Computer-Aided Framework for the Design of Freeze-Drying Cycles: Optimization of the Operating Conditions of the Primary Drying Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modeling of the Effect of the Operating Conditions on Dried Cake Resistance
2.2. Experimental Investigation
2.3. Calculation of the Design Space
- Selection of the minimum and maximum values of the fluid temperature (Tfluid,min and Tfluid,max) and of the pressure in the chamber (Pc,min and Pc,max) to be considered in the design space.
- Calculation of the arrays of values of Tfluid and Pc:where ∆Tfluid and ∆Pc are, respectively, the sampling intervals for Tfluid and Pc in the design space, and nT and nP are the length of the two arrays:A grid of nT x nP points with coordinates (Tfluid,k, Pc,j) is thus defined.
- Given a couple of values of Tfluid and Pc, the evolution of the product is simulated, as previously described, thus calculating the maximum value of its temperature. If this value is lower than the limit temperature, then the selected values of Tfluid and Pc belongs to the design space.
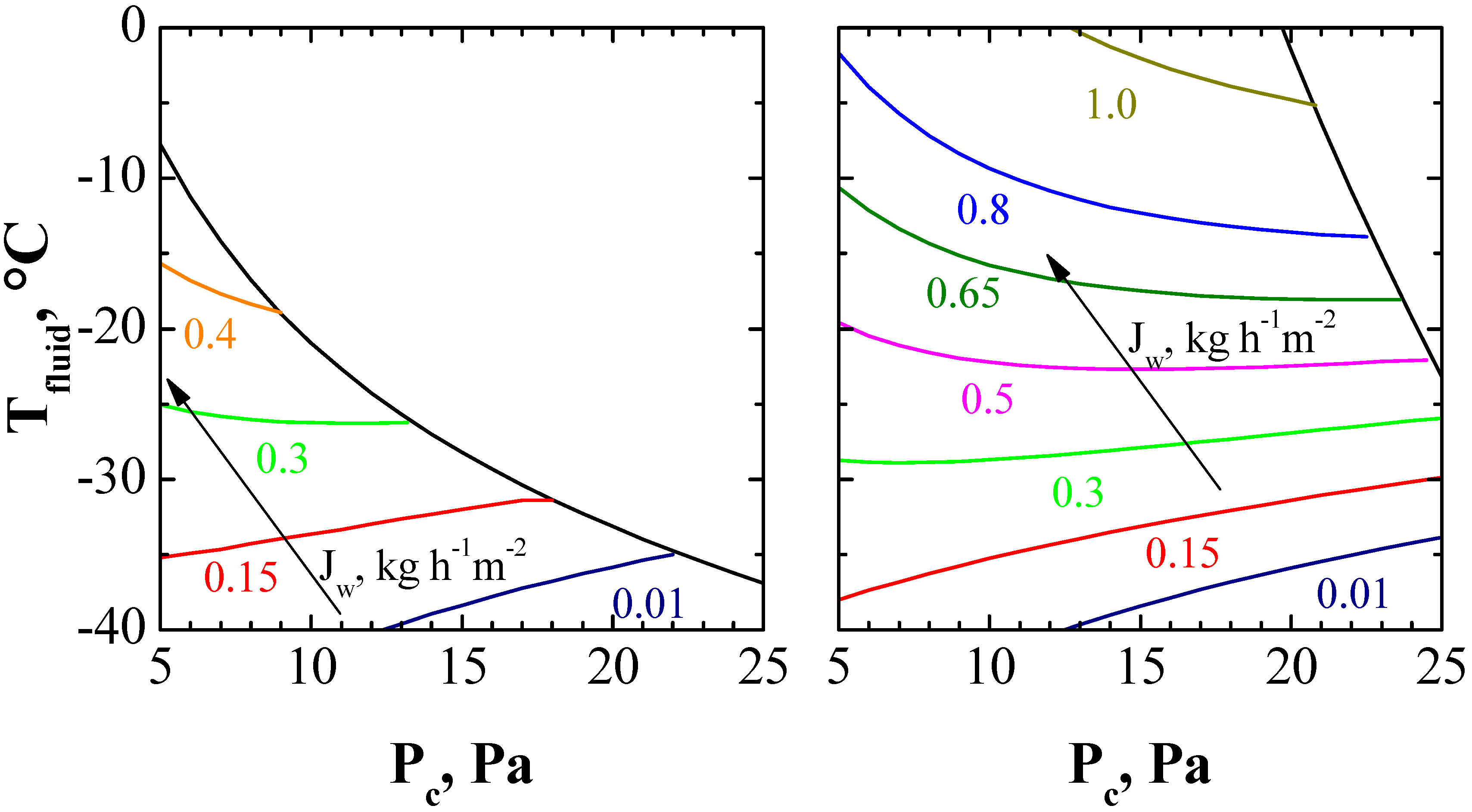
3. Results and Discussion








4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mellor, J.D. Fundamentals of Freeze-Drying; Academic Press: London, UK, 1978. [Google Scholar]
- Liapis, A.I.; Pikal, M.J.; Bruttini, R. Research and development needs and opportunities in freeze drying. Dry. Technol. 1996, 14, 1265–1300. [Google Scholar] [CrossRef]
- Jennings, T.A. Lyophilization: Introduction and Basic Principles; Interpharm/CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Oetjen, G.W. Freeze-Drying; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Franks, F. Freeze-Drying of Pharmaceuticals and Biopharmaceuticals; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Bellows, R.J.; King, C.J. Freeze-drying of aqueous