1. Introduction
Diabetes mellitus collectively denotes a group of metabolic diseases with a worldwide increasing prevalence over the last decades. Diabetes is characterised by abnormally high blood glucose concentrations (hyperglycaemia) resulting from a dysfunction of insulin secretion, insulin action, or both [
1]. In diabetes, hyperglycaemia is typically characterised by blood glucose concentrations beyond 126 mg/dL (7 mmol/L) in fasting state or beyond 200 mg/dL (11.1 mmol/L) in oral glucose tolerance tests and by increased HbA1c fraction (glycated haemoglobin >6.5%). According to a study on global estimates of diabetes prevalence [
2], 382 million people in 130 countries had diabetes in 2013. This number is estimated to increase to 592 million people by 2035. A subgroup of diabetes mellitus is type 1 diabetes mellitus (T1D), which is characterised by the destruction of pancreatic
β-cells (in healthy state about 65% to 80% of the islets of Langerhans) and a subsequent lack of the hormone insulin, which is important in the blood glucose regulation. About 10% of all diabetic patients are patients with T1D [
3]. Diabetes might induce long term secondary diseases that may affect heart, vascular system, peripheral nervous system, and can lead to blindness, heart disease or limb amputation.
To this day, manual control has to be used for patients with T1D to reduce increased blood glucose levels to the normophysiological range. This is achieved by (a) a measurement of the blood glucose concentration; (b) the determination of an appropriate size of subcutaneously injected insulin bolus; and (c) the subcutaneous insulin injection. An overview of the feedback control system is presented in
Figure 1. The patient can be seen as a feedback controller acting on its own metabolic dynamics. However, manual control of blood glucose bears several severe disadvantages. The glucose metabolic system is subject to external disturbance and internal dynamics, which affect the blood glucose level after insulin bolus application. Blood glucose predictions that are made by the patients rely heavily on expert knowledge and on sparsely available blood glucose measurements. In practice, the manual blood glucose control often leads to so-called hypo- or hyperglycaemic events. In both conditions, too low or too high blood glucose concentrations, respectively, can lead to acute problems due to too low or too high blood glucose levels.
A continuous research effort towards an automatic control of blood glucose, the so-called artificial pancreas (AP), was initiated in the early 1970s. The AP consists of three main components: (i) an insulin infusion pump; (ii) a blood glucose sensor; and (iii) a feedback control algorithm that computes insulin infusion rates based on glucose sensor measurements.
Note that other additional measurements such as meal size or physical activity might be measured and integrated into the control algorithm. An overview of the history of the AP is, for example, given in [
4]. First approaches relied on intravenous blood glucose measurements and insulin application, where the main challenge is the necessary catheterisation. More recently, the subcutaneous-to-subcutaneous AP have led to a tremendous increase in research. However, this approach is associated with multiple challenges, where it is commonly agreed that a large additional time-delay in the process dynamics is most prominent. Moreover, insulin storage in the subcutaneous compartment and sudden release is associated with a variable time-delay and the risk of severe hyperinsulinaemia by some researchers [
4]. A first promising scenario for the application of AP might therefore be the intensive care unit with respect to central venous catheterised patients with blood glucose imbalances [
5].
Over the last three decades, a number of AP approaches were proposed by different research groups. A recent review on the state-of-the-art T1D control strategies is presented in [
6]. Early controllers used, for example, proportional-integral-derivative (PID) strategies. An example is a nonlinear PD-type controller presented by Clemens [
7], which was extended by moving average filtering of the measured blood glucose. However, Hernjak and Doyle [
8] concluded that a simple PD controller structure is not able to stabilise the blood glucose level around a tight operating point as the nonlinear, time-varying metabolic system is subject to external disturbances. More recent approaches to AP rather focus on modern optimal or robust control methodologies [
9], model predictive control (MPC) [
10] or the combination of feedback and feedforward control [
11,
12] (which is sometimes called two-degrees-of-freedom control).
