeable fouling layer.
- (iv)
- Percolation theory: According to percolation theory, the porosity of the fouling layer gradually decreases due to the continuous filtration and material deposition within the deposit layer. At a critical condition, the fouling cake loses connectivity and resistance, resulting in a rapid increase in TMP. This model has been proposed for MBRs [115], but indicates a very rapid change (within minutes), which is not always observed in practice. However, the combination of percolation theory with the inhomogeneous fouling (area loss) model could satisfy the more typically gradual inclines observed for TMP transients. Similarly, fractal theory was successfully applied to describe cake microstructure and properties and to explain the cake compression observed during MBR operation.
- (v)
- Inhomogeneous fiber bundle model: Another manifestation of the TMP transient has been observed for a model fiber bundle where the flow from individual fibers was monitored [116]. The bundle was operated under suction at constant permeate flow, giving constant average flux and the flow was initially evenly distributed among the fibers. However, over the time the flows became less evenly distributed so that the standard deviation of the fluxes of individual fibers started to increase from the initial range of 0.1–0.15 up to 0.4. Consequently, the TMP rose to maintain the average flux across the fiber bundle, mirroring the increase in the standard deviation of the fluxes. At some point, both TMP and standard deviation rose rapidly. This is believed to be due to flow maldistribution within the bundle leading to local pore and flow channel occlusion. It was possible to obtain steadier TMP and standard deviation profiles when the flow regime around the fibers was more rigorously controlled by applying higher liquid and/or airflows.
2.3.2. Biomass Foulants
- Practically, based on permeability recovery,
- Mechanistically, based on fouling mechanism, and
- By material type, based on chemical or physical nature or on origin.
| Practical | Mechanism | Foulant material type |
|---|---|---|
Reversible/temporary:
Irreversible/permanent:
Irrecoverable */absolute:
| Pore blocking/filtration models (
Figure 3):
| Size:
Surface charge/chemistry:
Chemical type:
Origin:
|
2.3.2.1. Extracellular Polymeric Substances (EPS)

2.3.2.2. Selection and Evaluation of EPS Extraction Methods
2.3.2.3. EPS Quantification and Characterization
2.3.2.4. Soluble Microbial Products (SMP)
2.3.2.5. Organic Fouling by EPS
3. Prevention and Control Strategies
- 1.
- Applying appropriate pretreatment to the feedwater,
- 2.
- Employing appropriate physical or chemical cleaning protocols,
- 3.
- Reducing the flux,
- 4.
- Increasing the aeration,
- 5.
- Chemically or biochemically modifying the mixed liquor and
- 6.
- Membrane surface modification.
3.1. Feed Pretreatment
3.1.1. Screening
3.1.2. Other Feed Pretreatment Methods
3.2. Physical and Chemical Cleaning Protocols
| Definition (with Preferred Term) | Fouling Rate (mbar/min) | Time Interval | Cleaning Method Applied |
|---|---|---|---|
| Cake, reversible or removable fouling | 0.1–1 | 10 min | Physical cleaning (e.g., relaxation, backflush) |
| Residual fouling | 0.01–0.1 | 1–2 weeks | Maintenance cleaning (e.g., chemically enhanced backflush) |
| Irreversible fouling | 0.001–0.01 | 6–12 months | Chemical cleaning |
| Permanent, long-term or irrecoverable fouling | 0.0001–0.001 | Several years | Cannot be removed |
3.2.1. Physical Cleaning
3.2.2. Chemical Cleaning
- Maintenance cleaning at moderate chemical concentrations on a twice weekly to monthly basis, designed to remove residual fouling and
- Intensive (or recovery) chemical cleaning (once or twice a year), used to remove the so-called irreversible fouling.
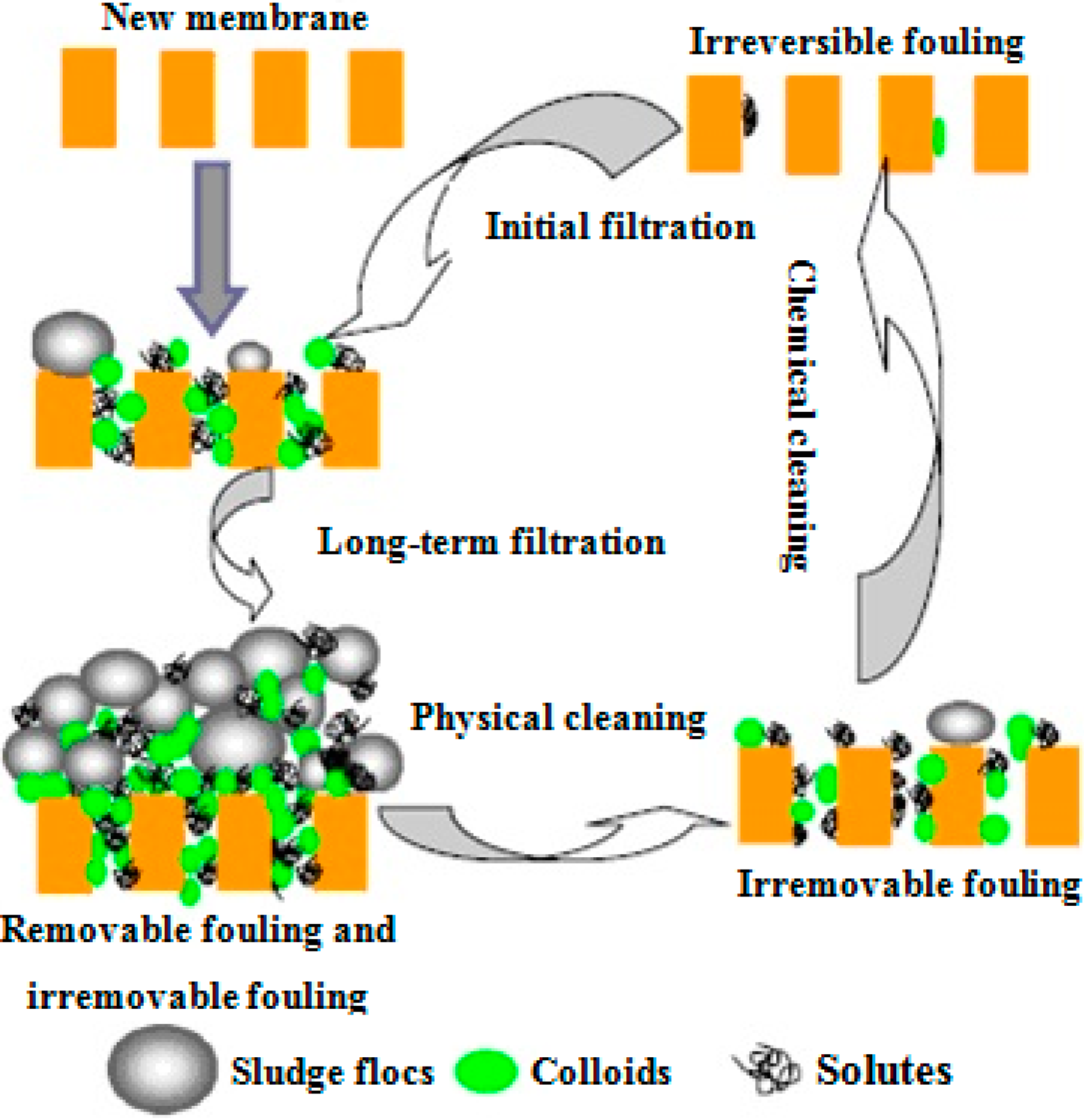
3.2.3. Feedback Control Systems
3.2.4. Chemical/Biochemical Mixed Liquor Modification
3.2.4.1. Coagulant Addition
3.2.4.2. Adsorbent Agents
3.2.4.3. Proprietary and Other Reagents
3.2.4.4. Quorum Sensing (QS)
3.2.5. Application of Ultrasound, Electric Field and Ozone
3.3. Optimal Operation of MBR Process
3.3.1. Flux Reduction
- Sustainable permeability operation: In this instance, the conditions are chosen so as to maintain stable operation (little or negligible increase in TMP at constant flux) over an extended period of time (i.e., several weeks or months) with only moderate remedial measures (namely relaxation), if any. Most immersed FS and all sidestream systems have traditionally operated under these conditions, with sMBRs operating continuously (i.e., without relaxation) between chemical cleans.
- Intermittent operation: In this mode of operation, the operational flux is above that which can be sustained by the filtration cycle operating conditions and, as a result, intermittent remedial measures are employed. These comprise relaxation supplemented with backflushing and usually some kind of maintenance chemical cleaning procedure. All immersed HF systems operate in this manner.
3.3.2. Aeration Increase
3.4. Membrane Surface Modification
3.4.1. Physical Coating/Adsorption on the Membrane Surface
3.4.1.1. Coating via Filtration
3.4.1.2. Coating via Adsorption
3.4.1.3. Coating via Casting
3.4.2. Development of Low-Fouling Polymer Membranes via Photoinitiated Grafting
3.4.3. Miscellaneous Grafting Methods on the Membrane Surface
3.4.4. Patterned Membranes
3.4.5. Plasma Treatment of Polymer Membranes
3.4.6. Chemical Reactions on the Membrane Surface for Fouling Reduction
3.4.7. Surface Modification with Nanoparticles
3.4.7.1. Membrane Modification with Deposited Nanoparticles
3.4.7.2. Phase Inversion Method

4. Conclusions
- New aeration systems
- New cleaning/fouling mitigation methods
- Emerging technologies (forward osmosis MBRs)Finally, the basic question “What should be the focus of research moving forward?”
- Will further research in the mechanisms of fouling shed some light in the efficient operation of MBRs?
- Or should research move to more macroscopic approaches such as mathematical modelling based on empirical relationships?