solutions: Maximum allowable operating temperature. Cryobiology 1972, 9, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Tsourouflis, S.; Flink, J.M.; Karel, M. Loss of structure in freeze-dried carbohydrates solutions: Effect of temperature, moisture content and composition. J. Sci. Food Agric. 1976, 27, 509–519. [Google Scholar] [CrossRef]
- Pikal, M.J.; Shah, S. The collapse temperature in freeze drying: Dependence on measurement methodology and rate of water removal from the glassy phase. Int. J. Pharm. 1990, 62, 165–186. [Google Scholar] [CrossRef]
- Adams, G.D.J.; Irons, L.I. Some implications of structural collapse during freeze-drying using Erwinia caratovora Lasparaginase as a model. J. Chem. Technol. Biotechnol. 1993, 58, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Pikal, M.J. Freeze-drying of proteins: Process, formulation, and stability. ACS Symp. Ser. 1994, 567, 120–133. [Google Scholar]
- Franks, F. Freeze-drying of bioproducts: Putting principles into practice. Eur. J. Pharm. Biopharm. 1998, 4, 221–229. [Google Scholar] [CrossRef]
- Wang, D.Q.; Hey, J.M.; Nail, S.L. Effect of collapse on the stability of freeze-dried recombinant factor VIII and α-amylase. J. Pharm. Sci. 2004, 93, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Sadikoglu, H.; Ozdemir, M.; Seker, M. Freeze-drying of pharmaceutical products: Research and development needs. Dry. Technol. 2006, 24, 849–861. [Google Scholar] [CrossRef]
- Barresi, A.A.; Ghio, S.; Fissore, D.; Pisano, R. Freeze drying of pharmaceutical excipients close to collapse temperature: Influence of the process conditions on process time and product quality. Dry. Technol. 2009, 27, 805–816. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Pharmaceutical Development Q8 (R2). 2009. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf (accessed on 3 December 2014).