An AP should reliably stabilise the blood glucose concentration while rejecting physical activity and meal uptake metabolic disturbances with satisfactory performance. The controlled system is nonlinear, has time-varying dynamics and shows intra-individual and inter-individual parameter uncertainties. In this article, we present a novel controller design procedure that is fully discrete. In contrast to [
9], our controller design procedure combines the advantages of classical frequency domain techniques with fixed structure controllers with modern internal model-based procedures for robust control, for example
- or
-design procedures. The central controller design is basically a discrete frequency domain disturbance rejection procedure. The design is conducted at a large number of operating points of the linearised process models. Gain scheduling of the discrete controllers is proposed on the measured blood glucose concentration and the estimated insulin sensitivity, thus guaranteeing an adaption to changing process conditions. The classical controllers are finally robustified by the discrete version of the Glover–McFarlane
-loop-shaping procedure [
13].
The AP approach in this paper is model-based and tested in an extensive in silico validation procedure with
Göttingen Minipig models that were obtained from previously conducted animal experimental studies:
In
Section 2, we briefly describe the mathematical model of a Göttingen Minipig, which is based on the Sorensen model [
14] and has already been published [
15,
16].
The controller design procedure is based on the Göttingen Minipig model and is introduced in
Section 3.
Section 4 describes the animal experimental study and the results of the model identification procedure that is the basis for the controller validation.
In
Section 5, the controller performance is evaluated in silico by means of the identified animal models and compared to the performance of a structural switching P/PI-controller that was validated in vivo [
17].
Discussion of the results and a summary are given in
Section 6.
3. Controller Design
The controller design presented in this section is based on a model linearisation, a model reduction and a discretisation. The novel design procedure consists of a classical loop-shaping design and a subsequent -loop shaping robustification. To form a gain scheduling controller by lookup table interpolation, the classical loop-shaping design is presented as an automatic procedure over a large number of operating points.
3.1. Model Linearisation
The nonlinear state space model given in Equation (
1) is trimmed and linearised with a first-order Taylor approximation. External disturbances and the full input vector are used in the trimming procedure. However, corresponding entries are neglected in the linearisation procedure. The trimmed dynamics result in
where the linearised state space model with state vector
, no external disturbances
and an input consisting only of subcutaneous insulin
is given by
with the initial state vector
and
given by the corresponding Jacobians. The model is trimmed over a range of blood glucose operating points
and insulin sensitivity values
. The set of blood glucose operating points is given by
mg/dL,
. Insulin sensitivities were varied in
mg/(min mU),
.
and
denote the integer sets of blood glucose and insulin sensitivity operating points, respectively. A total of 256 models are linearised for
and
, which are collected in set of plants
From the trimming procedure, the disturbance dynamics are obtained by linearisation around the same operating points. The linearised state space model with state
is given by
which are collected in the set of disturbance dynamics
3.2. Discrete Controller Design Prerequisites
In the first step, the set of plants
and
are discretised at a sampling time of
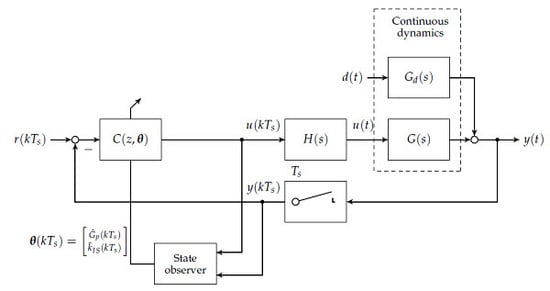
min. A block diagram showing the loop structure is given in
Figure 4.