Table of Symbols
| AA | acrylic acid | |
| AAG | 2-acrylamidoglycolic acid | |
| AAm | acrylamide | |
| ACH | aluminium chlorohydrate | |
| AFM | atomic force spectroscopy | |
| AMPS | 2-acrylamido-methylpropane sulfonic acid | |
| anMBR | anaerobic membrane bioreactor | |
| aniMBR | anaerobic immersed membrane bioreactor | |
| AS | activated sludge | |
| ASP | activated sludge process | |
| BAC | biologically activated carbon | |
| bEPS | bound extracellular polymeric substances | |
| BFM | Berlin filtration method | |
| BSA | bovine serum albumin | |
| CA | concentration of component A | mol·m−3 |
| CA | cellulose acetate | |
| CASP | conventional activated sludge plant | |
| CER | cation exchange resin | |
| CFV | cross flow velocity | m·s−1 |
| Cg | gel layer concentration | g·cm−3 |
| CIA | cleaning in air | |
| Cim1 | concentration of component i inside membrane wall on feed side | mol·m−3 |
| Cim2 | concentration of component i inside membrane wall on permeate side | mol·m−3 |
| CIP | cleaning in place | |
| CLSM | confocal laser scanning microscopy | |
| COD | chemical oxygen demand | mg·L−1 |
| Cp | permeate concentration | g·cm−3 |
| CP | concentration polarization | |
| Cr | retentate concentration | g·cm−3 |
| CS | chitosan | |
| CST | capillary suction time | s |
| D | inside diameter of the pipe | ft |
| Di | diffusivity of component i | m2·s−1 |
| DFCm | Delft filtration characterization method | |
| DO | dissolved oxygen | |
| DOC | dissolved organic carbon | |
| DTAB | dodecyltrimethyl ammonium bromide | |
| DW | dry weight | g |
| EDA | ethylene diamine | |
| EDTA | ethylenediaminetetraacetic acid | |
| EEM | excitation-emission matrix | |
| eEPS | extracted extracellular polymeric substances | |
| EGSB | expanded granular sludge bed | |
| EPS | extracellular polymeric substances | |
| EPSc | carbohydrate fraction of EPS | |
| EPSp | protein fraction of EPS | |
| ESEM | environmental scanning electron microscopy | |
| F/M | food to microorganisms | |
| FISH | fluorescence in situ hybridization | |
| FOG | fats, oil and grease | |
| FS | flat sheet | |
| FTIR | Fourier transform infrared spectroscopy | |
| GAMA | d-gluconamidoethyl methacrylate | |
| gDMAEM | quaternized 2 (dimethylamino) ethyl methacrylate | |
| GFC | gel filtration chromatography | |
| h | height of the channel | m |
| HA | humic acid | |
| HEMA | 2-hydroxyethyl methacrylate | |
| HF | hollow fiber | |
| HFRB | hair and fiber reinforced biomass | |
| HPSEC | high-pressure size exclusion chromatography | |
| iMBR | immersed membrane bioreactor | |
| IR | infrared spectroscopy | |
| J | membrane flux | m3·m−2·h−1 |
| Ji | flux of component i | mol·m−2·s−1 |
| K | membrane permeability | L·h−1·bar−1·m−2 |
| k | mass transfer coefficient | m3·m−2·s−1 |
| L | length of the flow channel | m |
| MBR | membrane bioreactor | |
| MC | methylcellulose | |
| MF | microfiltration | |
| MLSS | mixed liquor suspended solids | |
| MPDSAH | [(methacryloylamino)propyl]-dimethyl (3-sulfopropyl) ammonium hydroxide | |
| MW | molecular weight | |
| NF | nanofiltration | |
| NMR | nuclear magnetic resonance | |
| NOM | natural organic matter | |
| NVC | N-vinyl-caprolactam | |
| NVF | N-vinyl-formamide | |
| NVP | N-vinyl-2-pyrrolidone | |
| OC | organic carbon | |
| PA | polyamide | |
| PAC | powdered activated carbon | |
| PAN | polyacrylonitrile | |
| PCR-DGGE | polymerase chain reaction denaturing gradient gel electrophoresis | |
| PDA | 2,4-phenylenediamine | |
| PE | polyethylene | |
| PEGDA | poly(ethylene glycol) diacrylate | |
| PEGMA | poly(ethylene glycol) methacrylate | |
| PEI | polyethylenimine | |
| PEO | polyethylene oxide | |
| PI | polyimide | |
| PP | polypropylene | |
| PS | polysulfone | |
| PSS | poly(sodium 4-styrene sulfonated) | |
| PVA | polyvinyl alcohol | |
| PVP | polyvinylpyrrolidone | |
| PVS | polyvinyl sulfate-potassium salt | |
| QS | quorum sensing | |
| RAS | return activated sludge | |
| Rcol | resistance attributed to colloidal matter | m−1 |
| Re | Reynolds number | |
| Rm | resistance to flow through the membrane | psi·s·cm2·cm−3 |
| Rg | resistance to flow through the gel | psi·s·cm2·cm−3 |
| RI | refractive index | |
| RO | reverse osmosis | |
| Rsol | resistance attributed to soluble matter | m−1 |
| SBR | sequencing batch reactor | |
| SDS | sodium dodecyl sulfate | |
| sEPS | soluble EPS | |
| SLS | static light scattering | |
| sMBR | submerged membrane bioreactor | |
| SMP | soluble microbial product | |
| SMPc | carbohydrate fraction of soluble microbial product | |
| SMPp | protein fraction of soluble microbial product | |
| SPMA | 3-sulfopropyl methacrylate | |
| SRF | specific resistance to filtration | |
| SRT | sludge retention time | d |
| SS | suspended solids | |
| SUVA | spectrophotometer using ultraviolet | |
| tcrit | critical time | s |
| TFC | thin film composite | |
| TIPS | thermally induced phase separation | |
| tm | thickness of the membrane | m |
| TMC | trimesoyl chloride | |
| TMP | transmembrane pressure | bar |
| TOC | total organic carbon | |
| u | fluid velocity | ft·s−1 |
| ub | linear velocity through the channel | m·s−1 |
| UF | ultrafiltration | |
| UMFI | unified membrane fouling index | |
| UV | ultraviolet | |
| VFM | VITO fouling measurement | |
| VSS | volatile suspended solids | |
| WS2 | tungsten disulfide | |
| XPS | X-ray photoelectron spectroscopy | |
| ΔP or ΔPm | pressure difference | bar |
| η | viscosity | Pa·s |
| μ | fluid viscosity | lb·ft−1·s−1 |
| ν | fluid kinematic viscosity | ft2·s−1 |
| ρ | fluid density | lb·ft−3 |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akamatsu, K.; Lu, W.; Sugawara, T.; Nakao, S.-I. Development of a novel fouling suppression system in membrane bioreactors using an intermittent electric field. Water Res. 2010, 44, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Iversen, V.; Mehrez, R.; Horng, R.Y.; Chen, C.H.; Meng, F.; Drews, A.; Lesjean, B.; Ernst, M.; Jekel, M.; Kraume, M. Fouling mitigation through flocculants and adsorbents addition in membrane bioreactors: Comparing lab and pilot studies. J. Membr. Sci. 2009, 345, 21–30. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Stec, L.Z.; Field, R.W. The effect of the extracellular matrix on the microfiltration of microorganisms. In Proceedings of the Euromembrane’95, University of Bath, Bath, UK, 8–20 September 1995; pp. 402–405.
- Chang, I.S.; Lee, C.H. Membrane filtration characteristics in membrane-coupled activated sludge system-the effect of physiological states of activated sludge on membrane fouling. Desalination 1998, 120, 221–233. [Google Scholar] [CrossRef]
- Nagaoka, H.; Yamanishi, S.; Miya, A. Modelling of biofouling by extracellular polymers in a membrane separation activated sludge system. Water Sci. Technol. 1998, 38, 497–504. [Google Scholar] [CrossRef]
- Nagaoka, H.S.; Yamanishi, S.; Miya, A. Influence of organic loading rate on membrane fouling in membrane separation activated sludge process. In Proceedings of the Membrane Technology in Environmental Management, Tokyo, Japan, 1–4 November 1999; pp. 242–249.
- Laspidou, C.S.; Rittmann, B.E. A unified theory for extracellular polymeric substances, soluble microbial products and active and inert biomass. Water Res. 2002, 36, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Nielson, P.H.; Jahn, A. Extraction of EPS; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer-Verlag: Berlin, Germany, 1999. [Google Scholar]
- Judd, S. The MBR BOOK: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Judd, S., Judd, C., Eds.; Elsevier Ltd.: Oxford, UK, 2011. [Google Scholar]
- Grace, H.P. Resistance and compressibility of filter cakes. Chem. Eng. Prog. 1956, 49, 303–318. [Google Scholar]
- Jiang, T.; Kennedy, M.D.; Guinzbourg, B.F.; Vanrolleghem, P.A.; Schippers, J.C. Optimising the operation of a MBR pilot plant by quantitative analysis of the membrane fouling mechanism. Water Sci. Technol. 2005, 51, 19–25. [Google Scholar] [PubMed]
- Kraume, M.; Wedi, D.; Schaller, J.; Iversen, V.; Drews, A. Fouling in MBR: What use are lab investigations for full scale operation? Desalination 2009, 236, 94–103. [Google Scholar] [CrossRef]
- Wu, G.; Cui, L.; Xu, Y. A novel submerged rotating membrane bioreactor and reversible membrane fouling control. Desalination 2008, 228, 255–262. [Google Scholar] [CrossRef]
- Monclùs, H.; Zacharias, S.; Pidou, M.; Santos, A.; Judd, S. Criticality of flux and aeration for a hollow fiber membrane bioreactor. Sep. Sci. Technol. 2010, 45, 956–961. [Google Scholar] [CrossRef]
- Ramos, C.; Zecchino, F.; Ezquerra, D.; Diez, V. Chemical cleaning of membranes from an anaerobic membrane bioreactor treating food industry wastewater. J. Membr. Sci. 2014, 458, 179–188. [Google Scholar] [CrossRef]
- Vanysacker, L.; Bernshtein, R.; Vankelecom, I.F.J. Effect of chemical cleaning and membrane aging on membrane biofouling using model organisms with increasing complexity. J. Membr. Sci. 2014, 457, 19–28. [Google Scholar] [CrossRef]
- Zhang, H.F.; Sun, B.S.; Zhaoa, X.H.; Gao, Z.H. Effect of ferric chloride on fouling in membrane bioreactor. Sep. Purif. Technol. 2008, 63, 341–347. [Google Scholar] [CrossRef]
- Park, D.; Lee, D.S.; Park, J.M. Continuous biological ferrous iron oxidation in a submerged membrane bioreactor. Water Sci. Technol. 2005, 51, 59–68. [Google Scholar] [PubMed]
- Chen, W.; Liu, J. The possibility and applicability of coagulation-MBR hybrid system in reclamation of dairy wastewater. Desalination 2012, 285, 226–231. [Google Scholar] [CrossRef]
- Ivanovic, I.I.; Leiknes, T.O.L. Does reduction of SMPs by addition of inorganic coagulant lead to a better biofilm-MBR performance? Proc. Eng. 2012, 44, 921–922. [Google Scholar]
- Kimura, K.; Tanaka, K.; Watanabe, Y. Microfiltration of different surface waters with/without coagulation: Clear correlations between membrane fouling and hydrophilic biopolymers. Water Res. 2014, 49, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, L.; Roddick, F.A. Feedwater coagulation to mitigate the fouling of a ceramic MF membrane caused by soluble algal organic matter. Sep. Purif. Technol. 2014, 133, 221–226. [Google Scholar] [CrossRef]
- Hu, J.; Shang, R.; Deng, H.; Heijman, S.G.J.; Rietveld, L.C. Effect of PAC dosage in a pilot-scale PAC-MBR treating micro-polluted surface water. Bioresour. Technol. 2014, 154, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, B.K.; Roddick, F.A.; Fan, L. Effect of biological activated carbon pre-treatment to control organic fouling in the microfiltration of biologically treated secondary effluent. Water Res. 2014, 63, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-H.; Ravindran, V.; Williams, M.D.; Pirbazari, M. Forecasting the performance of membrane bioreactor process for groundwater denitrification. J. Environ. Eng. Sci. 2004, 3, 507–521. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, F.-L.; Meng, F.-G.; An, P.; Wang, D. Comparison of membrane fouling during short-term filtration of aerobic granular sludge and activated sludge. J. Environ. Sci. 2007, 19, 1281–1286. [Google Scholar] [CrossRef]
- Lade, H.; Paul, D.; Kweon, J.H. Quorum Quenching Mediated Approaches for Control of Membrane Biofouling. Int. J. Biol. Sci. 2014, 10, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xia, S.; Liang, J.; Zhang, Z.; Hermanowicz, S.W. Effect of quorum quenching on the reactor performance, biofouling and biomass characteristics in membrane bioreactors. Water Res. 2013, 47, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, Y.; Zhong, C.; Li, Y.; Hao, W.; Zhu, J. The effect of quorum sensing and extracellular proteins on the microbial attachment of aerobic granular activated sludge. Bioresour. Technol. 2014, 152, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.M.; Cheong, W.S.; Oh, H.S.; Lee, W.N.; Hwang, B.K.; Lee, C.H.; Beyenal, H.; Lewandowski, Z. Quorum sensing: A new biofouling control paradigm in a membrane bioreactor for advanced wastewater treatment. Environ. Sci. Technol. 2009, 43, 380–385. [Google Scholar] [CrossRef]