- Pikal, M.J. Use of laboratory data in freeze drying process design: Heat and mass transfer coefficients and the computer simulation of freeze drying. J. Parent. Sci. Techol. 1985, 39, 115–139. [Google Scholar]
- Sadikoglu, H.; Liapis, A.I. Mathematical modelling of the primary and secondary drying stages of bulk solution freeze-drying in trays: Parameter estimation and model discrimination by comparison of theoretical results with experimental data. Dry. Technol. 1997, 15, 791–810. [Google Scholar] [CrossRef]
- Hottot, A.; Vessot, S.; Andrieu, J. Determination of mass and heat transfer parameters during freeze-drying cycles of pharmaceutical products. PDA J. Pharm. Sci. Technol. 2005, 59, 138–153. [Google Scholar] [PubMed]
- Gan, K.H.; Crosser, O.K.; Liapis, A.I.; Bruttini, R. Lyophilisation in vials on trays: Effects of tray side. Dry. Technol. 2005, 23, 341–363. [Google Scholar] [CrossRef]
- Hottot, A.; Peczalski, R.; Vessot, S.; Andrieu, J. Freeze-drying of pharmaceutical proteins in vials: Modeling of freezing and sublimation steps. Dry. Technol. 2006, 24, 561–570. [Google Scholar] [CrossRef]
- Velardi, S.A.; Barresi, A.A. Development of simplified models for the freeze-drying process and investigation of the optimal operating conditions. Chem. Eng. Res. Des. 2008, 87, 9–22. [Google Scholar] [CrossRef]
- Milton, N.; Pikal, M.J.; Roy, M.L.; Nail, S.L. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilisation. PDA J. Pharm. Sci. Technol. 1997, 5, 7–16. [Google Scholar]
- Liapis, A.I.; Sadikoglu, H. Dynamic pressure rise in the drying chamber as a remote sensing method for monitoring the temperature of the product during the primary drying stage of freeze-drying. Dry. Technol. 1998, 16, 1153–1171. [Google Scholar] [CrossRef]
- Chouvenc, P.; Vessot, S.; Andrieu, J.; Vacus, P. Optimization of the freeze-drying cycle: A new model for pressure rise analysis. Dry. Technol. 2004, 22, 1577–1601. [Google Scholar] [CrossRef]
- Velardi, S.A.; Rasetto, V.; Barresi, A.A. Dynamic Parameters Estimation Method: Advanced Manometric Temperature Measurement approach for freeze-drying monitoring of pharmaceutical. Ind. Eng. Chem. Res. 2008, 47, 8445–8457. [Google Scholar] [CrossRef]
- Pisano, R.; Fissore, D.; Barresi, A.A. Innovation in monitoring food freeze-drying. Dry. Technol. 2011, 29, 1920–1931. [Google Scholar] [CrossRef]
- Fissore, D.; Pisano, R.; Barresi, A.A. On the methods based on the Pressure Rise Test for monitoring a freeze-drying process. Dry. Technol. 2011, 29, 73–90. [Google Scholar] [CrossRef]
- Gieseler, H.; Kessler, W.J.; Finson, M.; Davis, S.J.; Mulhall, P.A.; Bons, V.; Debo, D.J.; Pikal, M.J. Evaluation of Tunable Diode Laser Absorption Spectroscopy for in-process water vapor mass flux measurement during freeze drying. J. Pharm. Sci. 2007, 96, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Kuu, W.Y.; Nail, S.L.; Sacha, G. Rapid determination of vial heat transfer parameters using tunable diode laser absorption spectroscopy (TDLAS) in response to step-changes in pressure set-point during freeze-drying. J. Pharm. Sci. 2009, 98, 1136–1154. [Google Scholar] [CrossRef] [PubMed]
- Kuu, W.Y.; O’Bryan, K.R.; Hardwick, L.M.; Paul, T.W. Product mass transfer resistance directly determined during freeze-drying cycle runs using tunable diode laser absorption spectroscopy (TDLAS) and pore diffusion model. Pharm. Dev. Technol. 2011, 16, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Velardi, S.A.; Hammouri, H.; Barresi, A.A. In line monitoring of the primary drying phase of the freeze-drying process in vial by means of a Kalman filter based observer. Chem. Eng. Res. Des. 2009, 87, 1409–1419. [Google Scholar] [CrossRef]