Note that
is chosen in accordance to the animal experimental study presented in
Section 4. With respect to the linearised state space models given in minimum realisation
the discrete realisation of the plant is given by
where
denotes a zero-order hold element (
Figure 4) and
is the z-transform
of the
δ-sampled impulse response series
with the inverse Laplace-transform
. The disturbance transfer function is discretised by applying the z-transform to the impulse response of the disturbance dynamics
which corresponds to an impulse-invariant discretisation. After obtaining the discretised state space realisations
and
, the orders are subsequently reduced. Towards this end,
and
are brought to a balanced realisation by employing the state transformation
[
19], with respect to the discete-time controllability
and observability
gramians
where
is chosen such that
In Equation (
17), Λ denotes a diagonal matrix filled with (positive) square roots of the eigenvalues of
Consequently, balanced realised systems
and
are reduced to
and
, respectively, by truncation of all states that do not correspond to the maximum eigenvalue in Λ. The resulting discrete transfer functions are given by
with positive constants
,
,
and
.
Figure 5 shows a comparison of the linearised and discretised full order systems
and
and the reduced order systems
and
. The systems correspond to a blood glucose concentration of
mg/dL and an insulin sensitivity of
mg/(min mU).
3.3. Disturbance Rejection Design
This section presents the classical loop-shaping controller for disturbance rejection design. The presented procedure consists of three design stages, which are automatically computed over a large operating range of linearised and discretised models. A series interconnection of controllers , and , as a result of the three design stages, forms the classical loop-shaping controller.
3.3.1. Initial Controller Gain for Disturbance Rejection
The controller is designed for disturbance rejection. Therefore, the reference input is set to zero,
(see
Figure 4). Remaining control loop inputs and outputs are scaled with respect to their maximum possible (expected) magnitudes [
20]. Scaling factors are introduced for the error
, the process input
and the disturbance input
. The scaled and discretised plant of reduced order
and the scaled and discretised disturbance process of reduced order
are then given by
With respect to the discrete time disturbance signal
and the error of the control loop
, the objective of the controller is
with
. Assuming a worst case disturbance of
for some frequency
ω, one can obtain
which can be rewritten by introducing the sensitivity transfer function as
, with
. The requirement for disturbance rejection thus follows as
For frequencies
ω with
, it follows that
. The initial controller should, therefore, satisfy the following gain requirement
It follows from Equation (
19) that the initial controller is given by
where the superscript
s denotes the parameters of the scaled transfer functions.
3.3.2. Integral Action
In the second design phase, the initial controller is extended with integral action to reduce the remaining steady-state error of the disturbance rejection. A PI-controller
with proportional gain of
is discretised by using the Tustin approximation. With
, the discrete PI-controller is given by
The value of
is determined by employing the phase margin. Candidate integral gains
are obtained in the log space by the set
with the initial, possibly negative exponent
, the maximum exponent
and the number
of candidate integral gains to be evaluated. The phase margin
is then determined over the set of candidate gains
for the full order open-loop compensated plant
where the largest gain
with a minimum phase margin of
was chosen. The resulting controller has a relatively large bandwidth but an insufficiently low gain margin.
3.3.3. Lead–Lag Compensator
The third and final step of the classical loop-shaping procedure therefore consists of the design of a lead-lag compensator to increase the phase margin. A Tustin approximated lead-lag discrete compensator is used that has the form
Time constants
and
correspond to the lead and the lag pole, respectively. By denoting the frequency of the previously determined phase margin of Equation (
28)
, the time-constant of the lead part is set
. Furthermore, the time-constant of the lag part is set to
. The maximum phase increase occurs at
with a positive phase of
. Note that the phase margin is increased by this value. However, the GM is reduced by the additional increase in gain. The classical loop-shaping procedure is repeatedly executed over the whole set of plants
and
. Hence, the total number of 256 controllers for each
,
and
are determined automatically over the whole range of sampled operating points.