- Xu, M.; Wen, X.; Huang, X.; Li, Y. Membrane fouling control in an anaerobic membrane bioreactor coupled with online ultrasound equipment for digestion of waste activated sludge. Sep. Sci. Technol. 2010, 45, 941–947. [Google Scholar] [CrossRef]
- Sui, P.; Wen, X.; Huang, X. Feasibility of employing ultrasound for on-line membrane fouling control in an anaerobic membrane bioreactor. Desalination 2008, 219, 203–213. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Gao, B.; Yang, F.; Chellam, S. Fouling reductions in a membrane bioreactor using an intermittent electric field and cathodic membrane modified by vapor phase polymerized pyrrole. J. Membr. Sci. 2012, 394–395, 202–208. [Google Scholar] [CrossRef]
- Wu, J.; Huang, X. Use of ozonation to mitigate fouling in a long-term membrane bioreactor. Bioresour. Technol. 2010, 101, 6019–6027. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Roddick, F.A. Effects of ozonation and biological activated carbon filtration on membrane fouling in ultrafiltration of an activated sludge effluent. J. Membr. Sci. 2010, 363, 271–277. [Google Scholar] [CrossRef]
- Bruening, M.L.; Dotzauer, D.M.; Jain, P.; Ouyang, L.; Baker, G.L. Creation of functional membranes using polyelectrolyte multilayers and polymer brushes. Langmuir 2008, 24, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Boributh, S.; Chanachai, A.; Jiraratananon, R. Modification of PVDF membrane by chitosan solution for reducing protein fouling. J. Membr. Sci. 2009, 342, 97–104. [Google Scholar] [CrossRef]
- Du, J.R.; Peldszus, S.; Huck, P.M.; Feng, X. Modification of poly(vinylidene fluoride) ultrafiltration membranes with poly(vinyl alcohol) for fouling control in drinking water treatment. Water Res. 2009, 43, 4559–4568. [Google Scholar] [CrossRef] [PubMed]
- Sagle, A.C.; Van Wagner, E.M.; Ju, H.; McCloskey, B.D.; Freeman, B.D.; Sharma, M.M. PEG-coated reverse osmosis membranes: Desalination properties and fouling resistance. J. Membr. Sci. 2009, 340, 92–108. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, S.; Gao, C.; Feng, X. Surface modification of thin film composite polyamide membranes by electrostatic self-deposition of polycations for improved fouling resistance. Sep. Purif. Technol. 2009, 66, 287–294. [Google Scholar] [CrossRef]
- Ba, C.; Ladner, D.A.; Economy, J. Using polyelectrolyte coatings to improve fouling resistance of a positively charged nanofiltration membrane. J. Membr. Sci. 2010, 347, 250–259. [Google Scholar] [CrossRef]
- Gullinkala, T.; Escobar, I. A green membrane functionalization method to decrease natural organic matter fouling. J. Membr. Sci. 2010, 360, 155–164. [Google Scholar] [CrossRef]
- Susanto, H.; Balakrishnan, M.; Ulbricht, M. Via surface functionalization by photograft copolymerization to low-fouling polyethersulfone-based ultrafiltration membranes. J. Membr. Sci. 2007, 288, 157–167. [Google Scholar] [CrossRef]
- Reddy, A.V.R.; Trivedi, J.J.; Devmurari, C.V.; Mohan, D.J.; Singh, P.; Rao, A.P.; Joshi, S.V.; Ghosh, P.K. Fouling resistant membranes in desalination and water recovery. Desalination 2005, 183, 301–306. [Google Scholar] [CrossRef]
- Won, Y.-J.; Choi, D.-C.; Jang, J.H.; Lee, J.-W.; Chae, H.R.; Kim, I.; Ahn, K.H.; Lee, C.-H.; Kim, I.-C. Factors affecting pattern fidelity and performance of a patterned membrane. J. Membr. Sci. 2014, 462, 1–8. [Google Scholar] [CrossRef]
- Won, W.-J.; Lee, J.; Choi, D.-C.; Chae, H.R.; Kim, I.; Lee, C.-H.; Kim, I.-C. Preparation and application of patterned membranes for wastewater treatment. Environ. Sci. Technol. 2012, 46, 11021–11027. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Won, Y.-J.; Yoo, J.H.; Ahn, K.H.; Lee, C.-H. Flow analysis and fouling on the patterned membrane surface. J. Membr. Sci. 2013, 427, 320–325. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Fabrication and characterization of a surface-patterned thin film composite membrane. J. Membr. Sci. 2014, 452, 11–19. [Google Scholar]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Critical flux of surface-patterned ultrafiltration membranes during cross-flow filtration of colloidal particles. J. Membr. Sci. 2014, 471, 65–71. [Google Scholar] [CrossRef]
- Pozniak, G.; Gancarz, I.; Tylus, W. Modified poly(phenylene oxide) membranes in ultrafiltration and micellar-enhanced ultrafiltration of organic compounds. Desalination 2006, 198, 215–224. [Google Scholar] [CrossRef]
- Dong, B.; Jiang, H.; Manolache, S.; Lee Wong, A.C.; Denes, F.S. Plasma-mediated grafting of poly(ethylene glycol) on polyamide and polyester surfaces and evaluation of antifouling ability of modified substrates. Langmuir 2007, 23, 7306–7313. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, Y.S.; Xiang, C.B. Preparation of poly(vinylidene fluoride) (PVDF) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 2005, 46, 7701–7706. [Google Scholar] [CrossRef]
- He, X.C.; Yu, H.Y.; Tang, Z.Q.; Yan, M.G.; Liu, L.Q.; Wei, X.-W. Reducing protein fouling of a polypropylene microporous membrane by CO2 plasma surface modification. Desalination 2009, 244, 80–89. [Google Scholar]
- Reddy, A.V.R.; Patel, H.R. Chemically treated polyethersulfone/polyacrylonitrile blend ultrafiltration membranes for better fouling resistance. Desalination 2008, 221, 318–323. [Google Scholar] [CrossRef]
- Gonzalez-Munoz, M.P.; Navarro, R.; Saucedo, I.; Avila, M.; Prádanos, P.; Palacio, L.; Martínez, F.; Martín, A.; Hernández, A. Hydrofluoric acid treatment for improved performance of a nanofiltration membrane. Desalination 2006, 191, 273–278. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- Luo, M.J.; Zhao, J.Q.; Tang, W.; Pu, C.S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Bae, T.H.; Tak, T.M. Interpretation of fouling characteristics of ultrafiltration membranes during the filtration of membrane bioreactor mixed liquor. J. Membr. Sci. 2005, 264, 151–160. [Google Scholar] [CrossRef]
- Bae, T.H.; Kim, I.C.; Tak, T.M. Preparation of fouling-resistant TiO2 self-assembled nanocomposite membranes. J. Membr. Sci. 2006, 275, 1–5. [Google Scholar] [CrossRef]
- Lee, H.S.; Im, S.J.; Kim, J.H.; Kim, H.J.; Kim, J.P.; Min, B.R. Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 2008, 219, 48–56. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.J.; Patel, R.; Im, S.J.; Kim, J.H.; Min, B.R. Silver nanoparticles immobilized on thin film composite polyamide membrane: Characterization, nanofiltration, antifouling properties. Polym. Adv. Technol. 2007, 18, 562–568. [Google Scholar] [CrossRef]
- Li, J.B.; Zhu, J.W.; Zheng, M.S. Morphologies and properties of poly(phthala-zinone ether sulfone ketone) matrix ultrafiltration membranes with entrapped TiO2 nanoparticles. J. Appl. Polym. Sci. 2007, 103, 3623–3629. [Google Scholar] [CrossRef]
- Li, J.F.; Xu, Z.L.; Yang, H.; Yu, L.Y.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Li, J.H.; Xu, Y.Y.; Zhu, L.P.; Wang, J.H.; Du, C.H. Fabrication and characterization of a novel TiO2 nanoparticle self-assembly membrane with improved fouling resistance. J. Membr. Sci. 2009, 326, 659–666. [Google Scholar] [CrossRef]
- Kochkodan, V.; Tsarenko, S.; Potapchenko, N.; Kosinova, V.; Goncharuk, V. Adhesion of microorganisms to polymer membranes: A photobactericidal effect of surface treatment with TiO2. Desalination 2008, 220, 380–385. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar]
- Mansourpanah, Y.; Madaeni, S.S.; Rahimpour, A.; Farhadian, A.; Taheri, A.H. Formation of appropriate sites on nanofiltration membrane surface for binding TiO2 photo-catalyst: Performance, characterization, and fouling-resistant capability. J. Membr. Sci. 2009, 330, 297–306. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Van der Bruggen, V.B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Membrane Operations: Innovative Separations and Transformations; Drioli, E.; Giorno, L. (Eds.) Wiley-VCH Verlag: Weinheim, Germany, 2009.
- Rong, M.Z.; Zhang, M.Q.; Zheng, Y.X.; Zeng, H.M.; Walter, R.; Friedrich, K. Structure-property relationships of irradiation grafted nano-inorganic particle filled polypropylene composites. Polymer 2001, 42, 167–183. [Google Scholar]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar]
- Yan, L.; Li, Y.S.; Xiang, C.B.; Xianda, S. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by ammine-functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Jafarzadeh, J.; Yegani, R. Analysis of fouling mechanisms in TiO2 embedded high density polyethylene membranes for collagen separation. Chem. Eng. Res. Design. in press.
- Lin, J.; Zhang, R.; Ye, W.; Jullok, N.; Sotto, A.; Van Der Bruggen, B. Nano-WS2 embedded PES membrane with improved fouling and permselectivity. J. Coll. Interf. Sci. 2013, 396, 120–128. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Stephenson, T.; Judd, S.; Jefferson, B.; Brindle, K. Membrane Bioreactors for Wastewater Treatment; IWA Publishing: London, UK, 2000. [Google Scholar]
- Theodore, L.; Ricci, F. Mass Transfer Operations for the Practicing Engineer; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Romero, C.A.; Davis, R.H. Experimental verification of the shear-induced hydrodynamic diffusion model of crossflow microfiltration. J. Membr. Sci. 1991, 62, 247–273. [Google Scholar] [CrossRef]
- Fane, A.G. Ultrafiltration: Factors influencing flux and rejection. Prog. Filtr. Sep. 1986, 4, 101–179. [Google Scholar]
- Howell, J.A.; Nyström, M. Membranes in Bioprocessing Theory and Applications; Howell, S.F., Ed.; Chapman and Hall: Boston, MA, USA, 1993. [Google Scholar]
- Marshall, A.D.; Munro, P.A.; Trägårdh, G. The effect of protein fouling in microfiltration and ultrafiltration on permeate flux, protein retention and selectivity: A literature review. Desalination 1993, 91, 65–82. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behaviour of suspensions and macromolecular solutions in crossflow microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Palacek, S.P.; Zydney, A.L. Intermolecular electrostatic attractions and their effect on flux and protein deposition during protein filtration. Biotechnol. Progr. 1994, 10, 207–213. [Google Scholar] [CrossRef]
- Judd, S.J.; Till, S.W. Bacteria breakthrough in crossflow microfiltration of sewage. Desalination 2000, 127, 251–260. [Google Scholar] [CrossRef]
- Kim, K.J.; Fane, A.G.; Fell, C.J.D.; Joy, D.C. Fouling mechanisms of membranes during protein ultrafiltration. J. Membr. Sci. 1992, 68, 79–91. [Google Scholar] [CrossRef]
- Meuller, J.; Davis, R.H. Protein fouling of surface-modified polymeric microfiltration membranes. J. Membr. Sci. 1996, 116, 47–60. [Google Scholar] [CrossRef]
- Kelly, S.T.; Opong, W.S.; Fell, C.J.D. Modeling fouling mechanisms in protein ultrafiltration. J. Membr. Sci. 1993, 80, 175–187. [Google Scholar] [CrossRef]
- Marshall, A.D.; Munro, P.A.; Trägårdh, G. Influence of permeate flux on fouling during the microfiltration of β-lactoglobulin solutions under cross-flow conditions. J. Membr. Sci. 1997, 130, 23–30. [Google Scholar] [CrossRef]
- Pouet, M.F.; Grasmick, A. Microfiltration of urban wastewater: The roles of the different organic fractions in fouling the membrane. In Proceedings of Euromembrane’95, University of Bath, Bath, UK, 18–20 September 1995; pp. 482–486.