- Velardi, S.A.; Hammouri, H.; Barresi, A.A. Development of a high gain observer for in-line monitoring of sublimation in vial freeze-drying. Dry. Technol. 2010, 28, 256–268. [Google Scholar] [CrossRef]
- Bosca, S.; Fissore, D. Design and validation of an innovative soft-sensor for pharmaceuticals freeze-drying monitoring. Chem. Eng. Sci. 2011, 66, 5127–5136. [Google Scholar] [CrossRef]
- Bosca, S.; Fissore, D.; Barresi, A.A. Use of soft-sensors to monitor a pharmaceuticals freeze-drying process in vials. Pharm. Dev. Technol. 2014, 19, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Bosca, S.; Barresi, A.A.; Fissore, D. Fast freeze-drying cycle design and optimization using a PAT based on the measurement of product temperature. Eur. J. Pharm. Biopharm. 2013, 85, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Bosca, S.; Corbellini, S.; Barresi, A.A.; Fissore, D. Freeze-drying monitoring using a new Process Analytical Technology: Toward a “zero defect” process. Dry. Technol. 2013, 31, 1744–1755. [Google Scholar] [CrossRef]
- Bosca, S.; Barresi, A.A.; Fissore, D. Use of a soft-sensor for the fast estimation of dried cake resistance during a freeze-drying cycle. Int. J. Pharm. 2013, 451, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, J.; Shay, Y.H.M.; Hsu, C.C.; Sane, S.U. Design space development for lyophilization using DOE and process modeling. BioPharm. Int. 2010, 23, 26–36. [Google Scholar]
- Giordano, A.; Barresi, A.A.; Fissore, D. On the use of mathematical models to build the design space for the primary drying phase of a pharmaceutical lyophilization process. J. Pharm. Sci. 2011, 100, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Koganti, V.R.; Shalaev, E.Y.; Berry, M.R.; Osterberg, T.; Youssef, M.; Hiebert, D.N.; Kanka, F.A.; Nolan, M.; Barrett, R.; Scalzo, G.; et al. Investigation of design space for freeze-drying: Use of modeling for primary drying segment of a freeze-drying cycle. AAPS PharmSciTech 2011, 12, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Fissore, D.; Pisano, R.; Barresi, A.A. Advanced approach to build the design space for the primary drying of a pharmaceutical freeze-drying process. J. Pharm. Sci. 2011, 100, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Pisano, R.; Fissore, D.; Barresi, A.A.; Brayard, P.; Chouvenc, P.; Woinet, B. Quality by Design: Optimization of a freeze-drying cycle via design space in case of heterogeneous drying behavior and influence of the freezing protocol. Pharm. Dev. Technol. 2013, 18, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.F.H.; Roseman, T.J. Lyophilization of pharmaceutical injections: Theoretical physical model. J. Pharm. Sci. 1979, 68, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Overcashier, D.E.; Patapoff, T.W.; Hsu, C.C. Lyophilization of protein formulations in vials: Investigation of the relationship between resistance to vapor flow during primary drying and small-scale product collapse. J. Pharm. Sci. 1999, 88, 688–695. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fissore, D.; Pisano, R. Computer-Aided Framework for the Design of Freeze-Drying Cycles: Optimization of the Operating Conditions of the Primary Drying Stage. Processes 2015, 3, 406-421. https://doi.org/10.3390/pr3020406
Fissore D, Pisano R. Computer-Aided Framework for the Design of Freeze-Drying Cycles: Optimization of the Operating Conditions of the Primary Drying Stage. Processes. 2015; 3(2):406-421. https://doi.org/10.3390/pr3020406
Chicago/Turabian StyleFissore, Davide, and Roberto Pisano. 2015. "Computer-Aided Framework for the Design of Freeze-Drying Cycles: Optimization of the Operating Conditions of the Primary Drying Stage" Processes 3, no. 2: 406-421. https://doi.org/10.3390/pr3020406