3.4. Robust Loop-Shaping -Controller
The robust loop-shaping
-controller follows the approach of Walker [
13] and increases the robustness of the shaped loop with regard to discrete, not further specified, right coprime factor uncertainty. Given the discrete compensated plant in a normalised right coprime factorisation
a family of coprime factor uncertain plants can be described by
where
provide a fairly general type of dynamic uncertainty, which allows both poles and zeros to cross out of the unit circle in the z-domain. For the generalised plant P(z) given in the form of a normalised left coprime factorisation
the application of the small gain theorem (see for example [
21])
ensures robust stability with respect to the normalised coprime factor perturbed family of plants
. Here, the optimal achieved performance by the
-norm
provides a measure of tolerable right coprime factor with respect to Equation (
31). An internally stabilising controller is obtained given a minimal discrete state space realisation of
and the solution of an indefinite discrete time algebraic Riccati equation. The central loop-shaping
-controller
is employed in the overall mixed classical-robust controller
Figure 6 presents a bode plot comparing the open compensated loop with the classical controllers
from Equation (
30) and the compensated loop with the robust controller
. It can be clearly seen that the gain and phase margins are increased from
dB and
with the classical loop-shaping controller to
dB and
by the robustifying approach, respectively.
This corresponds to a reduction of peak sensitivity measure , with . The peak sensitivity measure is reduced from dB to dB. An was accomplished by the the robustification procedure.
Note that only one robust -compensator is designed. For the design, a model at an operating point corresponding to a blood glucose concentration of mg/dL and an insulin sensitivity of mg/(min mU) is chosen. Stability is determined by analysing classical gain and phase margin, as well as sensitivity peak measure at every operating point of . The resulting minimum gain and phase margin are computed as dB and . The maximum sensitivity peak was determined as dB. As a consequence, it is concluded that only one -compensator is enough to robustly stabilise the set of plants . Note that an additional sensor time-delay of about 2 min was neglected in the control design prodecure, as it was considered to be sufficiently small in comparison to the sampling time of 15 min. The additional time-delay is due to the time it takes from withdrawing venous blood until a sensor value is obtained. At the crossover frequency of the compensated open-loop of 0.02 rad/min, the time-delay leads to an additional phase lag of −2.3 deg.
6. Conclusions and Discussion
A novel discrete blood glucose control design procedure was developed that is focused on disturbance rejection of meal uptake. The procedure combines the advantages of classical loop-shaping control design with modern robust control design. Based on the linearisation of the detailed and accurate minipig model, the controller design is automatically conducted for a large operating range of trimmed models at varying blood glucose concentrations and insulin sensitivities. The family of gain scheduled controllers was then robustified with a single discrete loop-shaping controller. Classical gain and phase margin, as well as -norm peak sensitivity measures computed for the family of linear models, indicate strong stability properties.
The gain scheduled controller was tested in silico by using the five different full order nonlinear minipig models. Controller implementation was realised with the help of an augmented EKF that estimates insulin sensitivity as a system state. The controller was able to robustly stabilise the family of animal experimental models where additional uncertainty in terms of time-varying insulin sensitivity was included. A comparison with a previously designed classical PI-controller showed superior performance of the new control strategy.
The controller in its current form was implemented without anti-windup protection of the discrete integrator. Moreover, the performance of the gain scheduled controller depends on the accuracy of the insulin sensitivity estimator. As meal uptake and insulin sensitivity cannot be estimated at the same time, due to observability problems, the size of a meal uptake is considered in the estimator by regarding the forward dynamics. Since the subcutaneous application of insulin is considered as uncertain by different authors, a first suitable application of the proposed discrete blood glucose controller seems to be an intensive care unit glucose control scenario. Here, the intravenous-to-intravenous route minimises control input uncertainties, time-delay, and blood glucose sampling time. In its current form, the presented controller design procedure can be readily applied to this scenario, if a suitable model of the process dynamics is available, which can be linearised over the range of blood glucose operating points and insulin sensitivity values. Furthermore, the controller extension with a forward term regarding meal uptake disturbance compensation like, for example, in [
12] is possible. The application of the controller in a clinical scenario will be the subject of future work.