- Itokawa, H.; Thiemig, C.; Pinnekamp, J. Design and operating experiences of municipal MBRs in Europe. Water Sci. Technol. 2008, 58, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- APHA (American Public Health Association). American Water Works Association and Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Chang, I.-S.; Le Clech, P.; Jefferson, B.; Judd, S. Membrane fouling in membrane bioreactors for wastewater treatment. J. Environ. Eng. ASCE 2002, 128, 1018–1029. [Google Scholar] [CrossRef]
- Chang, S.; Fane, A.G.; Vigneswaran, S. Modeling and optimizing submerged hollow fiber membrane modules. AICHE J. 2002, 48, 2203–2212. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- McAdam, E.J.; Pawlett, M.; Judd, S.J. Fate and impact of organics in an immersed membrane bioreactor applied to brine denitrification and ion exchange regeneration. Water Res. 2010, 44, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAdam, E.J.; Eusebi, A.L.; Judd, S.J. Evaluation of intermittent air sparging in an anoxic denitrification membrane bioreactor. Water Sci. Technol. 2010, 69, 2219–2225. [Google Scholar] [CrossRef]
- Pollice, A.; Brookes, A.; Jefferson, B.; Judd, S. Sub-critical flux fouling in membrane bioreactors—A review of recent literature. Desalination 2005, 174, 221–230. [Google Scholar] [CrossRef]
- Zhang, J.; Chuan, H.C.; Zhou, J.; Fane, A.G. Factors affecting the membrane performance in submerged membrane bioreactors. J. Membr. Sci. 2006, 284, 54–66. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chuan, C.H.; Zhou, J.T.; Fane, A.G. Effect of sludge retention time membrane bio-fouling intensity in a submerged membrane bioreactor. Sep. Purif. Technol. 2006, 41, 1313–1329. [Google Scholar]
- Zhang, K.; Choi, H.; Dionysiou, D.D.; Sorial, G.A.; Oerther, D.B. Identifying pioneer bacterial species responsible for biofouling membrane bioreactors. Environ. Microbiol. 2006, 8, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, F.; Liou, Y.; Zhang, X.; Yamada, Y.; Furukawa, K. Performance of a metallic membrane bioreactor treating simulated distillery wastewater at temperatures of 30 to 45 °C. Desalination 2006, 194, 146–155. [Google Scholar] [CrossRef]
- Ognier, S.; Wisniewski, C.; Grasmick, A. Influence of macromolecule adsorption during filtration of a membrane bioreactor mixed liquor suspension. J. Membr. Sci. 2002, 209, 27–37. [Google Scholar] [CrossRef]
- Ognier, S.; Wisniewski, C.; Grasmick, A. Constant flux filtration in membrane bioreactors. Membr. Technol. 2002, 2002, 6–10. [Google Scholar]
- Choi, H.; Zhang, K.; Dionysiou, D.D.; Oerther, D.B.; Sorial, G.A. Effect of permeate flux and tangential flow on membrane fouling for wastewater treatment. Sep. Purif. Technol. 2005, 45, 68–78. [Google Scholar]
- Ma, L.; Li, X.; Du, G.; Chen, J.; Shen, Z. Influence of the filtration models on colloid adsorption on the membrane in submerged membrane bioreactor. Coll. Surf. A 2005, 264, 120–125. [Google Scholar] [CrossRef]
- Cho, B.D.; Fane, A.G. Fouling transients in nominally sub-critical flux operation of a membrane bioreactor. J. Membr. Sci. 2002, 209, 391–403. [Google Scholar] [CrossRef]
- Ye, Y.; Le-Clech, P.; Chen, V.; Fane, A.G. Evolution of fouling during crossflow filtration of model EPS solutions. J. Membr. Sci. 2005, 264, 190–199. [Google Scholar] [CrossRef]
- Ognier, S.; Wisniewski, C.; Grasmick, A. Membrane bioreactor fouling in sub-critical filtration conditions: A local critical flux concept. J. Membr. Sci. 2004, 229, 171–177. [Google Scholar] [CrossRef]
- Chang, S.; Fane, A.G.; Waite, T.D. Effect of coagulation within the cake-layer on fouling transitions with dead-end hollow fiber membranes. In Proceedings of the International Congress on Membranes and Membrane Processes (ICOM), Seoul, Korea, 21–26 August 2005.
- Hermanowicz, S.W. Membrane filtration of biological solids: A unified framework and its applications to membrane bioreactors. In Proceedings of the water environment-membrane technology conference, Seoul, Korea, 7–10 June 2004.
- Yeo, A.; Fane, A.G. Performance of individual fibers in a submerged hollow fiber bundle. Water Sci. Technol. 2005, 51, 165–172. [Google Scholar] [PubMed]
- Hwang, B.K.; Lee, W.N.; Yeon, K.M.; Park, P.K.; Lee, C.H.; Chang, I.S.; Drews, A.; Kraume, M. Correlating TMP increases with microbial characteristics in the bio-cake on the membrane surface in a membrane bioreactor. Environ. Sci. Technol. 2008, 42, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.H.; Tak, T.M. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membranes for activated sludge filtration. J. Membr. Sci. 2005, 249, 1–8. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, H.; Husain, H. Identification of wastewater sludge characteristics to predict critical flux for membrane bioreactor processes. Water Res. 2006, 40, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, F.; Hua, Z.; Du, G.; Chen, J. Treatment of synthetic wastewater by a novel MBR with granular sludge developed for controlling membrane fouling. Sep. Purif. Technol. 2005, 46, 19–25. [Google Scholar] [CrossRef]
- Itonaga, T.; Kimura, K.; Watanabe, Y. Influence of suspension viscosity and colloidal particles on permeability of membrane used in membrane bioreactor (MBR). Water Sci. Technol. 2004, 50, 301–309. [Google Scholar] [PubMed]
- Teychene, B.; Guigui, C.; Cabassud, C.; Amy, G. Toward a better identification of foulant species in MBR processes. Desalination 2008, 231, 27–34. [Google Scholar] [CrossRef]
- Sommariva, C.; Comite, A.; Capannelli, G.; Bottino, A. Relationship between biofouling and recovery ratio: The theoretical approach and one experimental case. Desalination 2007, 204, 175–180. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Garland, J.; Xu, S.; Levine, L.; Bisbee, P. Deposition of extracellular polymeric substances (EPS) and microtopographical changes on membrane surfaces during intermittent filtration conditions. J. Membr. Sci. 2006, 285, 225–231. [Google Scholar] [CrossRef]
- Marselina, Y.; Le-Clech, P.; Stuetz, R.M.; Chen, V. Monitoring and Visualizing Membrane Processes; Guell, F.L., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp. 305–325. [Google Scholar]
- Yun, M.-A.; Yeon, K.-M.; Park, J.-S.; Lee, C.-H.; Chun, J.; Lim, D.-J. Characterization of biofilm structure and its effect on membrane permeability in MBR for dye wastewater treatment. Water Res. 2006, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-H.; Yang, F.-L.; Wang, W.-J.; Chen, B. Preparation and characterization of hydrophilic modification of polypropylene non-woven fabric by dip-coating PVA (polyvinyl alcohol). Sep. Purif. Technol. 2008, 61, 276–286. [Google Scholar] [CrossRef]
- Meng, F.; Yang, F. Fouling mechanisms of deflocculated sludge, normal sludge and bulking sludge in membrane bioreactor. J. Membr. Sci. 2007, 305, 48–56. [Google Scholar] [CrossRef]
- Pan, J.R.; Su, Y.C.; Huang, C.P.; Lee, H.C. Effect of sludge characteristics on membrane fouling in membrane bioreactors. J. Membr. Sci. 2010, 349, 287–294. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.S. Causes and control of filamentous growth in aerobic granular sludge sequencing batch reactors. Biotechnol. Adv. 2006, 24, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Ueda, S.; Miya, A. Influence of bacterial extracellular polymers in the membrane separation activated sludge process. Water Sci. Technol. 1996, 34, 165–172. [Google Scholar] [CrossRef]
- Rosenberger, S.; Kraume, M. Filterability of activated sludge in membrane bioreactors. Desalination 2002, 146, 373–379. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPS)—Part 1: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [PubMed]
- Flemming, H.C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Ishiguro, K.; Imai, K.; Sawada, S. Effects of biological treatment conditions on permeate flux of UF membrane in a membrane/activated sludge wastewater treatment system. Desalination 1994, 98, 119–126. [Google Scholar] [CrossRef]
- Li, X.; Yang, M.; Zhang, Y.; Liu, X.; Gao, M.; Kamagata, Y. Comparison of nitrification performance and microbial community between submerged membrane bioreactor and conventional activated sludge system. Water Sci. Technol. 2005, 51, 193–200. [Google Scholar] [PubMed]
- Jang, N.; Ren, X.; Choi, K.; Kim, I.S. Comparison of membrane biofouling in nitrification and denitrification for the membrane bioreactor (MBR). In Proceedings of the IWA-Aspire, Singapore, 10–15 July 2005.
- Rosenberger, S.; Evenblij, H.; Te Poele, S.; Wintgens, T.; Laabs, C. The importance of liquid phase analysis to understand fouling in membrane assisted activated sludge processes—Six case studies of different European research groups. J. Membr. Sci. 2005, 263, 113–126. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H.P. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.P.; Yu, H.Q.; Yu, Z. Extraction of the extracellular polymeric substances from a photosynthetic bacterium Rhodopseudomonas acidophila. Appl. Microbiol. Biotechnol. 2005, 67, 125–130. [Google Scholar] [CrossRef] [PubMed]
- D’Abzac, P.; Bordas, F.; van Hullebusch, E.; Lens, P.N.; Guibaud, G. Extraction of extracellular polymeric substances (EPS) from anaerobic granular sludges: Comparison of chemical and physical extraction protocols. Appl. Microbiol. Biotechnol. 2010, 85, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Brown, M.J.; Lester, J.N. Comparison of bacterial extracellular polymer extraction methods. Appl. Environ. Microbiol. 1980, 40, 179–185. [Google Scholar] [PubMed]
- Raunkjær, K.; Hvitved-Jacobsen, T.; Nielsen, P.H. Measurement of pools of protein, carbohydrate and lipid in domestic wastewater. Water Res. 1994, 28, 251–261. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Ma, J.; Grasmick, A.; Wu, Z. Potential Foulants and Fouling Indicators in MBRs: A Critical Review. Sep. Sci. Technol. 2013, 48, 22–50. [Google Scholar] [CrossRef]
- Zuriaga-Agusti, E.; Bes-Pia, A.; Mendoza-Roca, J.A.; Alonso-Molina, J.L. Influence of extraction methods on proteins and carbohydrates analysis from MBR activated sludge flocs in view of improving EPS determination. Sep. Purif. Technol. 2013, 112, 1–10. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Beech, I.B.; Cheung, C.W.S.; Johnson, D.B.; Smith, J.R. Comparative studies of bacterial biofilms on steel surface using atomic force microscopy and environmental scanning electron microscopy. Biofouling 1996, 10, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Logan, B. Analysis of bacterial adhesion using a gradient force analysis method and colloid probe atomic force microscopy. Langmuir 2004, 20, 8817–8822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fang, H.H.P. Distribution of extracellular polysaccharides in anaerobic granular sludges. Water Environ. Manag. 2004, 503, 153–158. [Google Scholar]
- Staudt, C.; Horn, H.; Hempel, D.C.; Neu, T.R. Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechno. Bioeng. 2004, 88, 585–592. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q. Characterization of extracellular polymeric substances of aerobic and anaerobic sludge using 3-dimensional excitation and emission matrix fluorescence spectroscopy. Water Res. 2006, 40, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Omoike, A.; Chorover, J. Spectroscopic study of extracellular polymeric substances from Bacillus subtilis: Aqueous chemistry and adsorption effects. Biomacromolecules 2004, 5, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.P.; Zhang, M.L.; Yu, H.Q. Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge. Coll. Surf. B 2008, 62, 83–90. [Google Scholar] [CrossRef]
- Liao, B.Q.; Lin, H.J.; Langevin, S.P.; Gao, W.J.; Leppard, G.G. Effects of temperature and dissolved oxygen on sludge properties and their role in bioflocculation and settling. Water Res. 2011, 45, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Chellam, S.; Gassman, P.L.; Engelhard, M.H.; Lea, A.S.; Rosso, K.M. Role of extracellular polymeric substances in bioflocculation of activated sludge microorganisms under glucose-controlled conditions. Water Res. 2010, 44, 4505–4516. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, B.O.; Santiago-Garcia, J.L.; Chan-Bacab, M.J.; Moppert, X.; Miranda-Tello, E.; Faradeau, M.L.; Carrero, J.C.; Bartolo-Pérez, P.; Valadéz-González, A.; Guezennec, J. Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J. Appl. Microbiol. 2007, 102, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Satyawali, Y.; Balakrishnan, M. Effect of PAC addition on sludge properties in an MBR treating high strength wastewater. Water Res. 2009, 43, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.P.; Yu, H.Q. Relationship between the extracellular polymeric substances and surface characteristics of Rhodopseudomonas acidophila. Appl. Microbiol. Biotechnol. 2006, 72, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Kimura, K.; Miyoshi, T.; Naruse, T.; Yamato, N.; Ogyu, R.; Watanabe, Y. The difference in characteristics of foulants in submerged MBRs caused by the difference in the membrane flux. Desalination 2008, 231, 268–275. [Google Scholar] [CrossRef]
- Kimura, K.; Yamato, N.; Yamamura, H.; Watanabe, Y. Membrane fouling in pilot-scale membrane bioreactors (MBRs) treating municipal wastewater. Environ. Sci. Technol. 2005, 39, 6293–6299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Wu, Z.C.; Tang, S.J. Extracellular polymeric substances (EPS) properties and their effects on membrane fouling in a submerged membrane bioreactor. Water Res. 2009, 43, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.T.; Kang, S.T.; Chae, S.R.; Lee, C.Y.; Bae, B.U.; Shin, H.S. Simultaneous high-strength organic and nitrogen removal with combined anaerobic upflow bed filter and aerobic membrane bioreactor. Desalination 2007, 202, 114–121. [Google Scholar] [CrossRef]
- Ahn, Y.T.; Kang, S.T.; Chae, S.R.; Lim, J.L.; Lee, S.H.; Shin, H.S. Effect of internal recycle rate on the high-strength nitrogen wastewater treatment in the combined UBF/MBR system. Water Sci. Technol. 2005, 51, 241–247. [Google Scholar] [PubMed]
- Bourven, I.; Simon, S.; Guibaud, G. Influence of extraction method on size exclusion chromatography fingerprints of EPS from wastewater sludges. Environ. Technol. 2013, 34, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Teychene, B.; Guigui, C.; Cabassud, C. Engineering of an MBR supernatant fouling layer by fine particles addition: A possible way to control cake compressibility. Water Res. 2011, 45, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Wu, Z.C.; Yin, X.; Tian, L.M. Membrane fouling in a submerged membrane bioreactor (MBR) under sub-critical flux operation: Membrane foulant and gel layer characterization. J. Membr. Sci. 2008, 325, 238–244. [Google Scholar] [CrossRef]
- Ng, C.A.; Sun, D.; Zhang, J.; Chua, H.C.; Bing, W.; Tay, S.; Fane, A. Strategies to improve the sustainable operation of membrane bioreactors. In Proceedings of International Desalination Association Conference, Singapore, 11–16 September 2005.
- Lesjean, B.; Rosenberger, S.; Laabs, C.; Jekel, M.; Gnirss, R.; Amy, G. Correlation between membrane fouling and soluble/colloidal organic substances in membrane bioreactors for municipal wastewater treatment. Water Sci. Technol. 2005, 51, 1–8. [Google Scholar] [PubMed]
- Brookes, A.; Jefferson, B.; Le-Clech, P.; Judd, S. Fouling of membrane bioreactors during treatment of produced water. In Proceedings of the International Membrane Science and Technology Conference (IMSTEC), Sydney, Australia, 10–14 November 2003.
- Evenblij, H.; van der Graaf, J. Occurrence of EPS in activated sludge from a membrane bioreactor treating municipal wastewater. Water Sci. Technol. 2004, 50, 293–300. [Google Scholar] [PubMed]
- Gao, M.; Yang, M.; Li, H.; Yang, Q.; Zhang, Y. Comparison between a submerged membrane bioreactor and a conventional activated sludge system on treating ammonia-bearing inorganic wastewater. J. Biotechnol. 2004, 108, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Lyko, S.; Wintgens, T.; Al-Halbouni, D.; Baumgarten, S.; Tacke, D.; Drensia, K.; Janot, A.; Dott, W.; Pinnekamp, J.; Melin, T.; et al. Long-term monitoring of a full-scale municipal membrane bioreactor-characterisation of foulants and operational performance. J. Membr. Sci. 2008, 317, 78–87. [Google Scholar] [CrossRef]
- Shin, H.-S.; Kang, S.-T. Characteristics and fate of soluble microbial products in ceramic membrane bioreactor at various sludge retention times. Water Res. 2003, 37, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.; Liu, X.; Judd, S.J. Sludge characteristics and membrane fouling in full-scale submerged membrane bioreactors. Desalination 2008, 219, 240–249. [Google Scholar] [CrossRef]
- Brookes, A.; Judd, S.; Reid, E.; Germain, E.; Smith, S.; Alvarez-Vasquez, H.; Jefferson, B. Biomass characterisation in membrane bioreactors. In Proceedings of the International Membrane Science and Technology Conference (IMSTEC), Sydney, Australia, 10–14 November 2003.
- Spérandio, M.; Massé, A.; Espinosa-Bouchot, M.C.; Cabassud, C. Characteriztion of sludge structure and activity in submerged membrane bioreactor. Water Sci. Technol. 2005, 52, 401–408. [Google Scholar] [PubMed]
- Reid, E.; Judd, S.; Churchouse, S. Long term fouling in membrane bioreactors. In Proceedings of the Ninth World Filtration Congress, New Orleans, LA, USA, 18–22 April 2004.
- Evenblij, H.; Geilvoet, S.; van der Graaf, J.; van der Roest, H.F. Filtration characterisation for assessing MBR performance: Three cases compared. Desalination 2005, 178, 115–124. [Google Scholar] [CrossRef]
- Grelier, P.; Rosenberger, S.; Tazi-Pain, A. Influence of sludge retention time on membrane bioreactor hydraulic performance. In Proceedings of the International Congress on Membranes and Membrane Processes (ICOM), Seoul, Korea, 7–10 June 2005.
- Tarnacki, K.; Lyko, S.; Wintgens, T.; Melin, T.; Natau, F. Impact of extracellular polymeric substances on the filterability of activated sludge in membrane bioreactors for landfill leachate treatment. Desalination 2005, 179, 181–190. [Google Scholar] [CrossRef]
- Le-Clech, P.; Jefferson, B.; Judd, S.J. A comparison of submerged and sidestream tubular membrane bioreactor configuration. Desalination 2005, 173, 113–122. [Google Scholar] [CrossRef]
- Drews, A.; Vocks, M.; Iversen, V.; Lesjean, B.; Kraume, M. Influence of unsteady membrane bioreactor operation on EPS formation and filtration resistance. Desalination 2006, 192, 1–9. [Google Scholar] [CrossRef]
- Drews, A.; Vocks, M.; Bracklow, U.; Iversen, V.; Kraume, M. Does fouling in MBRs depend on SMP? Desalination 2008, 231, 141–149. [Google Scholar] [CrossRef]
- Hernandez Rojas, M.E.; Van Kaam, R.; Schetrite, S.; Albasi, C. Role and variations of supernatant compounds in submerged membrane bioreactor fouling. Desalination 2005, 179, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.P.; Li, X.Y. Membrane fouling in a membrane bioreactor (MBR): Sludge cake formation and fouling characteristics. Biotechnol. Bioeng. 2005, 90, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.W.; Song, K.G.; Lee, S.H.; Ahn, K.H. Sequencing anoxic/anaerobic membrane bioreactor (SAM) pilot plant for advanced wastewater treatment. Desalination 2005, 178, 219–225. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, W.-Y.; Lee, C.-H. Comparison of the filtration characteristics between attached and suspended growth microorganisms in submerged membrane bioreactor. Water Res. 2001, 35, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Metzger, U.; Le-Clech, P.; Stuetz, R.M.; Frimmel, F.H.; Chen, V. Characterisation of polymeric fouling in membrane bioreactors and the effect of different filtration modes. J. Membr. Sci. 2007, 301, 180–189. [Google Scholar] [CrossRef]
- Al-Halbouni, D.; Traber, J.; Lykos, S.; Wintgens, T.; Melin, T.; Tacke, D.; Janot, N.; Dott, W.; Hollender, J. Correlation of EPS content in activated sludge at different sludge retention times with membrane fouling phenomena. Water Res. 2008, 42, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.Q.; Allen, D.G.; Droppo, I.G.; Leppard, G.G.; Liss, S.N. Surface properties of sludge and their role in bioflocculation and settleability. Water Res. 2001, 35, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.S.; Furumai, H.; Fang, H.H.P. Yields of biomass and extracellular polymers in four anaerobic sludges. Environ. Technol. 1996, 173, 283–291. [Google Scholar] [CrossRef]
- Baek, S.H.; Pagilla, K.R. Aerobic and anaerobic membrane bioreactors for municipal wastewater treatment. Water Environ. Res. 2006, 78, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Li, Y.; Noike, T.; Cha, G. Behaviour of extracellular polymers and bio-fouling during hydrogen fermentation with a membrane bioreactor. J. Membr. Sci. 2008, 322, 13–18. [Google Scholar] [CrossRef]
- Fawehinmi, F. Anaerobic MBR Treatment of a Low Strength Municipal Wastewater. Ph.D. Thesis, Cranfield University, Cranfield, UK, August 2006. [Google Scholar]
- Chu, L.; Yang, F.; Zhang, X. Anaerobic treatment of domestic wastewater in a membrane-coupled expended granular sludge bed (EGSB) reactor under moderate to low temperature. Process. Biochem. 2005, 40, 1063–1070. [Google Scholar] [CrossRef]
- Barker, D.J.; Stuckey, D.C. Modeling of soluble microbial products in anaerobic digestion: The effect of feed strength and composition. Water Environ. Res. 2001, 73, 173. [Google Scholar] [CrossRef] [PubMed]
- Noguera, D.R.; Araki, N.; Rittmann, B.E. Soluble microbial products (SMP) in anaerobic chemostats. Biotechnol. Bioeng. 1994, 44, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.; Sneve, M.A.; Parkin, G.F. Formation of soluble microbial products during anaerobic treatment. Water Environ. Res. 1996, 68, 279–285. [Google Scholar] [CrossRef]
- Massé, A.; Spérandio, M.; Cabassud, C. Comparison of sludge characteristics and performance of a submerged membrane bioreactor and an activated sludge process at high solids retention time. Water Res. 2006, 40, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Aquino, S.F.; Hu, A.Y.; Akram, A.; Stuckey, D.C. Characterization of dissolved compounds in submerged anaerobic membrane bioreactors (SAMBRs). J. Chem. Technol. Biotechnol. 2006, 81, 1894–1904. [Google Scholar] [CrossRef]
- Van Voorthuizen, E.; Zwijenburg, A.; van der Meer, W.; Temmink, H. Biological black water treatment combined with water separation. Water Res. 2008, 42, 4334–4340. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.H.; Lee, C.H. Effect of anaerobic digestion broth composition on membrane permeability. Water Sci. Technol. 1996, 34, 173–179. [Google Scholar] [CrossRef]
- Choo, K.H.; Lee, C.H. Hydrodynamic behaviour of anaerobic biosolids during crossflow filtration in the membrane anaerobic bioreactor. Water Res. 1998, 32, 3387–3397. [Google Scholar] [CrossRef]
- Lant, P.; Hartley, K. Solids characterisation in an anaerobic migrating bed reactor (AMBR) sewage treatment system. Water Res. 2007, 42, 2437–2448. [Google Scholar] [CrossRef]
- Elmitwalli, T.A.; Soellner, J.; de Keizer, A.; Bruning, H.; Zeeman, G.; Lettinga, G. Biodegradability and change of physical characteristics of particles during anaerobic digestion of domestic sewage. Water Res. 2001, 35, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Wilén, B.; Keiding, K.; Nielsen, P.H. Anaerobic deflocculation and aerobic reflocculation of activated sludge. Water Res. 2000, 34, 3933–3942. [Google Scholar] [CrossRef]
- Ince, B.K.; Ince, O.; Sallis, P.J.; Anderson, G.K. Inert COD production in a membrane anaerobic reactor treating brewery wastewater. Water Res. 2000, 34, 3943–3948. [Google Scholar] [CrossRef]
- Stuckey, D.C. The submerged anaerobic membrane bioreactor (SAMBR): An intensification of anaerobic wastewater treatment. In Proceedings of the Presentation to the Department Civil Engineering at the University of Minnesota, Minneapolis, MN, USA, 10 September 2003.
- Harada, H.; Momonoi, K.; Yamazaki, S.; Takizawa, S. Application of anaerobic-UF membrane reactor for treatment of a wastewater containing high strength particulate organics. Water Sci. Technol. 1994, 30, 307–319. [Google Scholar]
- Hu, A.Y.; Stuckey, D.C. Treatment of dilute wastewaters using a novel submerged anaerobic membrane bioreactor. J. Environ. Eng. 2006, 132, 190–198. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Feasibility of submerged anaerobic membrane bioreactor (SAMBR) for treatment of low-strength wastewater. Water Sci. Technol. 2008, 58, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Huang, X.; Qian, Y. Domestic wastewater treatment using an anaerobic bioreactor coupled with membrane filtration. Process. Biochem. 1999, 35, 335. [Google Scholar] [CrossRef]
- Ho, J.; Sung, S. Methanogenic activities in anaerobic membrane bioreactors (AnMBR) treating synthetic municipal wastewater. Bioresour. Technol. 2010, 101, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choo, K.H.; Lee, C.H. Flux enhancement with powdered activated carbon addition in the membrane anaerobic bioreactor. Sep. Sci. Technol. 1999, 34, 2781. [Google Scholar] [CrossRef]
- Imasaka, T.; Kanekuni, N.; So, H.; Yoshino, H. Cross-flow of methane fermentation broth by ceramic membrane. J. Ferment. Bioeng. 1989, 68, 200. [Google Scholar] [CrossRef]
- Frechen, F.-B.; Schier, W.; Linden, C. Pre-treatment of municipal MBR applications. In Proceedings of the Fourth IWE International Membranes Conference for Water and Wastewater Treatment, Harrogate, UK, 15–17 May 2007.
- Thompson, B.; Marlow, R. National Screen Evaluation Facility, Inlet Screen Evaluation: Year 5 Comparative Report. Available online: http://pdfs.findtheneedle.co.uk/8055-491-ACE-Screener.pdf (accessed on 30 September 2014).
- Moustafa, M.A.E. Effect of the pre-treatment on the performance of MBR, Berghausen WWTP. Germany. Alex. Eng. J. 2011, 50, 197–202. [Google Scholar] [CrossRef]
- Schoeberl, P.; Brik, M.; Bertoni, M.; Brown, R.; Fuchs, W. Optimization of operational parameters for a submerged membrane bioreactor treating dyehouse wastewater. Sep. Purif. Technol. 2005, 44, 61–68. [Google Scholar] [CrossRef]
- Smith, P.J.; Vigneswaran, S.; Ngo, H.H.; Ben-Aim, R.; Nguyen, H. Design of a generic control system for optimising backflush durations in a submerged membrane hybrid bioreactor. J. Membr. Sci. 2005, 255, 99–106. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, X.; Chen, F.; Wen, X. A dual functional filtration/aeration membrane bioreactor for domestic wastewater treatment. In Proceedings of the Water Environment-Membrane Technology Conference, Seoul, Korea, 7–10 June 2004.
- Visvanathan, C.; Yang, B.S.; Muttamara, S.; Maythanukhraw, R. Application of air backflushing technique in membrane bioreactor. Water Sci. Technol. 1997, 36, 259–266. [Google Scholar] [CrossRef]
- Chua, H.C.; Arnot, T.C.; Howell, J.A. Controlling fouling in membrane bioreactors operated with a variable throughput. Desalination 2002, 149, 225–229. [Google Scholar] [CrossRef]
- Vallero, M.V.G.; Lettinga, G.; Lens, P.N.L. High rate sulfite reduction in a submerged anaerobic membrane bioreactor (SAMBAR) at high salinity. J. Membr. Sci. 2005, 253, 217–232. [Google Scholar] [CrossRef]
- Zhang, S.T.; Qu, Y.B.; Liu, Y.H.; Yang, F.L.; Zhang, X.W.; Furukawa, K.; Yamada, Y. Experimental study of domestic sewage treatment with a metal membrane bioreactor. Desalination 2005, 177, 83–93. [Google Scholar] [CrossRef]
- Lim, B.-R.; Ahn, K.-H.; Song, K.-G.; Cho, J.W. Microbial community in biofilm on membrane surface of submerged MBR: Effect of in-line cleaning chemical agent. In Proceedings of Water Environment-Membrane Technology Conference, Seoul, Korea, 7–10 June 2004.
- Lim, A.L.; Bai, R. Membrane fouling and cleaning in microfiltration of activated sludge wastewater. J. Membr. Sci. 2003, 216, 279–290. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Shi, X. Pore fouling of microfiltration membranes by activated sludge. J. Membr. Sci. 2005, 264, 161–166. [Google Scholar] [CrossRef]
- Masselin, I.; Chasseray, X.; Durand-Bourlier, L.; Lainé, J.-M.; Syzare, P.-Y.; Lemordant, D. Effect of sonication on polymeric membranes. J. Membr. Sci. 2001, 181, 213–220. [Google Scholar] [CrossRef]
- Choo, K.H.; Kang, I.J.; Yoon, S.H.; Park, H.; Kim, J.H.; Adlya, S.; Lee, C.H. Approaches to membrane fouling control in anaerobic membrane bioreactors. Water Sci. Technol. 2000, 41, 363. [Google Scholar]
- Lee, S.M.; Jung, J.Y.; Chung, Y.C. Novel method for enhancing permeate flux of submerged membrane system in two-phase anaerobic reactor. Water Res. 2001, 35, 471. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.J.; Yoon, S.H.; Lee, C.H. Comparison of the filtration characteristics of organic and inorganic membranes in a membrane-coupled anaerobic bioreactor. Water Res. 2002, 36, 1803. [Google Scholar] [CrossRef] [PubMed]
- Brepols, C.; Drensla, K.; Janot, A.; Trimborn, M.; Engelhardt, N. Strategies for chemical cleaning in large scale membrane bioreactors. Water Sci. Technol. 2008, 57, 457–463. [Google Scholar] [PubMed]
- Busch, J.; Marquardt, W. Model-based control of MF/UF filtration processes: Pilot plant implementation and results. Water Sci. Technol. 2009, 59, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Vigneswaran, S.; Ngo, H.H.; Nguyen, H.; Ben-Aim, R. Application of an automation system and a supervisory control and data acquisition (SCADA) system for the optimal operation of a membrane adsorption hybrid system. Water Sci. Technol. 2006, 53, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, B.; Peeters, J.; Pawloski, J. On-line fouling control for energy reduction in membrane bioreactors. In Proceedings of the WEF Membrane Technology Conference, Alexandria (VA), Egypt, 27–30 January 2008.
- Joss, A.; Boehler, M.; Wedi, D.; Siegrist, H. Proposing a method for online permeability monitoring in membrane bioreactors. Water Sci. Technol. 2009, 60, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Evenblij, H.; Verrecht, B.; van der Graaf, J.H.J.M.; van der Bruggen, B. Manipulating filterability of MBR activates sludge by pulsed substrate addition. Desalination 2005, 178, 193–201. [Google Scholar] [CrossRef]
- De La Torre, T.; Iversen, V.; Moreau, A.; Stuber, J. Filtration characterization methods in MBR systems: A practical comparison. Desalin. Water Treat. 2009, 9, 15–21. [Google Scholar] [CrossRef]
- Holbrook, R.D.; Higgins, M.J.; Murthy, S.N.; Fonseca, A.D.; Fleischer, E.J.; Daigger, G.T.; Grizzard, T.J.; Love, N.G.; Novak, J.T. Effect of alum addition on the performance of submerged membranes for wastewater treatment. Water Environ. Res. 2004, 76, 2699–2702. [Google Scholar] [PubMed]
- Lee, J.C.; Kim, J.S.; Kang, I.J.; Cho, M.H.; Park, P.K.; Lee, C.H. Potential and limitations of alum or zeolite addition to improve the performance of a submerged membrane bioreactor. Water Sci. Technol. 2001, 43, 59–66. [Google Scholar] [PubMed]
- Adham, S.; de Carolis, J.F.; Pearce, W. Optimization of Various MBR Systems for Water Reclamation-Phase III. Available online: https://www.usbr.gov/research/AWT/reportpdfs/report103.pdf (accessed on 30 September 2014).
- Zhang, Y.; Bu, D.; Liu, C.-G.; Luo, X.; Gu, P. Study on retarding membrane fouling by ferric salts dosing in membrane bioreactors. In Proceedings of Water Environment-Membrane Technology Conference, Seoul, Korea, 7–10 July 2004.
- Kim, J.S.; Lee, C.H. Effect of powdered activated carbon on the performance of an aerobic membrane bioreactor: Comparison between cross-flow and submerged membrane systems. Water Environ. Res. 2003, 75, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lesage, N.; Sperandio, M.; Cabassud, C. Performances of a hybrid adsorption/submerged membrane biological process for toxic waste removal. Water Sci. Technol. 2005, 51, 173–180. [Google Scholar] [PubMed]
- Li, Y.Z.; He, Y.L.; Liu, Y.H.; Yang, S.C.; Zhang, G.J. Comparison of the filtration characteristics between biological powdered activated carbon sludge and activated sludge in submerged membrane bioreactors. Desalination 2005, 174, 305–314. [Google Scholar] [CrossRef]
- Guo, W.S.; Vigneswaran, S.; Ngo, H.H. A rational approach in controlling membrane fouling problems: Pretreatment to a submerged hollow fiber membrane system. In Proceedings of Water Environment-Membrane Technology Conference, Seoul, Korea, 7–10 June 2004.
- Cao, J.-H.; Zhu, B.-K.; Lu, H.; Xu, Y.-Y. Study on polypropylene hollow fiber based recirculated membrane bioreactor for treatment of municipal wastewater. Desalination 2005, 183, 431–438. [Google Scholar] [CrossRef]
- Tay, J.H.; Yang, P.; Zhuang, W.Q.; Tay, S.T.L.; Pan, Z.H. Reactor performance and membrane filtration in aerobic granular sludge membrane bioreactor. J. Membr. Sci. 2007, 304, 24–31. [Google Scholar] [CrossRef]
- Yoon, S.H.; Collins, J.H.; Musale, D.; Sundararajan, S.; Tsai, S.P.; Hallsby, G.A.; Kong, J.F.; Koppes, J.; Cachia, P. Effects of flux enhcaning polymer on the characteristics of sludge in membrane bioreactor process. Water Sci. Technol. 2005, 51, 151–157. [Google Scholar] [PubMed]
- Guo, W.S.; Vigneswaran, S.; Ngo, H.H.; Kandasamy, J.; Yoon, S. The role of a membrane performance enhancer in a membrane bioreactor: A comparison with other submerged membrane hybrid systems. Desalination 2008, 231, 305–313. [Google Scholar] [CrossRef]
- Iversen, V.; Koseoglu, H.; Yigit, N.O.; Drews, A.; Kitis, M.; Lesjean, B.; Kraume, M. Impacts of membrane flux enhancers on activated sludge respiration and nutrient removal in MBRs. Water Res. 2009, 43, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-P.; Yang, C.-Z.; Zhou, J.-H.; Wang, X.-Y. Study of the influence of the electric field on membrane flux of a new type of membrane bioreactor. Chem. Eng. J. 2007, 128, 177–180. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J. Improvement of membrane filterability of the mixed liquor in a membrane bioreactor by ozonation. J. Membr. Sci. 2008, 318, 210–216. [Google Scholar] [CrossRef]
- Wen, X.; Sui, P.; Huang, X. Exerting ultrasound to control the membrane fouling in filtration of anaerobic activated sludge-mechanism and membrane damage. Water Sci. Technol. 2008, 57, 773–779. [Google Scholar] [CrossRef]
- Chai, X.J.; Kobayashi, T.; Fujii, N. Ultrasound effect on cross-flow filtration of polyacrylonitrile ultrafiltration membranes. J. Membr. Sci. 1998, 148, 129–135. [Google Scholar] [CrossRef]
- Mikko, O.L.; Harold, W.W.; Linda, K.W. Mechanisms and factors influencing the ultrasonic cleaning of particle-fouled ceramic membranes. J. Membr. Sci. 2004, 237, 213–223. [Google Scholar] [CrossRef]
- Juang, R.S.; Lin, K.H. Flux recovery in the ultrafiltration of suspended solutions with ultrasound. J. Membr. Sci. 2004, 243, 115–124. [Google Scholar] [CrossRef]
- Huang, H.; Svhwab, K.; Jacangelo, J. Pretreatment for low pressure membranes in water treatment: A review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.G.; Liu, L. Application of ceramic membranes with pre-ozonation for treatment of secondary wastewater effluent. Water Res. 2009, 43, 2020–2028. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Liu, Y.; Duan, W. Ozonation pretreatment for ultrafiltration of the secondary effluent. J. Membr. Sci. 2007, 287, 187–191. [Google Scholar] [CrossRef]
- Miyashita, S.; Honjyo, K.; Kato, O.; Watari, K.; Takashima, T.; Itakura, M.; Inoue, N. Gas Diffuser for Aeration Vessel of Membrane Assembly. US Patent 6,328,886, 11 December 2001. [Google Scholar]
- Côté, P. Inverted Air Box Aerator and Aeration Method for Immersed Membrane. US Patent 6,863,823, 17 June 2002. [Google Scholar]
- Rabie, H.R.; Côté, P.; Sigh, M.; Janson, A. Cyclic Aeration System for Submerged Membrane Modules. US Patent 6,881,343, 15 October 2003. [Google Scholar]
- Fufang, Z.; Jordan, J.E. Apparatus and Method for Cleaning Membrane Filtration Modules. US Patent 6,524,481, 23 March 2001. [Google Scholar]
- Sofia, A.; Ng, W.J.; Ong, S.L. Engineering design approaches for minimum fouling in submerged MBR. Desalination 2004, 160, 67–74. [Google Scholar] [CrossRef]
- Howell, J.A.; Chua, H.C.; Arnot, T.C. In situ manipulation of critical flux in a submerged membrane bioreactor using variable aeration rates, and effects of membrane history. J. Membr. Sci. 2004, 242, 13–19. [Google Scholar] [CrossRef]
- Stone, M.; Livingstone, D. Flat plate MBR energy consumption—Village of Dundee, MI. In Proceedings of Membrane technology 2008 Conference of the Water Environment Federation, Alexandria (VA), Egypt, 27–30 January 2008.
- Ueda, T.; Hata, K.; Kikuoka, Y.; Seino, O. Effects of aeration on suction pressure in a submerged membrane bioreactor. Water Res. 1997, 31, 489–494. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modification for anti-fouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Fane, A.G.; Fell, C.J.D. A review of fouling and fouling control in ultrafiltration. Desalination 1987, 62, 117–136. [Google Scholar]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods employed for control of fouling in MF and UF membranes: A comprehensive review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar]
- Al-Amoudi, A.S.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Kato, K.; Uchida, E.; Kang, E.T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar]
- Kochkodan, V. Membrane Modification: Technology and Applications; Hilal, N., Khayet, M., Wright, C.J., Eds.; Taylor & Francis Group: New York, NY, USA, 2012. [Google Scholar]
- Li, N.; Liu, Z.; Xu, S. Dynamically formed poly(vinyl alcohol) ultrafiltration membranes with good anti-fouling characteristics. J. Membr. Sci. 2000, 169, 17–28. [Google Scholar] [CrossRef]
- Maartens, A.; Jacobs, E.P.; Swart, P. UF of pulp and paper effluent: Membrane fouling-prevention and cleaning. J. Membr. Sci. 2002, 209, 81–92. [Google Scholar]
- Reddy, A.V.R.; Mohan, D.J.; Bhattacharya, A.; Shah, V.J.; Ghosh, P.K. Surface modification of ultrafiltration membranes by preadsorption of a negatively charged polymer I. Permeation of water soluble polymers and inorganic salt solutions and fouling resistance properties. J. Membr. Sci. 2003, 214, 211–221. [Google Scholar] [CrossRef]
- Asatekin, A.; Menniti, A.; Kang, S.; Elimelech, M.; Morgenroth, E.; Mayes, A.M. Antifouling nanofiltration membranes for membrane bioreactors from self-assembling graft copolymers. J. Membr. Sci. 2006, 285, 81–89. [Google Scholar] [CrossRef]
- Asatekin, A.; Olivetti, E.A.; Mayes, A.M. Fouling resistant, high flux nanofiltration membranes from polyacrylonitrile-graft-poly(ethylene oxide). J. Membr. Sci. 2009, 332, 6–12. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, H.; Wang, S.; Xu, Z. Improvement of antifouling characteristics in a bioreactor of polypropylene microporous membrane by the adsorption of Tween 20. J. Environ. Sci. 2007, 19, 1461–1465. [Google Scholar]
- Kim, K.J.; Fane, A.G.; Fell, C.J.D. The performance of ultrafiltration membranes pretreated by polymers. Desalination 1988, 70, 229–249. [Google Scholar] [CrossRef]
- Nystrom, M. Fouling of unmodified and modified polysulfone ultrafiltration membranes by ovalbumin. J. Membr. Sci. 1989, 44, 183–196. [Google Scholar] [CrossRef]
- Brink, L.E.S.; Romijn, D.J. Reducing the protein fouling of polysulfone surfaces and polysulfone ultrafiltration membranes: Optimization of the type of presorbed layer. Desalination 1990, 78, 209–233. [Google Scholar] [CrossRef]
- Sukhishvili, S.A.; Granick, S. Layered, erasable polymer multilayers formed by hydrogen-bonded sequential self-assembly. Macromolecules 2002, 35, 301–310. [Google Scholar]
- Kharlampieva, E.; Sukhishvili, S.A. Ionization and pH stability of multilayers formed by self-assembly of weak polyelectrolytes. Langmuir 2003, 19, 1235–1243. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Ulbricht, M.; Hicke, H.G. Photomodification of ultrafiltration membranes. 1. Photochemical modification of polyacrylonitrile ultrafiltration membranes with aryl azides. Angew. Makromol. Chem. 1993, 210, 69–95. [Google Scholar] [CrossRef]
- Yamagishi, H.; Crivello, J.V.; Belfort, G. Development of a novel photochemical technique for modifying poly(arylsulfone) ultrafiltration membranes. J. Membr. Sci. 1995, 105, 237–248. [Google Scholar] [CrossRef]
- Yamagishi, H.; Crivello, J.V.; Belfort, G. Evaluation of photochemically modified poly(arylsulfone) ultrafiltration membranes. J. Membr. Sci. 1995, 105, 249–259. [Google Scholar] [CrossRef]
- Ulbricht, M. Photograft-polymer-modified microporous membranes with environment-sensitive permeabilities. React. Funct. Polym. 1996, 31, 165–177. [Google Scholar] [CrossRef]
- Ulbricht, M.; Matuschewski, H.; Oechel, A.; Hicke, H.G. Photo-induced graft polymerization surface modifications for the preparation of hydrophilic and lowprotein-adsorbing ultrafiltration membranes. J. Membr. Sci. 1996, 115, 31–47. [Google Scholar] [CrossRef]
- Pieracci, J.; Crivello, J.V.; Belfort, G. Photochemical modification of 10 kDa polyethersulfone ultrafiltration membranes for reducing of biofouling. J. Membr. Sci. 1999, 156, 223–240. [Google Scholar] [CrossRef]
- Pieracci, J.; Wood, D.W.; Crivello, J.V.; Belfort, G. UV-assisted graft polymerization of N-vinyl-2-pyrrolidone onto poly(ether sulfone) ultrafiltration membranes: Comparison of dip versus immersion modification techniques. Chem. Mater. 2000, 12, 2123–2133. [Google Scholar] [CrossRef]
- Pieracci, J.; Crivello, J.V.; Belfort, G. UV-assisted graft polymerization of N-vinyl-2-pyrrolidinone onto poly(ether sulfone) ultrafiltration membranes using selective UV wavelengths. Chem. Mater. 2002, 14, 256–265. [Google Scholar] [CrossRef]
- Ma, H.; Bowman, C.N.; Davis, R.H. Membrane fouling reduction by backpulsing and surface modification. J. Membr. Sci. 2000, 173, 191–200. [Google Scholar] [CrossRef]
- Ma, H.; Davis, R.H.; Bowman, C.N. A novel sequential photoinduced living graft polymerization. Macromolecules 2000, 33, 331–335. [Google Scholar] [CrossRef]
- Kilduff, J.E.; Mattaraj, S.; Pieracci, J.P.; Belfort, G. Photochemical modification of poly(ether sulfone) and sulfonated poly(sulfone) nanofiltration membranes for control of fouling by natural organic matter. Desalination 2000, 132, 133–142. [Google Scholar] [CrossRef]
- Kaeselev, B.; Pieracci, J.; Belfort, G. Photoinduced grafting of ultrafiltration membranes: Comparison of poly(ether sulfone) and poly(sulfone). J. Membr. Sci. 2001, 194, 245–261. [Google Scholar] [CrossRef]
- Kaeselev, B.; Kingshott, J.; Jonsson, G. Influence of the surface structure on the filtration performance of UV-modified PES membranes. Desalination 2002, 146, 265–271. [Google Scholar] [CrossRef]
- Yang, B.; Yang, W. Photografting modification of PET nucleopore membranes. J. Macromol. Sci. Part. A 2003, 40, 309–320. [Google Scholar]
- Hilal, N.; Al-Khatib, L.; Atkin, B.P.; Kochkodan, V.; Potapchenko, N. Photochemical modification of membrane surfaces for (bio)fouling reduction: A nano-scale study using AFM. Desalination 2003, 158, 65–72. [Google Scholar] [CrossRef]
- Hilal, N.; Kochkodan, V.; Al-Khatib, L.; Levadna, T. Surface modified polymeric membranes to reduce (bio)fouling: A Microbiological study using E. coli. Desalination 2004, 167, 293–300. [Google Scholar] [CrossRef]
- Taniguchi, M.; Belfort, G. Low protein fouling synthetic membranes by UV-assisted surface grafting modification: Varying monomer type. J. Membr. Sci. 2004, 231, 147–157. [Google Scholar] [CrossRef]
- Hu, M.X.; Yang, Q.; Xu, Z.K. Enhancing the hydrophilicity of polypropylene microporous membranes by the grafting of 2-hydroxyethyl methacrylate via a synergistic effect of photoinitiators. J. Membr. Sci. 2006, 285, 196–205. [Google Scholar]
- Kochkodan, V.M.; Hilal, N.; Goncharuk, V.V.; Al-Khatib, L.; Levadna, T.I. Effect of the surface modification of polymer membranes on their microbiological fouling. Colloid J. 2006, 68, 267–273. [Google Scholar] [CrossRef]
- Gu, J.-S.; Yu, H.-Y.; Huang, L.; Tang, Z.-Q.; Li, W.; Zhou, J.; Yan, M.; Wei, X.-W. Chain-length dependence of the antifouling characteristics of the glycopolymer-modified polypropylene membrane in a SMBR. J. Membr. Sci. 2009, 326, 145–152. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Zereshki, S.; Mansourpanah, Y. Preparation and characterization of modified nano-porous PVDF membrane with high antifouling property using UV photo-grafting. Appl. Surf. Sci. 2009, 255, 7455–7461. [Google Scholar] [CrossRef]
- Yu, H.; Cao, Y.; Kang, G.; Liu, J.; Li, M.; Yuan, Q. Enhancing antifouling property of polysulfone ultrafiltration membrane by grafting zwitterionic copolymer via UV-initiated polymerization. J. Membr. Sci. 2009, 342, 6–13. [Google Scholar] [CrossRef]
- Zhang, M.; Nguyen, Q.T.; Ping, Z. Hydrophilic modification of poly(vinylidene fluoride) microporous membrane. J. Membr. Sci. 2009, 327, 78–86. [Google Scholar] [CrossRef]
- Abu Seman, M.N.; Khayet, M.; Bin Ali, Z.L.; Hilal, N. Reduction of nanofiltration membrane fouling by UV-irradiated graft polymerization technique. J. Membr. Sci. 2010, 355, 133–141. [Google Scholar] [CrossRef]
- Belfer, S.; Purison, Y.; Fanshtein, R.; Radchenko, Y.; Kedem, O. Surface modification of commercial composite polyamide reverse osmosis membranes. J. Membr. Sci. 1998, 139, 175–181. [Google Scholar] [CrossRef]
- Belfer, S.; Purison, Y.; Kedem, O. Surface modification of commercial polyamide reverse osmosis membranes by radical grafting: An ATR-FT-IR study. Acta Polym. 1998, 49, 574–582. [Google Scholar] [CrossRef]
- Belfer, S.; Gilron, J.; Purison, Y.; Fainshtain, R.; Daltrophe, N.; Priel, M.; Tenzer, B.; Toma, A. Effect of surface modification in preventing fouling of commercial SWRO membranes at Eilat seawater desalination plant. Desalination 2001, 139, 169–176. [Google Scholar] [CrossRef]
- Freger, V.; Gilron, J.; Belfer, S. TFC polyamide membranes modified by grafting of hydrophilic monomers: An FT-IR/AFM/TEM study. J. Membr. Sci. 2002, 209, 283–292. [Google Scholar] [CrossRef]
- Ulbricht, M.; Belfort, G. Surface modification of ultrafiltration membranes by low temperature plasma. II Graft polymerization onto polyacrylonitrile and polysulfone. J. Membr. Sci. 1996, 111, 193–215. [Google Scholar] [CrossRef]
- Bryjak, M.; Gancarz, I.; Pozniak, G. Surface evaluation of plasma-modified polysulfone (Udel P-1700) films. Langmuir 1999, 15, 6400–6404. [Google Scholar] [CrossRef]
- Chen, H.; Belfort, G. Surface modification of poly(ether sulfone) ultrafiltration membranes by low temperature plasma-induced graft polymerization. J. Appl. Polym. Sci. 1999, 72, 1699–1711. [Google Scholar] [CrossRef]
- Gancarz, I.; Bryjak, M.; Pozniak, G. Modification of polysulfone membrane. 1. CO2 plasma treatment. Eur. Polym. J. 1999, 35, 1419–1428. [Google Scholar] [CrossRef]
- Gancarz, I.; Pozniak, G.; Bryjak, M.; Frankiewiez, A. Modification of polysulfone membranes. 2. Plasma grafting and plasma polymerization of acrylic acid. Acta Polym. 1999, 50, 317–326. [Google Scholar] [CrossRef]
- Gancarz, I.; Pozniak, G.; Bryjak, M.; Tylus, W. Modification of polysulfone membranes. 5. Effect of n-butylamine and allylamine plasma. Eur. Polym. J. 2002, 38, 1937–1946. [Google Scholar] [CrossRef]
- Gancarz, I.; Bryjak, J.; Bryjak, M.; Pozniak, G. Plasma modified polymers as a support for enzyme immobilization. 1. Allyl alcohol plasma. Eur. Polym. J. 2003, 39, 1615–1622. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, K.H.; Cho, K.; Park, C.E. Surface modification of polysulfone ultrafiltration membrane by oxygen plasma treatment. J. Membr. Sci. 2002, 199, 135–145. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, Z.; Wang, B.; Ding, F. Modification of a membrane surface charge by a low temperature plasma induced grafting reaction and its application to reduce membrane fouling. Sep. Sci. Technol. 2004, 39, 2977–2995. [Google Scholar] [CrossRef]
- Dattatray, S.; Wavhal, D.S.; Fisher, E.R. Modification of polysulfone ultrafiltration membranes by CO2 plasma treatment. Desalination 2005, 172, 189–205. [Google Scholar] [CrossRef]
- Kull, K.R.; Steen, M.L.; Fisher, E.R. Surface modification with nitrogen containing plasmas to produce hydrophilic, low-fouling membranes. J. Membr. Sci. 2005, 246, 203–215. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Li, J.; Wang, D.; Chen, C.X. Nanofiltration membrane prepared from polyacrylonitrile ultrafiltration membrane by low-temperature plasma: 4. grafting of N-vinylpyrrolidone in aqueous solution. Desalination 2005, 184, 37–44. [Google Scholar] [CrossRef]
- Yu, H.Y.; Xie, Y.J.; Hu, M.X.; Wang, J. Surface modification of polypropylene microporous membrane to improve its antifouling property in a MBR: CO2 plasma treatment. J. Membr. Sci. 2005, 254, 219–227. [Google Scholar] [CrossRef]
- Yu, H.Y.; Xu, Z.K.; Xie, Y.J.; Liu, Z.M.; Wang, S.Y. Flux enhancement for polypropylene microporous membrane in a SMBR by the immobilization of poly(N-vinyl-2 pyrrolidone) on the membrane surface. J. Membr. Sci. 2006, 279, 148–155. [Google Scholar] [CrossRef]
- Yu, H.Y.; He, X.C.; Liu, L.Q.; Gu, J.S.; Wei, X.W. Surface modification of polypropylene microporous membrane to improve its antifouling characteristics in an SMBR: N2 plasma treatment. Water Res. 2007, 41, 4703–4709. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Liu, L.Q.; Tang, Z.Q.; Yan, M.G.; Gu, J.S.; Wei, X.W. Mitigated membrane fouling in an SMBR by surface modification. J. Membr. Sci. 2008, 310, 409–417. [Google Scholar] [CrossRef]
- Yu, H.Y.; Liu, L.Q.; Tang, Z.Q.; Yan, M.G.; Gu, J.S.; Wei, X.W. Surface modification of polypropylene microporous membrane to improve its antifouling characteristics in an SMBR: Air plasma treatment. J. Membr. Sci. 2008, 311, 216–224. [Google Scholar] [CrossRef]
- Tyszler, D.; Zytner, R.G.; Batsch, A.; Brügger, A.; Geissler, S.; Zhou, H.; Klee, D.; Melin, T. Reduced fouling tendencies of ultrafiltration membranes in wastewater treatment by plasma modification. Desalination 2006, 189, 119–129. [Google Scholar] [CrossRef]
- Yan, M.G.; Liu, L.Q.; Tang, Z.Q.; Huang, L.; Li, W.; Zhou, J.; Gu, J.-S.; Wei, X.-W.; Yu, H.-Y. Plasma surface modification of polypropylene microfiltration membranes and fouling by BSA dispersion. Chem. Eng. J. 2008, 145, 218–224. [Google Scholar] [CrossRef]
- Duputell, D.; Staude, E. Heterogeneous modification of ultrafiltration membranes made from poly(vinylidene fluoride) and their characterization. J. Membr. Sci. 1993, 78, 45–51. [Google Scholar] [CrossRef]
- Tremblay, A.Y.; Tam, C.M.; Guiver, M.D. Variations in the pore size of charged and noncharged hydrophilic polysulfone membranes. Ind. Eng. Chem. Res. 1992, 31, 834–838. [Google Scholar] [CrossRef]
- Bryjak, M.; Hodge, H.; Dach, B. Modification of porous polyacrylonitrile membranes. Angew. Makromol. Chem. 1998, 260, 25–29. [Google Scholar] [CrossRef]
- Combe, C.; Molis, E.; Lucas, P.; Riley, R.; Clark, M.M. The effect of CA membrane properties on adsorptive fouling by humic acid. J. Membr. Sci. 1999, 154, 73–87. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhang, D.R.; He, Y.; Zhao, X.S.; Bai, R. Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacteria approaches. J. Membr. Sci. 2010, 346, 121–130. [Google Scholar] [CrossRef]
- Zhao, J.G.; Stevens, E.S. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 1998, 11, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.Y.; Kim, S.H.; Kim, S.S. Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwak, S.Y.; Sohn, B.H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Yu, D.G.; Teng, M.Y.; Chou, W.L.; Yang, M.C. Characterization and inhibitory effect of antibacterial PAN-based hollow fiber loaded with silver nitrate. J. Membr. Sci. 2003, 225, 115–123. [Google Scholar] [CrossRef]
- Yan, L.; Hong, S.; Li, M.L.; Li, Y.S. Application of the Al2O3-PVDF nano-composite tubular ultrafiltration (UF) membrane for oily wastewater treatment and its antifouling research. Sep. Purif. Technol. 2009, 66, 347–352. [Google Scholar] [CrossRef]
- Chou, W.L.; Yu, D.G.; Yang, M.C. The preparation and characterization of silverloading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005, 16, 600–607. [Google Scholar] [CrossRef]
- Tiede, K.; Hassellov, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B.A. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkotsis, P.K.; Banti, D.C.; Peleka, E.N.; Zouboulis, A.I.; Samaras, P.E. Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies. Processes 2014, 2, 795-866. https://doi.org/10.3390/pr2040795
Gkotsis PK, Banti DC, Peleka EN, Zouboulis AI, Samaras PE. Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies. Processes. 2014; 2(4):795-866. https://doi.org/10.3390/pr2040795
Chicago/Turabian StyleGkotsis, Petros K., Dimitra Ch. Banti, Efrosini N. Peleka, Anastasios I. Zouboulis, and Petros E. Samaras. 2014. "Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies" Processes 2, no. 4: 795-866. https://doi.org/10.3390/pr2040795
APA StyleGkotsis, P. K., Banti, D. C., Peleka, E. N., Zouboulis, A. I., & Samaras, P. E. (2014). Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies. Processes, 2(4), 795-866. https://doi.org/10.3390/pr2040795







